Abstract
In recent years, the brain's “default network,” a set of regions characterized by decreased neural activity during goal-oriented tasks, has generated a significant amount of interest, as well as controversy. Much of the discussion has focused on the relationship of these regions to a “default mode” of brain function. In early studies, investigators suggested that, the brain's default mode supports “self-referential” or “introspective” mental activity. Subsequently, regions of the default network have been more specifically related to the “internal narrative,” the “autobiographical self,” “stimulus independent thought,” “mentalizing,” and most recently “self-projection.” However, the extant literature on the function of the default network is limited to adults, i.e., after the system has reached maturity. We hypothesized that further insight into the network's functioning could be achieved by characterizing its development. In the current study, we used resting-state functional connectivity MRI (rs-fcMRI) to characterize the development of the brain's default network. We found that the default regions are only sparsely functionally connected at early school age (7–9 years old); over development, these regions integrate into a cohesive, interconnected network.
Keywords: connectivity, development, fMRI, functional
A set of brain regions with consistently decreased neural activity during goal-oriented tasks was highlighted 10 years ago by Shulman et al. (1). In recent years, this collection of regions, often termed the “default network” (2, 3), has generated a significant amount of interest, as well as controversy (4–8). Much of the discussion has focused on the relationship of this set of regions to a “default mode” of brain function (2–4, 9).
In early studies, investigators suggested that in adults, the brain's default mode supports “self-referential” or “introspective” mental activity (3). Subsequently, regions of the default network, in particular the medial prefrontal cortex (mPFC), have been more specifically related to the internal “narrative” (3), the “autobiographical” self (3, 10), “stimulus independent thought” (3, 7), “mentalizing” (11, 12), and most recently “self-projection” (10), which includes aspects of prospection, episodic memory, and “theory of mind” (13). The variety of perspectives attributing specific functions to the default network underscores the present uncertainty in the field. Although many argue that the default network is in some way related to internally directed mental activity (3, 7, 10), this argument is not universally accepted (5, 6, 14, 15).
The extant literature on the function of the default system is driven by data derived from studying adults, i.e., after the system has reached maturity. We hypothesized that further insight into the functioning of this network could be achieved through characterizing its development. For example, if a functionally interconnected default system is required for internally directed mental activity, then it stands to reason that the default system should demonstrate a mature, or near mature, pattern of functional connectivity at a time in development when internally directed mental activity is demonstrable.
Although the developmental literature in cognitive psychology and neuroscience related to internally directed mental activity is still evolving, many studies suggest that some functions, such as episodic memory, theory of mind, and the ability to “mentalize,” are present by early school age (10, 12, 13, 16, 17). For example, although a child's capacity for episodic memory (e.g., encoding and retrieving information) continues to improve through adolescence, some suggest that such improvements are related to the incorporation of increasingly more complex strategies (16, 17)—meaning that early school-age children are capable of encoding and retrieving stored information. Although preschoolers “tend to be very poor at recalling or reconstructing both the fact and the content of their own recent or present thinking,” by the age of ≈8 years, such introspection is fairly routine (13). If the default network is required for such introspection (i.e., episodic memory, theory of mind, mentalizing, etc.), it should be relatively intact at an age when these abilities are observable. If the mature default network architecture has yet to emerge by early school age, then perhaps a functionally interconnected default network relates to, but is not required for, performing the above-proposed functions.
Greicius et al. (9) were the first to show that spontaneous blood–oxygen level-dependent (BOLD) activity in adults is highly correlated in the default network (i.e., the default regions are functionally connected). This result has been replicated multiple times in the mature system (18, 19) and is shown again here (for regions and coordinates, see Table 1).
Table 1.
Seed regions for default/task negative network (18)
| Regions | Abbreviations | Coordinates (x, y, z) |
|---|---|---|
| Medial prefrontal cortex (ventral) | mPFC (ventral) | −3, 39, −2 |
| Medial prefrontal cortex (anterior) | mPFC (anterior) | 1, 54, 21 |
| Posterior cingulate cortex | Post. cingulate | −2, −36, 37 |
| Left lateral parietal cortex | L lat. parietal | −47, −67, 36 |
| Right lateral parietal cortex | R lat. parietal | 53, −67, 36 |
| Left superior frontal cortex | L sup. frontal | −14, 38, 52 |
| Right superior frontal cortex | R sup. frontal | 17, 37, 52 |
| Left inferior temporal cortex | L inf.temporal | −61, −33, −15 |
| Right inferior temporal cortex | R inf.temporal | 65, −17, −15 |
| Left parahippocampal gyrus | L parahippocampus | −22, −26, −16 |
| Right parahippocamal gyrus | R parahippocampus | 25, −26, −14 |
| Cerebellar tonsils | Cerebellar tonsils | 7, −52, −44 |
| Retrosplenial | Retrosplenial | 3, −51, 8 |
However, in the current study, we used resting-state functional connectivity MRI (rs-fcMRI) and graph-theory methods to characterize the development of the default network [for a detailed description of this method and its utility, see supporting information (SI) Text and ref. 20].
Results
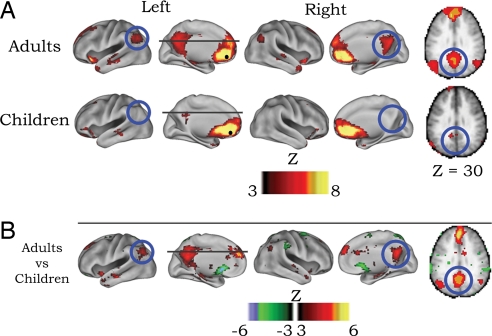
To investigate the emergence of temporally correlated neural activity between regions of the default network, two types of analyses were carried out. First, voxelwise functional connectivity maps were generated. This voxelwise approach involved cross-correlating the BOLD signal time series for a region of interest [ventral mPFC (−3, 39, −2); defined by Fox et al. (18); also see Table 1] with all other voxels in the brain for both children and adults. The voxelwise approach (see SI Text) provided three different types of statistical maps: an adult map, a child map, and a map representing the direct statistical comparison (i.e., random effects model) between children and adults for selected seed regions (see Fig. 1). Voxelwise functional connectivity maps for the ventral mPFC (−3, 39, −2), an important constituent of the default network, are presented in Fig. 1. These maps revealed two main findings. First, in adults, as previously described (9, 18, 19), the ventral mPFC was strongly correlated (i.e., functionally connected) with other regions of the default network, such as the posterior cingulate and lateral parietal cortex. Second, connections of the ventral mPFC with posterior cingulate and parietal default regions were minimal in the child group compared with the adult group.
Fig. 1.
Voxelwise resting-state functional connectivity maps for a seed region (solid black circle) in mPFC (ventral: −3, 39, −2). (A) Qualitatively, the rs-fcMRI map for the mPFC (ventral) seed region reveals the commonly observed adult connectivity pattern of the default network (9, 18, 19). The connectivity map in children, however, significantly deviates from that of the adults. Functional connections with regions in the posterior cingulate and lateral parietal regions (highlighted with blue open circles) are present in the adults but absent in children. (B) These qualitative differences between children and adults are confirmed by the direct comparison (random effects) between adults and children. mPFC (ventral) functional connections with the posterior cingulate and lateral parietal regions are significantly stronger in adults than children.
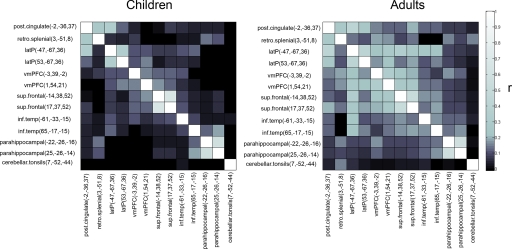
The second, more comprehensive, analysis used graph-theory methods (refs. 21 and 22). For the graph analyses, the resting-state BOLD time series for each of the 13 default and associated “task-negative” regions (18) was correlated with the resting-state BOLD time series of every other region. This approach generated one adult and one child correlation matrix (Fig. 2), which were then represented as graphs (Fig. 3). For direct between-group comparisons (i.e., child vs. adult), two-sample two-tailed t tests (assuming unequal variance; P ≤ 0.05; multiple comparisons corrected) were performed on all potential connections represented in the individual 13 × 13 correlation matrices. Connections that showed a significant difference between children and adults were also fit with LOWESS curves (a local regression smoothing procedure) (Fig. 3 and SI Fig. 4).
Fig. 2.
Correlation matrices representing default network functional connectivity for children and adults. Darker boxes represent low-correlation coefficients between regions, whereas lighter boxes represent stronger correlations. Qualitatively, adults have stronger correlation coefficients between regions of the default network than children do. The direct comparisons (see Fig. 3) between children and adults confirm this observation.
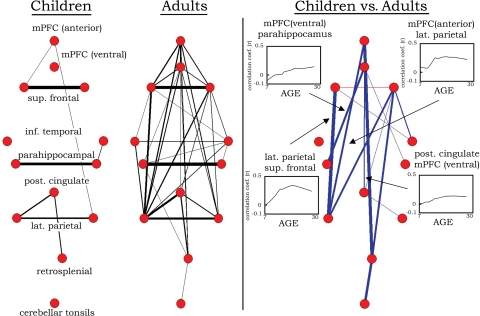
Fig. 3.
Graph visualization of the correlation matrices shown in Fig. 2 represented in a pseudoanatomical organization. Default regions (18) are only very sparsely connected in children aged 7–9 years. In adults aged 21–31 years, the default regions are highly integrated (strongly functionally connected). Only connections with r > 0.15 are shown at Left (line width is proportional to the connection strength). Statistically significant differences in functional connectivity between children and adults are highlighted on the Right. Blue lines represent significantly greater functional connectivity (r) in adults than in children. One connection (between superior frontal regions), although present in both groups, was significantly greater in children than in adults (red line). For the direct comparison, line width is proportional to the significance level (i.e., increased level of statistical significance; all lines meet P < 0.05 multiple comparisons corrected). Selected LOWESS curves for significant connections are also presented showing correlation coefficients (r) between regions as a function of age. For the full complement of LOWESS curves, see SI Fig. 4.
The graph approach revealed that the default network is only sparsely connected in children aged 7–9 years (see Figs. 2 and 3). In adults, however, the default network and associated regions are highly integrated (i.e., strongly functionally connected). Statistically significant differences between children and adults are shown in Fig. 3 along with selected LOWESS curves, which show the continuous increase in correlation strength over age between regions of the default network.
The developmentally dynamic nature of these connectional relationships is well illustrated by a movie that shows changes in functional connections over age (see SI Movie 1). The movie uses a spring-embedding algorithm (23) to demonstrate the evolving “natural” state of the network graph over developmental time.
Discussion
The principal finding in this report is that the default-network structure in children deviates significantly from the adult architecture. As in adults, interhemispheric functional connections between homotopic regions in the contralateral hemisphere appear to be relatively strong in children; yet, overall the network is only sparsely connected (i.e., fragmented). Over age, however, the default network becomes significantly more integrated.
One potential cause for the significant increase in correlation strengths in adults versus children is that correlation strengths in children simply tend to be more variable and less likely to be statistically significant. This explanation is unlikely to account for the present results for three reasons. First, a previous publication, using the same subjects, examining the brain's separate adaptive control and set-maintenance networks, has shown both increases in correlation strengths and decreases in correlation strength between regions over age (22). Second, the LOWESS curves (Fig. 3) show continued increases in correlation strength over age, suggesting that the significant differences between children and adults reported here are not solely the consequences of developmental changes in variability. Last, a recent publication by Fransson et al. (24) using independent component analysis in sleeping preterm infants (studied at term equivalent) failed to find a complete default network, suggesting that spontaneous activity in the default network does, indeed, undergo developmental change. However, despite this consistency, it should be noted that independent component analysis (ICA) and correlation analysis do not always reveal identical results (25). In addition, although these two methods (each with their own pros and cons; see ref. 20) often reveal similar rs-fcMRI findings in adults (25, 26), these findings remain to be demonstrated in children.
Of note, the default-network regions used in this study were all derived from adult imaging studies. Hence, it is possible that although the “adult” default network is not yet “assembled,” a distinct child version of the default network may exist. We think this caveat is rather unlikely, because the locations of task-induced deactivations (i.e., default regions) do not appear to differ substantially between children and young adults (e.g., ref. 27).
Assuming that the developmental changes described here represent real functional connectional change, the issue then becomes the neurobiological significance of the change. One likely contributor to the increases in correlation strength between distant regions over age is myelination. Although by ≈9 months of age long-range anatomical connectivity is adult-like (28), myelination continues into young adulthood (29). Increased efficiency of signal propagation with the elaboration of the myelin sheath may support the functional integration (i.e., stronger functional connectivity) of distant regions (22, 30, 31). Such findings are supported by resting EEG studies demonstrating that signal coherence in the alpha rhythm between posterior and anterior electrodes increases with age (32). However, our finding that changes in interhemispheric correlations were minimal from ≈7–9 years into adulthood, despite significant development of the corpus callosum into young adulthood (33, 34), suggests that elaboration of the myelin sheath is unlikely to be the sole variable involved. Consistent with this notion, as previously described (22), data by Honey and colleagues (35) suggest that spontaneous activity itself may, in part, account for the changes in interregional correlations (35). In their recent publication, Honey et al. (35) simulated spontaneous neuronal firing (millisecond timescale) for a network of nodes only constrained by the known anatomical connections of macaque neocortex. Complex spatial and temporal patterns of synchronous activity developed over time in the absence of external input and without changes in synaptic strengths (35). These findings suggest that a process of “integration through synchronization” may partially underlie the development of various brain networks (22, 36), including the default network described here.
A related account corresponds to the nature of correlated spontaneous BOLD fluctuations. A reasonable hypothesis regarding correlated BOLD activity is that it may, at least in part, reflect a longstanding history of regional coactivation (21, 22, 37). In this sense, coactivation, with experience and maturation, can lead to a Hebbian strengthening of the functional connections between brain regions (38, 39). Presumably, this strengthening is a gradual process, such that temporally correlated spontaneous activity in functionally intact, coactivating regions may only manifest in a “delayed” fashion (i.e., over several years).
Our next consideration regards the functional significance of the observed default-network development. As discussed in the Introduction, children are capable of performing many of the introspective abilities, such as episodic memory, mentalizing, and theory of mind, commonly attributed to the default network (10, 12, 13, 16, 17). As such, the present findings that demonstrate large-scale developmental changes in default-network architecture that continue well into young adulthood provoke fundamental questions: What does the development of correlated intrinsic activity within the default network signify? How does the development of the default network relate to currently held notions regarding this network's functions?
Potentially, although it may not be necessary for the default network to have a mature connectivity structure for it to carry out, at least partially, some of the functions attributed to it, the continued improvement over age of these functions may relate to the ongoing maturation of the default network architecture. For example, as mentioned in the Introduction, a child's basic ability to encode and retrieve stored information seems to be in place by early school age (16, 17); however, performance on episodic memory tasks continues to improve over age. Some have argued that this improvement is not solely due to improved abilities of encoding and retrieval per se, but is rather related to the incorporation of increasingly more complex strategies to encode and retrieve stored information (16, 17). Because task-induced deactivations resemble those of the default network in children (27), the results observed here may suggest that the default regions are intact and functioning at relatively young ages. However, the progressive integration of the default network may support the ability to incorporate a growing number of alternative strategies, or higher-order strategic organization, to improve memory processes over age (17). Future work that demonstrates a direct relationship with this behavior and the developmental trajectory seen here with rs-fcMRI will be required to confirm (or reject) this consideration (also see SI Text for a complementary speculation that relates these findings to schema development).
Another account concerns the relationship between the default network and a set of regions commonly activated during task states (40). In previous publications, we have noted that many of the regions that are negatively correlated with the default network (18, 19) belong to two separate networks (frontoparietal and cinguloopercular) proposed to implement task-level control of goal-oriented “external” behavior (refs. 21, 22, and 40; also see ref. 41 for review). The frontoparietal network includes, among other regions, the dorsolateral prefrontal cortex (dlPFC) and intraparietal sulcus (IPS). The cinguloopercular network includes the dorsal anterior cingulate/medial superior frontal cortex (dACC/msFC), anterior insula/frontal operculum (aI/fO), and anterior prefrontal cortex (aPFC). Over age, these task-level control networks show significant developmental change in functional connectivity (22) that, in some ways, parallels the development of the default network described here. In addition, recent work on the other end of the life span suggests that increased functional coherence between the regions of the default network is related to superior executive functions in healthy aging (42). Thus, the parallel development and close relationship (albeit negative) between the default and task control networks may be important for the age-related improvements in control processes such as alerting, inhibition, set switching, and set maintenance observed over development (30, 43–48).
Methods
Data Acquisition.
fMRI data were acquired on a Siemens 1.5 Tesla MAGNETOM Vision system. Structural images were obtained by using a sagittal magnetization-prepared rapid gradient echo (MP-RAGE) three-dimensional T1-weighted sequence. Functional images were obtained by using an asymmetric spin echo echo-planar sequence sensitive to BOLD contrast.
Functional Connectivity Preprocessing.
For rs-fcMRI analyses, several previously described (18, 49) preprocessing steps were implemented to reduce spurious variance unlikely to reflect neuronal activity. These steps included the following: (i) a temporal band-pass filter and spatial smoothing; and (ii) regression of head motion parameters, whole brain signal and ventricular signal, white matter signal, and each of their first-order derivative terms (see SI Text for details).
Extraction of Regionwise Resting-State Time Series.
Resting-state (fixation) data from 210 subjects (66 aged 7–9 years; 53 aged 10–15 years; 91 aged 19–31 years) were included in the analyses. For each subject, at least 555 s (9.25 min) of resting-state BOLD data were collected. For 13 regions derived from Fox et al. (18), a resting-state time series was extracted separately for each individual. For 10 adult subjects, resting fixation was continuous. For the remaining 200 subjects, resting periods were extracted from different interleaved experimental designs that also contained task periods (49). Our method for extracting resting periods from blocked fMRI designs was validated in a recent study that compared continuous resting data to interleaved resting data and task-related data (49).
Computation of Mean Regionwise Correlation Matrix for Graph.
The resting-state BOLD time series were correlated region by region for each subject across the full length of the resting time series. Because of the potential effects of head movement on rs-fcMRI data, even after movement correction (see SI Text), the child and adult groups were matched for movement. From the sample of 210 subjects, 96 movement-matched subjects (48 children aged 7–9 years; 48 adults aged 21–31 years) were used for the graph visualization and subsequent direct comparisons (see also SI Text and ref. 22 for details).
For each subject, then, we created a square (13 × 13) correlation matrix, resulting in 96 matrices. For each group (child and adult), the correlation coefficients (r) were combined across subjects by using the Schmidt–Hunter method for metaanalyses of r values (21, 22). For the LOWESS curves, the full complement of subjects (i.e., 210 subjects), which included adolescents, was used (for details, see SI Text).
Direct Comparisons Between Children and Adults.
We performed two-sample two-tailed t tests (assuming unequal variance; P ≤ 0.05) on all potential connections represented in the 13 × 13 correlation matrices between matched children and matched adults. Fischer z transformation was applied to the correlation coefficients to improve normality for the random effects analysis. To account for multiple comparisons, the Benjamini and Hochberg False Discovery Rate (50) was applied. Connections that were significantly different between groups, but r < 0.1 in both groups, were not displayed. For the voxelwise maps, a correction based on Monte Carlo simulation was implemented (51). To achieve P < 0.05 corrected for voxel clusters, a threshold of 17 contiguous voxels with a z value of >3.0 was used.
Application of LOWESS Smoothing.
Data on connection strength versus age were fit with LOWESS curves (a local regression smoothing procedure) for selected pairs, by using the full complement of subjects (210 subjects) (52). LOWESS smoothing is a weighted least-squares fit. Each smoothed value is determined by the neighboring data points defined within a span. The method makes no assumptions about the form of the bivariate relationship. This approach is qualitative and useful for identifying data patterns that may be overlooked with curve-fitting procedures that assume shape (e.g., ref. 53). Smooth lines were computed by using a tension of 50, which means that 50% of the adjacent data points were used to determine the LOWESS fit for each of the 210 data points across age. The data point being smoothed carries the largest weight.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Steven M. Nelson and Alecia C. Vogel for helpful discussions, and Mark McAvoy and Abraham Z. Snyder for help with data analysis. This work was supported by National Institutes of Health Grants NS053425 (to B.L.S.), NS41255 and NS46424 (to S.E.P.), and NS06833 (to M.E.R); the John Merck Scholars Fund; the Burroughs-Wellcome Fund; the Dana Foundation; the Ogle Family Fund (B.L.S.); the Washington University Chancellor's Graduate Fellowship; and the United Negro College Fund (UNCF)/Merck Graduate Science Research Dissertation Fellowship (D.A.F.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800376105/DC1.
References
- 1.Shulman GL, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 2.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 5.Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. NeuroImage. 2006;37:1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: The default network and stimulus-independent thought.”. Science. 2007;317 doi: 10.1126/science.317.5834.43. 43 and author reply 43. [DOI] [PubMed] [Google Scholar]
- 7.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner RL, Vincent JL. Unrest at rest: The importance of default activity and spontaneous network correlations. NeuroImage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cognit Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Frith CD, Frith U. Interacting minds–A biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- 12.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc London B Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flavell JH. Cognitive development: Children's knowledge about the mind. Annu Rev Psychol. 1999;50:21–45. doi: 10.1146/annurev.psych.50.1.21. [DOI] [PubMed] [Google Scholar]
- 14.Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cognit Sci. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kail RV. The Development of Memory in Children. New York: Freeman; 1990. [Google Scholar]
- 17.Schneider W, Pressley M. Memory Development Between Two and Twenty. Mahwah, NJ: Lawrence Erlbaum Assoc; 1997. [Google Scholar]
- 18.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 21.Dosenbach NU, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fair DA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender-deMoll S, McFarland DA. The art and science of dynamic network visualization. J Soc Struct. 2006;7:2. [Google Scholar]
- 24.Fransson P, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, Wang B, Chen X, Xiong J. Detecting functional connectivity in the resting brain: A comparison between ICA, CCA. Magn Reson Imaging. 2007;25:47–56. doi: 10.1016/j.mri.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Bartels A, Zeki S. Brain dynamics during natural viewing conditions–A new guide for mapping connectivity in vivo. NeuroImage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 27.Marsh R, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conel JL. The Postnatal Development of the Human Cerebral Cortex. Cambridge, MA: Harvard Univ Press; 1939–1963. [Google Scholar]
- 29.Giedd JN, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 30.Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann NY Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 31.Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasan R. Spatial structure of the human alpha rhythm: Global correlation in adults and local correlation in children. Clin Neurophysiol. 1999;110:1351–1362. doi: 10.1016/s1388-2457(99)00080-2. [DOI] [PubMed] [Google Scholar]
- 33.Giorgio A, et al. Changes in white matter microstructure during adolescence. NeuroImage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 34.Barnea-Goraly N, et al. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 35.Honey C, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varela F, Lachaux J-P, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 37.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 39.Bi G, Poo M. Distributed synaptic modification in neural networks induced by patterned stimulation. Nature. 1999;401:792–796. doi: 10.1038/44573. [DOI] [PubMed] [Google Scholar]
- 40.Dosenbach NU, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cognit Sci. 2008 doi: 10.1016/tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rueda MR, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Crone EA, Ridderinkhof KR, Worm M, Somsen RJ, van der Molen MW. Switching between spatial stimulus-response mappings: A developmental study of cognitive flexibility. Dev Sci. 2004;7:443–455. doi: 10.1111/j.1467-7687.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 45.Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diamond A. In: Principles of Frontal Lobe Function. Stuss DT, Knight RT, editors. Oxford: Oxford Univ Press; 2002. pp. 466–503. [Google Scholar]
- 47.Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Castellanos FX, et al. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fair DA, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 51.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 52.Cleveland WS. LOWESS: A program for smoothing scatterplots by robust locally weighted regression. Am Stat. 1981;35:54. [Google Scholar]
- 53.Fair DA, Brown TT, Petersen SE, Schlaggar BL. A comparison of analysis variance and correlation methods for investigating cognitive development with functional magnetic resonance imaging. Dev Neuropsychol. 2006;30:531–546. doi: 10.1207/s15326942dn3001_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.