Abstract
Although laboratory experiments document cooperative behavior in humans, little is known about the extent to which individual differences in cooperativeness result from genetic and environmental variation. In this article, we report the results of two independently conceived and executed studies of monozygotic and dizygotic twins, one in Sweden and one in the United States. The results from these studies suggest that humans are endowed with genetic variation that influences the decision to invest, and to reciprocate investment, in the classic trust game. Based on these findings, we urge social scientists to take seriously the idea that differences in peer and parental socialization are not the only forces that influence variation in cooperative behavior.
Keywords: behavioral genetics, cooperation, experimental economics
Many mutually beneficial transactions involve an element of interpersonal trust and may fail to materialize in the absence of an expectation that trust will be reciprocated. The prevalence of trust in a society has therefore been assigned primacy in a number of domains, for instance empirical (1) and theoretical (2) studies of economic growth. In recent years, the trust game (3) has emerged as a favorite instrument to elicit an individual's interpersonal trust and willingness to reciprocate trust. More generally, the game has been widely used to study cooperative behavior. In a trust game, an individual (the investor) decides how much money out of an initial endowment to send to another subject (the trustee). The sent amount is then multiplied by some factor, usually three, and the trustee decides how much of the money received to send back to the investor. The standard game-theoretic prediction for a single anonymous interaction between two purely self-interested individuals is for the investor to send nothing, rationally anticipating that the trustee will not reciprocate. Yet, experiments consistently show that cooperation flourishes in the trust game; the average investor sends a significant share of her endowment, and most trustees reciprocate (4). A voluminous body of theoretical and experimental work examines the mechanisms through which natural selection can favor cooperation, and proposed mechanisms include kin selection (5), reciprocity (6), indirect reciprocity (7), and group selection (8). These models offer different accounts for the ultimate explanation for the existence of cooperation and also generate different predictions about genotypic variation in equilibrium (9).
To investigate whether humans are endowed with genetic variation that could help account for individual differences in trust game behavior, two separate teams of researchers independently conceived and executed a very similar experiment on twins [see supporting information (SI) for experimental procedures]. These teams became aware of each other for the first time after all data had been collected. One team recruited 658 subjects from the population-based Swedish Twin Registry, and the other team recruited 706 subjects from the 2006 and 2007 Twins Days Festivals in Twinsburg, OH. Both teams administered the trust game to (identical) monozygotic (MZ) and (nonidentical) dizygotic (DZ) same-sex twin pairs. The game was played with real monetary payoffs and between anonymous partners.
Because MZ twins share the same genes, whereas the genes of DZ twins are only imperfectly correlated, MZ twins should exhibit higher correlation in their behavior than DZ twins if genetic differences help explain heterogeneity in strategies. Moreover, if we assume that MZ and DZ twins share comparable environments, then we can use these correlations to estimate explicitly the relative influence of genetic and environmental factors on behavioral variation. Specifically, we conduct a mixed-effects Bayesian ACE analysis that uses decisions in the trust game to estimate the degree to which variation in trust and trustworthiness is influenced by genetic factors (A), environmental factors shared or common to cotwins (C), and unshared environmental factors (E), which are specific to each twin (see SI). The analysis provides estimates of proportions of observed variance in cooperative behavior due to the respective effects of A, C, and E. This method generates 95% credible intervals (C.I.) that permit us to test the hypothesis that a factor plays no role (i.e., explains 0% of the variance; see SI).
Some scholars have objected to the assumption that MZ and DZ environments are comparable, arguing that MZ twins tend to be more influenced by one another and are treated more similarly by their parents than DZ twins (10). If so, then greater similarity in MZ twins might merely reflect that their shared environments cause them to become more similar than DZ twins. However, studies of twins raised together have been validated by studies of twins reared apart (11), suggesting that the shared environment does not exert substantially enhanced influence on MZ twins. Moreover, personality and cognitive differences between MZ and DZ twins persist even among DZ twins whose zygosity has been miscategorized by their parents (12), indicating that being mistakenly treated as an identical twin by one's parents is not sufficient to generate observed differences in similarity. Further, although MZ twins are sometimes in more frequent contact with each other than DZ twins, it appears that twin similarity may cause greater contact rather than vice versa (13).
To evaluate whether the MZ and DZ samples differ, we performed a series of tests on the mean difference between MZ and DZ twins for a number of variables (see Table 1). In the Swedish sample, high P values suggest that differences between types of twins are not significant for education (P = 0.95), gender (P = 0.35), trust (P = 0.35), trustworthiness (P = 0.53), cognitive ability (P = 0.64), emotional stability (P = 0.43), agreeableness (P = 0.54), extraversion (P = 0.63), and conscientiousness (P = 0.85). In the U.S., differences were insignificant for education (P = 0.26), gender (P = 0.91), trust (P = 0.87), and trustworthiness (P = 0.90). The only significant difference in either case is age (P = 0.03 in the Swedish sample and P = 0.02 in the U.S. sample). Thus, with the exception of age, there is no evidence that the MZ and DZ twins in our samples are drawn from different environments. In SI, we demonstrate that heritability estimates are not significantly different when we stratify the sample by age.
Table 1.
Summary statistics and twin comparisons
| Swedish sample |
P | U.S. sample |
P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MZ twins |
DZ twins |
MZ twins |
DZ twins |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Education | 0.69 | 0.46 | 0.70 | 0.46 | 0.95 | 0.65 | 0.48 | 0.71 | 0.45 | 0.26 |
| Female | 0.78 | 0.41 | 0.83 | 0.38 | 0.35 | 0.80 | 0.40 | 0.81 | 0.40 | 0.91 |
| Age | 32.4 | 7.4 | 34.6 | 7.5 | 0.03 | 36.9 | 16.7 | 32.4 | 14.8 | 0.02 |
| Trust | 0.77 | 0.28 | 0.80 | 0.25 | 0.26 | 0.52 | 0.27 | 0.51 | 0.25 | 0.87 |
| Trustworthiness | 0.39 | 0.15 | 0.40 | 0.16 | 0.53 | 0.45 | 0.25 | 0.45 | 0.24 | 0.90 |
| Cognitive ability | 0.01 | 0.98 | −0.04 | 1.05 | 0.64 | |||||
| Emotional stability | −0.02 | 1.00 | 0.07 | 1.01 | 0.43 | |||||
| Agreeableness | 0.02 | 0.98 | −0.05 | 1.07 | 0.54 | |||||
| Extraversion | 0.01 | 0.99 | −0.04 | 1.03 | 0.63 | |||||
| Conscientiousness | −0.00 | 1.00 | 0.02 | 0.99 | 0.85 | |||||
Education assumes the value 1 if the individual attended at least some college. Trust is the fraction sent, and trustworthiness is the fraction returned in the trust game. Psychological measures were adjusted to have mean 0 and standard deviation 1 for the whole sample. Tests of equality take into account the correlation between subjects by using the GEE cluster-robust variance–covariance matrix of Liang and Zeger (14). These data show that MZ and DZ twins differ significantly only in age, suggesting that MZ and DZ twins are drawn from very similar environments. To ensure that age is not a factor in the Swedish and U.S. experiments, we analyzed separate models for above- and below-median age subjects, and we find no significant differences in the estimates.
Results
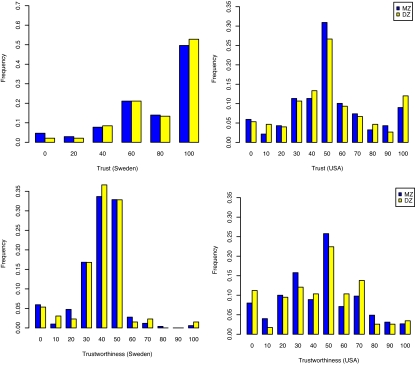
Fig. 1 shows histograms of behavior in the trust game for the Swedish and U.S. samples. The results are broadly consistent with prior work showing a substantial tendency to trust and to reciprocate trust in experimental settings. However, our primary goal is to investigate whether individual differences in trust and trustworthiness can, in part, be explained by genetic variation. A first indication comes from examining the difference in correlations between MZ and DZ twins. These results show that MZ twins are consistently more similar in their behavior than DZ twins. In the U.S. sample, nonparametric correlations are 0.13 vs. −0.07 for trust and 0.26 vs. 0.06 for trustworthiness. In the Swedish sample, the corresponding figures are 0.25 vs. −0.01 for trust and 0.29 vs. 0.18 for trustworthiness. Note, however, that the DZ correlations are estimated with less precision than the MZ correlations because of a smaller number of DZ twins in both of our samples.
Fig. 1.
Distribution of trust and trustworthiness responses, by zygosity. Blue bars indicate frequencies for MZ twins, and yellow bars indicate frequencies for DZ twins. Upper shows trust, which is the percentage of endowment sent by investor to trustee. Lower shows trustworthiness, which is the percentage returned by the trustee to the investor. Left shows results for Sweden, and Right shows results for the U.S. For amount sent, the Swedish experiment permitted only six choices, whereas the U.S. experiment permitted 11.
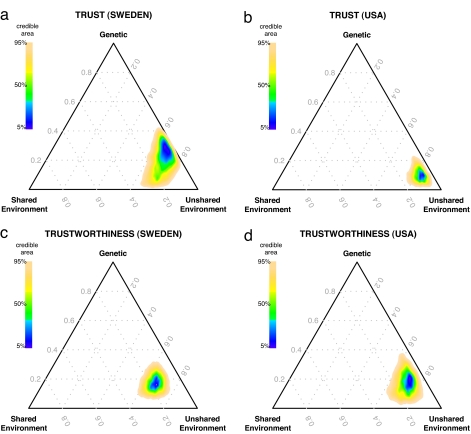
The results of our mixed-effects Bayesian ACE analysis suggest that variation in how subjects play the trust game is partially accounted for by genetic differences (Tables 2 and 3 and Fig. 2). In the ACE model of trust, the heritability estimate is 20% (C.I. 3–38%) in the Swedish experiment and 10% (C.I. 4–21%) in the U.S. experiment. The ACE model of trust also demonstrates that environmental variation plays a role. In particular, unshared environmental variation is a much more significant source of phenotypic variation than genetic variation (e2 = 68% vs. c2 = 12% in Sweden and e2 = 82% vs. c2 = 8% in the U.S.; P < 0.0001 in both samples). In the ACE model of trustworthiness, heritability (h2) generates 18% (C.I. 8–30%) of the variance in the Swedish experiment and 17% (C.I. 5–32%) in the U.S. experiment. Once again, environmental differences play a role (e2 = 66% vs. c2 = 17% in Sweden and e2 = 71% vs. c2 = 12% in the U.S.; P < 0.0001 in both samples).
Table 2.
Genetic models of trust (fraction sent in the trust game)
| Model | Heritability, h2 | Common environment, c2 | Unshared environment, e2 | DIC | Dbar | pD |
|---|---|---|---|---|---|---|
| Swedish sample | ||||||
| ACE | 0.20 (0.03, 0.38) | 0.12 (0.02, 0.31) | 0.68 (0.56, 0.81) | 13,087.54 | 12945.12 | 142.42 |
| AE | 0.32 (0.18, 0.45) | 0.68 (0.55, 0.82) | 13,084.79 | 12940.47 | 144.32 | |
| CE | 0.27 (0.14, 0.41) | 0.73 (0.60, 0.86) | 13,096.41 | 12974.09 | 122.32 | |
| E | 1.00 (1.00, 1.00) | 13,143.04 | 13141.02 | 2.02 | ||
| U.S. sample | ||||||
| ACE | 0.10 (0.04, 0.21) | 0.08 (0.03, 0.16) | 0.82 (0.72, 0.90) | 13,522.55 | 13409.79 | 112.75 |
| AE | 0.16 (0.06, 0.27) | 0.84 (0.73, 0.94) | 13,521.89 | 13421.22 | 100.67 | |
| CE | 0.13 (0.05, 0.22) | 0.87 (0.78, 0.95) | 13,529.61 | 13449.49 | 80.11 | |
| E | 1.00 (1.00, 1.00) | 13,542.53 | 13540.54 | 1.99 |
These results show that we consistently found a significant proportion of variance in trust is due to heritability, and that the best-fitting models are those that assume a role for genetic effects and the unshared environment (but not necessarily the common environment). The first column describes each model. ACE models estimate a parameter for genetic (A), common environment (C), and unshared environment (E); AE models assume the common environment has no effect, CE models assume there is no heritability, and E models assume only the unshared environment matters. Columns 2, 3, and 4 show the mean estimated proportion of total variance attributable to genetic effects (h2), common environment (c2), and unshared environment (e2), with 95% credible intervals indicated in parentheses below each estimate. Model fit is assessed using the deviance information criterion (DIC), which penalizes models for deviance (Dbar), capturing model fit, and the effective number of parameters (pD), capturing model complexity. The results show that the AE model generates the best fit for both the Swedish and U.S. samples. The empirical means, 95% credible intervals, and DICs are based on 1,000 draws from the posterior distribution.
Table 3.
Genetic models of trustworthiness (fraction returned in the trust game)
| Model | Heritability, h2 | Common environment, c2 | Unshared environment, e2 | DIC | Dbar | pD |
|---|---|---|---|---|---|---|
| Swedish sample | ||||||
| ACE | 0.18 (0.08, 0.30) | 0.17 (0.08, 0.28) | 0.66 (0.56, 0.75) | 11055.12 | 10889.78 | 165.34 |
| AE | 0.32 (0.18, 0.45) | 0.68 (0.55, 0.82) | 11057.50 | 10899.70 | 157.71 | |
| CE | 0.27 (0.14, 0.41) | 0.73 (0.60, 0.86) | 11064.40 | 10924.20 | 140.25 | |
| E | 1.00 (1.00, 1.00) | 11130.9 | 11128.90 | 2.00 | ||
| U.S. sample | ||||||
| ACE | 0.17 (0.05, 0.32) | 0.12 (0.04, 0.25) | 0.71 (0.60, 0.82) | 10626.82 | 10493.75 | 133.07 |
| AE | 0.28 (0.16, 0.40) | 0.72 (0.60, 0.84) | 10625.04 | 10494.13 | 130.91 | |
| CE | 0.24 (0.13, 0.34) | 0.76 (0.66, 0.87) | 10638.59 | 10529.65 | 108.94 | |
| E | 1.00 (1.00, 1.00) | 10680.27 | 10678.26 | 2.01 |
These results show that we consistently found a significant proportion of variability in trustworthiness is due to heritability, and that the best-fitting models are those that assume a role for genetic effects and the unshared environment (but not necessarily the common environment). For technical details, see Table 2 legend.
Fig. 2.
Heritable traits generate a significant fraction of the variation in trust and trustworthiness. Each triangle is a “ternary plot” that represents the proportions of three variance components (genetic, shared environment, and unshared environment) that sum up to one. A point in the center indicates all three components contribute equally, whereas a point at a vertex indicates that a single component fully explains the variance (i.e., a point at the top vertex indicates all variance is explained by heritability). Each ternary plot shows the posterior Bayesian distribution of estimated components of total variance in ACE models from independent experiments in Sweden and the U.S. Colors indicate credible areas calculated using 10,000 posterior draws to estimate a 2D kernel density of the most likely proportions given the data. The blue areas indicate the highest-density regions with the most credible estimates. The colored areas contain 95% of the draws (i.e., the probability that the true coefficients lie outside the colored regions is P = 0.05). Heritability (h2) of trust is estimated to be (a) 20% in Sweden and (b) 10% in the U.S. Heritability of trustworthiness is estimated to be (a) 18% in Sweden and (b) 17% in the U.S.
In Tables 2 and 3, we also provide fit statistics to compare results from an ACE model that includes all three factors with models that drop A, C, or both, to determine which factors significantly contribute to explaining the observed variance. These comparisons show that either the ACE or the AE model is the best-fitting model for trust and trustworthiness in both experiments, suggesting that heritability consistently and significantly contributes to variance in cooperative behavior. Additional analyses that separate the sample by age and gender do not generate significantly different results (see SI).
Despite differences‖ in these independently conducted experiments, the overall results are very similar. Moreover, we believe that the reported estimates indicate a lower bound on heritability and shared environment for two reasons. First, the estimate of the variance explained by the unshared environmental differences includes all idiosyncratic error, including measurement error. If our subjects had each played several rounds of the trust game with different individuals, our measures of trust and trustworthiness may have been more precise, which would have yielded higher estimates of heritability and common environmental influences. Second, one assumption of the ACE model is that there is no assortative mating with respect to the trait of interest. If preferences for cooperation are indeed heritable, and if people who cooperate tend to mate with other cooperative individuals, then this will increase the similarity in cooperative behavior in their children. This inflates the correlation of the genotypes of DZ siblings, making it harder to detect differences in MZ and DZ twins. As a result, the more assortativity, the more it biases downward the estimate of heritability.
Discussion
Our results are complementary to work on the neurological (15–17) and hormonal (18–20) substrates of behavior in the trust game and other similar social dilemma games. One imaging study demonstrates that people derive satisfaction from seeing cooperative norm violators being punished (17). Enhanced oxytocin levels have been documented in subjects who received a monetary transfer that reflected an intention of trust (18), and later work has demonstrated that exogenously administered oxytocin (19) increases trust. Scholars have also documented associations between cortisol (20) and trust. These hormonal studies, therefore, indicate that further study of polymorphisms of CYP11B1 (21), OXTR (22), and other genes involved in the expression and regulation of these hormones may explain part of the genetic effect on cooperation. In fact, one research team has already identified a polymorphism in the AVPR1a gene that is associated with related behavior in the dictator game (23).
There is by now a large literature on cooperation in the laboratory (4), but thus far, this literature has failed to address the heritability of interpersonal trust and trustworthiness. Two previous laboratory studies have examined cooperation between cotwins (24, 25) to test theories of inclusive fitness (5), whereas only one has focused on the question of how twins play an economic game with unrelated individuals (26). Our results thus suggest that humans are endowed with genetic variation that can partially account for differences in trust and trustworthiness when interacting with anonymous partners in the laboratory. Therefore, one might tentatively conclude this lends support to theories of human behavior in which cooperators and noncooperators coexist.
Genetic variation in human behavior is not always well understood, but recent work, motivated by consistent findings of heritability in a variety of personality traits, has argued that environmental heterogeneity could explain polymorphisms (28). If the fitness effects of a trait vary across time or space, then genetic variation can still be maintained in equilibrium (29). It is certainly possible to imagine that the fitness effects of trust and trustworthiness vary considerably according to context; although cooperators can achieve higher fitness when they interact with one another, they are also susceptible to predation by free-riding noncooperators. Although by no means conclusive, the finding of genetic variation in cooperative strategies would tend to favor evolutionary models that yield polymorphic equilibria over those that predict fixation on populations that always cooperate or always defect.**
Although we do find that genetic differences play a significant role for behavior in the classic trust game, the largest portion of the variance is explained by differences in unique environment. This is consistent with general results from the trust game that indicate behavior is more susceptible to state (unique mood, context) than trait (18). However, a result that may surprise some social scientists is that genetic differences appear to be a more important source of phenotypic variation than differences in common environment. This finding is in line with a broad consensus in the behavior genetics literature. Indeed, the second “law of behavior genetics” proposed by Turkheimer (30) is that the effect of being raised in the same family is generally smaller than the effect of genes.†† Thus, we urge social scientists to take seriously the idea that peer and parental socialization are not the only forces that explain differences in cooperative attitudes.
Materials and Methods
The Swedish study was undertaken in collaboration with the Swedish Twin Registry at Karolinska Institutet. The registry is the largest twin registry in the world and has been described in detail elsewhere (31). Same-sex twins born from 1960 to 1985 were solicited by e-mail and recruited in all major Swedish cities throughout the summer and autumn of 2006. A condition for participation was that both twins in a pair were able to attend the same experimental session. In total, 658 individuals (71 DZ and 258 MZ same-sex pairs of twins) participated. When the subjects arrived to the experimental session, they were seated apart and given oral general instructions about the experiments. Subjects played the trust game with other twins but never with their sibling. Zygosity was assigned by questionnaire items that have been shown to have a reliability of 95–98% (31).
The U.S. study had two phases. The first phase was conducted at the University of California, Davis, with 100 subjects. Subjects were not required to be twins; they were adults recruited from social science courses and were paid a $5 participation fee. They were also told they would be paid based on the outcome of a randomly selected game at the conclusion of the second phase of the study. The second phase was administered to same-sex adult twins in the research area at the annual Twins Days Festival in Twinsburg, OH, in August 2006 and 2007. This festival attracts ≈2,000 twins each year, many of whom volunteer for a number of studies. Same-sex twins 18 years of age and older were permitted to participate. A condition for participation was that both twins in a pair were able to attend the same experimental session. In total, 706 individuals participated (75 DZ and 278 MZ same-sex pairs of twins). Zygosity was determined by self-report, which has been used previously for studies of Twins Days participants (32). In particular, one study of 86 Twins Days subjects showed self-report to be 100% reliable compared with a genotypic assessment of zygosity (33). When the subjects arrived, they were given general instructions about the experiments and were asked not to communicate with one another. Subjects who had participated in 2006 were excluded from taking part in 2007.
Experimental instructions in their entirety and details on the estimation of model parameters are reported in SI.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Samuel Bowles, Terry Burnham, Nicholas Christakis, Eric Dickson, John Hibbing, Macartan Humphreys, Dominic Johnson, James Lee, Kevin McCabe, Rose McDermott, Richard McElreath, Moses Ndulu, Matt Notowidigdo, Steven Pinker, Pete Richerson, and Oleg Smirnov for helpful comments and discussions. A warm thank you to all the participants who made this work possible. The Swedish study was funded by the Jan Wallander and Tom Hedelius Foundation and the Swedish Research Council. The Swedish Twin Registry is supported by grants from the Swedish Research Council, the Ministry for Higher Education, and AstraZeneca. The U.S. study was funded by the Russell Sage Foundation; the Academic Senate Committee on Research at the University of California, San Diego; and the Institute of Government Affairs at the University of California, Davis. Patrik Ivert and Sandy Dawes provided research assistance, and the U.S. study thanks Eric Siry for the Twins Days festival artwork.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710069105/DC1.
There were several minor differences in implementation between the Swedish and U.S. experiments. In Sweden, subjects were paired with other twins (not their sibling) and paid several days later. Investors had six choices of how much to send the trustee, and trustees used the strategy method (they indicated how they would react to any possible amount sent prior to observing investor behavior). In contrast, subjects in the U.S. were paired with nontwins and paid immediately. Investors had 11 choices of the amount to send the trustee, and trustees observed investor behavior when deciding how much to send back.
Such a conclusion rests on the assumption that it is possible to draw some evolutionary inference from economic experiments. We believe this is likely to be the case but also acknowledge that the usual caveats are in order. For instance, weak ecological validity may render such inference difficult. For a careful discussion of the various methodological problems in eliciting trust experimentally, see Cox (27).
A relatively low estimate of common environmental influences means that in the two populations studied, differences in common environment are not an important source of phenotypic variation. This finding does not justify any conclusions about the influence of environmental manipulations in general. It is, for instance, possible that the reason why the estimated effect of common environment is so low is that there is little variation in how parents transmit trust attitudes to their children.
References
- 1.Knack S, Keefer P. Does social capital have an economic payoff? A cross-country investigation. Q J Econ. 1997;112:1251–1288. [Google Scholar]
- 2.Zak PJ, Knack S. Trust and Growth. Econ J. 2001;470:295–321. [Google Scholar]
- 3.Berg JE, Dickhaut J, McCabe K. Trust, reciprocity, and social history. Games Econ Behav. 1995;10:122–142. [Google Scholar]
- 4.Camerer CF. Behavioral Game Theory: Experiments in Strategic Interaction. Princeton, NJ: Princeton Univ Press; 2003. [Google Scholar]
- 5.Hamilton WD. The genetical theory of social behaviour. J Theor Biol. 1964;7:1–16. 17–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 6.Trivers R. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:35–57. [Google Scholar]
- 7.Nowak MA, Sigmund K. Evolution of indirect reciprocity. Nature. 2005;437:1291–1298. doi: 10.1038/nature04131. [DOI] [PubMed] [Google Scholar]
- 8.Boyd R, Gintis H, Bowles S, Richerson PJ. The evolution of altruistic punishment. Proc Natl Acad Sci USA. 2003;100:3531–3535. doi: 10.1073/pnas.0630443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurzban R, Houser D. Experiments investigating cooperative types in humans: A complement to evolutionary theory and simulations. Proc Natl Acad Sci USA. 2005;102:1803–1807. doi: 10.1073/pnas.0408759102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pam A, Kemker S, Ross CA, Golden R. The “equal environments assumption” in MZ-DZ twin comparisons: an untenable premise of psychiatric genetics? Acta Genet Med Gemellol (Roma) 1996;45:349–360. doi: 10.1017/s0001566000000945. [DOI] [PubMed] [Google Scholar]
- 11.Bouchard TJ. Genetic and environmental influences on adult intelligence and special mental abilities. Hum Biol. 1998;70:257–279. [PubMed] [Google Scholar]
- 12.Bouchard TJ, McGue M. Genetic and environmental influences on human psychological differences. J Neurobiol. 2003;54:4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- 13.Posner S, Baker LA, Martin NG. Social contact and attitude similarity in Australian twins. Behav Genet. 1996;26:123–134. doi: 10.1007/BF02359890. [DOI] [PubMed] [Google Scholar]
- 14.Liang K, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 15.de Quervain DJ, et al. The neural basis of altruistic punishment. Science. 2004;305:1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- 16.McCabe K, Houser D, Ryan L, Smith VL, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proc Natl Acad Sci USA. 2001;98:11832–11835. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer T, et al. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Kosfeld M, Heinrichs M, Zak P, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi TCA, et al. Interpersonal trust and social stress-induced cortisol elevation. NeuroReport. 2005;16:197–199. doi: 10.1097/00001756-200502080-00027. [DOI] [PubMed] [Google Scholar]
- 21.Hautanen A, et al. Associations between aldosterone synthase gene polymorphism and the adrenocortical function in males. J Intern Med. 1998;244:11–18. doi: 10.1046/j.1365-2796.1998.00308.x. [DOI] [PubMed] [Google Scholar]
- 22.Michelini S, Urbanek M, Dean M, Goldman D. Polymorphism and Genetic Mapping of the Human Oxytocin Receptor Gene on Chromosome 3. Am J Med Genet. 1995;60:183–187. doi: 10.1002/ajmg.1320600303. [DOI] [PubMed] [Google Scholar]
- 23.Knafo A, et al. Individual differences in allocation of funds in the dictator game associated with length of the arginine vasopressin 1a receptor (AVPR1a) RS3 promoter-region and correlation between RS3 length and hippocampal mRNA. Genes Brain Behav. 2007 doi: 10.1111/j.1601-183X.2007.00341.x. in press. [DOI] [PubMed] [Google Scholar]
- 24.Devi PU, Hossain M, Segal NL, Hershberger SL. Cooperation and competition between twins-peer and mother-child relationships. Evol Hum Behav. 1999;20:29–51. [Google Scholar]
- 25.Loh CY, Elliott JM. Cooperation and competition as a function of zygosity in 7- to 9-year-old twins. Evol Hum Behav. 1998;19:397–411. [Google Scholar]
- 26.Wallace B, Cesarini D, Lichtenstein P, Johannesson M. Heritability of ultimatum game responder behavior. Proc Natl Acad Sci USA. 2007;104:15631–15634. doi: 10.1073/pnas.0706642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox JC. How to identify trust and reciprocity. Games Econ Behav. 2004;46:260–281. [Google Scholar]
- 28.Penke L, Denissen JJA, Miller GF. The evolutionary genetics of personality. Eur J Person. 2007;21:549–587. [Google Scholar]
- 29.Roff DA. Evolutionary Quantitative Genetics. New York: Chapman & Hall; 1997. [Google Scholar]
- 30.Turkheimer E. Three laws of behavior genetics and what they mean. Curr Dir Psychol Sci. 2000;9:160–164. [Google Scholar]
- 31.Lichtenstein P, et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet. 2006;9:875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- 32.Ashenfelter O, Krueger A. Estimates of the economic return to schooling from a new sample of twins. Am Econ Rev. 1994;84:1157–1173. [Google Scholar]
- 33.Wise PM, Hansen JL, Reed DR, Breslin PAS. Twin study of the heritability of recognition thresholds for sour and salty taste. Chemical Senses. 2007;32:749–754. doi: 10.1093/chemse/bjm042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.