Abstract
Primary sensory cortices are remarkably organized in spatial maps according to specific sensory features of the stimuli. These cortical maps can undergo plastic rearrangements after changes in afferent (“bottom-up”) sensory inputs such as peripheral lesions or passive sensory experience. However, much less is known about the influence of “top-down” factors on cortical plasticity. Here, we studied the effect of a visceral malaise on taste representations in the gustatory cortex (GC). Using in vivo optical imaging, we showed that inducing conditioned taste aversion (CTA) to a sweet and pleasant stimulus induced plastic rearrangement of its cortical representation, becoming more similar to a bitter and unpleasant taste representation. Using a behavior task, we showed that changes in hedonic perception are directly related to the maps plasticity in the GC. Indeed imaging the animals after CTA extinction indicated that sweet and bitter representations were dissimilar. In conclusion, we showed that an internal state of malaise induces plastic reshaping in the GC associated to behavioral shift of the stimulus hedonic value. We propose that the GC not only encodes taste modality, intensity, and memory but extends its integrative properties to process also the stimulus hedonic value.
Keywords: chemical senses, map plasticity, taste coding
Sensory cortical processing is achieved in complex networks composed of millions of neurons. These networks are topographically organized in functional domains, reflecting the spatial distribution of peripheral sensory receptors. Consequently, cortical processing can be visualized in 2D functional maps at the cortical surface.
The capacity for plasticity of cortical areas is one of the most salient features to explain development, learning, or recovery of function. In sensory regions, plasticity changes have been shown to occur during both development and adulthood (1, 2). Most previous studies reporting map plasticity are associated with changes in afferent sensory inputs (or “bottom-up” inputs), such as peripheral input impairments or lesions (1), passive sensory experience (3), or sensory training (4, 5). Few reports indicated that topographical plasticity can occur while maintaining constant the bottom-up sensory inputs and varying “top-down” factors, such as task demands (6) or associative learning (7, 8). These forms of plasticity, however, have been much less investigated.
A specific form of associative learning plasticity that does not involve changes of the peripheral input is achieved by conditioned learning in which the response to a conditioned sensory stimulus (CS) is modulated by a strong unconditioned cue (US). An efficient paradigm of conditioned learning experience is given by conditioned taste aversion (CTA), where a subject learns to avoid a taste stimulus (CS) paired with visceral malaise (US) (9), even after a single learning procedure (10). The primary gustatory cortex (GC) is an important part of the brain circuitry involved in CTA acquisition, long-term retention, and extinction (10–12). The GC therefore constitutes an ideal region to investigate sensory maps plasticity after conditioned learning. Recently, we studied the spatial representation of taste modalities in the GC (13). Taste maps differ across modalities and seem to contain also information about the pleasantness (or hedonic value) of the stimulus. Electrophysiological recordings from small ensembles of cortical neurons in awake rodents have shown that GC neurons are modulated by stimulus pleasantness in their late responding phase (14). However, it is still unknown whether the hedonic value might be represented in the activation of a large population of GC neurons.
Here, we addressed two important questions. First, we wondered whether a specific internal state of malaise associated to a taste stimulus induced sensory map plasticity. Second, we explored whether this potential plasticity could be directly related to the behavioral shift of the stimulus hedonic value induced by the internal state. These questions on taste maps plasticity and hedonic value representation were addressed by using a CTA learning and subsequent extinction paradigm coupled with intrinsic signal imaging (15). We conditioned the aversion to a pleasant stimulus, saccharin (used as a CS), and compared its cortical representation to the response elicited by a reference aversive bitter compound, quinine. We show that the CS taste maps are plastic and that they rearrange according to the shift of the CS hedonic value, both in the CTA acquisition (where CS becomes aversive) and extinction (CS is attractive again) learning phase.
Results
Conditioning the Aversion to a Pleasant Taste Stimulus.
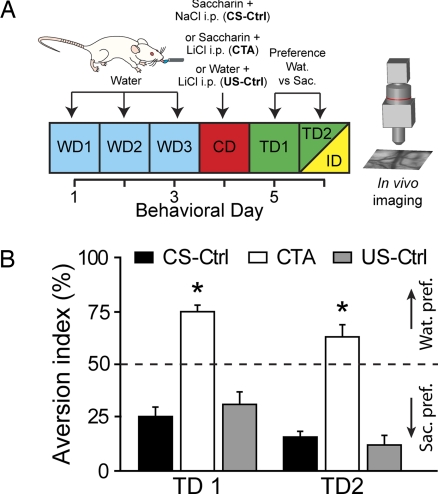
The artificial sweetener saccharin is greatly attractive to rodents and proves to be a very efficient CS in CTA paradigm when it is paired with a lithium chloride (LiCl) injection as an US (12). To test the strength of the conditioning in our animals, after 3 days of training with distilled water, saccharin was presented to the rats and coupled 30 min later with i.p. injection of LiCl (CTA group, Fig. 1A) or saline (CS-Ctrl group). To study the possible effects of the malaise inducing agent on its own, we added another control group of animals injected with LiCl but with no saccharin pairing (US-Ctrl group). In the next 2 days after conditioning (TD1 and TD2), the rats of the CTA group displayed a robust aversion to saccharin (Fig. 1B), whereas the control rats strongly preferred saccharin to water (Fig. 1B). Therefore, an evident behavioral shift of saccharin pleasantness (or hedonic value) was only produced in the CTA group.
Fig. 1.
Acquisition of conditioned taste aversion (CTA) to saccharin. (A) Schema of the behavioral paradigm. Rats were water-deprived and trained for 3 days [water days (WD)] to drink distilled water over a 10-min access period. On the conditioning day (CD), either saccharin (CTA and CS-Ctrl) or distilled water (US-Ctrl) is given. Thirty minutes later, either LiCl (0.15 M, 2% body weight) was injected i.p. to induce visceral malaise (CTA and US-Ctrl groups) or NaCl (0.9%, 2% b.w.) was injected i.p. (CS-Ctrl group). In the following test days (TD1 and TD2), a preference test for distilled water (Wat.) over saccharin (Sac.) was performed. On TD2, after the preference evaluation, animals were anesthetized, and in vivo gustatory cortex imaging was conducted (ID, yellow). (B) Aversion index (water over total fluid intake) histograms proving CTA induction to saccharin. The dotted line represents no preference between the two solutions. Control animals showed a strong preference for saccharin, whereas the CTA animals associated saccharin with the malaise and exhibited a strong aversion to it (*, unpaired t test comparison with control groups at least P < 0.006).
Plasticity of Cortical Representation After CTA.
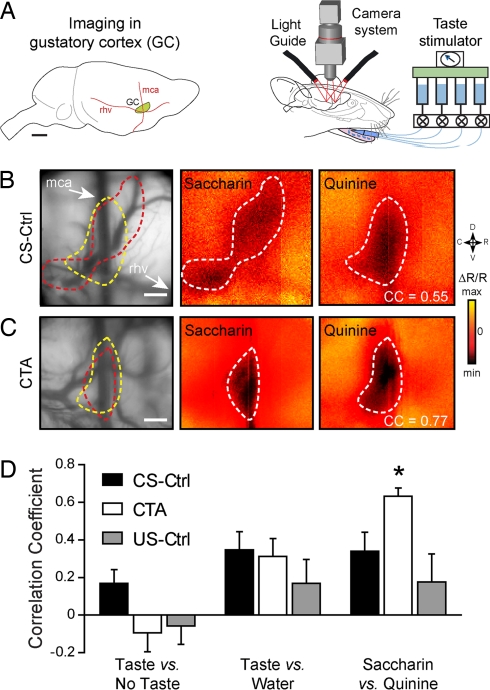
After inducing a shift in the hedonic value (from positive to negative) of the CS, we examined whether the CS-US pairing would affect the cortical representation of the tastant used as CS. We exposed the area of the insular cortex that was functionally defined as the primary GC (Fig. 2A Left) and we conducted in vivo intrinsic optical imaging to map functional activity in the GC (Fig. 2A Right) (13). Rats of CTA (n = 8), CS-Ctrl (n = 8), and US-Ctrl (n = 5) groups were imaged on test day 2 (however, similar results were also obtained on TD1, n = 4, data not shown) when there was a significant difference of the saccharin preference among the groups (Fig. 1B, two-tailed t test, PCTA/CS-Ctrl < 0.002, PCTA/US-Ctrl < 0.006). Each animal was delivered repeated applications of two tastants of opposed hedonic values (if not conditioned): saccharin, sweet and palatable, and quinine, as a reference bitter unpalatable stimulus. We studied tastant-evoked cortical representation of these two taste stimuli in the GC in CS-Ctrl and CTA rats (Fig. 2 B and C respectively). As shown (13), we found in the CS-Ctrl rats that sweet and bitter tastants are represented by distinctive spatial patterns with some common cortical territories (Fig. 2B). Interestingly, in the CTA group the same taste stimuli induced more similar activated regions (Fig. 2C).
Fig. 2.
CTA alters tastant representation in the gustatory cortex (GC). (A) (Left) Approximate size and location of the primary GC with respect to anatomical landmarks (blood vessels). mca, middle cerebral artery; rhv, rhinal veins. (Scale bar, 2 mm.) (Right) Schematic drawing of the stimulation and imaging systems. Fluids are delivered through pressurized reservoirs, using a computer-driven valve system, and the images are acquired through a CCD camera while illuminating the exposed cortex with light guides. (B and C) (Left) vascular pattern taken with a 546-nm illumination filter. The following images represent intrinsic signal responses averaged >16 presentations of 5 mM saccharin and 20 mM quinine in a CS-Ctrl (B) or a CTA animal (C). Correlation coefficients (CC) between quinine and saccharin images of the same animal are indicated. Tastant-activated cortical areas are outlined in white on the responses, and, for each example, the two different outlines are reported on the blood vessel pattern image. Minimum (×10−4) = −0.8 (Sac) (B), −1.5 (Qui) (B), and −4.5 (Sac and Qui) (C); maximum (×10−4) = 0.8 (Sac) (B), 1.5 (Qui) (B), and 4.5 (Sac and Qui) (C). (Scale bars, 500 μm.) (D) Maps correlation coefficient histograms for the CS-Ctrl (black, n = 8), the CTA (white, n = 8), and the US-Ctrl (gray, n = 5) groups. Three conditions are plotted: taste (Sac. and Qui.) versus no stimulus images, taste versus distilled water, and saccharin versus quinine. Only the Sac. versus Qui. correlation was statistically different between the CTA and CS-Ctrl or US-Ctrl groups (PCTA/CS-Ctrl < 0.03; PCTA/US-Ctrl < 0.005).
For quantification, we compared quinine and saccharin maps similarity in each animal, computing the correlation coefficient (CC) between the two tastant-evoked cortical representations. We found that the CC was significantly higher in the CTA group than in the control groups (Fig. 2D; CS-Ctrl, 0.34 ± 0.10; CTA, 0.63 ± 0.05; US-Ctrl, 0.17 ± 0.14 paired two-tailed t test, PCTA/CS-Ctrl < 0.03, PCTA/US-Ctrl < 0.005, and PCS-Ctrl/US-Ctrl = 0.38). As a control of plasticity specificity, the correlations between tastants (all applications of saccharin and quinine) and no stimulus (PCTA/CS-Ctrl = 0.10, PCTA/US-Ctrl = 0.83, and PCTA/US-Ctrl = 0.18) or between tastants and distilled water (PCTA/CS-Ctrl = 0.69, PCTA/US-Ctrl = 0.38, and PCTA/US-Ctrl = 0.23) were not significantly different in the three groups considered.
In conclusion, GC taste maps of saccharin are modified by the association with internal malaise. Interestingly, consistent with previous studies showing that LiCl injection did not induce the expression of the activation marker c-fos in the GC (16–18), the malaise itself does not induce significant changes in the correlations between saccharin and quinine patterns (PCTA/US-Ctrl = 0.38). On the contrary, the higher correlation between saccharin and quinine in the CTA group indicates that the cortical territories activated by the two tastants are more similar and this might directly be associated to the shift of tastant (CS) hedonic value, supporting the view of the GC as an important site for processing taste hedonics (13, 14, 19).
Extinction of CTA Induces a New Rearrangement of Cortical Maps.
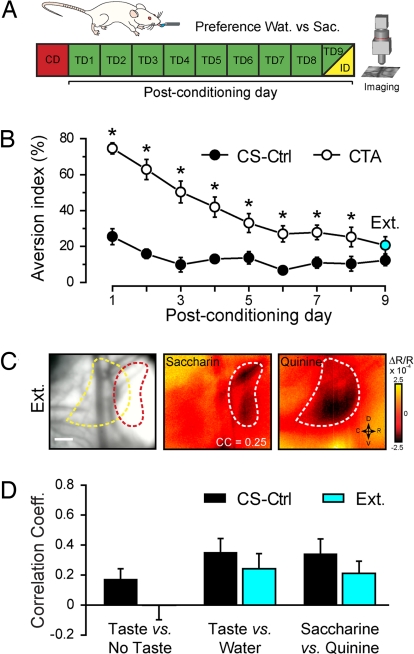
Experimental extinction represents the behavioral decline of the conditioned response when the subject is repeatedly exposed to the CS without US reinforcement. Under these conditions, the animal learns that the CS is not noxious. To test the hypothesis that cortical maps could change again, we first exposed the animals to the choice between saccharin and distilled water for 9 consecutive days after conditioning (Fig. 3A). We found, as expected, that the CTA group had a higher aversion index when compared with the CS-Ctrl group (ANOVA F(1,6) = 9.41, P < 0.02). However, we observed a progressive decline of saccharin aversion in the CTA group (Fig. 3B; ANOVA F(8,48) = 3.57, P < 0.003). By test day 9 (TD9), we observed >80% recovery of natural saccharin preference, the aversion index being no longer significantly different when compared with the CS-Ctrl group (Fig. 3B; posthoc LSD test, P = 0.14).
Fig. 3.
Cortical representation after CTA extinction. (A) Schema of the behavioral paradigm. After the conditioning day (CD), CS-Ctrl and CTA rats were subjected to a preference test for water over saccharin for 9 consecutive days (TD1 to TD9) until at least 80% of Sac reacceptance was reached (with respect to the average CS-Ctrl intake of Sac). On TD9, after the preference evaluation, an animal was anesthetized, and in vivo gustatory cortex imaging was conducted (ID). (B) Aversion index curves for CS-Ctrl (black circle) and CTA (white circle) rats. Note the progressive extinction of saccharin aversion in the CTA group (ANOVA F(8,48) = 3.57, P < 0.003). After 9 days, CTA rats displayed a similar preference toward saccharin compared with the control group (blue circle). *, posthoc LSD test, at least P < 0.05. (C) Example of intrinsic responses averaged >24 presentations of saccharin and quinine in an animal that experienced CTA extinction. Tastant-activated area is outlined in white on the responses, and the two different outlines are reported in the blood vessel pattern (left histogram). Correlation coefficient (CC) between quinine and saccharin images is indicated. (Scale bar, 500 μm.) (D) Correlation coefficient histograms for the control (black, n = 8) and the extinction (cyan, n = 8) groups. None of the comparisons between CS-Ctrl and EXT groups were statistically different.
After 9 days, CTA rats exhibited a complete extinction of the aversion to saccharin and are referred as extinction group (EXT). Rats of the extinction group (n = 8) were imaged at TD9 to determine how this learning process affected cortical taste representation. Similar to the CTA and the control groups, we compared the saccharin and quinine responses. In the example shown (Fig. 3C), the two taste stimuli induced quite distinct patterns of activation in the GC. The CC between saccharin and quinine were calculated for all of the animals belonging to the EXT group and the values compared with the ones of the CS-Ctrl and CTA groups. The results show that there was no significant difference between the control unconditioned animals and the ones that experienced extinction after CTA acquisition (right histograms of Fig. 3D, two-tailed t test, P = 0.61). In contrast, the patterns of two stimuli were significantly less correlated in the EXT than in the CTA group (two-tailed t test, P < 0.008). As a control, no difference between the groups resulted from the CC of either taste versus no stimulus (CS-Ctrl/EXT P = 0.18; CTA/EXT P = 0.53) or taste versus water (CS-Ctrl/EXT P = 0.37; CTA/EXT P = 0.62) comparisons. We also observed changes in the saccharin pattern between CS-Ctrl and EXT group, but the interanimal variability typical of GC intrinsic signals could not allow establishing whether extinction is clearly represented by a different topographical configuration with respect to the unlearned state.
In summary, after CTA extinction cortical representations of saccharin and quinine are more distinct, and this could be again related to the new shift in the hedonic value of the CS.
Discussion
In the present study, we combined CTA learning paradigm with intrinsic signal imaging to investigate how conditioned learning (coupling a taste stimulus with an internal state of malaise) impacts directly on primary sensory maps (taste maps). We took advantage of the unique features of the central taste system where changes in internal body states, such as satiety (20) or visceral malaise (16, 19) have a direct influence on primary GC neuronal responses.
CTA Acquisition and GC Maps Plasticity.
Compared with other forms of learning induced plasticity, the induction of CTA to saccharin by coupling the first delivery of the stimulus with visceral malaise caused relatively fast cortical topographical remapping (21). In fact, we observed that a single CS-US coupling could induce changes in the CS cortical intrinsic responses.
Our results are in general agreement with learning-induced cortical map plasticity reported in other sensory areas (1, 22). Training involving peripheral stimulation usually modifies the relative representation in primate auditory cortex (5) or in somatosensory cortices of both humans (23), nonhuman primates (24), and rodents (4, 25). However, unlike the above cited studies, in our experiments maps rearrangement is caused not by a modification in the stimulus-receptor interaction at the periphery (the bottom-up inputs) but rather by viscero-gustatory interaction that might occur from top-down modulations in early brainstem relays, such as the nucleus of solitary tract (26, 27), the parabrachial nucleus (28, 29), or CTA-related forebrain centers, such as basolateral amygdala (10) and primary GC (11, 12).
Only a few studies report plasticity of sensory maps after conditioned learning. In rat auditory cortex, Weinberger et al. (8) reported a shift of the best responding frequencies of neurons in the direction of the CS frequency (frequency paired with electrical shock) and observed long-term retention of the receptive field plasticity. In another study, a stimulation of a whiskers row (CS) was paired with a tail shock (US) (7). The representation of these whiskers in the primary somatosensory cortex was expanded with respect to the unconditioned animals. In the gustatory maps, we reported a rearrangement of the saccharin (CS) pattern with respect to the quinine reference rather than a simple expansion/shrinking.
The anatomical substrates responsible for CTA were established in ref. 10. The association between CS and US is likely to happen at the brainstem level, in the parabrachial nucleus, the US-relevant (visceral) information is transmitted to the amygdala and then to the cortex via glutamatergic projections (30), whereas taste-related information follows a classical brainstem–thalamic pathway (31). The role of the insular cortex (containing primary GC) in CTA has been the topic of several studies (12, 21). Electrophysiological recordings in the GC showed that some neurons vary their response pattern before and after CTA (19, 32). Cortical lesions studies demonstrated that the insular cortex (that contains the GC) is important for CTA acquisition (11, 33), although some particular insular lesions only partially disrupt CTA (34). However, the consensus is that GC is necessary for an effective acquisition of CTA (12). Approaches aimed to unravel the molecular machinery of taste learning established that GC is fundamental for the formation of short- and long-term aversion memories (35–40). The changes we observed in the saccharin activated patterns between the control and the CTA rats might therefore be explained by a long-term retention of saccharin aversion.
CTA Extinction and GC Maps Plasticity.
When presented for several days with the CS (saccharin) in the absence of US (LiCl), the animals resumed the normal level of saccharin consumption, thus extinguishing the aversion (Fig. 3). Under this condition, we found that the activation pattern of saccharin and quinine again became decorrelated. We note, however, that the rearrangement of the GC maps after extinction may not represent a reversal of the CTA and thus a return to the unlearned (CS-Ctrl) state. Indeed, a debate has emerged regarding the extent to which extinction represents unlearning or new learning (41, 42). Interestingly, the two studies that reported sensory map plasticity after conditioned learning in auditory (8) and somatosensory (7) cortices, both observed a complete reversal in the neural responses with extinction. In CTA, responses in the nucleus of solitary tract examined by using c-fos-like immunoreactivity (c-FLI) showed a reversal of c-FLI expression after extinction of sucrose aversion (27). Nevertheless, recent reports presented strong evidence for extinction as a learning process that provides the CS with a new meaning. The effects of CTA to saccharin in neurons recorded from the nucleus of solitary tract were not fully reversed with extinction: a burst of activity (typical of the CTA state) was still present in a subset of cells of the EXT but not of the CS-Ctrl animals (43). In addition, at the GC level, both molecular machinery involved (44) and c-FLI expression (45, 46) characterized extinction as a specific learning state. The changes we observed between the CS-Ctrl and EXT saccharin patterns might support this second interpretation of the extinction state, but the inter-animal variability was too high to reach a significant conclusion on this point.
GC and Hedonic Value Representation.
CTA is a very effective paradigm that can be used to modify the pleasantness (or hedonic value) of a taste stimulus. A sweet and innately preferred compound such as saccharin can therefore become aversive after its coupling with visceral malaise. By comparing the CS activation pattern to the response produced by a reference aversive compound in the same animal, we could directly assess whether the spatial responses contained some information related to tastant hedonic value. In a previous study, we measured cortical intrinsic responses in naive rats after taste stimulation with four compounds, two eliciting preference behavior (salt and sweet) and two provoking rejection (bitter and sour). We found that similar hedonic value compounds induced more similar responses than compounds with a different hedonic value, suggesting a role of the GC in processing tastant hedonics (13).
The present results confirm and integrate this view. The relative cortical response to saccharin changes in the three conditions examined (CS-Ctrl, CTA, and EXT, see Fig. 4 for a schematic view) and presents the highest correlation with the pattern of the aversive compound in the CTA group (Fig. 2D). Because the taste modality should remain unchanged (saccharin will interact with the same receptors at the periphery), changes in correlation are directly related to shifts of the perceived hedonic value of the compound. Therefore, our results suggest that spatial cortical patterns in the GC are important for taste modality discrimination (13) and contain direct information on the palatability of the compound. Evidence for hedonic processing in the GC comes from early electrophysiological recordings in behaving rats, suggesting that the GC contains both taste-modality and taste-hedonic responding neurons (19). In addition, analyzing the temporal firing patterns of GC neurons from awake rats, Katz et al. (14) found that some neurons could contain somatosensory, taste modality, and hedonics information according to the specific response time window with the palatability component having a late onset (after 1.2 s from the stimulus) and a long duration (>1 sec). We included this entire time window in our averaged cortical response, and we show that a shift in the hedonic value induces a change in the activation of a large neuronal population in the GC (Fig. 4). It is important to note that intrinsic signals do not permit the distinction among the fast somatosensory, the chemosensory responses, and slower hedonic or adaptive components that are known to be present in the GC. Intrinsic signals are characterized by a decrease in light reflectance over time that reaches its peak between 2 s and 4 s from stimulus onset (13, 15, 47) and have a very different time-course from that of the evoked electrical activity (15). Of course, its main advantage is that it gives very good spatial information on functional activation, but it is not suitable to investigate temporal dynamics.
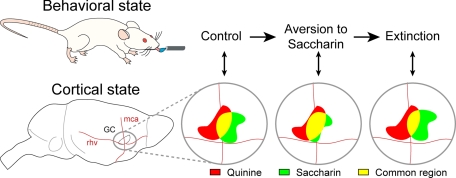
Fig. 4.
Relationship between behavioral state and cortical state in the gustatory cortex. In a naïve (i.e., control) rat, cortical representations of the hedonically positive (saccharin) and negative (quinine) tastants are quite different, although common activated cortical territories exist (in yellow). After pairing a malaise to the ingestion of saccharin, the rat displays a strong aversion to saccharin. The change in hedonic value of saccharin is associated to a change of its cortical representation that becomes more similar (high correlation) to the quinine one. After saccharin aversion extinction, the hedonic value of saccharin is positive again, and its cortical map is again less similar (low correlation) to quinine. Note that the new representation of saccharin after extinction may not be a simple return to the one before conditioning.
Strong evidence suggests that GC is not the first site in the taste pathway where hedonic value is assessed. In CTA experiments done in rats with GC lesions (48), animals could still generate aversive responses to avoid unpleasant tastes. In a series of studies using c-FLI expression, Yamamoto et al. (29) conditioned rats to saccharin aversion and individuated subnuclei in the parabrachial nucleus responding only to the hedonic value of the stimulus, with other regions responding to the pure taste modality. Despite the evidence that hedonic value might be determined in the brainstem, altogether the imaging and electrophysiological approaches demonstrate that the GC distinguishes between positive and negative hedonics, although it does not seem to possess an area exclusively dedicated to this processing.
In general, our study adds an important evidence for the view of the GC as a processing site of multiple taste attributes (modality, texture, and viscosity) (49), including taste hedonics and taste memories (21, 50, 51). Modulation of GC responses by internal states was recently reported at a single cell level (20). By the means of intrinsic imaging, we show that visceral modulations induce a large neuronal ensemble plasticity that signals a shift in tastant hedonic value associated to behavioral shift of the stimulus hedonic value. We propose that general changes in internal body state, such as visceral malaise, stress, or hormonal levels, may also affect cortical representations and functions that may be the source of some food intake disorders.
Materials and Methods
Animals.
The experiments were performed on Wistar rats (Charles River Laboratories) housed in a temperature-controlled room and maintained on a 12-h/12-h light/dark cycle. All animal protocols conformed to the Swiss Federal laws.
Behavior.
To assess the potential impact of visceral modulations on cortical representations, we used a conditioned taste aversion (CTA) learning paradigm. In CTA, the animal learns to avoid ingesting a taste solution that has been paired with malaise (12). Saccharin (Sac) (0.1% wt/vol, representing a 5 mM concentration, sodium salt) was used as conditioned stimulus (CS) paired to LiCl (0.15 M, 2% body weight, i.p.) as the malaise inducing agent, also named unconditioned stimulus (US). The choice of a novel taste such as saccharin was made because CTA is more effective when the CS is unfamiliar (39, 52).
The subjects were divided into four main groups: the animals that acquired aversion through CS pairing (CTA group, n = 12), the nonconditioned group for which the US was paired with saline injection (CS-Ctrl group, n = 17), another control group with animals that experienced the malaise without coupling to the CS (US-Ctrl group, n = 5), and the animals that acquired the aversion and subsequently extinguished it by repeated daily applications of the CS stimulus (EXT group, n = 16).
The behavioral paradigm for CTA acquisition is described in Fig. 1A. Rats [postnatal day 21 (P21) on first behavioral day] were water-deprived for 24 h and then pretrained over 3 days to get their daily distilled water ratio [water day (WD)] for 10 min from three pipettes of 3 ml each [see supporting information (SI) Text]. On day 4 [conditioning day (CD)], saccharin (CTA, CS-Ctrl) or distilled water (US-Ctrl) only were given in similar pipettes, and 30 min after the end of the stimulus presentation, either LiCl (CTA, US-Ctrl) or 0.9% NaCl (CS-Ctrl) was injected i.p.
On the subsequent test days (TD), rats were presented for 10 min once a day, with a multiple choice of six pipettes of 3 ml each, three filled with distilled water, and three with saccharin. Small volume pipettes ensured a more equal exposure to both fluids and prevented the satiation effect (53). Preference for one solution over the other was assessed by measuring the aversion index: AI = (milliliters of water) × 100/(milliliters of water + milliliters of Sac).
Gustatory cortex intrinsic signals were measured after test day 2 in the CTA and CS-Ctrl groups (see Intrinsic Signals Imaging and Stimulation). Some animals of the CS-Ctrl group were not imaged (n = 4) and were used to create a baseline of the AI over the entire period of testing days (TD1 to TD9, AIbase = 13.16 ± 1.77). The EXT animals were imaged when the recovery of the preference with respect to the CS-Ctrl baseline was at least 80% for 2 consecutive days (or, conversely, only 20% of maximal aversion maintained). This occurred generally after TD7 (Fig. 3B) and corresponds to the following: AIrecovery = AIbase + (AICTA(TD1) − AIbase) × 20/100 = 26. All paired statistical comparisons between conditions were performed by using a two-tailed unpaired t test.
Intrinsic Signals Imaging and Stimulation.
Eight animals per group were imaged (five in the US-Ctrl group); the others were either included only for the behavior analysis or died during the imaging experiments. The surgical and imaging procedures are described in detail in ref. 13. The stimulation procedure is described in SI Text.
Data Analysis.
All analyses were performed by using custom programmed Matlab scripts. The images obtained for presentations of the same tastant were averaged in each individual rat. Frames were averaged over a 2-s period, 1 s after stimulus onset (13). The relative amplitude responses were obtained by dividing the average of the first 15 blank frames of each data acquisition period (representing a respiratory cycle of ≈500 ms). Unspecific and diffused darkening of the cortical region after stimulus application (DC shift) was treated with a 2D Gaussian high pass filter (1/2πσ = 50 pixels), whereas a temporal low pass elliptic filter (fstop = 2 Hz; fpass = 1 Hz; Rstop = 50 dB; Rpass = 1 dB) was used to reduce the effect of heart beat and respiratory artifacts.
The correlation indices between the amplitude maps to saccharin and quinine were calculated for every animal considering the responses averaged over a 2-s period, 1 s after the stimulus.
Supplementary Material
ACKNOWLEDGMENTS.
We thank R. Shema and Y. Dudai for useful details and advices on CTA protocols and C. C. H. Petersen for comments on the manuscript. This work was supported by the Brain Mind Institute, Ecole Polytechnique Fédérale de Lausanne, and the network of European Neuroscience Institutes from the European Union.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708927105/DC1.
References
- 1.Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 2.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 3.Welker E, Rao SB, Dorfl J, Melzer P, van der Loos H. Plasticity in the barrel cortex of the adult mouse: effects of chronic stimulation upon deoxyglucose uptake in the behaving animal. J Neurosci. 1992;12:153–170. doi: 10.1523/JNEUROSCI.12-01-00153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polley DB, Kvasnak E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. doi: 10.1038/nature02469. [DOI] [PubMed] [Google Scholar]
- 5.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siucinska E, Kossut M. Short-lasting classical conditioning induces reversible changes of representational maps of vibrissae in mouse SI cortex: A 2DG study. Cereb Cortex. 1996;6:506–513. doi: 10.1093/cercor/6.3.506. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci USA. 1993;90:2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- 10.Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res. 1994;65:123–137. doi: 10.1016/0166-4328(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 11.Kiefer SW, Cabral RJ, Garcia J. Neonatal ablations of the gustatory neocortex in the rat: Taste aversion learning and taste reactivity. Behav Neurosci. 1984;98:804–812. doi: 10.1037//0735-7044.98.5.804. [DOI] [PubMed] [Google Scholar]
- 12.Bures J, Bermudez-Rattoni F, Yamamoto T. Conditioned Taste Aversion. Memory of a Special Kind. Oxford: Oxford Univ Press; 1998. [Google Scholar]
- 13.Accolla R, Bathellier B, Petersen CC, Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci. 2007;27:1396–1404. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grinvald A, et al. In: Modern Techniques in Neuroscience Research. Windhorst U, Johansson H, editors. Berlin: Springer; 1999. pp. 893–969. [Google Scholar]
- 16.Ferreira G, Ferry B, Meurisse M, Levy F. Forebrain structures specifically activated by conditioned taste aversion. Behav Neurosci. 2006;120:952–962. doi: 10.1037/0735-7044.120.4.952. [DOI] [PubMed] [Google Scholar]
- 17.Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behav Neurosci. 2005;119:388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- 18.St Andre J, Albanos K, Reilly S. C-fos expression in the rat brain following lithium chloride-induced illness. Brain Res. 2007;1135:122–128. doi: 10.1016/j.brainres.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste responses of cortical neurons in freely ingesting rats. J Neurophysiol. 1989;61:1244–1258. doi: 10.1152/jn.1989.61.6.1244. [DOI] [PubMed] [Google Scholar]
- 20.de Araujo IE, et al. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 22.Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascual-Leone A, Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- 24.Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci. 1994;14:1710–1721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siucinska E, Kossut M. Experience-dependent changes in cortical whisker representation in the adult mouse: A 2-deoxyglucose study. Neuroscience. 2004;127:961–971. doi: 10.1016/j.neuroscience.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Chang FC, Scott TR. Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. J Neurosci. 1984;4:1850–1862. doi: 10.1523/JNEUROSCI.04-07-01850.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houpt TA, Philopena JM, Wessel TC, Joh TH, Smith GP. Increased c-fos expression in nucleus of the solitary tract correlated with conditioned taste aversion to sucrose in rats. Neurosci Lett. 1994;172:1–5. doi: 10.1016/0304-3940(94)90648-3. [DOI] [PubMed] [Google Scholar]
- 28.Shimura T, Tanaka H, Yamamoto T. Salient responsiveness of parabrachial neurons to the conditioned stimulus after the acquisition of taste aversion learning in rats. Neuroscience. 1997;81:239–247. doi: 10.1016/s0306-4522(97)00188-7. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T, Shimura T, Sakai N, Ozaki N. Representation of hedonics and quality of taste stimuli in the parabrachial nucleus of the rat. Physiol Behav. 1994;56:1197–1202. doi: 10.1016/0031-9384(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 30.Miranda MI, Ferreira G, Ramirez-Lugo L, Bermudez-Rattoni F. Glutamatergic activity in the amygdala signals visceral input during taste memory formation. Proc Natl Acad Sci USA. 2002;99:11417–11422. doi: 10.1073/pnas.182200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. San Diego: Academic; 1995. pp. 751–771. [Google Scholar]
- 32.Yasoshima Y, Yamamoto T. Short-term and long-term excitability changes of the insular cortical neurons after the acquisition of taste aversion learning in behaving rats. Neuroscience. 1998;84:1–5. doi: 10.1016/s0306-4522(97)00636-2. [DOI] [PubMed] [Google Scholar]
- 33.Nerad L, Ramirez-Amaya V, Ormsby CE, Bermudez-Rattoni F. Differential effects of anterior and posterior insular cortex lesions on the acquisition of conditioned taste aversion and spatial learning. Neurobiol Learn Mem. 1996;66:44–50. doi: 10.1006/nlme.1996.0042. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T, Fujimoto Y, Shimura T, Sakai N. Conditioned taste aversion in rats with excitotoxic brain lesions. Neurosci Res. 1995;22:31–49. doi: 10.1016/0168-0102(95)00875-t. [DOI] [PubMed] [Google Scholar]
- 35.Berman DE, Hazvi S, Neduva V, Dudai Y. The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: Activation of ERK1–2 and formation of a memory trace. J Neurosci. 2000;20:7017–7023. doi: 10.1523/JNEUROSCI.20-18-07017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira G, Gutierrez R, De La Cruz V, Bermudez-Rattoni F. Differential involvement of cortical muscarinic and NMDA receptors in short- and long-term taste aversion memory. Eur J Neurosci. 2002;16:1139–1145. doi: 10.1046/j.1460-9568.2002.02174.x. [DOI] [PubMed] [Google Scholar]
- 37.Gutierrez R, Tellez LA, Bermudez-Rattoni F. Blockade of cortical muscarinic but not NMDA receptors prevents a novel taste from becoming familiar. Eur J Neurosci. 2003;17:1556–1562. doi: 10.1046/j.1460-9568.2003.02608.x. [DOI] [PubMed] [Google Scholar]
- 38.Rosenblum K, Berman DE, Hazvi S, Lamprecht R, Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci. 1997;17:5129–5135. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenblum K, Meiri N, Dudai Y. Taste memory: The role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- 40.Yasoshima Y, Sako N, Senba E, Yamamoto T. Acute suppression, but not chronic genetic deficiency, of c-fos gene expression impairs long-term memory in aversive taste learning. Proc Natl Acad Sci USA. 2006;103:7106–7111. doi: 10.1073/pnas.0600869103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rescorla RA. Experimental extinction. In: Mowrer RR, Klein SB, editors. Handbook of Contemporary Learning Theories. Mahwah, NJ: Lawrence Erlbaum; 2001. pp. 119–154. [Google Scholar]
- 42.Richards WG, Farley J, Alkon DL. Extinction of associative learning in Hermissenda: Behavior and neural correlates. Behav Brain Res. 1984;14:161–170. doi: 10.1016/0166-4328(84)90185-2. [DOI] [PubMed] [Google Scholar]
- 43.McCaughey SA, Giza BK, Nolan LJ, Scott TR. Extinction of a conditioned taste aversion in rats: II. Neural effects in the nucleus of the solitary tract. Physiol Behav. 1997;61:373–379. doi: 10.1016/s0031-9384(96)00412-x. [DOI] [PubMed] [Google Scholar]
- 44.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- 45.Mickley GA, et al. Spontaneous recovery of a conditioned taste aversion differentially alters extinction-induced changes in c-Fos protein expression in rat amygdala and neocortex. Brain Res. 2007;1152:139–157. doi: 10.1016/j.brainres.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 46.Mickley GA, et al. Dynamic processing of taste aversion extinction in the brain. Brain Res. 2004;1016:79–89. doi: 10.1016/j.brainres.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 47.Bathellier B, Van De Ville D, Blu T, Unser M, Carleton A. Wavelet-based multi-resolution statistics for optical imaging signals: Application to automated detection of odour activated glomeruli in the mouse olfactory bulb. Neuroimage. 2007;34:1020–1035. doi: 10.1016/j.neuroimage.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 48.Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behav Neurosci. 1992;106:140–146. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- 49.Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85:45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol. 2006;69:243–255. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- 52.Lubow RE. Latent Inhibition and Conditioned Attention Theory. London: Cambridge Univ Press; 1989. [Google Scholar]
- 53.Bures J, Buresova O, Krivanek J. Brain and Behaviour: Paradigms for Research in Neural Mechanisms. New York: Wiley; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.