Abstract
Domestication of the donkey from the African wild ass transformed ancient transport systems in Africa and Asia and the organization of early cities and pastoral societies. Genetic research suggests an African origin for the donkey, but pinpointing the timing and location of domestication has been challenging because donkeys are uncommon in the archaeological record and markers for early phases of animal domestication are hard to determine. We present previously undescribed evidence for the earliest transport use of the donkey and new paleopathological indicators for early phases of donkey domestication. Findings are based on skeletal data from 10 ≈5,000-year-old ass skeletons recently discovered entombed in an early pharaonic mortuary complex at Abydos, Middle Egypt, and a concurrent study of 53 modern donkey and African wild ass skeletons. Morphometric studies showed that Abydos metacarpals were similar in overall proportions to those of wild ass, but individual measurements varied. Midshaft breadths resembled wild ass, but midshaft depths and distal breadths were intermediate between wild ass and domestic donkey. Despite this, all of the Abydos skeletons exhibited a range of osteopathologies consistent with load carrying. Morphological similarities to wild ass show that, despite their use as beasts of burden, donkeys were still undergoing considerable phenotypic change during the early Dynastic period in Egypt. This pattern is consistent with recent studies of other domestic animals that suggest that the process of domestication is slower and less linear than previously thought.
Keywords: morphometrics, osteopathologies, African wild ass, Egypt
The domestication of plants and animals starting ≈11,000 years ago (1, 2) transformed human interactions with the natural world and resulted in a trajectory of intensification that led to contemporary urbanism. Domestication has often been thought of as a rare, intentional, short-term process, but recent genetic, zooarchaeological, and behavioral studies have shown that, although few large mammals were ever domesticated, many were domesticated more than once (1–5). More than one wild ancestor was often involved, and in many species gene flow from the wild continued for long periods (2, 6, 7). Moreover, unintentional human selection played an important role, particularly in the early phases of domestication and husbandry (8, 9). As a result of this new knowledge there is increasing emphasis on domestication as a nonlinear microevolutionary process influenced by the behavior of individual species, the nature of human environments, and management practices. To investigate the length and course of this complex process, though, species-specific indicators that identify early phases of domestication are needed (2, 4–6, 8–11).
Ten articulated donkey (Equus asinus) skeletons recently discovered in three brick tombs adjacent to the mortuary complex of one of the founder dynasty Egyptian kings (ca. 3000 B.C.) at Abydos, Egypt, represent the earliest and most numerous articulated donkey skeletons ever found and a unique opportunity to investigate these issues in the donkey (Fig. 1). This species is an especially interesting case because multiple domestication events may have occurred within one geographic area, northeast Africa (12, 13), and because little is known about the course of domestication of transport animals (7, 14). Domestication of donkeys ≈6,000 years ago transformed early pastoral societies and ancient states. Donkeys are tough desert-adapted animals, and their ability to carry heavy loads through arid lands enabled pastoralists to move farther and more frequently and to transport their households with their herds. Domestication of the donkey also allowed large-scale food redistribution in the nascent Egyptian state and expanded overland trade in Africa and western Asia. Today donkeys and mules are essential for transport in arid, rugged, and poorer regions of the globe (15). There is, however, little direct evidence on the length of the process and the timing of donkey domestication, or on when they were first used for transport rather than for food. We report here on the recently discovered skeletons and on a concurrent study of modern African wild ass and donkey metacarpals that was designed to complement analysis of the Abydos skeletons and to develop markers for the process of donkey domestication.
Fig. 1.
Abydos donkeys in situ within brick tombs.
The African wild ass (Equus africanus) is the wild ancestor of the donkey. Because the earliest donkeys were found in ancient Egypt, archaeologists concluded that they were domesticated from resident Nubian wild ass (E. africanus africanus) by villagers inhabiting the Egyptian Nile Valley. One of the bases for this view was Sir Flinders Petrie's unusual discovery of three donkey skeletons in a First Dynasty tomb at Tarkhan in Egypt (ca. 2850 B.C.) (16). More recent size-based analyses of bones from the sites of Maadi and Hierakonpolis have provided additional evidence for the presence of early donkeys in ancient Egypt (17, 18).
In the 1980s zooarchaeologists working in southwestern Asia found bones attributable to donkey from sites in Syria, Iran, and Iraq dating to ca. 2800–2500 B.C. (19–21). Identifications are difficult in this region because of the ubiquitous presence of Asian wild ass (Equus hemionus), but these finds and the discovery of bones identified as African wild ass (22, 23) raised the alternate hypothesis that donkeys were domesticated in this part of the Old World.
New genetic research on mitochondrial DNA of modern donkeys, though, suggests that not one but two subspecies of African wild ass were domesticated. Two clades of domestic donkeys have been identified, one that groups with the Somali wild ass and the other with Nubian wild ass (12, 13). Variability in both clades is greatest in Africa. As a result of these findings and recent archaeological research it has been argued that African pastoralists domesticated the wild ass as a response to increasing aridity in the Sahara ≈6,000 years ago (12, 24). Testing these hypotheses for ancient Egyptian, southwest Asian, or African pastoral domestication of Equus africanus requires identifying the earliest donkeys in their cultural context. Unfortunately, the initial stages of domestication are when donkeys are most similar to their wild ancestor and therefore hardest to distinguish.
Egyptian nobility hunted African wild ass long after donkeys were domesticated, so both occur on Dynastic Egyptian sites. Equus africanus has been identified from archaeological sites and from depictions of wild ass hunts such as that from King Tutankhamun's tomb. Because many domestic ungulates are smaller than their wild ancestors and bone fragments from Predynastic and Dynastic sites show a trend toward size decrease in Egyptian donkeys through time, osteologically large ass are usually considered wild and small individuals are usually considered domestic (18). The earliest remains thought to be donkeys were identified on the basis of size and archaeological context and are thought to date to late 5th millennium and the first half of the 4th millennium B.C. contexts in the Egyptian prehistoric settlements of El-Omari (ca. 4600–4400 B.C.), Maadi (first half of 4th millennium B.C.), and Hierakonpolis (ca. 3600 B.C.) (18, 25–28).
Comparative studies of the morphology of a wide range of skeletal elements and contemporary equids by the paleontologist Vera Eisenmann suggest that the metacarpals of these cursorial mammals are especially responsive to changes in body size and life habits. She argues that metacarpal morphometrics and particularly measures such as midshaft depth are better indicators of donkey domestication than element size alone (29), but this requires more complete specimens than are commonly found in settlement debris. Complete donkey skeletons are rare, and since Petrie's discovery at Tarkhan relatively complete early donkey skeletons have been excavated at only one Egyptian site, Abusir (17), where three donkey skeletons were discovered among the contents of a mastaba dating to the time of King Den, fourth king of the First Dynasty ca. 3000 B.C. In southwest Asia, three finds of donkey skeletons date clearly later, between 2400 and 2200 B.C. (19, 30–32). No ancient wild ass skeletons have ever been found. As a result of the rarity of whole skeletons, morphometric approaches have not been widely applied to archaeological material and the identification of early donkeys.
The whole skeletons from Abydos provide a morphological context for each bone, unobtainable from isolated fragments from Maadi and Hierakonpolis, and an exceptional opportunity to apply metacarpal metrics and new paleopathological approaches to the problem of distinguishing hunted wild ass from domestic donkeys. By providing information on the whole skeleton and the physical and social setting for domestication the Abydos burials may also help to identify early management practices and intentional selection.
Archaeological Context
Four hundred eighty kilometers south of Cairo, the site of Abydos, situated in a great embayment of high cliffs that form the western edge of the Nile Valley, is famous as the burial place of the earliest Egyptian kings and as the cult place of the god Osiris, himself a mythic king of Egypt and ruler of the land of the dead. One and a half kilometers north of, and functionally linked to, the tombs of the kings lies a series of royal mortuary monuments. Overlooking the ancient town, each king buried at Abydos also built a monumental cult enclosure, in which ceremonial focused on the divine king and relating to the royal funeral appears to have been conducted. In the 1st Dynasty, both the tombs and the cult enclosures were surrounded by the tombs of courtiers and retainers. The exact relationship of the occupants of the graves to the kings is uncertain, but some, at least, appear to have been of very high status, and physical proximity to the king may have, in itself, constituted such status. Not all of these “subsidiary” graves were occupied by human burials. Graves next to the tomb of one early king contained the remains of lions, symbolically associated with the power of kingship, and one of the enclosures was accompanied by graves containing 14 large wooden boats. Regardless of their specific contents, the most likely basic purpose of the subsidiary graves was to ensure that the occupants or contents would accompany and be available to the king in the next world. Along with the elaborate and richly appointed royal burial, the presence of the subsidiary graves adjacent to the royal tomb and cult enclosure appears to have been seen as a defining component of kingship in this early period.
The Abydos donkeys were buried in three contiguous subsidiary grave chambers, in a part of the site known today as the North Cemetery, adjacent to the cult enclosure of one of the earliest kings to build at the site. They were found in situ in the sealed tombs. The construction and location of the graves are consistent with those of the graves containing human burials adjacent to other enclosures. The discovery is dated to the Early Dynastic period by its architectural context and evidence from seal impressions. Unfortunately none of the seal impressions bore a royal name identifying the king to whom this monument was erected, but the iconography of the seals and the configuration of the architecture suggest a date in the beginning of the Early Dynastic period, late Dynasty 0, or early 1st Dynasty (ca. 3000 B.C.), the time of the earliest Egyptian Kings such as Narmer and Aha.
The grave chambers were constructed in pits dug into sand, with sidewalls built of mud bricks. They were roofed with wood, which was capped with mud brick masonry. There was no evidence for superstructures above the grave chambers. All animals were oriented on their left sides on reed mats, parallel to each other and facing southeast (Fig. 1). The tombs were used only for the burial of the asses and did not contain human remains or mortuary goods. The skeletons were largely, but not entirely, complete. Five crania were missing, possibly as a result of ancient probing by grave robbers. Preservation was excellent, and in places remnants of soft tissue and hair adhered to the excavated bones.
Results and Discussion
Four kinds of information were analyzed for this study: age, sex, osteometrics, and skeletal pathology. Approaches taken to collection of these data are detailed in Materials and Methods. Age data including fully erupted dentitions with moderate wear (4/4 analyzable individuals) and fully fused long bone epiphyses (10/10 individuals) show that the Abydos animals were prime adults. Incisor wear suggests ages between 8 and 13 years. The presence of prominent canines (4/4 analyzable individuals) and pelvic characteristics of two additional individuals, including the semielliptical proportions of the pelvic inlet and the thickness of the pubic symphysis and body, suggest that all sexable animals (6/10) were male.
Metacarpal Osteometrics.
Osteometric analysis focused on metacarpals. Measures used and sampling of variation are discussed in Materials and Methods. Twelve measurements taken on six Abydos animals, 21 African wild ass, and 32 donkey metacarpals are listed in supporting information (SI) Table 3 and summarized in Table 1. Osteometric data were analyzed by using two contrasting approaches to gain insights into different aspects of the group patterns. The first approach examined patterns in individual measurements by broad group, Abydos ancient ass, African wild ass, and donkeys, to explore specific changes with early domestication. The second approach combined individual animals into groups with hierarchical cluster analysis using 11 variables to explore individual variation and its relationship to group membership in our sample. In exploring relationships among morphology, size, taxonomy, and life habits, it was useful to distinguish between Nubian and Somali wild ass and between wild living and confined zoo wild ass (see SI Table 3).
Table 1.
Metacarpal measurements (mean ± SD) by broad group, with results of statistical testing
| Measure | Abydos (n = 6) | Donkey (n = 32) | Nubian (n = 13) | Somali (n = 8) | P value (ANOVA) |
|---|---|---|---|---|---|
| GL (E1) greatest length | 195.7 ± 6.5 | 183.2 ± 14.4 | 190.5 ± 6.7 | 207.1 ± 8.4 | <.0001 |
| SD (E3) smallest shaft breadth | 27.4 ± 0.9* | 24.6 ± 2.5 | 25.8 ± 1.8 | 27.0 ± 1.4 | 0.0055 |
| E4 midshaft depth | 20.8 ± 0.4* | 19.4 ± 1.9 | 21.3 ± 1.8 | 22.7 ± 2.0* | 0.0002 |
| Bp (E5) proximal breadth | 43.3 ± 1.2 | 38.3 ± 3.6 | 44.0 ± 2.3 | 45.8 ± 1.8 | <.0001 |
| E6 depth of proximal articular surface | 27.7 ± 1.5 | 25.2 ± 2.1 | 29.2 ± 1.6 | 29.2 ± 1.6 | <.0001 |
| E7 diameter of facet Os C III | 35.0 ± 2.2 | 31.9 ± 2.9 | 36.2 ± 1.8 | 36.9 ± 4.3 | <.0001 |
| E8 diameter of anterior facet Os C IV | 12.2 ± 1.0 | 10.8 ± 1.4 | 12.1 ± 1.3† | 12.2 ± 1.0† | 0.0104 |
| Bd (E10) distal supra-articular breadth | 38.2 ± 1.5 | 35.7 ± 2.7* | 39.6 ± 1.9 | 41.2 ± 2.3 | <.0001 |
| E11 distal articular breadth | 37.8 ± 1.0 | 35.2 ± 2.9 | 38.5 ± 2.1* | 40.2 ± 1.4 | <.0001 |
| Dd (E12) depth distal end | 28.2 ± 1.2 | 26.3 ± 2.5 | 28.8 ± 1.7* | 28.9 ± 1.2 | 0.0011 |
| E13 smallest depth medial condyle | 22.8 ± 1.2 | 21.8 ± 1.9 | 23.8 ± 1.1* | 24.1 ± 1.1 | 0.0004 |
| E14 greatest depth medial condyle | 25.3 ± 1.8 | 23.4 ± 2.3 | 25.2 ± 1.3* | 26.1 ± 1.2 | 0.0012 |
*One missing data point.
†Two missing data points.
The general trend in the measurements was for donkeys to be smallest, Somali to be largest, and for Nubian to be larger than Abydos (Table 1). The mean of the donkey measurements was always the smallest of the four groups. As might be expected, given that specimens were domestic, came from three continents, and included animals bred for different purposes (see Materials and Methods), their variability, as measured by standard deviation, was usually the largest (10/12 measurements). The means of the Somalis were largest, or tied for largest, for 11/12 measurements, and the means of the Nubians were larger than the means of the Abydos for 8/12 measurements.
Donkeys were the group most different from the others: the difference between the donkey and Somali means was statistically significant for 11/12 measurements, the difference between donkey and Nubian means was statistically significant for 9/12 measurements, and the difference between donkey and Abydos means was statistically significant for 3/12 measurements. Nubian and Abydos differed very little from Somalis, or from each other: the difference between Somali and Nubian means was statistically significant for 1/12 measurements, and the difference between Somali and Abydos means, and between Nubian and Abydos means, was never statistically significant.
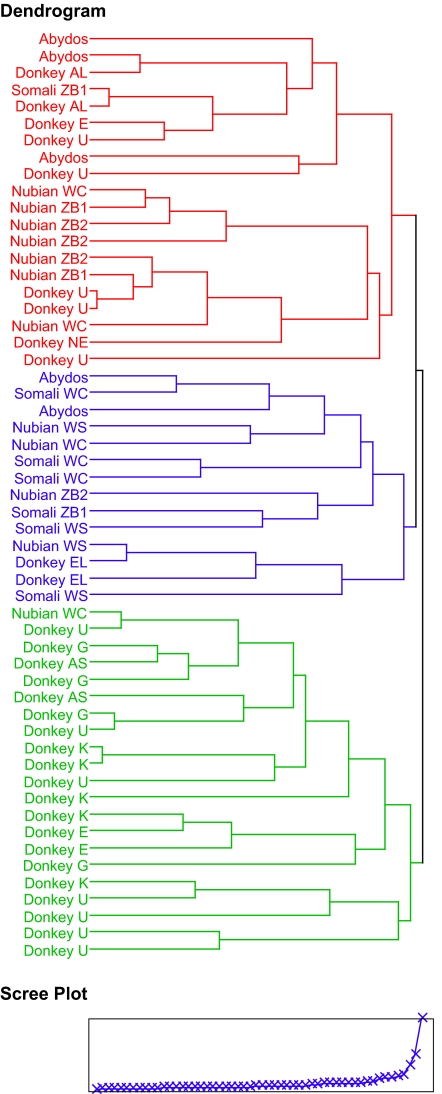
To explore these patterns further, individuals were analyzed by hierarchical cluster analysis (Fig. 2; statistics discussed in Materials and Methods). For this analysis individuals were classified into narrower groups that reflect taxonomy as well as size and life habits (SI Table 3). Lifelong wild animals or wild-shot animals (Nubian WS 3/13, Somali WS 2/8) were differentiated from wild-caught but zoo-raised wild ass (Nubian WC 8/13, Somali WC 2/8); other groups included first-generation zoo-born (Nubian ZB1, 2/13) and second-generation zoo-born (Nubian ZB2, 4/13 Nubian) wild ass. Donkeys were divided into size groups; the smaller group included Kenyan donkeys (K 5/32), northeast African Egyptian, Sudanese, and Ethiopian donkeys (E 3/32), Greek donkeys (G 4/32), and small American donkeys (AS 2/32), and the larger group included northern European donkeys (NE 1/32), large American donkeys (AL 2/32), large Egyptian riding ass (EL 2/32), and unknown (U 13/32) (see SI Table 3).
Fig. 2.
Hierarchical cluster analysis dendrogram of metacarpal variables.
The scree plot at the base of the dendrogram showed that the distance bridged to join clusters began to increase markedly at the transition from six to five clusters, but using three clusters was most informative for our analysis. One cluster, highlighted in green, was clearly the “small, donkey” group. It included a majority (20/31) of the donkeys and one exceptionally small wild-caught Nubian. Another cluster, highlighted in blue, was clearly the “large, wild” group. It included 6/7 Somalis, 4/12 Nubians (generally the animals with less history of confinement: 2/2 wild-shot, 1/4 wild-caught, and 1/6 zoo-born), a minority (2/5) of the Abydos, and two Egyptian riding donkeys specially bred for size. Finally, there was a “middle” group, highlighted in red. It included 7/12 of the Nubians (generally the zoo animals: 2/4 wild-caught and 5/6 zoo-born), the majority (3/5) of the Abydos, and a minority (9/31) of the donkeys.
Paleopathology.
Despite the fact that the Abydos animals were prime adults and not aged animals, we observed multiple developmental stages of spondylo-arthropathies on the Abydos skeletons (Table 2). These are a posttraumatic response to microfractures arising in the vertebral bodies with overloading and strain. The initial symptom is inflammation of the ventral ligament (33), visible in animals II, IV, V, and X, followed by periarticular osteophyte formation at the intervertebral space resulting in spondylosis deformans (34) present in animals II, V, and X (Fig. 3). Strain-induced spondylosis is accompanied by partial to almost complete degeneration of intervertebral discs, the consequences of which we observed on the articular facets of the entire vertebral column and in all individuals studied, along with hypertrophy of bone along the margins of these articulations. Compression and inclination of neural spines toward the right side were repeatedly observed in the caudal thoracic and cranial lumbar segments of the vertebral column of individuals I, II, IV, V, VI, VII, and VIII, possibly the result of the animals' being ridden (31). In all skeletons except IX, the neural spines of the first five thoracic vertebrae were compressed against each other (Fig. 3). This is consistent with considerable external pressure exerted just over the shoulder blade. The joints of the appendicular skeleton of all animals showed heavy wear implying serious damaging of the articular cartilage. In several instances the condition of the acetabular joint approached eburnation. Joint wear was distributed fairly evenly among the different parts of the extremities, including the feet, which we believe results from loading rather than pulling. Finally, we also noted several examples of individual trauma, including an inflamed mandibular diastema, one dislocated rib, and one rib with a healed fracture.
Table 2.
Distribution of skeletal pathology among Abydos animals, confined African wild ass from zoos, and free-living African wild ass and zebra
| Pathology | Abydos (E. asinus) | Zoo (E. africanus) | Free-living (Equus sp.) |
|---|---|---|---|
| Spavin (arthropathia deformans et ankylopoetica tarsi) | 0/10 | 2/15 | 0/8 |
| Spondylosis deformans | 3/10 | 3/15 | 0/8 |
| Compression/inclination of neural spines | 7/10 | 0/15 | 0/8 |
| Compression of neural spines of first thoracic vertebrae | 9/10 | 0/15 | 0/8 |
| Joint wear | 10/10 | 0/15 | 0/8 |
Fig. 3.
Vertebral arthropathies of Abydos donkey skeletons. (Upper) Osteophyte formation. (Lower) Compression remodeling of spine.
We observed no pathologies on skeletons of modern free-living wild equids (0/8), but five of 16 modern African wild ass from zoos exhibited some form of pathology (Table 2). Four of these were aged animals, but the fifth was only 11 years old. Three animals showed vertebral spondylo-arthropathies, two suffered from spavin (arthropathia deformans et ankylopoetica tarsi) (33), and one animal had dislocated a rib. None of the African wild ass from zoos exhibited the lipping, eburnation, and extensive wear of appendicular joints shown by the Abydos animals or any traces of spinal compression.
Conclusions
An analysis of Abydos, modern Nubian, and Somali wild ass and donkey metacarpal measurements shows that Abydos animals were closest in shape to the Nubian wild ass but were more similar to Somali wild ass than to modern donkey. Examination of individual traits shows that metacarpal morphology in Abydos animals is a mosaic of wild and domestic characters. These Abydos data provide the first insights into the sequence of morphological change in the African wild ass with domestication. Reductions in metacarpal midshaft depth and distal breadth occur earlier than other metrical changes and are good indicators of ongoing selection processes. Metacarpal midshaft breadth, proximal breadth, and proximal depth in the Abydos metacarpals are, on the other hand, conservative and similar to those of the African wild ass. The degree of overall size reduction (metacarpal length) in the Abydos metacarpals is difficult to gauge, being more dependent than other measures on whether ancient Egyptian donkeys were domesticated from the tall Somali or the shorter Nubian wild ass. If, as seems likely on geographic grounds, the Nubian wild ass was the wild ancestor of Egyptian donkeys, then size reduction was minimal at this stage of donkey husbandry.
The overall similarity of the shapes of Abydos and African wild ass metacarpals could be interpreted as suggesting that the Abydos animals were wild. Alternately, the fact that some measurements of metacarpal architecture suggest wild ass and others suggest donkey might point to initial stages of domestication and unintentional selection processes in an anthropogenic environment before confinement by humans or introgression between wild and domestic populations. But the context of and the skeletal pathologies on the Abydos animals are consistent with confinement and prolonged use for heavy transport. All Abydos long bones show abrasion of cartilage, osteophyte formation along the margins of the articular surfaces, and scouring of the joint surface consistent with wear resulting from transport of heavy loads. All vertebral columns exhibit neural spine compression and remodeling consistent with confinement or suspension of loads across the back. Regardless of morphology, all of these animals were used as beasts of burden.
These First Dynasty donkeys from Abydos provide the earliest direct evidence of use of donkeys for transport rather than for meat. They also provide the earliest secure, non-size-based evidence for domestic donkeys. Unlike size, behavior-induced bone pathology is an unambiguous indicator of how donkeys were actually used by humans. The Abydos finds demonstrate that bone pathology can be used archaeologically to identify domestic status in transport animals (35) and to recognize early stages of management before genetic or potential osteomorphological and metrical changes.
The Abydos data establish pathology as an early signal of domestication of the donkey, followed by decreases in metacarpal midshaft depth and distal breadth measurements, and finally by overall size decreases and shape changes. Together these indicators will allow identification of different phases of the domestication process and development of a more nuanced understanding of early use and management of the donkey. This sequence supports arguments that domestication may be first seen through indicators of management rather than through a decrease in body size (9–11).
It used to be thought that animal domestication was a quick and deliberate process involving intense initial selection. Recent genetic and zooarchaeological studies emphasize long-term evolutionary change in the process of domestication (2, 4, 5, 36). But the time scale and nature of selection processes during domestication are not well understood. The Abydos data show that the Abydos donkeys still bore important similarities to their wild ancestors at least 1,000 years after the earliest possible evidence for domestication. Their burial and its location in the high status area of the North Cemetery indicate that the animals were highly valued and may have been used to provision the royal household. This elite status reinforces perspectives on the economic importance of the donkey to the first pharaohs, land-based transport, and integration of the early Egyptian state (37). Morphology shows that, despite their value and intensive use as beasts of burden, the process of domestication of the donkey was lengthy, with significant phenotypic changes still ongoing during the early Dynastic period in Egypt.
Materials and Methods
The Abydos skeletons are curated in the magazine of the Penn–Yale–IFA Expedition House. Modern comparative wild ass skeletons were studied at the Field Museum in Chicago; the natural history museums in London, Basel, Berlin, and Bern, Switzerland; the Bavarian State Collections for Zoology and Anthropology and Palaeoanatomy in Munich; and the Powell Cotton Museum in Kent, U.K. Domestic donkey skeletons were also studied at the Natural History Museum in Geneva; the Julius Kühn Museum in Halle, Germany; and the National Museums of Kenya (Table 1 and SI Table 3). All bones were measured to the nearest millimeter following conventions established for zooarchaeologists by von den Driesch (38) and specialist equid measurements developed by Eisenmann and Beckouche (29). The 12 metacarpal measurements detailed in Table 1 were taken by using digital calipers and an osteological measuring box. Age and sex were estimated for the Abydos animals on the basis of dental eruption and wear, epiphyseal fusion, presence and size of canines, and pelvis morphology (39). Six Abydos metacarpals were complete enough for morphometric study; five crania, one pelvis, and long bones from 10 animals were used for estimation of age and sex.
Few African wild ass skeletons exist in museums worldwide, but all known skeletons were measured (n = 21). There was considerable variability because wild ass measurements are drawn from two subspecies; 13 Nubian wild ass (E. a. africanus, extinct in the wild) and eight of the larger Somali wild ass (Equus africanus somaliensis, critically endangered) were studied (SI Table 3). A third group, the Atlas wild ass (extinct), is known from Saharan rock art and Roman mosaics, but not from skeletons. In this analysis, therefore, it was possible to sample the majority, but not the full range, of past variability in the skeleton of the African wild ass.
Donkeys are common, but their skeletons are rare in world collections. For comparison with wild ass and archaeological specimens we measured 16 donkeys from Africa, Asia, the Mediterranean, and Europe and obtained measurements for 16 additional animals from published sources (29) (n = 32) (SI Table 3). Donkeys are not as phenotypically variable as domesticates such as horses or dogs, but because of selective breeding all modern domestic animals are more variable than their wild ancestors (8). This sample excludes modern giant and dwarf breeds, but two Egyptian White Riding asses, bred for height (29), were included in addition to donkeys used as pack animals.
We used a photographic record, color-coded diagram, scoring system, and qualitative description of individual skeletal features and abnormalities to record paleopathologies and wear (40). All elements from the 10 Abydos skeletons were examined for the paleopathological study. Two Somali wild ass and three Nubian wild ass, representing all known skeletons collected for museums from the wild, and one Burchell's (Equus burchelli) and two Grevy's zebra (Equus grevyi) skeletons were studied to provide a baseline for age-related wear in free-living wild equids. Nine Nubian wild ass and six Somali wild ass skeletons from zoo animals were examined for age-related wear in confined wild ass.
Statistical analysis of the measurement data was performed in two phases. The first was comparison of the group means. Differences in the overall pattern were tested for statistical significance with ANOVA. When the overall pattern was statistically significant, all pairs of means were tested by using the Tukey–Kramer honestly significant difference test. The threshold of statistical significance for all tests was P < 0.05. The second phase was hierarchical cluster analysis. All variables except E8, which was statistically weakest with most missing data (four individuals), were used to cluster all individuals with complete data (55/59) using Ward's minimum variance method. All analysis was performed by using JMP 6.0 (SAS Institute).
Supplementary Material
ACKNOWLEDGMENTS.
For their role in facilitating the Abydos research we thank the Egyptian Supreme Council of Antiquities. We are grateful to Louis Chaix, Richard Meadow, and the museums listed in Materials and Methods (SI Table 3) and their curators for facilitating collection of metrical and pathological data. We also thank Richard Redding, David Browman, and anonymous reviewers of the manuscript for their comments. This research was supported by National Science Foundation Grant BCS-0447369.
Footnotes
This paper is dedicated to the memory of S.R. (1975–2007), who died in a tragic hiking accident shortly after the manuscript was submitted.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709692105/DC1.
References
- 1.Tanno K, Willcox G. Science. 2006;311:1886. doi: 10.1126/science.1124635. [DOI] [PubMed] [Google Scholar]
- 2.Peters J, von den Driesch A, Helmer D. In: The First Steps of Animal Domestication. Vigne JD, Peters J, Helmer D, editors. Oxford: Oxbow Books; 2005. pp. 96–124. [Google Scholar]
- 3.Bradley DG, MacHugh DE, Cunningham P, Loftus RT. Proc Natl Acad Sci USA. 1996;93:5131–5135. doi: 10.1073/pnas.93.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobney K, Larson G. J Zool. 2006;269:261–271. [Google Scholar]
- 5.Zeder MA, Emshwiller E, Smith BD, Bradley DG. Trends Genet. 2006;22:139–155. doi: 10.1016/j.tig.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Larson G, Dobney K, Albarella U, Meiying F, Matisoo-Smith E, Robins J, Lowden S, Finlayson H, Brand T, Willerslev E, et al. Science. 2005;307:1618–1621. doi: 10.1126/science.1106927. [DOI] [PubMed] [Google Scholar]
- 7.Olsen SL. In: Documenting Domestication. Zeder M, Bradley D, Emshwiller E, Smith BD, editors. Berkeley, CA: Univ of California Press; 2006. pp. 245–369. [Google Scholar]
- 8.Hemmer H. Domestication. Cambridge, UK: Cambridge Univ Press; 1990. [Google Scholar]
- 9.Zohary D, Tchernov E, Horwitz LK. J Zool. 1999;245:129–135. [Google Scholar]
- 10.Lösch S, Grupe G, Peters J. Am J Phys Anthropol. 2006;131:181–193. doi: 10.1002/ajpa.20395. [DOI] [PubMed] [Google Scholar]
- 11.Zeder MA, Hesse B. Science. 2000;287:2254–2257. doi: 10.1126/science.287.5461.2254. [DOI] [PubMed] [Google Scholar]
- 12.Beja-Pereira A, England PE, Ferrand N, Jordan S, Bakhiet AO, Abdalla MA, Mashkour M, Jordana J, Taberlet P, Luikart G. Science. 2004;304:1781. doi: 10.1126/science.1096008. [DOI] [PubMed] [Google Scholar]
- 13.Vilà C, Leonard JA, Beja-Pereira A. In: Documenting Domestication. Zeder MA, Bradley DG, Emshwiller E, Smith BD, editors. Berkeley: Univ of California Press; 2006. pp. 342–353. [Google Scholar]
- 14.Wing ES. In: High Altitude Tropical Biogeography. Vuilleumier F, Monasterio M, editors. Oxford: Oxford Univ Press; 1986. pp. 246–264. [Google Scholar]
- 15.Starkey P. In: The Origins and Development of African Livestock: Archaeology, Genetics, Linguistics and Ethnography. McDonald KC, Blench RM, editors. London: Univ College London Press; 2000. pp. 478–502. [Google Scholar]
- 16.Petrie WMF. Tarkhan II. London: Quarritch; 1914. [Google Scholar]
- 17.Boessneck J, von den Driesch A, Eissa A. Mitt Dtsch Archäol Inst Abt Kairo. 1992;48:1–11. [Google Scholar]
- 18.von den Driesch A. Archaeofauna. 1997;6:23–39. [Google Scholar]
- 19.Boessneck J, von den Driesch A. Mitt Dtsch Orientgesellschaft. 1986;118:45–50. [Google Scholar]
- 20.Vila E. In: Equids in Time and Space. Mashkour M, editor. Oxford: Oxbow Books; 2006. pp. 101–123. [Google Scholar]
- 21.Zeder MA. In: Equids in the Ancient World. Meadow RH, Uerpmann HP, editors. Wiesbaden, Germany: Dr. Ludwig Reichert Verlag; 1986. pp. 366–412. [Google Scholar]
- 22.Ducos P. In: Equids in the Ancient World. Meadow RH, Uerpmann HP, editors. Wiesbaden, Germany: Dr. Ludwig Reichert Verlag; 1986. pp. 237–245. [Google Scholar]
- 23.Uerpmann HP. In: Equids in the Ancient World. Meadow RH, Uerpmann HP, editors. Vol II. Wiesbaden, Germany: Dr. Ludwig Reichert Verlag; 1991. pp. 12–33. [Google Scholar]
- 24.Marshall F. In: Rethinking Agriculture: Archaeological and Ethnoarchaeological Perspectives. Denham TP, Iriarte J, Vrydaghs L, editors. Walnut Creek, CA: Left Coast Press; 2007. pp. 371–407. [Google Scholar]
- 25.Boessneck J, von den Driesch A, Ziegler R. In: Maadi III. Rizkana I, Seeher J, editors. Mainz, Germany: Philipp von Zabern; 1989. pp. 87–128. [Google Scholar]
- 26.Boessneck J, von den Driesch A. In: El Omari. Debono F, Mortensen B, editors. Mainz, Germany: Philipp von Zabern; 1998. pp. 99–101. [Google Scholar]
- 27.McArdle J. In: The Followers of Horus. Friedman R, Adams B, editors. Oxford: Egyptian Studies Assoc Publication No. 2, Oxford Monograph 20; 1992. pp. 53–56. [Google Scholar]
- 28.Van Neer W, Linseele V, Friedman R. In: Egypt at Its Origins. Hendrickx S, Friedman RF, Cialowicz KM, Chlodnicki M, editors. Leuven, Belgium: Peeters; 2004. pp. 67–130. [Google Scholar]
- 29.Eisenmann V, Beckouche S. In: Equids in the Ancient World. Meadow RH, Uerpmann HP, editors. Wiesbaden, Germany: Dr. Ludwig Reichert Verlag; 1986. pp. 117–163. [Google Scholar]
- 30.Clutton-Brock J. In: Equids in the Ancient World. Meadow RH, Uerpmann HP, editors. Wiesbaden, Germany: Dr. Ludwig Reichert; 1986. pp. 207–229. [Google Scholar]
- 31.Clutton-Brock J. In: Prehistoric Steppe Adaptation and the Horse. Levine M, Renfrew C, Boyle K, editors. Cambridge, UK: McDonald Institute Monographs; 2003. pp. 126–127. [Google Scholar]
- 32.Clutton-Brock J, Davis S. Iraq. 1993;55:209–221. [Google Scholar]
- 33.Dahme E, Weiss E. Grundriss der Speziellen Pathologischen Anatomie der Haustiere. 5th ed. Stuttgart, Germany: Enke; 1999. [Google Scholar]
- 34.Daugnora L, Thomas R. In: Diet and Health in Past Animal Populations. Davies J, Fabiš M, Mainland I, Richards M, Thomas R, editors. Oxford: Oxbow Books; 2002. pp. 68–74. [Google Scholar]
- 35.Levine MA, Bailey GN, Whitwell KE, Jeffcott LB. In: Human Ecodynamics. Winder N, Bailey GN, Charles R, editors. Oxford: Oxbow Books; 2000. pp. 123–133. [Google Scholar]
- 36.Zeder MA. Evol Anthropol. 2006;15:105–117. [Google Scholar]
- 37.Hassan F. In: The Archaeology of Africa: Food, Metals and Towns. Shaw TP, Sinclair BA, Okpoko A, editors. London: Routledge; 1993. pp. 551–569. [Google Scholar]
- 38.von den Driesch A. A Guide to the Measurement of Animal Bones From Archaeological Sites. 1, Harvard Univ, Cambridge MA: Peabody Museum Bulletins; 1996. [Google Scholar]
- 39.Misk NA, Semieka MMA. Equine Pract. 1997;19:23–29. [Google Scholar]
- 40.Bartosiewicz L, Van Neer W, Lentacker A. Draught Cattle: Their Osteological Identification and History. Tervuren, Belgium: Annales Musée Royale de l'Afrique Centrale, Annales Sciences Zoologiques 281; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.