Abstract
P-glycoprotein (MDR-1) is a well-known transporter that mediates efflux of chemotherapeutic agents from the intracellular milieu and thereby contributes to drug resistance. MDR-1 also is expressed by nonmalignant cells, including leukocytes, but physiologic functions for MDR-1 are poorly defined. Using an initial screening assay that included >100 mAbs, we observed that neutralizing mAbs MRK16, UIC2, and 4E3 against MDR-1 specifically and potently blocked basal-to-apical transendothelial migration of mononuclear phagocytes, a process that may mimic their migration into lymphatic vessels. Antagonists of MDR-1 then were used in a model of authentic lymphatic clearance. In this model, antigen-presenting dendritic cells (DC) migrate out of explants of cultured human skin and into the culture medium via dermal lymphatic vessels. DC and T cells derived from skin expressed MDR-1 on their surfaces. Addition of anti-MDR-1 mAbs MRK16, UIC2, or the MDR-1 antagonist verapamil to skin explants at the onset of culture inhibited the appearance of DC, and accompanying T cells, in the culture medium by approximately 70%. Isotype-matched control mAbs against other DC molecules including CD18, CD31, and major histocompatibility complex I did not block. In the presence of MDR-1 antagonists, epidermal DC were retained in the epidermis, in contrast to control conditions. In summary, this work identifies a physiologic function for MDR-1 during the mobilization of DC and begins to elucidate how these critical antigen-presenting cells migrate from the periphery to lymph nodes to initiate T lymphocyte-mediated immunity.
Immune responses in a peripheral organ like skin are initiated after antigen-presenting cells, particularly dendritic cells (DC), capture antigens locally and migrate via afferent lymphatic vessels to draining lymph nodes. T lymphocytes continually recirculate through lymph nodes, so the newly arrived, antigen-bearing DC become positioned to select lymphocytes that bear receptors for the presented antigens (1). For example, when skin is transplanted (2), or a contact allergen such as poison ivy is applied (3), epidermal DC (also termed Langerhans cells) migrate from their epithelial locations and carry antigens or allergens to the lymph node. If the lymphatic conduits to the draining lymph nodes are severed, the immune system does not become primed to the antigen (2). As they migrate, the DC undergo maturation, increasing their expression of molecules involved in antigen presentation, including major histocompatibility complex (MHC) II products, CD80 (B7–1), and CD86 (B7–2) (4, 5).
Models for the induced emigration of DC and T lymphocytes from the periphery have been developed by using cultured explants of skin. Beginning within 1–2 days after the onset of culture, DC in the explanted skin migrate into dermal lymphatics (6) and emerge via these conduits into the culture medium, along with T lymphocytes (7, 8). Here we describe that antagonism of p-glycoprotein (MDR-1), a molecule well known for its ability to transport of a broad spectrum of xenobiotics out of cells and thereby induce drug resistance (for review see ref. 9), blocks the migration of DC and T lymphocytes out of cultured human skin explants. Thus, these data identify a unique physiologic function for MDR-1.
MATERIALS AND METHODS
Transendothelial Migration Assays.
By using a method detailed previously (10, 11), freshly isolated peripheral blood mononuclear cells (PBMC), approximately one-fourth of which are monocytes, were added to confluent monolayers of unstimulated human umbilical vein endothelial cells (HUVEC) grown on type I bovine collagen gels with or without addition of mAb. Cultures were incubated for 1.5 hr; during this time, monocytes, but very few lymphocytes, transmigrate into the collagen (10). To examine their subsequent basal-to-apical transendothelial migration, monocytes were allowed to accumulate beneath the endothelium in the absence of added mAb. Then monolayers were washed twice in Medium 199 (Life Technologies, Grand Island, NY) to remove nonadherent cells from the apical surface, and individual wells received aliquots of Medium 199 containing 20% heat-inactivated human serum with or without added mAbs as indicated. After 24 hr of incubation, cultures were washed twice in Medium 199, and control medium or medium containing mAb was replenished. After a total time of 48 hr in culture, samples were fixed for microscopic analysis. Differential interference contrast optics were used to quantitate the number of mononuclear phagocytes (MP) that were beneath the endothelial monolayer (11) in at least five high-power fields per sample. Each experiment was conducted with six replicates per parameter tested.
Skin Cultures.
Human split-thickness skin was obtained from the New York Firefighter’s Skin Bank (New York Hospital-Cornell Medical Center) from cadavers within 24 hr of death, or from patients undergoing plastic surgery, and was authorized for use in research. Generally, dermatomes were approximately 300 μm thick, including both epidermis (about 100 μm) and a portion of the dermis. Skin was prepared and cultured, and emigrated cells were quantitated as previously described (7). Each explant was trimmed to 400-mm2 and floated in 3–6 ml of culture medium. When used, mAbs or verapamil (Sigma) were added to the culture medium at the onset of culture, and cultures were incubated undisturbed until the indicated day of analysis. Verapamil was prepared as a concentrated stock in methanol. Final methanol concentration in cultures was 0.03% (vol/vol).
Immunostaining and Flow Cytometry.
Skin explants were separated into epidermal and dermal sheets by treatment with 0.5 M ammonium thiocyanate for 20 min at 37°C (12). Alternatively, skin samples were snap-frozen in OCT compound (Miles) and then used for the preparation of thick sections (approximately 100 μm) cut parallel to the epidermis (6) or thin cross-sections (10 μm). After either procedure, sheets of skin were fixed in acetone for 5 min before staining. Sheets of epidermis or thick sections were incubated overnight at 4°C in Eppendorf tubes with Cy3-conjugated goat anti-mouse IgG (1:200; Jackson ImmunoResearch), 1:20 fluorescein isothiocyanate-conjugated anti-MHC II (Becton Dickinson), or anti-MHC II 9.3C9 hybridoma supernatant. Tissues were washed three times in PBS containing 1% Tween 20, 15 min each at room temperature. For detection of 9.3C9 when it was used, Cy3-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) was added for 2 hr at 4°C, followed by three washes as just described. Staining for flow cytometry was carried out as previously detailed (7).
In some experiments, explants were incubated in dispase II (Boehringer Mannheim) for 45 min at 37°C to separate the epidermis from dermis. Pieces of epidermis were submerged in 0.05% trypsin/0.53 mM EDTA for 45 min. Single cell suspensions were generated by gently pressing epidermal pieces through a fine mesh. Aliquots of digested cells were incubated with fluorescein isothiocyanate-labeled anti-MHC II for 30 min on ice, washed twice, and analyzed by flow cytometry. Some samples were labeled for two-color flow cytometry using R-phycoerythrin-conjugated anti-CD86 (PharMingen).
Immunoblots.
Emigrated DC were purified by negative selection. Anti-CD3 mAb was incubated with emigrated cells, and T lymphocytes were removed by using anti-mouse IgG-conjugated magnetic beads (Dynal). The purity of the resulting population was verified by flow cytometric evaluation after staining with phycoerythrin-conjugated anti-MHC II (Becton Dickinson). Microsomal membranes from 5 million thus purified DC, 25 million PBMC, or 5 million HUVEC were prepared as described (13), subjected to SDS/PAGE (4–12% gradient gel) under reducing conditions, and electroblotted onto a nitrocellulose membrane. The membrane was probed with 5 μg/ml of C219 anti-MDR-1 mAb (Centocor) and visualized by using peroxidase-conjugated anti-mouse IgG, followed by ECL substrate (Amersham).
Efflux Assays.
A previously published method to detect functional MDR-1 (14) was used with minor modifications. Emigrated cells were incubated in RPMI medium 1640 containing 20 ng/ml of DiOC2 (Molecular Probes) for 15 min at 37°C, washed twice in ice-cold medium, resuspended in 10% fetal bovine serum/RPMI medium 1640 containing no additives or with addition of 10 μg/ml of UIC2 mAb (Coulter). After 90 min of incubation at 37°C, cells were washed and immediately placed on ice for labeling with phycoerythrin-conjugated anti-MHC II mAb and analysis by flow cytometry.
RESULTS
Role of MDR-1 in Basal-to-Apical Transendothelial Migration of MP.
Our attention was drawn to MDR-1 by a set of observations with blood monocytes, now known to be a precursor of DC when appropriately stimulated with cytokines (15–19). In these initial experiments, we examined the migratory behavior of monocytes in an in vitro model of the blood vessel wall consisting of HUVEC grown on type I collagen gels (10, 11). Human blood monocytes, which express MDR-1 (ref. 20 and data not shown), transmigrate across the confluent endothelium and enter the collagenous substrate. This initial migration into the collagen, which is completed within 1 hr, was unaffected by the addition of anti-MDR-1 mAb, but was partially blocked by mAbs against the β2 chain of integrins (CD18) and CD31, as described (10, 11) (Fig. 1A).
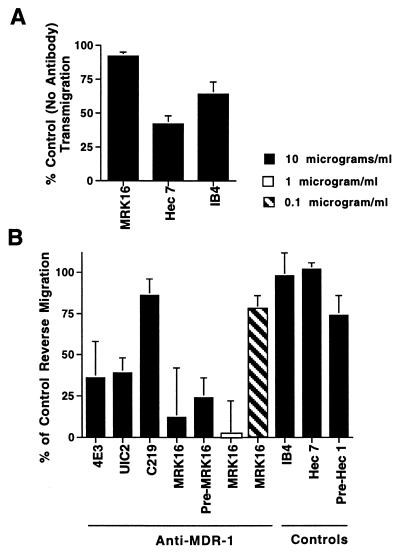
Figure 1.
Effect of anti-MDR-1 mAbs on transendothelial migration of MP. (A) Monocytes were allowed to migrate across endothelial monolayers with or without addition of anti-MDR-1 mAb MRK16, or mAb to the β2 integrin (IB4) or CD31 (hec 7). Cultures were incubated for 1.5 hr. (B) In experiments to examine their subsequent basal-to-apical transmigration, monocytes were allowed to accumulate beneath the endothelium in the absence of added mAb. Then monolayers were washed, and individual wells received aliquots of culture medium with or without added mAbs [all IgG2a; MRK16 used as F(ab′)2 fragments] at the indicated concentrations. In some samples, PBMC were preincubated with MRK16 or isotype-matched control mAb hec 1 against cadherin 5 (Pre-MRK16 and Pre-Hec 1) before addition to the endothelium. After 48 hr, cultures were fixed for analysis. Data are plotted relative to control levels of reverse transmigration (no mAb added), in which 50% of the subendothelial MP retraverse the endothelium in 48 hr. Bars represent means ± SD from 3–10 experiments.
After a brief residence in the collagen, a majority of subendothelial MP retraverse the overlying, intact endothelium with a t1/2 of 24–48 hr (21). This reverse transendothelial migration was not prevented by mAb to CD18 or CD31 (Fig. 1B). However, screening of mAbs (>100) submitted to the VIth International Workshop for Human Leukocyte Differentiation Antigens revealed that neutralizing mAbs to MDR-1 strongly inhibited reverse transmigration. UIC2 (22), 4E3 (23), and MRK16 (24) against extracellular domains of MDR-1 inhibited reverse transmigration by 61%, 64%, and 78%, respectively (Fig. 1B). MRK16, used here as F(ab′)2 fragments, was the most potent blocking mAb, exhibiting maximal levels of inhibition using as little as 0.5–1.0 μg/ml. C219 (25), which binds an intracellular epitope of MDR-1, had no effect. Preincubation of monocytes with MRK16 and subsequent removal of unbound mAb before addition of these cells to the endothelium blocked reverse transmigration as well as when the mAb was present continuously during the assay (Pre-MRK16, Fig. 1B). No other mAbs screened inhibited reverse transmigration, except one against tissue factor, whose possible specific role in reverse transmigration is currently under investigation.
Role of MDR-1 in Mobilization of DC and T Lymphocytes from Skin.
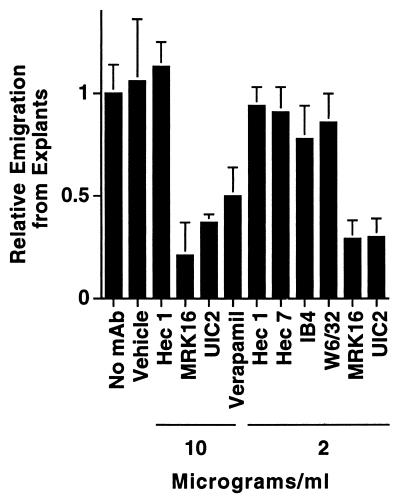
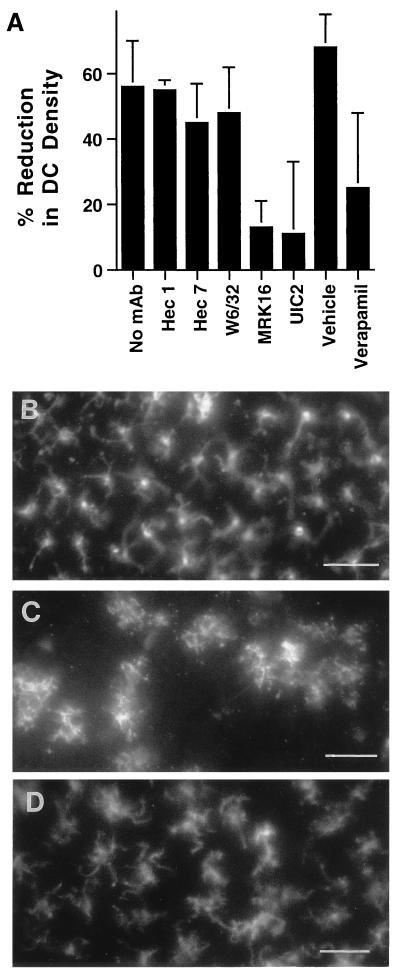
During reverse transmigration, MP migrate across endothelium in a basal-to-apical direction, a movement that is reminiscent of the normal trafficking of DC from the periphery into afferent lymphatics. We therefore applied a panel of mAbs, including those against MDR-1, to cultured human skin explants. Appearance of DC and T lymphocytes in the culture medium of the explants was reduced by 71% ± 9%, and 79% ± 16% when the skin was incubated in the presence of MRK16 at concentrations of 2 and 10 μg/ml, respectively (Fig. 2). Verapamil, a drug that antagonizes MDR-1 transport (26) reduced DC and T lymphocyte accumulation in the culture medium by 50% ± 13% at 10 μg/ml (20 μM) (Fig. 2). The anti-MDR-1 mAb UIC2 also blocked migration, by 70% ± 9% when used at 2 μg/ml (Fig. 2). Isotype-matched mAbs that did not inhibit migration included nonbinding control mAb hec1 against cadherin 5 (11) and binding control mAb W6/32 against MHC I. DC express CD18 and CD31, but mAbs against these established leukocyte-endothelial cell adhesion molecules did not block their migration (Fig. 2). Under all conditions, the cellular content of emigres ranged from 40% to 60% DC, with the remaining population being T lymphocytes. Thus, antagonists of MDR-1 blocked migration of DC and T lymphocyte uniformly. Cell viability in all groups was >90%, as assessed by trypan blue exclusion.
Figure 2.
Effect of anti-MDR-1 mAb on emigration of DC and T lymphocytes from skin explants. Explants of human skin were floated in culture medium without added mAb (n = 10) or in medium containing anti-MDR-1 mAbs MRK16 (n = 10) or UIC2 (n = 3), anti-cadherin 5 mAb hec 1 (n = 5), anti-CD31 mAb hec 7 (n = 5), anti-MHC I mAb W6/32 (n = 3), anti-CD18 mAb IB4 (n = 2), verapamil (n = 5), or the vehicle control for verapamil 0.03% methanol (Vehicle, n = 3). After 3 days of incubation, DC and T lymphocytes that appeared in the culture medium were collected and counted as previously described (7). Each condition was tested in triplicate; n = number of experiments in which condition was included. The number of emigrated cells recovered from individual control explants was typically 5 × 105. To compare data from different experiments, the mean number of emigrated cells in the absence of added mAb in each experiment was set equal to 1.0, and relative values were obtained for the remaining data.
Expression of MDR-1 by DC.
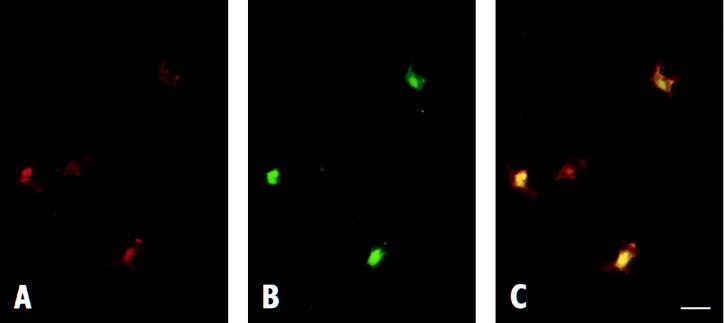
T lymphocytes are known to express MDR-1 (14, 20, 27), but its expression by DC has not been reported previously. In some experiments, skin was cultured in the presence of MRK16 for 2 days, as for migration analysis. Then staining was carried out on sections prepared from snap-frozen skin, applying only anti-mouse Cy3-conjugated detection antibody (Fig. 3). Immunostaining for MDR-1 was observed on DC in the epidermis (Fig. 3 A and C) and dermis (not shown). The same cells also stained positively for the DC marker MHC II (Fig. 3 B and C). A minority of MHC II+ cells in the epidermis and dermis were found that did not clearly express MDR-1. A similar pattern of MDR-1 expression was observed in explants of skin that were not cultured (day 0, not shown). No cell types in the skin other than DC or T cells, such as keratinocytes or fibroblasts, showed positive staining for MDR-1. This approach illustrates not only the presence of MDR-1 on epidermal DC in situ, but also demonstrates that mAb applied during culture gains sufficient access to the epidermis. Skin incubated with isotype-matched control mAb hec 1 did not show positive staining.
Figure 3.
Expression of MDR-1 in situ by DC. (A) Epidermis derived from explants cultured with anti-MDR-1 mAb MRK16 were fixed, and Cy3-conjugated anti-mouse IgG was added to detect expression of MDR-1 (red). Addition of Cy3-labeled detection antibody to skin incubated in the presence of nonbinding control mAb (hec1) showed no staining (not shown). The section shown was cut parallel to the epidermal basement membrane. (B) Immunostaining of same sample using fluorescein isothiocyanate-conjugated anti-MHC II mAb (green). (C) Doubly exposed frame to examine colocalization (yellow) of MDR-1 and MHCII. (Bar is 10 μm.)
Flow cytometric analysis of the cells that emigrated from control explants using double labeling with anti-MDR-1 (using either MRK16 or UIC2) and anti-MHC II mAbs demonstrated high levels of MDR-1 on all DC in some experiments (Fig. 4A) or on a majority, but not all, DC in other experiments (Fig. 4B). The fraction of DC staining positively was always greater than the fraction of positively stained subset of T lymphocytes (Fig. 4C).
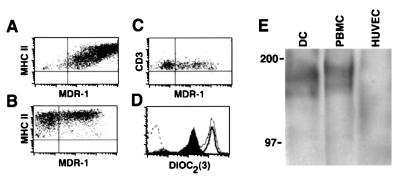
Figure 4.
Expression of functional MDR-1 by emigrated DC. (A-C) MDR-1 expression in emigrated skin cells was examined by flow cytometry by using double staining with anti-MDR-1 mAb and the DC lineage marker MHC II (A and B) or the T lymphocyte lineage marker CD3 (C). Quadrants were marked based on the level of fluorescence intensity observed in samples stained with negative control mAbs. (D) For studies measuring efflux, emigrated cells were loaded with the dye DiOC2 (dashed line, no dye added) and cultured at 37°C for 90 min without addition of mAb (filled profile), in the presence of UIC2 mAb (bold line), or kept at 4°C for this duration (thin line). Analysis of efflux in the DC fraction of emigrants was assessed by double labeling with phycoerythrin-conjugated anti-MHC II mAb for flow cytometry. Figure shown was gated on MHC II+ cells. (E) Membranes prepared from purified, emigrated DC, PBMC, or HUVEC were immunoblotted with anti-MDR-1 mAb C219. Numbers to the left of bands are molecular weight markers.
Expression of authentic MDR-1 by DC was evident in two assays. To document expression of functional MDR-1 (28), DC were loaded with a synthetic, fluorescent substrate for MDR-1, DiOC2. At 37°C, but not 4°C, DC transported this substrate into the surrounding medium, showing a log decrease in fluorescence intensity (Fig. 4D), and this efflux was inhibited by anti-MDR-1 mAb UIC2 (Fig. 4D). To identify MDR-1 molecules in DC, crude membranes were prepared and immunoblotted by using anti-MDR-1 mAb C219. Two bands of approximately 170–180 kDa, the reported molecular mass of MDR-1, were identified (Fig. 4E). This pattern of reactivity was similar to that seen in membranes from PBMC and was not observed in HUVEC (Fig. 4E), which do not express MDR-1.
Neither extracellular expression of MDR-1 nor efflux of DiOC2 were observed in fully mature DC (19) that were differentiated in culture from monocytes (data not shown). Thus, expression of MDR-1 by DC appears to be regulated by environmental or maturational events.
Effect of MDR-1 Antagonists on Maturation and Retention of DC in Epidermis.
To examine the step at which emigration of DC from the explants was inhibited by anti-MDR-1 mAb, epidermal and dermal sheets prepared from cultured skin explants were stained for MHC II (29, 30). Compared with the number present at the onset of culture (Fig. 5 A and B), the number of DC remaining in the epidermis after three days of culture under control conditions was decreased by 56% (Fig. 5 A and C). In explants cultured in the presence of MRK16 (Fig. 5 A and D), UIC2 (Fig. 5A), or verapamil (Fig. 5A), a more limited reduction in DC density of 13%, 11%, and 25%, respectively, were observed. Thus, antagonism of MDR-1 results in retention of DC in the epidermis.
Figure 5.
Retention of DC in the epidermis after treatment with MDR-1 antagonists. (A) Epidermal sheets were stained with anti-MHC II mAb to enumerate DC before the onset of culture or after 3 days of culture in the absence of mAb (no mAb, n = 7), or in the presence of anti-cadherin 5 mAb hec 1 (n = 3); anti-MHC I mAb W6/32 (n = 3); anti-CD31 mAb hec 7 (n = 3); anti-MDR-1 mAb MRK16 (n = 6); anti-MDR-1 mAb UIC2 (n = 1); verapamil (n = 2); or the vehicle control for verapamil 0.03% methanol (Vehicle, n = 1). n = number of experiments in which each condition was examined. DC were counted from en face examinations of epidermal sheets in 16–20 high-power fields per experiment. Percent reduction in DC density was calculated by comparing the number of DC in cultured explants to the mean number present in a portion of the same skin sample before culture (typically 75 cells/field). (B–D) Photomicrographs show the distribution of DC within the epidermis before culturing of explants (B), after 3 days of culture under control conditions (no mAb) (C), and after 3 days of culture in the presence of MRK16 (D). (Bar is 50 μm.)
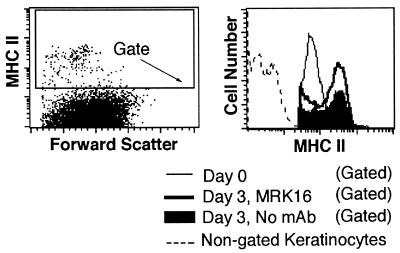
Maturation accompanies migration of DC from the epidermis. In cultures treated with anti-MDR-1 mAb, some DC retained in the epidermis appeared morphologically similar to epidermal DC before the onset of culture (compare Fig. 5 B and D), but others showed evidence of maturation resembling that observed under control conditions (Fig. 5C and data not shown), including an increase in cell size and greater intensity of staining for MHC II (4, 29, 30). Flow cytometric analysis of DC enzymatically removed from the epidermis after culture in the presence of anti-MDR-1 mAb MRK16 or under control conditions indicated that the maturation of DC was not inhibited by anti-MDR-1, as exemplified by the up-regulation of MHC II (Fig. 6) and CD86 (not shown). Thus, antagonism of MDR-1 strongly impedes the migration of DC, but only inconsistently or neglibly suppresses their maturation. However, we cannot exclude the possibility that some aspects of maturation not examined here were affected by antagonists of MDR-1.
Figure 6.
Effect of anti-MDR-1 mAb on the maturation of DC. The levels of MHC II expressed by epidermal DC were analyzed in gated epidermal suspensions by flow cytometry before the onset of culture (Day 0) or after 3 days of culture in the absence of mAb or in the presence of MRK16. Single cell suspensions of the epidermis were prepared by digestion with dispase followed by trypsin. Keratinocytes and other skin cells were excluded from the analysis by setting a gate to include only MHC II+ cells.
DISCUSSION
In this study, we initially identified mAbs to MDR-1 as potent inhibitors of basal-to-apical transendothelial migration of MP in an in vitro model of a vessel wall. The profound effect of anti-MDR-1 mAbs in this model contrasted with the lack of inhibition observed when approximately 100 other mAbs against HUVEC or MP surface proteins were applied, underscoring the specificity of the effect seen with the use of anti-MDR-1 reagents. A previous study postulated that reverse transmigration in this model may mimic the migration of leukocytes into lymphatic vessels (21), because both processes involve basal-to-apical transmigration from tissues across endothelium. Accordingly, when we added MDR-1 antagonists to cultured explants of human skin, from which DC emigrate via lymphatic vessels (6), we observed a specific inhibition of their mobilization. The close correlation between our findings in the two models suggest that the vessel wall model may be a simple, useful tool for future use in further identifying molecular events that regulate migration into authentic lymphatic vessels.
The mechanism by which MDR-1 acts to facilitate migration remains unclear. It is unlikely that MDR-1 acts as a mediator of adhesion. Before leaving the epidermis, DC down-regulate E-cadherin (31), allowing retraction from neighboring keratinocytes. In the presence of anti-α6 mAb, epidermal DC up-regulate MHC II and retract from neighboring keratinocytes (32), but fail to migrate out of the epidermis. Another adhesion molecule, CD44, also is used by DC during mobilization from the epidermis (33). As with anti-α6, we found that up-regulation of MHC II was not blocked by anti-MDR-1. However, our finding that epidermal DC generally maintained their interdigitating appearance in the presence of anti-MDR-1 mAb, in contrast to our control cultures and the effect of anti-α6 mAb (32), suggests that anti-MDR-1 may interfere with a signal that directs DC to release from the epidermis.
Based on its well-known action as a membrane transporter, MDR-1 likely mediates the translocation of a soluble, endogenous substrate(s), as yet unidentified, that regulates migration. MDR-1 can transport a wide variety of synthetic membrane lipids from the inner to the outer leaflet of the plasma membrane (34). However, the identity of a possible natural lipid substrate is still uncertain. Two candidate substrates, ceramide and prostaglandin E2, are known to affect maturation and activation of DC (35, 36). Another proposed substrate (34), platelet-activating factor, is chemotactic for DC in vitro (37).
MDR-1 also may transport endogenous polypeptides, as do a number of its homologs (38). In accord with a role in protein trafficking, functional MDR-1 appears to be required for optimal secretion of the polypeptide cytokine interleukin (IL) 2, and possibly other cytokines (39, 40). IL-1β mediates the migration of epidermal DC from human skin explants in response to contact allergens (41). In murine models of contact sensitivity, the cytokines IL-1β and tumor necrosis factor (TNF) α are required for the migration of epidermal DC to lymph nodes (42, 43). In these animals, neutralizing anti-TNF-α mAb blocks the migration of epidermal DC, but not up-regulation of MHC II. Moreover, just as in our studies, DC retained in the epidermis by anti-TNF-α remained interdigitated among surrounding keratinocytes (43). The possibility that MDR-1 activity is necessary for the secretion of IL-1β or TNF-α is under investigation.
Induction or up-regulation of MDR-1 in cancerous cells leads to drug resistance, a major obstacle in chemotherapeutic treatment strategies (9). Antagonists of MDR-1 are used clinically to combat the drug-transport activity of MDR-1 with the aim of improving the treatment of xenobiotic-resistant cancers (44). Prophylactic treatment with anti-MDR-1 reagents even in the absence of concurrent chemotherapy also has been proposed (44). Recently, other approaches that harness the antigen-presenting capacity of DC to enhance immune rejection of cancerous cells have emerged as potentially potent anticancer regimens (45, 46). Our findings, implicating a role for MDR-1 in the mobilization of DC to lymph nodes, highlight the wisdom of temporally segregating anti-MDR-1 treatments to suppress drug resistance from immunotherapy targeting DC, because antagonism of MDR-1 may limit successful initiation of immune responses.
This conclusion contrasts with the findings of Schinkel et al. (47), who report that transgenic mice lacking mdr-1-type p-glycoproteins (mdr1a/1b−/−) have no apparent physiologic defects and, therefore, suggest that antagonism of p-glycoproteins during cancer treatments should not interfere with physiologic functions. However, DC function and migration were not specifically analyzed in these mice. Routine histologic analysis of lymph nodes appeared normal. Our findings suggest that immunologic function in mdr1a/1b−/− mice needs further assessment. It is possible, too, that important differences in function and representation by functionally redundant molecules exist between human and murine p-glycoproteins.
Based on the present work, we propose that MDR-1 acts as an upstream component in a cascade of events that regulates traffic of leukocytes, particularly DC, out of tissues via lymphatic conduits. These data, in addition to demonstrating a role for MDR-1 beyond protection against xenobiotic compounds, stand to serve as a springboard in the identification of physiologic substrates for MDR-1 and in furthering the study of mechanisms that initiate T lymphocyte-dependent immune responses.
Acknowledgments
We are grateful to the New York Firefighter’s Skin Bank, Dr. John Goncalves, and Mr. Darren Esposito at Cornell University for preparing skin specimens. This research was supported by Grants HL46849, AI13013, and AI40045 from the National Institutes of Health. G.J.R. was supported by National Research Service Award HL09722. S.B. was the recipient of a fellowship from the National Health and Research Development Program; AIDS, Health and Welfare, Canada. W.A.M. is an Established Investigator of the American Heart Association.
ABBREVIATIONS
- DC
dendritic cells
- HUVEC
human umbilical vein endothelial cells
- MDR-1
MDR-1-type p-glycoprotein
- MHC
major histocompatibility complex
- MP
mononuclear phagocytes
- PBMC
peripheral blood mononuclear cells
References
- 1.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Barker C F, Billingham R E. J Exp Med. 1968;128:197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frey J R, Wenk P. Int Arch Allergy Appl Immunol. 1957;11:81–100. doi: 10.1159/000228405. [DOI] [PubMed] [Google Scholar]
- 4.Aiba S, Katz S I. J Immunol. 1990;145:2791–2796. [PubMed] [Google Scholar]
- 5.Larsen C P, Ritchie S C, Hendrix R, Linsley P S, Hathcock K S, Hodes R J, Lowry R P, Pearson T C. J Immunol. 1994;152:5208–5219. [PubMed] [Google Scholar]
- 6.Lukas M, Stössel J, Hefel L, Imamura S, Fritsch P, Sepp N T, Schuler G, Romani N. J Invest Dermatol. 1996;106:1293–1299. doi: 10.1111/1523-1747.ep12349010. [DOI] [PubMed] [Google Scholar]
- 7.Pope M, Betjes M G H, Hirmand H, Hoffman L, Steinman R M. J Invest Dermatol. 1995;104:11–17. doi: 10.1111/1523-1747.ep12613452. [DOI] [PubMed] [Google Scholar]
- 8.Steinman R, Hoffman L, Pope M. J Invest Dermatol. 1995;105:2S–7S. doi: 10.1111/1523-1747.ep12315162. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 10.Muller W A, Weigl S A. J Exp Med. 1992;176:819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller W A, Weigl S A, Deng X, Phillips D M. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lennart J, Shelley W B. Acta Dermatoverner. 1977;57:289–296. [Google Scholar]
- 13.Gerlach J H, Bell D R, Karakousis C, Slocum H K, Kartner N, Rustum Y M, Ling V, Baker R M. J Clin Oncol. 1987;5:1452–1460. doi: 10.1200/JCO.1987.5.9.1452. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary P M, Mechetner E B, Roninson I B. Blood. 1992;80:2735–2739. [PubMed] [Google Scholar]
- 15.Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch P O, Steinman R M, Schuler G. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1117. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiertscher S M, Roth M D. J Leukocyte Biol. 1996;59:208–218. doi: 10.1002/jlb.59.2.208. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L J, Tedder T F. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 20.Drach D, Zhao S, Drach J, Mahadevia R, Gattringer C, Huber H, Andreeff M. Blood. 1992;80:2729–2734. [PubMed] [Google Scholar]
- 21.Randolph G J, Furie M B. J Exp Med. 1996;183:451–462. doi: 10.1084/jem.183.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mechetner E B, Roninson I B. Proc Natl Acad Sci USA. 1992;89:5824–5828. doi: 10.1073/pnas.89.13.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arceci R J, Stieglitz K, Bras J, Schinkel A, Baas F, Croop J. Cancer Res. 1993;53:310–317. [PubMed] [Google Scholar]
- 24.Hamada H, Tsuruo T. Proc Natl Acad Sci USA. 1986;83:7785–7789. doi: 10.1073/pnas.83.20.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kartner N, Evernden-Porelle D, Bradley G, Ling V. Nature (London) 1985;316:820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- 26.Ford J M, Hait W N. Pharmacol Rev. 1990;42:155–199. [PubMed] [Google Scholar]
- 27.Klimecki W T, Futscher B W, Grogan T M, Dalton W S. Blood. 1994;83:2451–2458. [PubMed] [Google Scholar]
- 28.Chaudhary P M, Roninson I B. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 29.Larsen C P, Steinman R M, Witmer-Pack M, Hankins D F, Morris P J, Austyn J M. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierre P, Turley S J, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman R M, Mellman I. Nature (London) 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 31.Schwarzenberger K, Udey M C. J Invest Dermatol. 1996;106:553–558. doi: 10.1111/1523-1747.ep12344019. [DOI] [PubMed] [Google Scholar]
- 32.Price A A, Cumberbatch M, Kimber I, Ager A. J Exp Med. 1997;186:1725–1735. doi: 10.1084/jem.186.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss J M, Sleeman J, Renkl A C, Dittmar H, Termeer C C, Taxis S, Howells N, Hofmann M, Köhler G, Schöpf E, et al. J Cell Biol. 1997;137:1137–1147. doi: 10.1083/jcb.137.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Helvoort A, Smith A J, Sprong H, Fritzsche I, Schinkel A H, Borst P, van Meer G. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Nicolò C, De Maria R, Corinti S, Testi R. J Exp Med. 1996;184:2411–2416. doi: 10.1084/jem.184.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieser C, Böck G, Klocker H, Bartsch G, Thurnher M. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sozanni S, Longoni D, Bonecchi R, Luini W, Bersani L, D’Amico G, Borsatti A, Bussolino F, Allavena P, Mantovani A. FEBS Lett. 1997;418:98–100. doi: 10.1016/s0014-5793(97)01358-6. [DOI] [PubMed] [Google Scholar]
- 38.Leveille-Webster C R, Arias I M. J Membr Biol. 1995;143:89–102. doi: 10.1007/BF00234655. [DOI] [PubMed] [Google Scholar]
- 39.Drach J, Gsur A, Hamilton G, Zhao S, Angerler J, Fiegl M, Zojer N, Raderer M, Haberl I, Andreeff M, Huber J. Blood. 1996;88:1747–1754. [PubMed] [Google Scholar]
- 40.Raghu G, Park S W, Roninson I B, Mechetner E B. Exp Hematol. 1996;24:1258–1264. [PubMed] [Google Scholar]
- 41.Rambukkana A, Pistoor F H M, Bos J D, Kapsenberg M L, Das P K. Lab Invest. 1996;74:422–435. [PubMed] [Google Scholar]
- 42.Cumberbatch M, Kimber I. Immunology. 1995;84:31–35. [PMC free article] [PubMed] [Google Scholar]
- 43.Cumberbatch M, Dearman R J, Kimber I. Immunology. 1997;92:388–395. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sikic B I. Semin Hematol. 1997;34:40–47. [PubMed] [Google Scholar]
- 45.Hsu F J, Benike C, Fagnoni F, Liles T M, Czerwinski D, Taidi B, Engleman E G, Levy R. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 46.Hsu F J, Caspar C B, Czerwinski D, Kwak L W, Liles T M, Syrengelas A, Taidi-Laskowski B, Levy R. Blood. 1997;89:3129–3135. [PubMed] [Google Scholar]
- 47.Schinkel A H, Mayer U, Wagenaar E, Mol C A A M, van Deemter L, Smit J J M, van der Valk M A, Voordouw A C, Spits H, van Tellingen O, et al. Proc Natl Acad Sci USA. 1997;94:4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]