Abstract
The corticotropin-releasing factor (CRF) receptor CRFR2 is expressed widely in peripheral tissues and in the vasculature, although its functional roles in those tissues have only recently begun to be elucidated. Previously we found that genetic deletion of CRFR2 resulted in profound postnatal hypervascularization in mice, characterized by both an increase in total vessel number and a dramatic increase in vessel diameter. These data strongly suggested that ligands for CRFR2 act to limit tissue vascularity, perhaps as a counterbalance to factors that promote neovascularization. Urocortin 2 (Ucn2) is a specific ligand for the CRFR2. We hypothesized that activation of CRFR2 by Ucn2 might thus suppress tumor vascularization and consequently limit tumor growth. Here, we show that viral-mediated expression of Ucn2 strikingly inhibits the growth and vascularization of Lewis Lung Carcinoma Cell (LLCC) tumors in vivo. Further, we found that this effect on tumor growth inhibition was independent of whether exposure to Ucn2 occurred before or after establishment of measurable tumors. In vitro, Ucn2 directly inhibited the proliferation of LLCC, suggesting that the tumor-suppressing effects of CRFR2 activation involve a dual mechanism of both a direct inhibition of tumor cell cycling and the suppression of tumor vascularization. These results establish that Ucn2 inhibits tumor growth, suggesting a potential therapeutic role for CRFR2 ligands in clinical malignancies.
Keywords: anti-angiogenic, cancer, CRF, peptide, CRFR2
The corticotropin-releasing factor (CRF) pathway, initially defined as a hypothalamic neuroendocrine pathway controlling the release of ACTH from the pituitary, is now known to involve at least two distinct G protein-linked receptors, several receptor splice variants, and an assortment of unique peptide ligands and to be present in multiple peripheral organs and tissues (1–6). The peripheral roles of this pathway and its various components are only recently becoming recognized, and much is not known or understood. It has become apparent, however, that this pathway and its components are involved in a wide array of physiological and potentially pathophysiological processes, and the CRF pathway presents, therefore, a very promising clinical therapeutic target (4). Global deletion of the CRFR2 receptor causes a heightened stress response in mice, a finding attributed to CNS activities of this pathway (5, 7). These same mice, however, are hypertensive, exhibit defects in cardiac function, and are markedly hypervascularized (2, 5, 8, 9). This hypervascularity is quite distinct, featuring dramatic increases in both the number of vessels and the diameters of conductance vessels.
The mechanisms whereby loss of the CRFR2 receptor leads to such profound vascular changes remains unclear, although these receptors are found ubiquitously in the vasculature (10). Ligands for this receptor, including the 38-aa peptide urocortin2 (Ucn2), are very potent vasodilators, mediating vasodilation that can be sustained for relatively long periods after exposure to the ligand (11). The combination of this vasodilator effect and the ability of Ucn2 to augment cardiac contractility has, in fact, led to ongoing clinical assessments of Ucn2 therapy for the treatment of heart failure (8, 12). The vasodilation has been attributed to a variety of mechanisms, including involvement of protein kinase A activation, nitric oxide, and the modulating effects of CRFR2 activation on calcium-activated vascular potassium channels (10, 13, 14). Whether any of these mechanistic pathways are involved in mediating the effects of CRFR2 on tissue vascularity is unknown, but the increase in vessel size and number that occurs in the absence of CRFR2 is profound and strongly suggests that ligands of CRFR2 play a role in suppressing or modulating tissue vascularization. We have previously shown that CRFR2 activation can suppress the proliferation of vascular cells and alter the phosphorylation state of Rb (2). Although this direct influence on the cell cycle may contribute significantly to the profound vascular effects of CRFR2, it is likely that additional mechanisms are involved and that the relationship is more complex.
Identification of CRFR2 ligands, including Ucn2, as potential suppressors of tissue vascularization has important clinical implications, especially in the context of human malignancies and other pathologies that involve neovascularization. Anti-angiogenic therapies have been sufficiently successful in treating human cancers such that there are now approved therapeutics with this mechanism of action. The challenge has been, however, to develop therapeutics that can efficiently suppress neovascularization, but that do not have significant collateral toxicity. Ucn2 has been experimentally used in humans and appears to be well tolerated (15). The possibility that Ucn2, as a CRFR2 ligand, could suppress vascularization and potentially act therapeutically in malignancies is therefore intriguing. To test this potential action of Ucn2 we evaluated the effects of Ucn2 on tumor growth and vascularization in a well established murine model.
Results
Urocortin 2 Inhibits the Growth of Lewis Lung Carcinoma Cell Tumors in Vivo.
Tumors were created in the flanks of C57BL/6 mice by s.c. injection of C57BL/6-derived Lewis Lung Carcinoma Cells (LLCCs). To determine the ability of Ucn2 to suppress tumor growth and vascularization a recombinant adenovirus encoding Ucn2 was used to either transduce the LLCC in culture 24 h before injection, or to transduce the LLCC tumors in situ after they had been established in the mice. RT-PCR analysis documented that nontransduced LLCCs and those transduced with a control adenovirus did not express appreciable levels of Ucn2 at baseline, but efficiently expressed Ucn2 after transduction by the Ad-Ucn2 recombinant adenovirus (Fig. 1A). When LLCCs were transduced before injection there were no significant differences in the volume of tumors derived from Ad-LacZ- or saline-treated cells, but by day 3 postinjection the volume of the tumors derived from Ad-Ucn2-transduced cells was already reduced >1.5-fold compared with Ad-LacZ- and saline-treated controls (Fig. 1B; P < 0.05 Ad-Ucn2 vs. Ad-LacZ or saline controls; n ≥ 6 per group). These differences peaked at ≈day 9 postinjection (day 10 post-Ad-Unc2 transduction) with a >2.8-fold reduction in Ad-Ucn2 tumor volume vs. saline control tumors, and a 2.5-fold reduction vs. Ad-LacZ tumors. During this interval, which coincides with the known peak period for adenovirus-based transgene expression, the rate of tumor growth in the Ad-Ucn2 group was markedly reduced (3-fold vs. controls; P < 0.005), reflecting the peak exposure to Ucn2. Although after 11 days, at a time coinciding with an expected diminution/loss of adenovirus-mediated Ucn2 expression, the rate of tumor growth increased in the Ad-Ucn2 group, it nonetheless remained >2-fold less than controls. Finally, 15 days after injection the Ucn2 effect was still markedly apparent, with a 2.1-fold lesser tumor volume in Ad-UCN2 vs. Ad-LacZ tumors, and a >2.5-fold lesser volume vs. saline control tumors (P < 0.05 for both comparisons). To ensure that the measured differences in tumor volumes were not due to a difference in peritumoral inflammation or hematoma we corroborated the tumor volume results with in vivo ultrasound assessment and with analysis of terminal tumor weights. As shown (Fig. 1C) tumor weights corroborated the in vivo volume assessments with a profound reduction in the Ucn2-exposed tumors. Ultrasound analysis confirmed that the differences in measured volumes reflected actual differences in tumor size, without a significant contribution by a peritumoral process (Fig. 1D). Of note is that, by definition, the tumor volume data were restricted to those tumors that actually grew to an assessable size. Interestingly, there was a significant reduction in the number of Ucn2-treated LLCCs that resulted in tumor formation after injection (44% of Ucn2 LLC injections vs. 89% of controls). Lack of growth was confirmed at the time of killing.
Fig. 1.
Urocortin2 suppresses tumor growth. C57BL/6 mouse-derived Lewis lung carcinoma cells (LLCCs) were injected into the flanks of syngeneic mice to produce s.c. tumors and assess the effects of urocortin2 (Ucn2) on tumor growth. (A) LLCCs were transduced with a recombinant adenovirus vector encoding Ucn2 (Ad-Ucn2), a control adenovirus vector (Ad-EGFP), or exposed to vehicle only (CT). RT-PCR analysis revealed robust Ucn2 expression in Ad-Ucn2-transduced cells, but undetectable levels in the controls. (B) LLCCs were transduced in vitro with Ad-Ucn2 or Ad-LacZ (encoding β-galactosidase), or exposed to saline vehicle (PBS) and injected s.c. 24 h later. Tumor volumes were measured every other day for 15 days, demonstrating a marked reduction in tumor growth in the Ad-Ucn2-transduced group, and a correlating decrease in tumor weights when excised at the end of this period (n ≥ 6 per group) (C). (D) Ultrasound assessment of tumors in vivo confirmed the volume measurements and that the measured differences in tumor volumes were not due to peritumoral inflammatory infiltrates or hemorrhage. (E) To assess the effects of Ucn2 on the growth of established tumors, Ad-Ucn2, PBS, or Ad-LacZ were injected in situ into established tumors at day 3 after implantation. Tumor volumes were significantly decreased by Ad-Ucn2 treatment on days 4–12 of examination (n ≥ 6).
Although Ucn2 significantly reduced tumor growth when present in the LLCCs before injection, it remained unclear whether Ucn2 exposure could decrease the growth of LLCC-derived tumors after they were established in vivo. To address this question, we injected LLCC-derived tumors in situ with Ad-Ucn2 after tumors were apparent in vivo (day 3 postinjection of LLCCs). This intervention also significantly reduced tumor growth compared with tumors injected with control Ad-LacZ or saline (Fig. 1E; n ≥ 6). In the first 6 days after in situ adenoviral transduction (tumor age, 9 days) there was a near-4-fold reduction in the growth of Ad-Ucn2-injected tumors vs. controls, again consistent with the expected peak period of adenovirus-mediated expression of Ucn2.
Ucn2 Reduces Tumor Vascularization and Has Antiproliferative Effects.
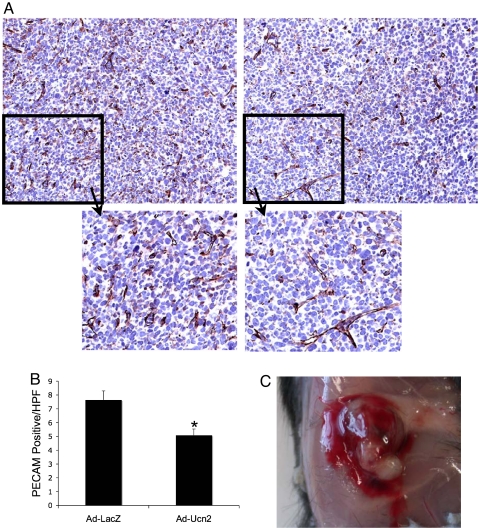
We have previously reported a marked increase in tissue vascularity in mice with an absence of the major Ucn2 receptor, CRFR2, and showed direct effects of CRFR2 activation on vascular cell proliferation in vitro (2). On the basis of these data, we hypothesized that exposure to Ucn2 could suppress tumor growth by both a reduction in tumor vascularization and an additional direct effect on tumor cell proliferation. Tumor vessel counts based on PECAM immunohistochemistry were consistent with this proposed mechanism of suppressed tumor growth, showing a 1.5-fold (± 0.12) reduction in Ad-Ucn2 tumor vascularity vs. Ad-LacZ controls (Fig. 2 A and B; P = 0.009). Interestingly, in addition to this significant reduction in tumor vascularity, Ucn2-treated tumors displayed an increased peritumoral hemorrhage that was apparent at the time of excision (Fig. 2C), a finding consistent with previous observations we have made with implanted urocortin-impregnated sponges (unpublished data).
Fig. 2.
Urocortin2 suppresses tumor vascularization. LLCC-derived tumors were excised from the flanks of mice after completion of tumor growth studies. Tumor vascularity was assessed by PECAM immunohistochemistry and digital determination of vessel density per high-power field (HPF). (A and B) PECAM staining demonstrates a 1.5-fold reduction in total PECAM-positive vessels in Ad-Ucn2-treated tumors (×40; Insets, ≈×100). (C) At excision, Ad-Ucn2-treated tumors exhibited peritumoral hemorrhage not apparent in the control tumors. Ad-Ucn2 and Ad-LacZ connote recombinant adenovirus encoding either urocortin2 or β-galactosidase, respectively.
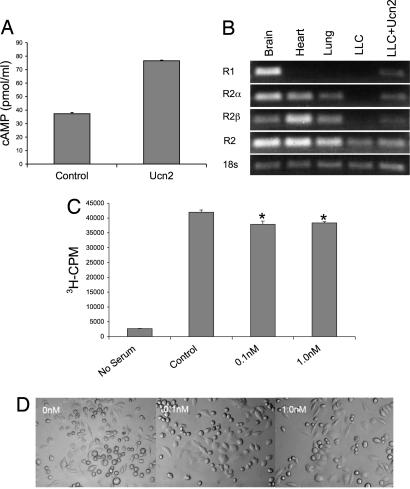
To assess the direct effects of Ucn2 exposure on tumor cell proliferation and morphometry, we exposed LLCC to graduated concentrations of Ucn2 in vitro. Before assessing these effects, we documented the biological activity of Ucn2 in culture by demonstrating an expected 2-fold increase in cAMP levels after exposure to 50 nM concentration of Ucn2 (Fig. 3A). Next we assessed by RT-PCR the expression pattern of CRF receptors on LLCCs in the absence and presence of Ucn2, and compared these expression patterns with those measured in representative central CNS (brain) and peripheral tissues (heart, lung). As expected the predominant receptor expressed in the brain was CRFR1, whereas in heart and lung only CRFR2 isoforms were detected (Fig. 3B). LLCCs expressed only low levels of CRFR2 at baseline, unlike normal lung tissue, and no CRFR1. In fact, basal expression of CRFR2 was only detected in LLCC when using primers that detect total CRFR2, including both α- and β-isoforms. This basal expression was sufficient, however, to facilitate a Ucn2-mediated induction of CRFR2, which included increased expression of both the α- and β-receptor isoforms.
Fig. 3.
Urocortin2 directly suppresses tumor cell proliferation, induces distinct morphological changes, and induces expression of its own receptor CRFR2. Treatment of LLCCs with urocortin2 (Ucn2) was used to assess effects on cell proliferation and morphology. (A) The biological activity of the Ucn2 peptide was documented in vitro by assessing the ability of Ucn2 exposure to induce cAMP generation. (B) Expression of CRF receptors in LLCCs, brain, heart, and lung was assessed by RT-PCR. As shown, LLCCs do not basally express CRFR1, express low basal levels of CRFR2, but demonstrate inducible expression of CRFR2 after exposure to Ucn2. (C) Exposure of LLCC to graduated concentrations of Ucn2 induced a mild, but significant diminution of cellular proliferation rates, as assessed by [3H]thymidine incorporation (cpm = cpm/g protein). (D) Exposure to Ucn2 induces morphological alterations in LLCCs, including an apparent increase in average cell size.
Having established the expression of CRFR2 in LLCCs, we next evaluated the effects of graduated doses of Ucn2 on the proliferation rate of LLCC in culture by using a standard [3H]thymidine incorporation assay. Although the effect was modest, and non-dose-dependent within the range tested (0.1–100 nM), there was a consistent and significant reduction in LLCC proliferation rate in response to Ucn2 exposure, as opposed to vehicle controls, similar to the effects we have shown previously in vascular cells in response to Ucn1 (Fig. 3C). Interestingly, there was also a consistent effect of Ucn2 on the morphology of the LLCCs, including an increase in cell size, cell flattening/spreading, and the formation of sporadic multinuclear cells (Fig. 3D).
Discussion
Our previous findings demonstrated a role for CRFR2 in regulation of tissue vascularization (2). Because neovascularization is thought to be a critical component in growth of most tumors, we hypothesized that specific activation of CRFR2 by its known ligand, Ucn2, could inhibit tumor growth. By use of a Ucn2-expressing adenovirus transfected into Lewis Lung Carcinoma cells before or after transplantation and tumor development, we examined the effects on tumor growth and vascularization. Further, we have explored the possible involvement of Ucn2 in tumor cell proliferation as an additional mechanism whereby CRFR2 may regulate tumor growth.
Our results showed that LLCC tumors infected with Ad-Ucn2 before implantation had a significant and profound decrease in both tumor volume and tumor weight. These effects were significant by day 3 after transplantation and were maintained through day 15 at excision. Ultrasound analysis verified that the difference in tumor volume was not caused peritumoral processes. These differences peaked at day 9 postinjection. During this interval, which coincides with the known peak period for adenovirus-based transgene expression, the rate of tumor growth in the Ad-Ucn2 group was markedly reduced, reflecting the peak exposure to Ucn2. Fifteen days after injection the Ucn2 effect was still markedly apparent. To ensure that the measured differences in tumor volumes were not caused by a difference in peritumoral inflammation or hematoma, we corroborated the tumor volume results with in vivo ultrasound assessment and with analysis of terminal tumor weights. Tumor weights corroborated the in vivo volume assessments with a profound reduction in the Ucn2-exposed tumors. Ultrasound analysis confirmed that the differences in measured volumes reflected actual differences in tumor size, without a significant contribution by a peritumoral processes. Of note, by definition, the tumor volume data were restricted to those tumors that actually grew to an assessable size. Interestingly, there was a significant reduction in the number of Ucn2-treated LLCC that resulted in tumor formation after injection (44% of Ucn2 LLC injections vs. 89% of controls). Lack of growth was confirmed at the time of killing.
Although Ucn2 significantly reduced tumor growth when present in the LLCCs before injection, it remained unclear whether Ucn2 exposure could decrease the growth of LLCC-derived tumors after they were established in vivo. To assess this, we injected LLCC-derived tumors in situ with Ad-Ucn2 after tumors were apparent in vivo. This intervention also significantly reduced tumor growth compared with tumors injected with control Ad-LacZ or saline. In the first 6 days after in situ adenoviral transduction (tumor age, 9 days), there was a near-4-fold reduction in the growth of Ad-Ucn2-injected tumors, again consistent with the expected peak period of adenovirus-mediated expression of Ucn2.
We have previously reported a marked increase in tissue vascularity in mice with an absence of the Ucn2 receptor, CRFR2, and showed direct effects of CRFR2 activation on vascular cell proliferation in vitro (2). On the basis of these data, we hypothesized that exposure to Ucn2 could suppress tumor growth by both a reduction in tumor vascularization and an additional direct effect on tumor cell proliferation. Tumor vessel counts based on PECAM immunohistochemistry were consistent with this proposed mechanism of suppressed tumor growth, showing a reduction in Ad-Ucn2 tumor vascularity. Interestingly, in addition to this significant reduction in tumor vascularity, Ucn2-treated tumors displayed an increased peritumoral hemorrhage that was apparent at the time of excision, a finding consistent with previous observations we have made with implanted urocortin-impregnated sponges (unpublished results).
To assess the direct effects of Ucn2 exposure on tumor cell proliferation and morphometry, we exposed LLCCs to graduated concentrations of Ucn2 in vitro. Before assessing these effects, we documented the biological activity of Ucn2 in culture by demonstrating an expected increase in cAMP production after Ucn2 treatment. To assess the endogenous expression of CRF receptors in the LLCCs, we compared levels by RT-PCR in the absence and presence of Ucn2, and compared these expression patterns to those measured in representative central CNS (brain) and peripheral tissues (heart, lung). As expected the predominant receptor expressed in the brain was CRFR1, whereas in heart and lung only CRFR2 isoforms were detected. LLCCs expressed only low levels of CRFR2 at baseline, unlike normal lung tissue, and no CRFR1. In fact, basal expression of CRFR2 was only detected in LLCCs when using primers that detect total CRFR2, including both α- and β-isoforms. This basal expression was sufficient, however, to facilitate a Ucn2-mediated induction of CRFR2, which included increased expression of both the α- and β-receptor isoforms.
Having established the expression of CRFR2 in LLCCs, we next evaluated the effects of Ucn2 on the proliferation rate of LLCCs in vitro by using a standard [3H]thymidine incorporation assay. Although the effect was modest, there was a consistent and significant reduction in LLCC proliferation rate in response to Ucn2 exposure, similar to the effects we have shown previously in vascular cells in response to Ucn1. Interestingly, there was also a consistent effect of Ucn2 on the morphology of the LLCCs, including an increase in cell size, cell flattening/spreading, and the formation of sporadic multinuclear cells.
Mechanistically, it is interesting to note that, although Ucn2 is a potent vasodilator and thus might be expected to increase tumor blood flow by dilation of feeder vessels, its overall effect is to decrease the vascularity of tumors and, by extrapolation, to decrease tumor blood flow. This disconnect between vascularization and vascular tone was definitively apparent in initial studies of CRFR2 knockout mice in which profound hypervascularization was accompanied by increased systemic blood pressure, presumably reflecting the loss of the CRFR2-mediated peripheral vasodilator function (2, 5, 7, 9). In the current study we did not monitor blood pressure, and it is possible that transgene-mediated local expression of Ucn2 could have had a systemic effect on blood pressure and that this contributed to the observed effects on tumor growth, but we feel that this is unlikely. First, the amount of Ucn2 secreted locally by cells containing the Ucn2 transgene is unlikely to reach the peripheral circulation in levels sufficient to alter systemic hemodynamic parameters. Second, the vasodilation effects of Ucn2 are accompanied by concomitant increases in cardiac output, thus tissue, and tumor perfusion should be maintained or increase (8, 15). The decrease in tumor vascularization noted is therefore likely due to a direct effect of Ucn2 on the vascularization process, consistent with our data from CRFR2-deficient mice (2).
The noted suppressive effects of Ucn2 on LLCC proliferation were not profound, but were significant and mirror what we have previously shown in vascular smooth muscle cells and has been shown in other cell types (2, 16). These findings support our contention that Unc2 can inhibit tumor growth by dual mechanisms, although our current study suggests that the anti-angiogenic effect is predominant. Interestingly, previous work has shown that CRFR1 activation can directly inhibit the proliferation of malignant cells (17–19). Ucn2 is not a ligand for CRFR1, and therefore the effects of Ucn2 we report here are likely CRFR2-mediated. Although the relative biological effects of CRF receptor activation on the behavior of transformed cells requires further study, the fact that CRFR2 is highly expressed in the vasculature and mediates suppressive effects on neovascularization would appear to delineate CRFR2-specific ligands, such as Ucn2, as superior anticancer agents. Whereas there are now several published reports showing expression of CRF-R1 and its ligands in human malignancies, currently there are limited clinical data regarding the expression of CRFR2 in cancer. We demonstrated here that CRFR2 is expressed basally in the malignant murine LLCCs we studied, and that this expression is actually inducible by exposure to Ucn2. Additional studies to define the expression of this receptor and its ligands in various human malignancies are required, as is work to clarify what appears to be a positive feedback loop whereby Ucn2 induces its own receptor. Although only observational in this study, we did delineate two interesting Ucn2-related phenomena that we and others have previously observed in response to CRF-pathway modulation; altered cellular morphology and evidence of altered vascular permeability (20–24). Specifically, we observed enlargement and flattening/spreading of LLCCs when exposed to Ucn2. Although these findings were not quantified, they are consistent with what has been reported in the literature in other cell types (23, 24), and are further evidence that the effects of Ucn2 on tumor biology go beyond Ucn2 effects on vascularization. We also saw an increase in peritumoral hemorrhage in Ucn2-treated tumors, a finding we saw in matrigel studies (2), which could be attributed to increased vascular permeability. It has been reported that urocortin increases vascular permeability in the lung (21) and skin (25), and that both CRFR1 and CRFR2 may be involved in defining mucosal permeability in the intestine (20). There are data demonstrating urocortin and CRF pathway-mediated mast cell activation (25), and involvement in the modulation of inflammation (26), but a definitive mechanistic explanation for effects on vascular permeability has not been elucidated.
How Ucn2 exerts its suppressive effects on tissue vascularization is unclear and might involve more than one mechanism. Interestingly, protein kinase A (PKA) activation has been shown to inhibit angiogenesis and promote endothelial cell apoptosis (27). Ucn2, through CRFR2, signals at least partially by a PKA-dependent pathway, and this provides one potential mechanistic tie-in that warrants study. Also of interest is that inhibition of α5β1 integrin ligation increases PKA activation and may be an important mechanism whereby agents that alter intregrin function can inhibit angiogenesis (27). Recently we showed that endothelial cell-specific loss of the β1 integrin causes embryonic lethality and profound abnormalities in vascular development (28). It is interesting to consider that a PKA-mediated final common pathway may be involved in the vascular effects of Ucn2 and of altered integrin function. PKA-independent functions of cAMP might also be involved in defining Ucn2 effects. For example, it was recently shown that cAMP defines endothelial cell adhesion characteristics in both a PKA-dependent and -independent manner involving exchange protein activated by cAMP (EPAC) (29). The mechanisms underlying the effects of CRFR2 activation on tissue vascularization need to be further elucidated. Efforts directed to this area are definitively warranted because further delineation of this pathway may result in an entirely new category of therapeutics.
In summary, we have demonstrated that the CRFR2 ligand, Ucn2, can suppress tumor growth predominantly by inhibition of tumor vascularization, but also potentially through direct effects on tumor cell proliferation. Additional contributory mechanisms were not excluded in the present study and future studies will be required to more definitively elaborate the precise mechanisms involved in the Ucn2 antitumor effect. Our current findings build on our previous work that established a crucial role of the CRFR2 receptor in determining tissue vascularization (2), and raise the possibility that CRFR2 ligands could be used therapeutically to inhibit neovascularization in malignancies. The clinical application of Ucn2 for this purpose is particularly appealing given that the physiological effects of Ucn2 in humans has already been evaluated (15) and that, in human trials of Ucn2 treatment for heart failure, the peptide appears well tolerated and nontoxic (12).
Methods
Tumor Implantation and Growth Assessment.
LLCCs were cultured in DMEM containing 2 mM l-glutamine and penicillin/streptomycin (Pen/Strep). Cells were transduced with adenovirus encoding either Ucn2 or β-gal or vehicle. Twenty-four hours later, cells were washed, trypsinized, and resuspended in serum-free, l-glutamine and Pen/Strep-free DMEM and were injected s.c. in the flanks of 6- to 8-week-old C57BL/6 male mice (1 × 106 cells in 100 μl).
Alternatively, tumors were established by implantation of 1 × 106 cells into the right hind flank of C57BL/6 mice. When the tumors reached 100 mm3 in volume, tumor-bearing mice were treated with intratumoral injection of Ad-Ucn2 or Ad-β-gal at a dose of 1 × 1010 pfu per animal. Tumor growth was monitored by caliper measurement. Tumor volume was calculated from the formula (volume = 0.52 × [width]2 × [length]) to approximate the volume of a spheroid. Tumor weights were measured 2 weeks after implantation.
To assess tumor morphology in vivo and evaluate any potential contributions of peritumoral fluid/inflammation to the caliper-based volume determinations, ultrasound analysis of tumors were made in vivo with a high-resolution ultrasound console (Vevo 770, Visualsonics Inc.). Mice were anesthetized with inhaled isoflurane by a nose cone apparatus, their flanks surrounding the tumor shaved, and 2D images of the tumors and surrounding tissues obtained in real time.
Immunohistochemistry and Vessel Counts.
Tumors were harvested and fixed in 10% formalin for 1 day and transferred to 70% ethanol overnight followed by paraffin embedding. These tissues were sectioned and stained for PECAM-1 (PharMingen) and visualized by using the Vectorstain ABC kit (Vector Laboratories) followed by incubation with NovaRed substrate (Vector Laboratories). For vessel counts, the immunohistochemistry microscopy images were captured at high magnification and were quantified by an investigator who was blinded to treatment allocation by using ImageJ image-analysis freeware. PECAM-positive staining was expressed as a percentage of the total image area. Five images were analyzed per tumor (n = 3–5).
Cell Proliferation and cAMP Measurement.
LLCCs were plated in 24-well plate at a density of 1.0 × 105 cells per well. After they were incubated in DMEM containing 10% FBS for 48 h, they were further incubated with serum-free DMEM for 24 h. Cells were then incubated in DMEM containing 0.5% FBS with or without (control) a specified concentration of Ucn2 for 24 h. For proliferation determination, 1 μCi/ml of [3H]thymidine (Life Science Products) was added to LLCCs and incubated for 6 h. After incubation, cells were rinsed three times with ice-cold PBS and 10% TCA, and lysed with 0.25 N NaOH. Incorporation of radioactivity was measured by a liquid scintillation counter. Each experiment was performed in quadruplicate. cAMP concentration was measured by a commercial ELISA-based cAMP assay (ParametercAMP Assay; R&D Systems).
RT-PCR.
To determine the endogenous expression of CRF receptors in LLCCs and peripheral tissues, we examined gene expression by reverse-transcriptase PCR. Total RNA was extracted by using RNeasy extraction kit with DNase I treatment (Qiagen). Real-time reactions were performed by using StrataScript First Strand Synthesis System (Stratagene). Primer sequences were as follows: CRFR1 forward: 5′-GCCAGGAGATTCTCAACG-3′, CRFR1 reverse: 5′-AAGAGGACAAAGGCCACC-3′. CRFR2 forward: 5′-GCTGCCTGAGGAATGTGA-3′, CRFR2 reverse: 5′-TCGTGGTCGATGAGTTGC-3′. Ucn2 forward: 5′-GACAACTCCTAGCTCTGTGA-3′, Ucn2 reverse: 5′-CTGTTCCAGTAAGATCCGTA-3′. Primers were also designed to distinguish CRFR2α and CRFR2β, primer sequences are as follows: CRFR2 α forward: 5′-CACTCCCACTCCCTCTGCG-3′, CRFR2α reverse: 5′-AGCAGCTCTTCGGCCAGC-3′, CRFR2β forward: 5′-CTCCTCTGCCTGTTTTCC-3′, CRFR2β reverse: 5′-GTGTCCACAGGGGCTGGT-3′. PCRs were performed by using 50 ng of total RNA in a 25-μl volume. The product were run on a 2% agarose gel and stained with ethidium bromide. Ucn2 concentration was 10 nm for 24 h before assessment of CRFR2 expression.
Footnotes
Conflict of interest statement: W.W.V. is a member of the Board of Directors and a shareholder of Neurocrine Biosciences (NBI), a company that is developing antagonists and agonists of CRF receptors for pharmaceutical purposes. Urocortin 2 has been licensed to NBI by the Salk Institute and the Clayton Foundation. This work was supported by the National Institutes of Health and private sources, and is independent of Neurocrine Biosciences. T.L.B. is also a shareholder of NBI.
References
- 1.Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Bale TL, et al. Corticotropin-releasing factor receptor 2 is a tonic suppressor of vascularization. Proc Natl Acad Sci USA. 2002;99:7734–7739. doi: 10.1073/pnas.102187099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale TL. Sensitivity to stress: Dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bale TL, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 6.Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144:2580–2587. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 8.Bale TL, et al. The cardiovascular physiologic actions of urocortin II: Acute effects in murine heart failure. Proc Natl Acad Sci USA. 2004;101:3697–3702. doi: 10.1073/pnas.0307324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coste SC, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 10.Wiley KE, Davenport AP. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. Br J Pharmacol. 2004;143:508–514. doi: 10.1038/sj.bjp.0705985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leitch IM, et al. Vasodilator actions of urocortin and related peptides in the human perfused placenta in vitro. J Clin Endocrinol Metab. 1998;83:4510–4513. doi: 10.1210/jcem.83.12.5356. [DOI] [PubMed] [Google Scholar]
- 12.Davis ME, et al. Urocortin 2 infusion in human heart failure. Eur Heart J. 2007;28:2589–2597. doi: 10.1093/eurheartj/ehm340. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, et al. Urocortin-induced endothelium-dependent relaxation of rat coronary artery: Role of nitric oxide and K+ channels. Br J Pharmacol. 2002;135:1467–1476. doi: 10.1038/sj.bjp.0704587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, et al. Roles of cyclic AMP, Ca2+-activated K+ channels in endothelium-independent relaxation by urocortin in the rat coronary artery. Cardiovasc Res. 2003;57:824–833. doi: 10.1016/s0008-6363(02)00773-3. [DOI] [PubMed] [Google Scholar]
- 15.Davis ME, et al. Urocortin 2 infusion in healthy humans: hemodynamic, neurohormonal, and renal responses. J Am Coll Cardiol. 2007;49:461–471. doi: 10.1016/j.jacc.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 16.Krause K, Schnitger A, Fimmel S, Glass E, Zouboulis CC. Corticotropin-releasing hormone skin signaling is receptor-mediated and is predominant in the sebaceous glands. Horm Metab Res. 2007;39:166–170. doi: 10.1055/s-2007-961811. [DOI] [PubMed] [Google Scholar]
- 17.Graziani G, et al. CRH inhibits cell growth of human endometrial adenocarcinoma cells via CRH-receptor 1-mediated activation of cAMP-PKA pathway. Endocrinology. 2002;143:807–813. doi: 10.1210/endo.143.3.8694. [DOI] [PubMed] [Google Scholar]
- 18.Carlson KW, et al. Inhibition of mouse melanoma cell proliferation by corticotropin-releasing hormone and its analogs. Anticancer Res. 2001;21:1173–1179. [PubMed] [Google Scholar]
- 19.Wang J, Li S. Corticotropin-releasing factor family and its receptors: tumor therapeutic targets? Biochem Biophys Res Commun. 2007;362:785–788. doi: 10.1016/j.bbrc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Xu Y, Zhou H, Tao J, Li S. Expression of urocortin in rat lung and its effect on pulmonary vascular permeability. J Endocrinol. 2006;189:167–178. doi: 10.1677/joe.1.06607. [DOI] [PubMed] [Google Scholar]
- 22.Slominski AT, et al. Corticotropin releasing hormone and related peptides can act as bioregulatory factors in human keratinocytes. In Vitro Cell Dev Biol Anim. 2000;36:211–216. doi: 10.1290/1071-2690(2000)036<0211:CRHARP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol. 2005;203:118–126. doi: 10.1002/jcp.20209. [DOI] [PubMed] [Google Scholar]
- 24.Davidson SM, et al. The transcriptional coactivator p300 plays a critical role in the hypertrophic and protective pathways induced by phenylephrine in cardiac cells but is specific to the hypertrophic effect of urocortin. ChemBioChem. 2005;6:162–170. doi: 10.1002/cbic.200400246. [DOI] [PubMed] [Google Scholar]
- 25.Singh LK, et al. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J Pharmacol Exp Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- 26.Moss AC, et al. Urocortin II mediates proinflammatory effects in human colonocytes via corticotropin-releasing hormone receptor 2alpha. Gut. 2007;56:1210–1217. doi: 10.1136/gut.2006.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Bakre M, Yin H, Varner JA. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest. 2002;110:933–941. doi: 10.1172/JCI14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei L, et al. Endothelial expression of beta-1 integrin is required for embryonic vascular patterning and postnatal vascular remodeling. Mol Cell Biol. 2008;28:794–802. doi: 10.1128/MCB.00443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A, exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res. 2007;101:768–776. doi: 10.1161/CIRCRESAHA.106.146159. [DOI] [PubMed] [Google Scholar]