Abstract
Interstitial flow in and around tumor tissue affects the mechanical microenvironment to modulate tumor cell growth and metastasis. We investigated the roles of flow-induced shear stress in modulating cell cycle distribution in four tumor cell lines and the underlying mechanisms. In all four cell lines, incubation under static conditions for 24 or 48 h led to G0/G1 arrest; in contrast, shear stress (12 dynes/cm2) induced G2/M arrest. The molecular basis of the shear effect was analyzed, and the presentation on molecular mechanism is focused on human MG63 osteosarcoma cells. Shear stress induced increased expressions of cyclin B1 and p21CIP1 and decreased expressions of cyclins A, D1, and E, cyclin-dependent protein kinases (Cdk)-1, -2, -4, and -6, and p27KIP1 as well as a decrease in Cdk1 activity. Using specific antibodies and small interfering RNA, we found that the shear-induced G2/M arrest and corresponding changes in G2/M regulatory protein expression and activity were mediated by αvβ3 and β1 integrins through bone morphogenetic protein receptor type IA-specific Smad1 and Smad5. Shear stress also down-regulated runt-related transcription factor 2 (Runx2) binding activity and osteocalcin and alkaline phosphatase expressions in MG63 cells; these responses were mediated by αvβ3 and β1 integrins through Smad5. Our findings provide insights into the mechanism by which shear stress induces G2/M arrest in tumor cells and inhibits cell differentiation and demonstrate the importance of mechanical microenvironment in modulating molecular signaling, gene expression, cell cycle, and functions in tumor cells.
Keywords: mechanical force, BMP, differentiation, Runx2
Mechanical microenvironment plays important roles in modulating tissue development, maintenance, and remodeling and in cellular responses and functions (1). Interstitial fluid flow in and around tissue affects the mechanical microenvironment, including the shear stress and pressure force acting on the cell surface and the tethering force acting on cell–matrix connections (2). These interstitial flow-induced forces can modulate tumor metastasis and invasion as well as anticancer drug delivery (3).
Although the influence of interstitial fluid flow on tumor pathobiology and drug delivery has been studied, the effect of the flow-induced shear force on tumor cells has not been much explored. Compressive forces have been shown to inhibit tumor cell growth (4) and up-regulate adhesion molecules (5). A recent study reported that tumor cell proliferation is affected by intratumoral pressure and that activation of mitogen-activated protein kinases and nuclear antigen Ki-67 is involved in this mechanical modulation (6). Although these results show that mechanical forces can modulate tumor cell responses, the detailed mechanisms by which mechanical stimuli are transduced into cellular signaling to regulate tumor cell gene expression and functions remain unclear.
Integrins have been implicated as mechanosensors in many types of cells seeded on extracellular matrix (ECM) (7), but their role in modulating mechanical responses of tumor cells remains unclear. Smad proteins, including bone morphogenetic proteins (BMPs) receptor-regulated Smads (R-Smads, i.e., Smad1/5/8), play important roles in modulating tumorigenesis (8). BMPs transduce signals by means of heteromeric complex formation of cognate type I (i.e., BMPRIA or BMPRIB) and type II serine/threonine kinase receptors, leading to phosphorylation of Smads and activation of runt-related transcription factor 2 (Runx2), which plays essential roles in mesenchymal lineage cell differentiation (9). Smad proteins can modulate cell proliferation and cell cycle, which is controlled by its regulatory proteins, including cyclin-dependent protein kinases (Cdks) and their regulatory subunits cyclins, as well as inhibitors such as p27KIP1 and p21CIP1 (10). The functional significance of Smad proteins in modulating mechanical responses of tumor cells has not been reported.
We investigated the regulatory effects of shear stress on cell cycle distribution in four tumor cell lines and the underlying mechanism. In these tumor cells, shear stress induces a G2/M cell cycle arrest that is mediated by αvβ3 and β1 integrins through BMPRIA-specific Smad1/5 and accompanied by corresponding changes in cell cycle regulatory protein expression and activity. Shear stress down-regulates the Runx2 binding activity in MG63 cells and inhibits their differentiation. Our findings provide evidence for the importance of mechanical microenvironment in modulating tumor cell responses and tumor pathobiology and generate insights into mechanisms of shear-regulation of tumor cell cycle and differentiation.
Results
Shear Stress Induces G2/M Arrest and Corresponding Changes in Cell Cycle Regulatory Protein Expression in Tumor Cells.
Effects of shear stress in cell cycle distribution were studied in four tumor cell lines (human MG63 and Saos2 osteosarcoma cells, SCC25 oral squamous carcinoma cells, and SW1353 chondrosarcoma cells). Cells were kept as controls or subjected to shear stress (12 dynes/cm2 for 24 and 48 h). Flow cytometry showed that incubation of these tumor cells under static conditions for 24 or 48 h led to increases in cell percentage in G0/G1 phases and decreases in synthetic and/or G2/M phases (Table 1), indicating a G0/G1 arrest. Application of shear stress to these tumor cells caused significant increases in cell percentage in the G2/M phases and decreases in the G0/G1 phases compared with cells under static conditions for the same periods.
Table 1.
Shear stress induces a G2/M cell cycle arrest in tumor cells
| Cell type | Duration, h | Control, % cells (mean ± SEM) |
Shear, % cells (mean ± SEM) |
||||
|---|---|---|---|---|---|---|---|
| G0 /G1 | Synthetic | G2/M | G0 /G1 | Synthetic | G2/M | ||
| MG63 | |||||||
| 0 | 58.4 ± 7.2 | 31.1 ± 2.8 | 10.5 ± 1.2 | − | − | − | |
| 24 | 75.7 ± 5.4 | 16.9 ± 2.0* | 7.4 ± 1.1 | 64.4 ± 7.3 | 15.9 ± 1.2 | 19.7 ± 2.4† | |
| 48 | 84.5 ± 4.7* | 9.0 ± 1.3* | 6.5 ± 1.1* | 65.2 ± 4.9† | 8.2 ± 0.9 | 26.6 ± 3.4† | |
| Saos2 | |||||||
| 0 | 65.9 ± 5.2 | 14.6 ± 1.7 | 19.5 ± 2.1 | − | − | − | |
| 24 | 82.6 ± 3.7* | 9.0 ± 0.8* | 8.4 ± 0.9* | 50.9 ± 5.3† | 22.2 ± 3.2† | 26.9 ± 1.5† | |
| 48 | 86.5 ± 6.1* | 8.8 ± 0.6* | 4.7 ± 0.9* | 51.7 ± 6.5† | 12.7 ± 2.1 | 35.6 ± 4.2† | |
| SCC25 | |||||||
| 0 | 43.9 ± 3.2 | 39.9 ± 2.8 | 16.2 ± 1.3 | − | − | − | |
| 24 | 61.3 ± 2.3* | 19.9 ± 1.1* | 18.8 ± 1.2 | 54.7 ± 2.6 | 17.9 ± 1.5 | 27.4 ± 1.4† | |
| 48 | 64.3 ± 4.8* | 18.6 ± 2.5* | 17.1 ± 1.5 | 50.8 ± 2.2† | 17.4 ± 2.1 | 31.8 ± 2.9† | |
| SW1353 | |||||||
| 0 | 52.9 ± 3.8 | 32.9 ± 2.4 | 14.2 ± 2.1 | − | − | − | |
| 24 | 65.3 ± 5.8 | 26.7 ± 2.3 | 8.0 ± 1.0* | 53.1 ± 3.9 | 16.4 ± 2.1† | 30.5 ± 2.3† | |
| 48 | 66.7 ± 2.3* | 23.6 ± 1.7* | 9.7 ± 0.9* | 52.2 ± 2.7† | 11.5 ± 0.8† | 36.3 ± 4.3† | |
Four different tumor cell lines, i.e., human MG63 and Saos2 osteosarcoma cells, SCC25 oral squamous carcinoma cells, and SW1353 chondrosarcoma cells, were kept in static conditions as controls or subjected to a shear stress of 12 dynes/cm2 for 24 and 48 h. The cells were stained with propidium iodide and analyzed for DNA content by flow cytometry to show percentages of cells in G0/G1, synthetic, or G2/M phases of the cell cycle. Data are mean ± SEM from three independent experiments. *, P < 0.05 vs. static control cells at 0 h; †, P < 0.05 vs. static control cells at the corresponding time; −, no sample
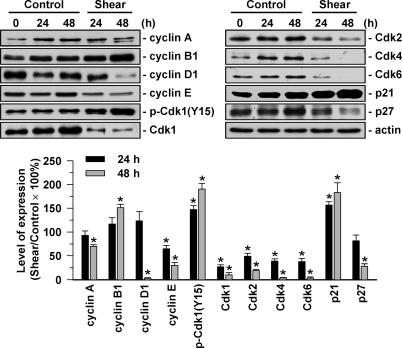
We investigated the molecular basis of this shear effect, and the presentation is focused on human MG63 osteosarcoma cells. Application of shear stress to MG63 cells for 24 or 48 h increased cyclin B1 and p21CIP1 expressions and decreased Cdk1 expression (Fig. 1). The decrease in Cdk1 expression was accompanied by an increase in its tyrosine 15 phosphorylation, indicating a shear-induced decrease in Cdk1 activity (11). Shear stress also decreased expressions of cyclins A, D1, and E, Cdk-2, -4, and -6, and p27KIP1.
Fig. 1.
Shear stress regulates expressions of cell cycle regulatory proteins in MG63 cells. MG63 cells were kept as controls or subjected to shear stress (12 dynes/cm2) for 24 and 48 h. Expression of cell cycle regulatory proteins was determined by Western blot analysis. Data are means ± SEM from three independent experiments. *, P < 0.05 vs. static control cells.
Shear Stress Induces Sustained Phosphorylations of Smad1/5 in MG63 Cells Through BMPRIA.
Application of shear stress to MG63 induced a rapid increase (within 10 min) in Smad1/5 phosphorylation, which reached a maximal level ≈10 times static controls within 1 h, and then declined but remained elevated after 24 h of shearing (Fig. 2A). The increases of Smad1/5 phosphorylation were similar with shear stresses of 2, 12, and 20 dynes/cm2, indicating that the shear-induced Smad1/5 activations were shear dose-independent over the range tested (Fig. 2B). Pretreating MG63 cells with Noggin, a specific antagonist that binds to BMPs to block their binding to the BMP receptors, did not inhibit the shear-induced Smad1/5 phosphorylation; hence the shear-activations of Smad1/5 were not mediated by BMPs (Fig. 2C). As a positive control, pretreatment of MG63 cells with Noggin did cause an inhibition of the Smad1/5 phosphorylation induced by BMP-4.
Fig. 2.
Shear stress induces sustained phosphorylation of Smad1/5 in MG63 cells through BMPRIA. (A) MG63 cells were kept as controls or subjected to shear stress (12 dynes/cm2) for 10 min, 30 min, 1 h, 3 h, 6 h, and 24 h, and their Smad 1/5 phosphorylation was determined by Western blot analysis. (B) MG63 cells were exposed to shear stress of 2, 12, and 20 dynes/cm2 for 30 min. (C) MG63 cells were kept as controls (−) or pretreated with Noggin (100 ng/ml) for 1 h (+N) and then subjected to flow or BMP-4 (100 ng/ml) for 30 min. (D) MG63 cells were transfected with siRNAs at various concentrations (5, 15, 30, and 40 nM), and their BMPRIA and BMPRIB protein expressions were examined by Western blot analysis. (E) MG63 cells were transfected with control siRNA (siCL) or specific siRNA of BMPRIA (siRIA) or BMPRIB (siRIB) (40 nM each) for 48 h and then kept as controls (C) or exposed to flow (S) for 30 min. Data in A and B are means ± SEM from three independent experiments and presented as percentage changes in band density from control cells normalized to Smad1/5 protein levels. The results in C–E are representative of triplicate experiments with similar results. *, P < 0.05 vs. static control cells.
To study the types of BMP receptor responsible for the shear-activation of Smad1/5, MG63 cells were transfected with BMPRIA- or BMPRIB-specific siRNA (40 nM), which reduced the expressions of corresponding receptor proteins by ≈2/3 of that with control siRNA (Fig. 2D), and then exposed to shear stress for 30 min. The shear-induced Smad1/5 phosphorylation was abolished by BMPRIA-specific siRNA, but not inhibited by BMPRIB-specific siRNA (Fig. 2E).
Shear-Induced G2/M Arrest in Tumor Cells Is Mediated by Smad1/5.
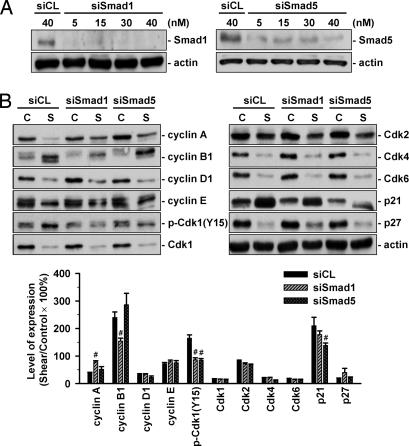
MG63 cells were transfected with Smad1- or Smad5-specific siRNA (40 nM), which caused 80–90% reductions in expressions of the corresponding Smad (Fig. 3A), and then kept under static conditions or exposed to flow for 48 h. Under static condition, MG63 cells transfected with Smad1- or Smad5-specific siRNA (compared with control siRNA, 40 nM) did not alter their cell cycle distribution (Table 2). After shear stress, MG63 cells transfected with either Smad5- or Smad1-specific siRNA had significantly higher cell percentage in G0/G1 and synthetic phases and a lower cell percentage in G2/M phases, when compared with cells transfected with control siRNA (Table 2). Transfection with Smad1- or Smad5-specific siRNA (compared with control siRNA) resulted in significant inhibitions of the shear-induced up-regulation of Cdk1 tyrosine 15 phosphorylation (Fig. 3B). Smad1-specific and Smad5-specific siRNAs have some differential actions, with the former inhibiting the shear-induced cyclin A down-regulation and cyclin B1 up-regulation and the latter the shear-induced p21CIP1 up-regulation. Neither Smad-specific siRNA had significant effects on the shear-induced changes of the other cell cycle regulatory proteins (Fig. 3B).
Fig. 3.
Shear-induced G2/M arrest in MG63 cells is mediated by Smad1/5. (A) MG63 cells were transfected with Smad1- or Smad5-specific siRNA at 5, 15, 30, or 40 nM for 48 h, and Smad1 and Smad5 protein expressions were determined by Western blot analysis. Results are representative of triplicate experiments with similar results. (B) MG63 cells were transfected with control siRNA (siCL) or specific siRNA of Smad1 (siSmad1) or Smad5 (siSamd5) (40 nM each) and then kept under static conditions (C) or exposed to flow (S) for 48 h. The expression of cell cycle regulatory proteins was determined by Western blot analysis. Data are means ± SEM from three independent experiments and are presented as percentage changes in band density from control cells normalized to Smad1/5 protein levels. #, P < 0.05 vs. cells transfected with control siRNA.
Table 2.
Shear-induced G2/M arrest in tumor cells is mediated by αvβ3 and β1 integrins and Smad1/5
| siRNA and antibody | Control, % cells (mean ± SEM) |
Shear, % cells (mean ± SEM) |
||||
|---|---|---|---|---|---|---|
| G0 /G1 | Synthetic | G2/M | G0 /G1 | Synthetic | G2/M | |
| siCL | 89.2 ± 3.8 | 5.7 ± 0.7 | 5.1 ± 1.2 | 65.9 ± 5.1* | 2.3 ± 0.5* | 31.8 ± 4.2* |
| siSmad1 | 86.8 ± 4.9 | 6.0 ± 1.0 | 7.2 ± 1.2 | 67.1 ± 6.1* | 16.2 ± 1.3*† | 16.7 ± 2.4*† |
| siSmad5 | 90.0±3.2 | 4.3 ± 0.7 | 5.7 ± 1.0 | 82.7 ± 7.3† | 12.9 ± 0.8*† | 4.4 ± 1.1† |
| IgG | 83.1 ± 4.9 | 7.2 ± 1.4 | 9.7 ± 0.8 | 63.2 ± 8.1* | 6.7 ± 1.1 | 30.1 ± 2.6* |
| Anti-αvβ3 | 86.1 ± 5.3 | 5.5 ± 0.9 | 8.4 ± 1.0 | 85.9 ± 6.8† | 8.6 ± 1.3 | 5.5 ± 1.1† |
| Anti-β1 | 83.2 ± 7.6 | 7.4 ± 0.8 | 9.4 ± 0.8 | 74.8 ± 9.8 | 13.9 ± 2.2*† | 11.3 ± 1.5† |
MG63 cells were transfected with control siRNA (siCL) or specific siRNA of Smad1 (siSmad1) or Smad5 (siSamd5) (40 nM for each) for 48 h or pretreated with control IgG or a specific antibody against αvβ3 (Anti-αvβ3) or β1 (Anti-β1) (10 μg/ml for each) for 2 h and then were kept under static conditions (Control) or exposed to flow (Shear) for 48 h. The cells were stained with propidium iodide and analyzed for DNA content by flow cytometry to show percentages of cells in G0/G1, synthetic, or G2/M phases of the cell cycle. Data are mean ± SEM from three independent experiments. *, P < 0.05 vs. static control cells; †, P < 0.05 vs. the cells transfected with control siRNA or pretreated with control IgG.
αvβ3 and β1 Integrins Mediate Shear-Induced Smad1/5 Phosphorylation and G2/M Arrest in Tumor Cells.
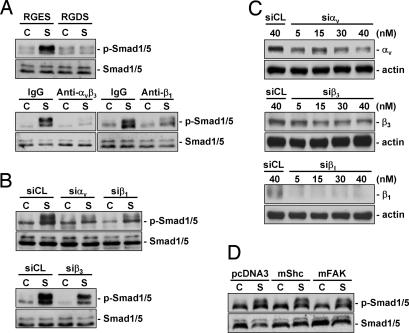
MG63 cells were pretreated with RGDS (Arg-Gly-Asp-Ser), which blocks the cell-ECM interaction mediated by the integrin-recognition sequence RGD (Arg-Gly-Asp) on ECM proteins, or with specific antibodies against αvβ3 and β1 integrins and then kept under static condition or exposed to flow for 30 min. Pretreatments with RGDS and integrin antibodies significantly inhibited the shear-induced Smad1/5 phosphorylation compared with cells pretreated with control RGES (Arg-Gly-Gla-Ser) or IgG (Fig. 4A). The inhibitions of shear-induced Smad1/5 phosphorylation by blocking αvβ3 and β1 integrins were substantiated by transfections of cells with αv-, β1-, and β3-specific siRNAs (40 nM for each), which also showed significant inhibitory effects on shear-induced Smad1/5 phosphorylation (Fig. 4B). αv- and β3-specific siRNAs had 50–60% blocking effects on their respective integrin expression; the β1-specific siRNA almost totally abolished the β1 expression (Fig. 4C). Transfections with dominant-negative mutants of Shc and focal adhesion kinase (FAK) (compared with empty vector pcDNA3) did not have significant effects on shear-induced Smad1/5 phosphorylation (Fig. 4D). Pretreatment of MG63 cells with specific antibodies against αvβ3 and β1 did not affect cell cycle distribution in static control cells, but under shear stress they caused significant increases in cell percentage in G0/G1 or synthetic phases and decreases in G2/M phases (Table 2).
Fig. 4.
Shear-induced Smad1/5 activations are mediated by αvβ3 and β1 integrins in MG63 cells. (A) MG63 cells were pretreated with RGDS peptides (500 μg/ml) or specific antibodies against β1 (Anti-β1) or αvβ3 (Anti-αvβ3) for 2 h (10 μg/ml each) and kept under static conditions (C) or subjected to shear stress (12 dynes/cm2) (S) for 30 min. As controls, the cells were pretreated with RGES (500 μg/ml) and nonspecific control IgG (10 μg/ml). (B) MG63 cells were transfected with control siRNA (siCL) or specific siRNA of αv (siαv), β1 (siβ1), or β3 (siβ3) (40 nM each) for 48 h before exposure to flow. (C) MG63 cells were transfected with specific siRNAs of integrins at 5, 15, 30, or 40 nM, and their integrin protein expressions were examined. (D) MG63 cells were transfected with 3 μg of Shc-SH2 (mShc), FAK(F397Y) (mFAK), or pcDNA3 empty vector for 48 h before exposure to flow (S). Results are representative of triplicate experiments with similar results.
Shear Stress Inhibits MG63 Cell Differentiation and Runx2 Binding Activity in the Nucleus.
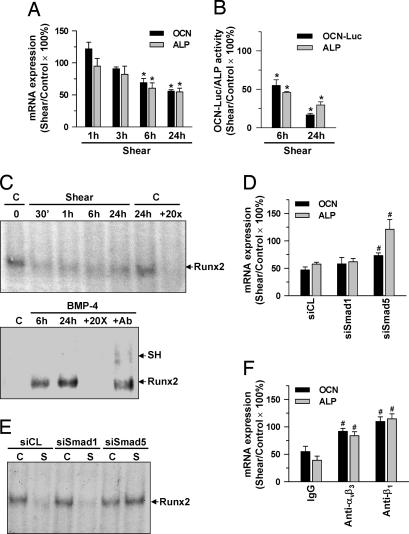
MG63 cells were kept as controls or exposed to shear stress for 1, 3, 6, and 24 h, and their expressions of differentiation marker genes, i.e., osteocalcin (OCN) and alkaline phosphatase (ALP), were examined. Shearing for 6 and 24 h resulted in significant decreases in OCN and ALP gene expressions (Fig. 5A). These effects of shearing on MG63 cells were substantiated by the shear-induced decreases of the activities of ALP and luciferase, which was determined by transfection of cells with OCN-Luc containing the promoter region of the human OCN gene in front of the luciferase gene (Fig. 5B). Because the promoter regions of the OCN and ALP genes contain the Runx2-binding domain that is responsible for the modulation of these genes (9), we tested whether shear stress regulates the Runx2 binding activity in MG63 cells. The results of electrophoretic mobility shift assay (EMSA) obtained by incubating nuclear protein extracts of the cells with oligonucleotides corresponding to the Runx2-binding sequences of the OCN promoter showed that shear stress caused sustained decreases in the Runx2 binding activity over the period tested (24 h of flow) (Fig. 5C). As positive controls, treatment of MG63 cells with BMP-4 for 6 and 24 h induced Runx2 binding activity. The specificity of this binding for Runx2 was shown by its abolition by coincubation of nuclear proteins with 20-fold unlabeled oligonucleotides and by supershifting in the gel mobility after preincubation of nuclear proteins with an antibody to Runx2.
Fig. 5.
MG63 cell differentiation and Runx2 binding activity in the nucleus were inhibited by shear stress acting through αvβ3 and β1 integrins and Smad5. (A–C) MG63 cells were kept as controls (C) or subjected to shear stress (12 dynes/cm2) for 30 min to 24 h, as indicated. (B, D–F) The cells were transfected with empty vector control PSRα or OCN-Luc (1 μg/ml each) (B), transfected with siRNA (siCL for control, or specific siRNA of Smad1 or Smad5) (40 nM each) (D and E) for 48 h, or pretreated with control IgG or a specific antibody against αvβ3 or β1 (10 μg/ml each) for 2 h (F) and then were kept under static conditions or subjected to shear stress for 6 and 24 h (B) or 24 h (D–F). The OCN and ALP mRNA expressions (A, D, and E), OCN promoter and ALP activities (B), and Runx2-DNA binding activity (C and E) were determined by real-time PCR, luciferase and ALP activity assays, and EMSA, respectively. In C, total nuclear extracts of cells and 32P-labeled oligonucleotides containing human OCN Runx2-binding sites were used. Nuclear extracts were preincubated with 20-fold excess unlabeled oligonucleotides (+20×). As positive controls, MG63 cells were treated with BMP-4 (100 ng/ml) for 6 and 24 h (C). Nuclear extracts preincubated with the Runx2 antibody (+Ab) show a super shift band (SH). Results in A, B, D, and F are means ± SEM from three to four independent experiments. Results in C and E are representative of two or three independent experiments with similar results. *, P < 0.05 vs. static control cells (A and B); #, P < 0.05 vs. control treatments (D and F).
αvβ3 and β1 Integrins and Smad5 Mediate Shear-Induced Inhibition of MG63 Cell Differentiation.
Transfection of MG63 cells with specific siRNA of Smad5 (compared with control siRNA), but not Smad1, inhibited the shear-induced down-regulations of OCN and ALP expressions (Fig. 5D) and nuclear binding activity for Runx2 (Fig. 5E). The shear-induced down-regulations of OCN and ALP expressions were also inhibited by pretreating MG63 cells with a specific antibody against αvβ3 or β1 (Fig. 5F). These results suggest that the shear-induced inhibition of MG63 cell differentiation was mediated by αvβ3 and β1 integrins through Smad5, but not Smad1.
Discussion
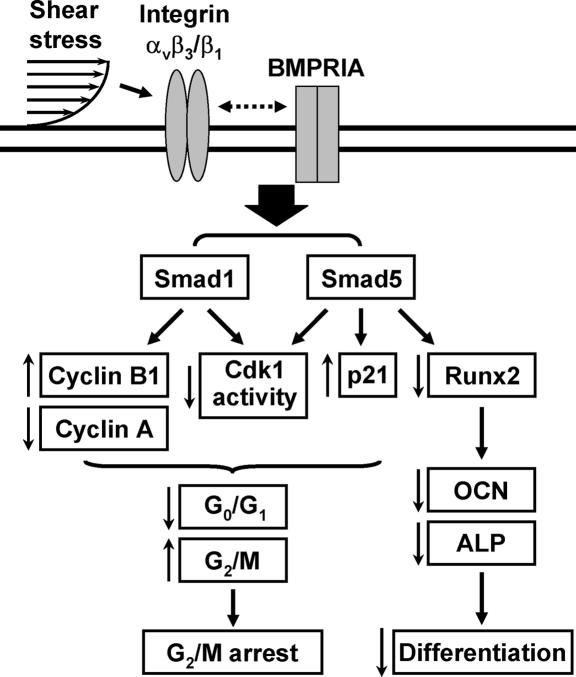
The aim of this study was to investigate the roles of shear stress in cell cycle distribution in tumor cells and the underlying mechanism. In a series of systematic studies, we have characterized the mechanisms by which shear stress regulates cell cycle in tumor cells through specific integrins and their modulations of BMP receptor-specific Smads, as summarized in Fig. 6. Our study has generated the following findings: (i) This work shows that shear stress induces a G2/M cell cycle arrest in several types of tumor cells. This shear-induced G2/M arrest is associated with corresponding changes in the expression and activity of G2/M regulatory proteins. (ii) Our study showed a signaling pathway from integrins to BMPRIA-specific Smads (independent of BMPs) that modulates the expression of cell cycle regulatory proteins and hence cell cycle distribution in tumor cells in response to mechanical forces. (iii) In addition to regulating cell cycle, shear stress can inhibit the differentiation of osteosarcoma cells by inhibiting Runx2 binding activity in the nucleus. These shear-induced inhibitions in Runx2 activity and cell differentiation are mediated by αvβ3 and β1 integrins through Smad5 but not Smad1 (Fig. 6). Thus, our findings provide insights into the molecular mechanisms by which flow-induced shear force regulates cell cycle and differentiation in tumor cells.
Fig. 6.
Schematic representation of the signaling pathways regulating cell cycle and differentiation in tumor cells in response to shear stress. ↑, up-regulation by shear; ↓, down-regulation by shear; dotted double-arrow line represents the interaction pathway that has not been defined.
Cell cycle arrest is a major cellular response to DNA damage before the decision to repair or die. In cell cycle progression, increasing accumulations of cyclin D-Cdk4/6 and cyclin A/E-Cdk2 complexes regulate the transition through G1 and synthetic phases. The subsequent G2–M transition is mainly regulated by cyclin B-Cdk1 activity, which is suppressed by Cdk1 phosphorylation on tyrosine 15 during G2/M (11). Recent studies showed that tumor cell cycle arrest at G2/M is associated with an increase in cyclin B1 (12). In addition to inhibiting Cdk-2, -4, and -6 activities and promoting G1-synthetic phase transition, p21CIP1 can accumulate in nuclei near the G2/M boundary and cause a transient block in late G2 phase (10). These reports suggest that a decrease in Cdk1 activity and increases in cyclin B1 and p21CIP1 expressions can promote cell cycle arrest at the G2/M phases. In the present study, we found that shear stress caused sustained decreases in Cdk1 activity (due to Cdk1 tyrosine 15 phosphorylation) and increases in cyclin B1 and p21CIP1 expressions; these shear-induced modulations of G2/M regulatory protein expression and activity would contribute to the shear-induced G2/M arrest in tumor cells.
BMPRIA-specific Smad signaling has been shown to suppress tumorigenesis at gastric epithelial junctional zones (8). Mice with a conditional inactivation of the BMPRIA or overexpressing a BMP antagonist in the intestine develop intestinal tumors. Mice with Smad4-deficient T cells develop epithelial cancers in the intestinal tract (13). These results suggest that the BMPRIA-specific Smad signaling may play tumor-suppressive roles. Using specific siRNAs of different BMP receptors (i.e., BMPRIA and BMPRIB), our study showed that the shear-induced Smad1/5 activation was mediated by BMPRIA. This shear-induced Smad1/5 activation was not due to autocrine effect of BMPs released from sheared cells, because it is not inhibited by a specific BMP antagonist Noggin. Our results also demonstrated that the shear-induced BMPRIA-specific Smad1/5 activation contributes to the shear-induced G2/M arrest in tumor cells by modulating the expression and activity of G2/M regulatory proteins. Thus, shear stress may play tumor-suppressive roles by inducing a G2/M cell cycle arrest in tumor cells through the BMP-independent activation of BMPRIA-specific Smad signaling pathway.
The blockade of the shear-induced Smad1/5 phosphorylation by using RGD peptide, specific integrin antibodies, and specific siRNAs provides evidence that αvβ3 and β1 integrins act as upstream signaling molecules for the shear-induced Smad1/5 activation in tumor cells. The detailed mechanism by which αvβ3 and β1 mediate shear-induced Smad1/5 activation remains unclear. It is not likely that Shc and FAK participate in the modulation of shear-induced Smad1/5 activation by integrins, because transfection of MG63 cells with their dominant-negative mutants did not inhibit the Smad1/5 response to shear. There is evidence that integrins may be cooperative with receptors of several growth factors, including insulin receptor and platelet-derived growth factor-β receptor, to form integrin–receptor heteromeric complexes in mediating downstream signaling cascades (14). Our coimmunoprecipitation experiments using an antibody against αvβ3 or β1, followed by Western blot analysis using an antibody against BMPRIA or BMPRIB, however, did not show increases in the formation of integrin-BMP receptor complexes in the sheared MG63 cells (data not shown). The mechanism by which integrins mediate BMPRIA-specific Smad signaling in tumor cells in response to shear stress warrants further investigations.
In addition to being an important regulatory transcription factor for mesenchymal lineage cell differentiation (9), Runx2 has been shown to activate expressions of adhesion proteins, matrix metalloproteinases, and angiogenic factors in tumor cells and promote tumor metastasis (15). Inhibition of Runx2 in MDA-MB-231 cells transplanted to bone inhibits tumorigenesis and prevents osteolysis (16). These results suggest that inhibition of Runx2 activity may have therapeutic potential against tumor development. Our demonstration that shear stress induces a down-regulation of Runx2 activity in human osteosarcoma cells support the notion that shear stress may act as a tumor suppressor by inhibiting Runx2 activity in tumor cells, thereby inhibiting tumor growth and metastasis.
It is difficult to directly measure interstitial flow velocities and the resulting shear stresses in vivo because of their slowness and heterogeneity (2). Measured velocities have been reported to vary between 0.1 and 4.0 μm/s (17, 18). Using a mathematical model of interstitial pressure-fluid flow, Jain et al. (19) showed that the interstitial fluid velocity in tumors is nearly zero in the center and increases rapidly in the periphery. In the present study, we studied one carcinoma line (i.e., human SCC25 oral squamous carcinoma cells) and three sarcoma lines (i.e., human MG63 and Saos2 osteosarcoma cells and SW1353 chondrosarcoma cells). These sarcoma cells are originated from tumors in bone and cartilage, whose formation and development are highly influenced by mechanical microenvironment. Because the interstitial flow-induced shear stress on bone cells in response to mechanical loading has been found to be 8–30 dynes/cm2 in vivo (20), we used a shear stress level of 12 dynes/cm2, which would be more relevant to the periphery of tumors (19), to investigate the roles of shear stress in modulating tumor cell signaling, gene expression, cell cycle, and differentiation. Our results suggest that mechanical forces play significant roles in modulating tumor cell responses and functions (cell cycle and differentiation). Normalization of tumor vasculature has emerged as an important concept in antiangiogenic therapy, and this may alter interstitial flow environment and enhance drug delivery (3, 19). Our findings suggest that the response of tumor cells to changes in interstitial flow-induced shear force should be considered in the management of the disease.
Another implication of our study is the potential role of shear stress in tumor metastasis. Tumor cells leaving a primary tumor are subjected to shear stress in blood and lymphatic vessels. There is evidence that tumor cell metastasis occurs mainly to some specific tissues/organs, e.g., liver (21), but not to others, e.g., heart and large arterial wall (22). It is possible that such region-specificity for tumor metastasis is related to the different shear stress/flow patterns. The present findings suggest that tissues/organs receiving laminar shear stress with a large forward component (12 dynes/cm2) would result in G2/M arrest of the metastatic tumor cells; such G2/M arrest would facilitate the removal of tumor cells by the immune system (23). There is evidence that disturbed flow with a very low net shear stress has effects on intracellular signaling and cell cycle that are opposite to those of shear stress with a large forward flow (24, 25). Therefore, it is possible that tumor cells in regions receiving disturbed/low shear flow would not undergo G2/M arrest and their metastasis/invasion would thus be promoted. Thus, different flow patterns may have differential actions on tumor cell metastasis or invasion, and the effects of disturbed flow on tumor cell cycle deserve further investigations.
In summary, the present study demonstrated that shear stress induces a G2/M cell cycle arrest in tumor cells and inhibits cell differentiation. These shear-induced responses are mediated by αvβ3 and β1 integrins through their modulations of BMPRIA-specific Smad activation, which leads to changes in expression and activity of cell cycle regulatory protein and inhibition of Runx2 binding activity. It is well accepted that mechanical microenvironment is a fundamental determinant of cell behavior and tissue remodeling (1). The current study advances the new notion that mechanical forces are natural regulators of tumor biology. Our data on shear-modulations of cell cycle and differentiation in tumor cells suggest that mechanical microenvironment of tumor cells may play important roles in tumor development and pathology, and should be taken into account in tumor therapy and management.
Materials and Methods
Materials.
Mouse monoclonal antibodies (mAbs) against cyclin E and Cdk2, goat polyclonal antibodies (pAbs) against Smad1/5 and Runx2, and BMPRIA- and BMPRIB-specific siRNAs were purchased from Santa Cruz Biotechnology. Mouse mAbs against cyclins A, B1, D1, Cdk-4 and -6, and p21CIP1, and rabbit pAbs against p27KIP1, Cdk1, phospho-Cdk1(Y15), and phospho-Smad1/5 were purchased from Cell Signaling Technology. Mouse mAbs against human αvβ3 and β1 integrins were purchased from Chemicon. The dominant-negative mutants of Shc (Shc-SH2) and FAK [FAK(F397Y)] were described (26, 27). The control siRNA and specific siRNAs of Smad1, Smad5, and αv, β1, and β3 integrins were purchased from Invitrogen. The OCN promoter construct was a gift from Leland W. K. Chung (Department of Urology, Emory University School of Medicine, Atlanta, GA). All other chemicals of reagent grade were obtained from Sigma, unless otherwise noted.
Cell Culture.
MG63, Saos2, SCC25, and SW1353 tumor cell lines were obtained from American Type Culture Collection and cultured in DMEM (GIBCO) supplemented with 10% FBS (GIBCO). Cells (≈1–2 × 105 cells per cm2) were trypsinized and seeded onto glass slides (75 × 38 mm; Corning) precoated with type I collagen (30 μg/ml). The medium was then exchanged with DMEM containing only 2% FBS for incubating the cells for 24 h before the experiment.
Statistical Analysis.
Results are given as mean ± SEM. Statistical tests were performed with an independent Student t test for two groups of data and ANOVA, followed by Scheffé's test for multiple comparisons. P value <0.05 was considered significant.
The following procedures are provided in supporting information (SI) Text: flow apparatus, primer designs, flow cytometry, ALP activity assay, RNA isolation and real-time PCR, Western blots, immunoprecipitation, treatments with RGD peptides and antibodies, reporter gene construct, DNA plasmids, siRNA, transfection, and luciferase assays, and EMSA.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. J. Y. Chang for helpful discussion and support. This work was supported by National Health Research Institutes (Taiwan) Grant ME-096-PP-06 (to J.-J.C.); National Science Council (Taiwan) Grants 96-3112-B-400-009 and 96-2628-B-400-002-MY3 (to J.-J.C.), 96-2627-B-010-002 (to C.-K.C.), 95-2113-M-009-025 (to C.A.C.), and 96-3112-B-400-013 (to J. Y. Chang); and National Heart, Lung, and Blood Institute Grants HL080518 and HL085159 (to S.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712353105/DC1.
References
- 1.Nerem RM. Tissue engineering: The hope, the hype, and the future. Tissue Eng. 2006;12:1143–1150. doi: 10.1089/ten.2006.12.1143. [DOI] [PubMed] [Google Scholar]
- 2.Rutkowski JM, Swartz MA. A driving force for change: Interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 4.Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 5.Koike C, et al. Solid stress facilitates spheroid formation: Potential involvement of hyaluronan. Br J Cancer. 2002;86:947–953. doi: 10.1038/sj.bjc.6600158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmann M, et al. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 8.Bleuming SA, et al. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149–8155. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 9.Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357:1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graña X, Reddy EP. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 11.Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: A membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- 12.Kuo CC, et al. BPR0L075, a novel synthetic indole compound with antimitotic activity in human cancer cells, exerts effective antitumoral activity in vivo. Cancer Res. 2004;64:4621–4628. doi: 10.1158/0008-5472.CAN-03-3474. [DOI] [PubMed] [Google Scholar]
- 13.Kim BG, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 14.Schneller M, Vuori K, Ruoslahti E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratap J, et al. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol. 2005;25:8581–8591. doi: 10.1128/MCB.25.19.8581-8591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javed A, et al. Impaired intranuclear trafficking of Runx2 (AML3/CBFA1) transcription factors in breast cancer cells inhibits osteolysis in vivo. Proc Natl Acad Sci USA. 2005;102:1454–1459. doi: 10.1073/pnas.0409121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci USA. 1989;86:5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dafni H, Israely T, Bhujwalla ZM, Benjamin LE, Neeman M. Overexpression of vascular endothelial growth factor 165 drives peritumor interstitial convection and induces lymphatic drain: Magnetic resonance imaging, confocal microscopy, and histological tracking of triple-labeled albumin. Cancer Res. 2002;62:6731–6739. [PubMed] [Google Scholar]
- 19.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 21.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 22.Raaf HN, Raaf JH. Sarcomas related to the heart and vasculature. Semin Surg Oncol. 1994;10:374–382. doi: 10.1002/ssu.2980100511. [DOI] [PubMed] [Google Scholar]
- 23.Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis: Do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007;8:444–448. doi: 10.1016/S1470-2045(07)70140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao H, et al. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: In-vivo and in-vitro investigations. J Vasc Res. 2005;42:77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
- 25.Wang N, et al. Shear stress regulation of Krüppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006;341:1244–1251. doi: 10.1016/j.bbrc.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 26.Chen KD, et al. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 27.Li S, et al. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem. 1997;272:30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.