Abstract
Understanding transdifferentiation—the conversion of one differentiated cell type into another—is important from both basic science and clinical perspectives. In Caenorhabditis elegans, an epithelial cell named Y is initially part of the rectum but later appears to withdraw, migrate, and then become a motor neuron named PDA. Here, we show that this represents a bona fide transdifferentiation event: Y has epithelial hallmarks without detectable neural characteristics, and PDA has no residual epithelial characteristics. Using available mutants and laser microsurgery, we found that transdifferentiation does not depend on fusion with a neighboring cell or require migration of Y away from the rectum, that other rectal epithelial cells are not competent to transdifferentiate, and that transdifferentiation requires the EGL-5 and SEM-4 transcription factors and LIN-12/Notch signaling. Our results establish Y-to-PDA transdifferentiation as a genetically tractable model for deciphering the mechanisms underlying cellular plasticity in vivo.
Keywords: cell plasticity, motor neuron, rectum, hindgut
Although it is commonly believed that commitment and differentiation are stable events, in fact, under some circumstances, committed or differentiated cells have the ability to change their fates (1). Various examples of cell plasticity, from the reprogramming of a nucleus through cloning to the reprogramming of tissue stem cells, have suggested that the final identity of a cell is not locked. Transdifferentiation, the process by which one differentiated cell type changes into another directly (2, 3), is one kind of cell plasticity.
Classic work on the complete cell lineage of Caenorhabditis elegans is consistent with the possibility that transdifferentiation occurs naturally during C. elegans development: Observation of nuclear division and morphology using Nomarski microscopy suggests that a few cells seem to change identity during larval development (4). However, for any of these apparent identity changes to be true examples of transdifferentiation, it must be established that the cell is fully differentiated into different cell types both before and after the apparent transdifferentiation event (2).
Here, we have focused on a cell called “Y,” which is born in the embryo and forms part of the rectum until the second larval stage, when it rescinds from the rectum, migrates anteriorly, and becomes a motor neuron named PDA (4–6). PDA has a characteristic axonal process and synaptic connections that have been described at the ultrastructural level (5, 6). Here, we demonstrate the epithelial nature of Y by ultrastructural and molecular criteria and show that it does not express neuronal markers, establishing it as a fully differentiated rectal epithelial cell. We also show that PDA lacks expression of epithelial markers and has specific neuronal characteristics. Thus, the Y-to-PDA change appears to be a bona fide example of transdifferentiation. We also perform an initial characterization of this process, using genetics and cell ablation to explore factors pertaining to competence, lineage, and local environment.
Results
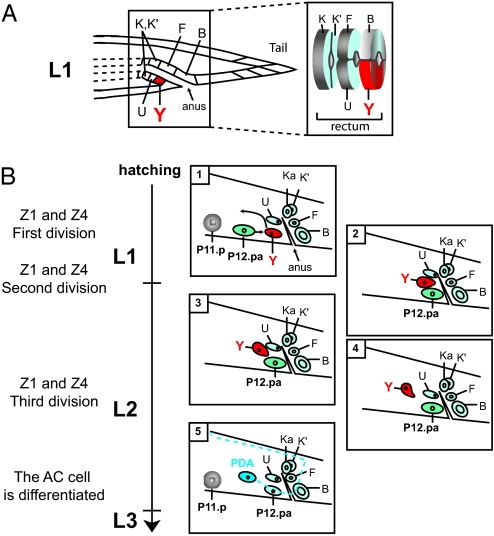
The rectum is a vital organ formed during embryogenesis and is made of three pairs of cells, named K and K′, U and F, and Y and B. Each pair forms a toroid of the rectal epithelium (Fig. 1A). Based on nuclear morphology and position, Sulston et al. (4) reported that during the second larval stage (L2), Y rescinds from the rectum and migrates anterodorsally. Another cell, named P12.pa, born at the end of the L1 stage just anterior to the position of Y, replaces Y in the rectum, completing the toroid with B. Y subsequently differentiates as the PDA motor neuron (Fig. 1B), with a characteristic axonal process that extends ventrally toward the posterior end past the rectum, makes a right-handed commissure and extends along the dorsal cord toward the anterior of the worm (5, 6). By contrast, the rectal cells B, U, F, and K′ remain in the rectum at all stages. We note that Y migration appears to involve the whole cell, not just its nucleus as previously observed by using Nomarski optics (4), because marker expression (see below) shows that Y and PDA have a totally different cell shape and position.
Fig. 1.
The Y-to-PDA transition in wild type and mutants. (A) Y is part of the rectum in the L1 stage. The rectum is composed of three rings of two cells each. In the L1 stage, the most anterodorsal ring is made of the cells K and K′, the middle ring is made of U and F, and the posteroventral-most ring is made of Y and B. (B) Stages and timing of Y-to-PDA transdifferentiation relative to somatic gonad development. The somatic precursors, Z1 and Z4, undergo three rounds of divisions, and the differentiated anchor cell is evident when the primordium forms (41). We correlated milestones in Y-to-PDA transdifferentiation with somatic gonadal development. Depicted is Y's nucleus migration and morphology change during the process. The position of the nuclei (and nucleoli) of the rectal cells, PDA, and P11.p, the other epithelial cell found near the anterior rectum, as seen by Nomarski optics, are represented as well as the PDA axon (blue dotted line). In this figure and all others, anterior is to the left, and ventral is to the bottom.
The timing of these morphological events is stereotyped; different phases can be correlated with the presumably independent events of somatic gonad development (7) (Fig. 1B), facilitating the analysis of mutants with defects in Y-to-PDA plasticity). Despite the stereotyped timing, the heterochronic genes lin-4, lin-14, and lin-28, which control the timing of many L1 and L2 stage-specific events (8), do not affect the Y-to-PDA change [supporting information (SI) Table 4].
In the next three sections, we use multiple markers (summarized in SI Table 5), several identified expressly for this purpose here, and ultrastructural features to show that Y has only epithelial character while it is part of the rectum and that these features are completely lost and replaced with neuronal characteristics when it becomes the PDA neuron, supporting the view that the Y-to-PDA change is a transdifferentiation event. We then examine environmental and genetic factors that might influence this event.
Y Displays the Hallmark Ultrastructural Characteristics of Rectal Epithelial Cells.
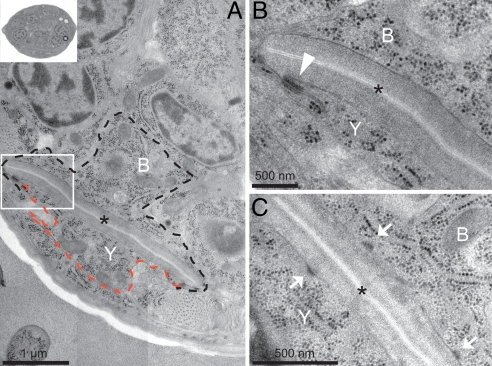
We reconstructed the rectal area of newly hatched L1 and L4 hermaphrodites using serial section electron microscopy. We compared L1 Y to the other L1 rectal cells and to L4 P12.pa, the cell that replaced it (Fig. 2 and SI Fig. 3). All of these cells had identical epithelial characteristics, including C. elegans junctions at the apical side with the surrounding epithelial cells and fibrous organelles with the cuticle. L1 Y and L4 P12.pa also have an identical train-rail shape (Fig. 2 and SI Fig. 3). In sum, Y has the hallmark epithelial features as the other rectal epithelial cells that do not change their identity.
Fig. 2.
Ultrastructural characteristics of Y. (A) Electron micrograph of the rectal area of a newly hatched L1 hermaphrodite showing the B and the Y cells (outlined in black and red, respectively) wrapped around the rectum, both displaying a train-rail-like shape. (Inset) The section of the whole worm from which the rectal area is magnified in A. (B) Blow-up of the boxed area in A, illustrating a C. elegans junction (arrowhead) between the apical membranes of Y and B. (C) Another section of the same L1 animal illustrating a junction between the apical side of the Y cell, or the B cell, and the cuticle of the rectum (arrows). This structure is called a fibrous organelle in C. elegans. An asterisk indicates the rectal slit.
Epithelial Markers Are Expressed in Y but Not in PDA.
Three markers associated with epithelial apical–basolateral polarity are expressed in Y during the L1 stage, consistent with the ultrastructure analysis: the apical marker CHE-14::GFP (9) (40 of 40 L1s), and the C. elegans junction markers AJM-1 (10) (41 of 41 L1s) and DLG-1 (11) (27 of 27 L1s) (see also SI Fig. 4). If a true transdifferentiation event occurs, PDA should have no epithelial vestiges. Accordingly, in L3 and older animals, all three of these markers are absent from PDA (CHE-14::GFP, 0 of 40; AJM-1::GFP, 0 of 39; DLG-1::GFP, 0 of 48 animals).
Reporters for transcription factors involved in epithelial fate specification (lin-26, refs. 12, 13) or differentiation (egl-26, ref. 14) are expressed in Y in the L1 stage and in P12.pa and B but not in PDA in later larvae (SI Table 5 and data not shown). Expression of both lin-26 and egl-26 disappears as Y migrates anteriorly to become the neuron PDA (data not shown). In addition, ceh-6 and peb-1, which are required for proper differentiation or function of the rectal cells, are expressed in Y in the L1 larvae but disappear from Y as it withdraws from the rectum (15, 16).
Neuronal Markers Are Not Expressed in Y.
Two previous studies establish PDA as being a motor neuron based on its ultrastructural features (5, 6). To test whether Y has any neuronal characteristics while functioning and appearing epithelial in the L1 stage, we first looked at the pan-neuronal makers unc-119::gfp (17) and F25B3.3::gfp (18). The panneuronal markers are absent in Y in the L1 stage (0 of 26 and 0 of 32 animals expressed unc-119 or F25B3.3 respectively) and, as expected, appear present in PDA neurons (data not shown). To assay specifically for PDA identity, we examined a number of transgenes (see SI Text) and found that cog-1 (42) and ace-3/4 (43) are useful PDA markers. In particular, cog-1::gfp and ace-3/4::gfp are strongly and reliably expressed in PDA (59 of 59 cog-1::gfp and 48 of 51 ace-3/4::gfp L3 or older hermaphrodites) but are never expressed in Y (0 of 30 cog-1::gfp and 0 of 45 ace-3/4::gfp L1 hermaphrodites).
Our results strongly support the view that the Y-to-PDA identity change is a bona fide example of transdifferentiation: Y has epithelial hallmarks and no evident neuronal character, whereas PDA has neuronal hallmarks and no residual epithelial character.
Assessment of the Role of the Cellular Environment on Y Transdifferentiation.
We investigated whether the microenvironment, sometimes referred to as “niche,” could influence Y-to-PDA transdifferentiation.
Grafted bone marrow cells change their fates after fusion with differentiated cells of the host (19). Thus, we asked whether the Y-to-PDA identity change is triggered by the fusion of Y with a neuron or a cell with neural potential in the local environment. Although all neurons initially present in the neighborhood of Y remain intact throughout development (4–6), there are transient prospective neurons that normally undergo apoptosis and are engulfed by nearby hypodermal cells (4). We found that Y-to-PDA transdifferentiation occurs normally when apoptosis or engulfment is defective (SI Table 6); furthermore, Y does not express a marker associated with engulfment (ced-1::gfp, data not shown). These results suggest that fusion with prospective neurons is unlikely to initiate Y-to-PDA transdifferentiation.
We also examined the effect of removing cells that are in close contact with Y (Table 1). Laser ablation of the rectal cells B, U, or F or phasmid sheath cells in newly hatched egl-26::gfp L1 larvae did not affect Y migration and PDA formation (Table 1). Furthermore, ablation of P12 or P12.p, the precursors to P12.pa, does not affect the formation of PDA (Table 1). These ablation results suggest that no single neighboring cell acts as a source of a putative signal required for Y-to-PDA transdifferentiation. However, the fragility of operated animals prohibited the scoring of individuals that had undergone ablation of multiple cells to assess potential cellular redundancy.
Table 1.
Cell ablation experiments
| Genotype operated | Ablated cell(s) | No. of Y-to-PDA per total no. |
|---|---|---|
| cog-1::gfp | B | 13 of 15* |
| egl-26::gfp | B | 28 of 29† |
| egl-26::gfp | U | 16 of 19† |
| egl-26::gfp | F | 13 of 14† |
| egl-26::gfp | Phasmid sheath‡ | 13 of 13† |
| cog-1::gfp | P12 | 0 of 10§ |
| cog-1::gfp | P12.p | 2 of 6§ |
| cog-1::gfp | P12.pa | 11 of 11§ |
| egl-5(0); cog-1::gfp | Extra P11 | 0 of 6* |
| egl-5(0); cog-1::gfp | Extra P11.p | 0 of 8* |
| egl-38(0); egl-26::gfp | Y | 9 of 10¶ |
Ablations were performed in newly hatched L1 larvae or as soon as the relevant cell was born; operated animals were scored when they reached the L3 stage or older.
*Presence of a PDA neuron (i.e., WT phenotype; cog-1::gfp scored).
†Absence of a persistent Y cell (i.e., WT phenotype; egl-26::gfp scored, confirmed by Nomarski scoring).
‡The two phasmid sheath cells seem to extend toward B and Y and hence were candidates for a PDA-inducing signal.
§WT position of a cog-1::gfp-expressing PDA neuron scored; a PDA motor-neuron was always found.
¶Presence of the egl-26::gfp-expressing extra Y-like cell in the rectum scored.
Interestingly, although ablation of P12 did not affect transdifferentiation, it blocked Y migration: In 10 of 10 animals in which P12 and 4 of 6 in which P12.p had been ablated, PDA formed ventrally near the rectum—the position of Y in unoperated L1 animals (Table 1). Wnt or EGF pathway mutants that lack a P12 cell (20) display a similar phenotype (SI Fig. 5 and SI Table 7). Thus, P12 and/or P12.p may provide a signal that promotes or sustains Y migration, but migration per se does not appear to provide a microenvironment necessary for transdifferentiation.
egl-5 and sem-4 Are Required for an Early Step of the Y-to-PDA Identity Change.
egl-5 and sem-4 mutations affect the fates of many adjacent cells in the tail that are related by position but not lineage (21, 22), and, in these mutants, Y has been reported to sustain an epithelial appearance in later larvae, as judged by Nomarski optics. We have confirmed this inference by using epithelial and PDA markers.
In egl-5(n945) null mutants, the epithelial marker ajm-1::gfp is expressed in Y when the worms hatch (38 of 38 animals) and continues to be so in older animals (Table 2), and the PDA marker ace-3/4::gfp is not expressed (Table 2). Thus, in egl-5 null mutants, Y stays part of the rectum, suggesting that transdifferentiation is not initiated. We note that P12 adopts the fate of P11 so that no P12.pa cell is formed, and two cells that have nuclei with epithelial appearance in Nomarski are found in the anterior rectum area (21). Laser ablation of the “extra P11” or “extra P11.p” did not restore a PDA motor neuron in operated egl-5 hermaphrodites (Table 1), and, because the absence of P12 does not impair Y transdifferentiation (Table 1), the altered cellular neighborhood of Y in egl-5(n945) does not appear to be responsible for the block in transdifferentiation.
Table 2.
Transdifferentiation of Y to PDA does not occur in egl-5 and sem-4 mutants
| Relevant genotype | 2 P11.p, % (n) | Persistent Y, % (n) | No PDA, % (n) |
|---|---|---|---|
| Wild type | 0 (34) | 0 (34) | 5.8 (51) |
| egl-5(n945) | 97.5 (41) | 100 (41) | 100 (34) |
| sem-4(n1971) | 12.5 (32) | 84.8 (79)* | 100 (38) |
(n), total number of L3 and older hermaphrodites scored; 2 P11.p, percentage of animals with 2 P11.p-like cells; persistent Y, percentage of animals in which Y remained at its initial position with an epithelial appearance and expressed an epithelial or a Y marker. ajm-1::gfp was used to score egl-5 mutants and egl-26::gfp was used for sem-4 mutants; No PDA, percentage of animals that did not express ace-3/4::gfp.
*In 15% sem-4 mutants, P12.pa is not formed or does not differentiate as a rectal epithelial cell, as assessed by using both egl-26::gfp and egl-5::gfp reporters. This result suggests that hermaphrodites scored here as lacking a persistent Y probably had one but no recognizable P12.pa consistent with the results obtained with a PDA marker.
Approximately 85% of sem-4(n1971) null mutants appear to exhibit a persistent Y phenotype by morphological criteria or continued expression of a Y marker (egl-26::gfp, Table 2 and SI Fig. 5). We also observed a weakly penetrant P12.pa defect in sem-4 mutants, accounting for the remaining animals that have two cells in the rectum, appearing as if Y were not affected. In addition, the PDA marker was never observed to be expressed in sem-4 mutants, consistent with a complete failure of Y-to-PDA transdifferentiation (Table 2). The rectal epithelial character of the persistent Y, found at its original location, was confirmed by egl-5 and ajm-1 expression (23 of 26 and 26 of 26 L3 and older animals, respectively), indicating that it was blocked at an early step in transdifferentiation. We note that sem-4 regulates the expression of various hox genes, including egl-5, during C. elegans development (23, 24); however, an egl-5::gfp reporter is expressed normally in the rectal area of sem-4 null mutants (data not shown), suggesting that such a regulation is not the basis of their similar Y phenotype.
In summary, Y expresses epithelial markers and remains as part of the rectum in egl-5 and sem-4 mutants. We conclude that the transdifferentiation of Y-to-PDA is affected at a very early step, which, assuming cell-autonomy, may reflect compromised competence or defective reception/implementation of a hypothetical transdifferentiation signal.
Ectopic Y Cells Generated by Transformation of Other Rectal Cells Are Not Competent to Transdifferentiate.
We first tested whether there is a “counting mechanism” in the rectal epithelium by asking whether an extra rectal-bound P12.pa can differentiate as PDA. We examined lin-15(n765ts) animals grown at 25°C, under which conditions P11 is sometimes transformed into a supernumerary P12 cell, resulting in two P12.pa cells (20). Although 32% of the time, P11.p has undergone such a transformation (n = 112), we never observed an additional cell expressing a PDA marker (0%, n = 37), suggesting that an extra rectal cell per se does not become competent to transdifferentiate as PDA.
We next assessed the fate of “supernumerary Y” cells made at the expense of other rectal epithelial cells. We considered a cell to be a supernumerary Y if it had the appropriate morphology and marker expression in the L1 stage and then assessed its ability to transdifferentiate into PDA in parallel with the “real Y.”
In egl-38 loss-of-function mutant males, U is transformed into Y, and expression of genes normally expressed in U are lost in hermaphrodites (25, 26). We confirmed that in egl-38 mutant hermaphrodites, as in males, U is transformed into Y: In an egl-38 mutant, in contrast to wild type, the cell at U's position ectopically expresses egl-26::gfp in L1 (Table 3 and SI Fig. 5) and continues to do so in older larvae (0% WT L4 versus 74% egl-38 L4 mutants express egl-26::GFP, Table 3). Although there is apparently a supernumerary Y, only one PDA cell is observed (Table 3), suggesting that only the “real Y” transdifferentiates into PDA. The supernumerary Y appears instead to be part of the rectum, displays an epithelial appearance and expresses egl-26::gfp (Table 3). Furthermore, when Y is ablated in egl-38 mutant L1 hermaphrodites, the cell at the U position keeps its epithelial morphology and stays at its position in the rectum in older larvae or adult (Table 1). In a mab-9 null mutant male, B adopts the identity of Y (27). In hermaphrodites, the cell at the B position has normal nuclear morphology in the L1 stage and expresses the epithelial marker egl-26::gfp (Table 3 and SI Fig. 5). Although in some older mab-9 mutants, the B nucleus appears smaller and sometimes lacks a nucleolus (24 of 51 animals), B continues to express egl-26::gfp in most L4 hermaphrodites, suggesting that it retains epithelial characteristics, and fails to express the PDA marker (Table 3). These results suggest that the ectopic Y does not undergo transdifferentiation. In sum, in both egl-38 and mab-9 mutants, the normal Y transdifferentiates into PDA but the extra Y-like cell does not. Because the extra Y-like looks like the true Y in all other respects, we believe that the results suggest that other rectal cells are lineally or otherwise intrinsically restricted such that they lack the potential to transdifferentiate or to respond to a hypothetical transdifferentiation-inducing signal.
Table 3.
The ectopic Y cell in lin-12 mutants, but not in egl-38 or mab-9 mutants, undergoes transdifferentiation
| Genotype | 2 PDA in L4, % (n) | B GFP + in L1, % (n) | B GFP + in L4, % (n) | U GFP + in L1, % (n) | U GFP + in L4, % (n) |
|---|---|---|---|---|---|
| egl-26::gfp (Y marker) | — | 100 (39) | 100 (34) | 25 (39) | 0 (34) |
| mab-9(e2410);egl-26::gfp | — | 93 (30)* | 88 (51)* | — | — |
| egl-38(sy294);egl-26::gfp | — | — | — | 100 (21)† | 74 (23)† |
| cog-1::gfp (PDA marker) | 0 (59) | — | 0 (59) | — | — |
| mab-9(e2410);cog-1::gfp | 0 (37) | — | 0 (37)* | — | — |
| ace-3/4::gfp (PDA marker) | 0 (131) | — | — | — | 0 (51) |
| egl-38(sy294); ace-3/4::gfp | 0 (95) | — | — | — | 0 (95)† |
| lin-12(n137); ace-3/4::gfp | 76 (54)‡ | — | — | — | — |
The percentage of newly hatched (L1) or L3 to adult (collectively called L4) hermaphrodites were scored for the presence of a GFP-positive cell at the position of the extra Y; (n), total number of animal scored.
*The cell at B position forming the extra Y cell was scored.
†The cell at U position forming the extra Y cell was scored.
†In some lin-12(n137) mutant hermaphrodites (20/54), the axon of one or both of the neurons expressing the PDA marker went more posteriorly than it does in wild type before joining the dorsal cord, perhaps reflecting an effect of elevated LIN-12/Notch activity on axon guidance.
Ectopic Y Cells Caused by Activation of lin-12/Notch Transdifferentiate.
In the wild-type embryo, ABprpppaaaa is the future Y, and its contralateral lineal homolog ABplpppaaaa is the future neuron DA9 (28). In lin-12(n137) mutants, LIN-12 is constitutively active, and, in males, both of these cells adopt the Y fate (29). Both cells appear to adopt the Y fate in hermaphrodites, too, as in 53% of lin-12(n137) L1 hermaphrodites (19 of 36), an extra epithelial cell is found in the anterior rectal area (as seen by anatomy or expression of egl-26::gfp). Furthermore a PDA marker is expressed in two adjacent neurons in L4 hermaphrodites (SI Fig. 5 and Table 3), suggesting that both the normal and the extra Y cell transdifferentiated into PDA neurons.
To assess when lin-12 activity is required for transdifferentiation, we used a temperature-sensitive partial loss-of-function allele, lin-12(n676n930). lin-12 activity is required for a Y cell to be formed: When lin-12(n676n930) mutants are grown at 25°C, no L1 hermaphrodites have a Y cell; at 15°C, approximately two-thirds of them do (30). To remove LIN-12 function shortly after, or around the time of Y cell specification, we allowed lin-12(n676n930); cog-1::gfp embryos to develop at 15°C until embryonic stages ranging from just before Y cell birth to the 3-fold stage (a time window of ≈250 min at 25°C), at which point, embryos were shifted to 25°C. Half of the newly hatched L1s were then scored for successful Y cell formation (based on anatomy), whereas the other half were scored as L4s or adults for the presence of a PDA motor-neuron (using cog-1::gfp expression). We observed that 72% (43 of 60) of newly hatched L1s had a Y cell, and 60% of older animals (33 of 55) had a PDA motor-neuron, statistically indistinguishable numbers (P = 0.4589, Fisher's exact test). There appears to be little perdurance of active LIN-12 protein upon temperature shift, because only 3 of 23 embryos that were transferred at 25°C within 100 min of ABprpppaaaa birth had a cell with Y characteristics. We interpret these results as suggesting that lin-12 acts at the same time that Y is specified to endow it with the competence to transdifferentiate and is not required at the time of transdifferentiation per se.
Discussion
In this study, we have provided evidence that the apparent change in fate of the rectal epithelial cell Y into the motor neuron PDA is a bona fide transdifferentiation event. We have also investigated the effect of various cellular and genetic factors on transdifferentiation. The results presented here, together with the many virtues of C. elegans for genetic and other experimental manipulations, establish Y-to-PDA transdifferentiation as a compelling model to characterize cellular plasticity in vivo.
Observations of wild-type and mutant hermaphrodites suggest that there are at least five phases in the Y-to-PDA transdifferentiation process: establishment of the Y epithelial identity; establishment of competence to undergo transdifferentiation; retraction from the rectum, in a process that resembles epithelial-to-mesenchyme transition; migration of Y away from the rectum; and establishment of neural identity as PDA (4–6). Altering the cellular environment of Y and varying the number, identity, and position of cells in the rectum suggest that transdifferentiation does not appear to require Y to migrate to the PDA position, to fuse with prospective neurons or other cells, or to interact with specific single neighboring rectal cells. Furthermore, the onset of transdifferentation does not appear to require the activity of heterochronic genes that control many other L1 or L2-specific events, raising the possibility that the onset of transdifferentiation is controlled by an unidentified developmental timer acting independently of the heterochronic pathway (31).
The egl-5/Abd-B or sem-4/spalt genes are required for transdifferentiation. In these mutants, Y remains a rectal epithelial cell, raising the possibility that egl-5 and sem-4 set or maintain the competence of Y to become PDA or are involved in triggering the transdifferentiation program. If so, neither gene activity is sufficient to promote transdifferentiation, because both genes are expressed in other rectal cells that do not transdifferentiate [egl-5 in U, F and B (32); sem-4, U, F, and B (ref. 23 and this study)]. Alternatively, egl-5 and sem-4 activities might be important in Y neighboring cell(s) to establish a necessary “niche” for Y transdifferentiation.
LIN-12/Notch signaling appears to act during hermaphrodite embryogenesis both to specify Y and also to endow Y with the potential to transdifferentiate. In lin-12(d) mutants, when LIN-12 is constitutively active, the normal Y is formed, as well as an “extra Y” instead of the prospective neuron DA9 (29); both the normal and the supernumerary Y cells transdifferentiate into PDA neurons. In contrast, in other mutants in which a supernumerary Y cell is created, egl-38 (U transformed into Y) and mab-9 (B transformed into Y), only the normal Y transdifferentiates, whereas the supernumerary Y remains an epithelial cell. Y differs from the other rectal cells in that it alone must experience lin-12 activity at the time it is born to differentiate as a rectal cell: In the absence of lin-12 activity, no epithelial Y cell is formed (29). Together, these observations suggest that the competence to transdifferentiate is specified in parallel to the distinct Y epithelial fate, because lack of the competence to transdifferentiate does not involve loss of Y rectal epithelial identity. A simple model is that activation of lin-12 in the future Y results in activation of two distinct sets of genes, one needed to ensure that the right contralateral homolog becomes a Y epithelial cell and one giving it the competence to change its identity.
We note two salient features of Y-to-PDA transdifferentiation in the context of other developmental phenomena or putative or confirmed transdifferentiation events. First, the Y-to-PDA epithelial–neuronal transdifferentiation phenomenon does not involve cell division, unlike, for example, neurogenesis during Drosophila development, which involves the generation of neuroblasts from a transient, proliferating epithelium. Cell division is also a feature of other transdifferentiation models, such as regeneration in urodeles (33–35) or the presumptive transdifferentiation of astrocytes into neurons during adult neurogenesis (36, 37). It is not clear, however, whether cell division per se is needed for transdifferentiation in these contexts. If so, transdifferentiation of Y may involve a distinct mechanism. In another C. elegans model for transdifferentiation, female germ cells can differentiate into various somatic cell types in mutants lacking certain translational regulators. In this model, entry into meiosis is critical for transdifferentiation (38), so the mechanistic relationship of this interesting phenomenon to somatic transdifferentiation is not clear.
Second, transdifferentiation during regeneration in urodeles, in cell culture or adult neurogenesis (33, 34, 37, 39), appears to involve, at least partially, progression through a proliferative dedifferentiated state. It is interesting to note that Y undergoes what superficially resembles an epithelial-to-mesenchymal transition, suggesting a transition through an intermediary state. However, we do not yet know whether Y undergoes a transition to a dedifferentiated state, or whether loss of its epithelial identity happens in parallel to gain of the neural one.
The tractability of C. elegans to genetic analysis should allow us to explore in a systematic way the genetic circuitry and consequent molecular cascades underlying transdifferentiation in vivo. Indeed, a pilot screen initiated to isolate transdifferentiation mutants has yielded 10 mutants in which a Y cell is initially found but no PDA is made (V. Pavet, N. Vaucamps, and S.J., unpublished data). The understanding of the factors that permit a differentiated cell to change its identity has significant consequences for our understanding of the appearance and progression of various cancers and for our ability to reprogram cells for therapeutic purposes.
Materials and Methods
Genetics.
Experiments were conducted at 20°C unless otherwise indicated. The wild-type parent for most strains used in this study is the C. elegans var. Bristol strain N2. The relevant mutations used in this study are: LG I: sem-4(n1971), lin-44(n1792), lin-28(n719), ced-1(n1735), ced-12(k149); LG II: mab-9(e2410), lin-4(e912); LG III: egl-5(n945), lin-12(n676n930ts), lin-12(n137); LG IV: egl-38(sy294), ced-3(n717), ced-3(n1286), ced-10(n3246), let-60(sy93dn); and LG X: lin-15(n765ts), bar-1(ga80), lin-14(n179ts).
Information about these alleles can be obtained from Wormbase, www.wormbase.org. See SI Text for details about the epithelial markers mcEx242 [CHE-14::GFP], jcIs1 [AJM-1::GFP], mcIs47 [DLG-1::GFP], mcEx [LIN-26::GFP]; the rectal epithelial markers bxIs7 [egl-5::gfp], kuIs36 [egl-26::gfp]; kuIs34 [sem-4::gfp]; the PDA markers fpIs1 [ace-3/4::gfp], syIs63 [cog-1::gfp], and arEx627 [exp-1::gfp]; and the other markers used or assessed for expression in Y or PDA.
Anatomy and Laser Ablation.
Methods used for electron microscopy are described in SI Text. For live-animal analyses, cells were identified based on their characteristic morphology and position by Nomarski optics or GFP expression from transgenes on a Zeiss Z1 Axio imager. A Micropoint laser beam (4) was used to ablate Y, B, U, F, or the phasmid sheath cells in newly hatched L1 hermaphrodites. Ablations of P11 or P12 were performed on L1 hermaphrodites after these cells had entered the ventral cord (6–9 h after hatching). Ablations of P11.p, P12.p, or P12.pa were performed on older L1 hermaphrodites in which the somatic gonadal precursor cells had divided at least twice.
Antibody Staining and Expression in the Y Cell.
Synchronized L1 worms expressing CHE-14::GFP (mcEx242) or DLG-1::GFP (mcIs47) were stained as described (40) by using antibodies against GFP protein (Molecular Probes) and Cy3- or FITC-conjugated secondary antibodies, together with DAPI. The C. elegans junction marker MH27 antibody against AJM-1 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) was also used on the fixed animals. Worms were mounted in a drop of antifade (80% glycerol, 20% PBS, 5% propylgalate) and analyzed with a Zeiss Z1 IMAGER 2 microscope or a Leica SP2 AOBS confocal microscope. Alternatively, live worms were anesthetized in 10 mM sodium azide and analyzed with a Zeiss Z1 IMAGER 2 microscope.
Mutant Analysis and Scoring.
The Y nucleus has a characteristic morphology and appearance in wild-type hermaphrodites and, in the L1 stage, Y expresses the epithelial markers ajm-1, dlg-1, che-14, lin-26, and egl-26; it also expresses egl-5 and sem-4. After transdifferentiation into PDA, its nucleus has a different morphology characteristic of neurons and a characteristic axon and expresses the PDA markers ace-3/4, cog-1, and exp-1.
To assess whether Y is present and transdifferentiates normally in mutant backgrounds, all or a subset of the following criteria were used. For Y identity, epithelial appearance, and epithelial and/or Y marker gene expression in the early L1 stage. For PDA identity, neuronal appearance and PDA marker gene expression in the L3 stage and later was used. A mutant phenotype can be inferred from an altered nuclear position and/or morphology and marker gene expression patterns. For Nomarski optics scoring, we relied on the characteristic rectal cell and P11.p morphologies. In L3 and older wild-type animals, there are three cells with epithelial appearance in the anterior rectum area: U, P12.pa, and P11.p. Mutants in which Y does not transdifferentiate have four epithelial cells in the same area: U, Y, P12.pa, and P11.p or, if a P12 cell is not made, U, Y, and 2 P11.p. Note that some of the mutants used in this study are constipated, making scoring by Nomarski microscopy less reliable and GFP scoring the method of choice.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Scott Emmons (Albert Einstein College of Medicine, New York, NY), Andy Fire (Stanford University, Stanford, CA), Wendy Hanna-Rose (Pennsylvania State University, University Park, PA), Mike Krause (National Institutes of Health, Bethesda), Michel Labouesse (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France), Zheng Zhou (Baylor College of Medicine, Houston), Theresa Stiernagle (University of Minnesota, Minneapolis), and the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources, for providing useful plasmids or nematode strains; Scott Clark, Caroline Goutte, Oliver Hobert, Michel Labouesse, Xiajun Li, and Sophie Quintin for a critical reading of the manuscript; Dave Hall, Renaud Legouis, Michel Labouesse, and Erik Jorgensen for helpful discussions; Valeria Pavet for the initial scoring of cog-1 expression in L1 larvae; Konstantinos Kagias and Jai Richard for integrating ace-3/4::gfp; and Nadine Fisher and Xinlan Zhou for valuable technical assistance. This work was supported by an Action Thématique Incitative sur Programme Centre National de la Recherche Scientifique grant, an Association pour la Recherche contre le Cancer grant and a Fondation pour la Recherche Médicale equipment grant (to S.J.). S.J. performed some of this work while a Postdoctoral Associate of the Howard Hughes Medical Institute at Columbia University. S.J. is an Investigator of the Centre National de la Recherche Scientifique, Y.S. is a Research Engineer of the Institut National de la Santé et de la Recherche Médicale, and I.G. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712159105/DC1.
References
- 1.Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187–194. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- 2.Eguchi G, Kodama R. Transdifferentiation. Curr Opin Cell Biol. 1993;5:1023–1028. doi: 10.1016/0955-0674(93)90087-7. [DOI] [PubMed] [Google Scholar]
- 3.Thowfeequ S, Myatt EJ, Tosh D. Transdifferentiation in developmental biology, disease, and in therapy. Dev Dyn. 2007;236:3208–3217. doi: 10.1002/dvdy.21336. [DOI] [PubMed] [Google Scholar]
- 4.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 5.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of Caenorhabditis elegans. Philos Trans R Soc London Ser B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 6.Hall DH, Russell RL. The posterior nervous system of the nematode Caenorhabditis elegans: Serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci. 1991;11:1–22. doi: 10.1523/JNEUROSCI.11-01-00001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimble J, Hirsh D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- 8.Rougvie AE. Control of developmental timing in animals. Nat Rev Genet. 2001;2:690–701. doi: 10.1038/35088566. [DOI] [PubMed] [Google Scholar]
- 9.Michaux G, Gansmuller A, Hindelang C, Labouesse M. CHE-14, a protein with a sterol-sensing domain, is required for apical sorting in C. elegans ectodermal epithelial cells. Curr Biol. 2000;10:1098–1107. doi: 10.1016/s0960-9822(00)00695-3. [DOI] [PubMed] [Google Scholar]
- 10.Koppen M, et al. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol. 2001;3:983–991. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- 11.McMahon L, Legouis R, Vonesch JL, Labouesse M. Assembly of C. elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J Cell Sci. 2001;114:2265–2277. doi: 10.1242/jcs.114.12.2265. [DOI] [PubMed] [Google Scholar]
- 12.Labouesse M, Hartwieg E, Horvitz HR. The Caenorhabditis elegans LIN-26 protein is required to specify and/or maintain all non-neuronal ectodermal cell fates. Development. 1996;122:2579–2588. doi: 10.1242/dev.122.9.2579. [DOI] [PubMed] [Google Scholar]
- 13.Quintin S, Michaux G, McMahon L, Gansmuller A, Labouesse M. The Caenorhabditis elegans gene lin-26 can trigger epithelial differentiation without conferring tissue specificity. Dev Biol. 2001;235:410–421. doi: 10.1006/dbio.2001.0294. [DOI] [PubMed] [Google Scholar]
- 14.Hanna-Rose W, Han M. The Caenorhabditis elegans EGL-26 protein mediates vulval cell morphogenesis. Dev Biol. 2002;241:247–258. doi: 10.1006/dbio.2001.0514. [DOI] [PubMed] [Google Scholar]
- 15.Burglin TR, Ruvkun G. Regulation of ectodermal and excretory function by the C. elegans POU homeobox gene ceh-6. Development. 2001;128:779–790. doi: 10.1242/dev.128.5.779. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez AP, Gibbons J, Okkema PG. C elegans peb-1 mutants exhibit pleiotropic defects in molting, feeding, and morphology. Dev Biol. 2004;276:352–366. doi: 10.1016/j.ydbio.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Maduro M, Pilgrim D. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics. 1995;141:977–988. doi: 10.1093/genetics/141.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altun-Gultekin Z, et al. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C elegans. Development. 2001;128:1951–1969. doi: 10.1242/dev.128.11.1951. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Dolado M, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 20.Jiang LI, Sternberg PW. Interactions of EGF, Wnt and HOM-C genes specify the P12 neuroectoblast fate in C elegans. Development. 1998;125:2337–2347. doi: 10.1242/dev.125.12.2337. [DOI] [PubMed] [Google Scholar]
- 21.Chisholm A. Control of cell fate in the tail region of C. elegans by the gene egl-5. Development. 1991;111:921–932. doi: 10.1242/dev.111.4.921. [DOI] [PubMed] [Google Scholar]
- 22.Basson M, Horvitz HR. The Caenorhabditis elegans gene sem-4 controls neuronal and mesodermal cell development and encodes a zinc finger protein. Genes Dev. 1996;10:1953–1965. doi: 10.1101/gad.10.15.1953. [DOI] [PubMed] [Google Scholar]
- 23.Grant K, Hanna-Rose W, Han M. sem-4 promotes vulval cell-fate determination in Caenorhabditis elegans through regulation of lin-39 Hox. Dev Biol. 2000;224:496–506. doi: 10.1006/dbio.2000.9774. [DOI] [PubMed] [Google Scholar]
- 24.Toker AS, Teng Y, Ferreira HB, Emmons SW, Chalfie M. The Caenorhabditis elegans spalt-like gene sem-4 restricts touch cell fate by repressing the selector Hox gene egl-5 and the effector gene mec-3. Development. 2003;130:3831–3840. doi: 10.1242/dev.00398. [DOI] [PubMed] [Google Scholar]
- 25.Sewell ST, Zhang G, Uttam A, Chamberlin HM. Developmental patterning in the Caenorhabditis elegans hindgut. Dev Biol. 2003;262:88–93. doi: 10.1016/s0012-1606(03)00352-x. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlin HM, et al. The PAX gene egl-38 mediates developmental patterning in Caenorhabditis elegans. Development. 1997;124:3919–3928. doi: 10.1242/dev.124.20.3919. [DOI] [PubMed] [Google Scholar]
- 27.Chisholm AD, Hodgkin J. The mab-9 gene controls the fate of B, the major male-specific blast cell in the tail region of Caenorhabditis elegans. Genes Dev. 1989;3:1413–1423. doi: 10.1101/gad.3.9.1413. [DOI] [PubMed] [Google Scholar]
- 28.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 29.Greenwald IS, Sternberg PW, Horvitz HR. The lin-12 locus specifies cell fates in Caenorhabditis elegans. Cell. 1983;34:435–444. doi: 10.1016/0092-8674(83)90377-x. [DOI] [PubMed] [Google Scholar]
- 30.Delattre M, Felix MA. Development and evolution of a variable left–right asymmetry in nematodes: the handedness of P11/P12 migration. Dev Biol. 2001;232:362–371. doi: 10.1006/dbio.2001.0175. [DOI] [PubMed] [Google Scholar]
- 31.Ruaud AF, Bessereau JL. Activation of nicotinic receptors uncouples a developmental timer from the molting timer in C. elegans. Development. 2006;133:2211–2222. doi: 10.1242/dev.02392. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira HB, Zhang Y, Zhao C, Emmons SW. Patterning of Caenorhabditis elegans posterior structures by the Abdominal-B homolog, egl-5. Dev Biol. 1999;207:215–228. doi: 10.1006/dbio.1998.9124. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Velloso CP, Imokawa Y, Brockes JP. Plasticity of retrovirus-labelled myotubes in the newt limb regeneration blastema. Dev Biol. 2000;218:125–136. doi: 10.1006/dbio.1999.9569. [DOI] [PubMed] [Google Scholar]
- 34.Echeverri K, Clarke JD, Tanaka EM. In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev Biol. 2001;236:151–164. doi: 10.1006/dbio.2001.0312. [DOI] [PubMed] [Google Scholar]
- 35.Echeverri K, Tanaka EM. Ectoderm to mesoderm lineage switching during axolotl tail regeneration. Science. 2002;298:1993–1996. doi: 10.1126/science.1077804. [DOI] [PubMed] [Google Scholar]
- 36.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 38.Ciosk R, DePalma M, Priess JR. Translational regulators maintain totipotency in the Caenorhabditis elegans germline. Science. 2006;311:851–853. doi: 10.1126/science.1122491. [DOI] [PubMed] [Google Scholar]
- 39.Means AL, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 40.Jarriault S, Greenwald I. Suppressors of the egg-laying defective phenotype of sel-12 presenilin mutants implicate the CoREST corepressor complex in LIN-12/Notch signaling in C. elegans. Genes Dev. 2002;16:2713–2728. doi: 10.1101/gad.1022402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimble J, Hirsh D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- 42.Palmer RE, Inoue T, Sherwood DR, Jiang LI, Sternberg PW. Caenorhabditis elegans cog-1 locus encodes GTX/Nkx6.1 homeodomain proteins and regulates multiple aspects of reproductive system development. Dev Biol. 2002;252:202–213. doi: 10.1006/dbio.2002.0850. [DOI] [PubMed] [Google Scholar]
- 43.Combes D, Fedon Y, Toutant J-P, Arpagaus M. Multiple ace genes encoding acetylcholinesterases of Caenorhabditis elegans have distinct tissue expression. Eur J Neurosci. 2003;18:497–512. doi: 10.1046/j.1460-9568.2003.02749.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.