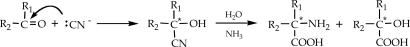Abstract
The nonracemic amino acids of meteorites provide the only natural example of molecular asymmetry measured so far outside the biosphere. Because extant life depends on chiral homogeneity for the structure and function of biopolymers, the study of these meteoritic compounds may offer insights into the establishment of prebiotic attributes in chemical evolution as well as the origin of terrestrial homochirality. However, all efforts to understand the origin, distribution, and scope of these amino acids' enantiomeric excesses (ee) have been frustrated by the ready exposure of meteorites to terrestrial contaminants and the ubiquitous homochirality of such contamination. We have analyzed the soluble organic composition of a carbonaceous meteorite from Antarctica that was collected and stored under controlled conditions, largely escaped terrestrial contamination and offers an exceptionally pristine sample of prebiotic material. Analyses of the meteorite diastereomeric amino acids alloisoleucine and isoleucine allowed us to show that their likely precursor molecules, the aldehydes, also carried a sizable molecular asymmetry of up to 14% in the asteroidal parent body. Aldehydes are widespread and abundant interstellar molecules; that they came to be present, survived, and evolved in the solar system carrying ee gives support to the idea that biomolecular traits such as chiral asymmetry could have been seeded in abiotic chemistry ahead of life.
Keywords: carbonaceous chondrites, chemical evolution, chiral asymmetry, diastereomer amino acids
Carbonaceous chondrites (CC) are fragments of primitive asteroids that contain abiotic organic material and offer a unique record of the chemical evolution that came to precede terrestrial life in the solar system. These meteorites have a complex organic composition and contain structures as diverse as kerogen-like macromolecules and simpler soluble compounds ranging from polar amino acids and polyols to nonpolar hydrocarbons (1). Within this complex suite, some meteoritic compounds have identical counterparts in the biosphere; for example, eight of the protein amino acids as well as nicotinic and other carboxylic acids are indigenous to meteorites, as verified by their extraterrestrial isotopic signature. In addition, some chiral amino acids of CC display enantiomeric excesses (ee) that, although not as extensive, have the same configuration (l) as terrestrial amino acids (2, 3). To date, these nonracemic amino acids of meteorites provide the only example of molecular asymmetry measured outside the biosphere.
Because extant life depends on chiral homogeneity for the structure and function of biopolymers, these findings imply the possibility that prebiotic properties became established in abiotic chemical evolution and aided in the origin of life (4, 5). In turn, however, they also raise new questions. What is the scope of chiral asymmetry in meteorites? Where did it originate? Did its delivery help in molecular evolution? All are difficult to answer, linked as they are to the uncertainties surrounding the origins of life. Even understanding the molecular scope of CC asymmetry, which would appear within analytical reach, has been frustrated by the ready exposure of meteorites to terrestrial contamination and the pervasive homochirality of such contaminants. We report here on the soluble organic composition of a carbonaceous meteorite that was collected in the Graves Nunataks Antarctica ice fields, stored in the Johnson Space Center curatorial facilities (as GRA 95229), and seems exceptional in having escaped most of terrestrial contamination. This meteorite is classified as CR2, i.e., to belong to a recently discovered family of chondrites that are compositionally similar to Renazzo, a meteorite fallen in 1824 and long kept unclassified. The mineralogy of these CCs is not known in detail, but it is believed that their asteroidal parent bodies experienced a liquid phase of high water/rock ratio (6) at temperature ≤20°C (hence the type 2). The insoluble organic materials (IOM) of meteorites of this type have been analyzed and appear to be compositionally primitive (7, 8).
Results and Discussion
Molecular and Isotopic Analyses.
We found that the GRA 95229 meteorite contains an abundant and diverse suite of soluble organic compounds that, while reflecting in the overall number of molecular species those of other CC, is unique for having a definite preponderance of water-soluble species (>90% overall). By comparison, all other meteorites analyzed so far have sizeable amounts of hydrocarbons (Table 1). The relative distributions of these CR2 compounds are also novel, with N-containing amino acids and amines being significantly more abundant than O-containing hydroxy-, mono-, and dicarboxylic acids. In the Murchison meteorite, for example, monocarboxylic acids are 10 times more abundant than amino acids, whereas in the GRA 95229, it is the amino acids that are the most abundant soluble compounds, followed closely by amines. In addition, ammonia's presence was found to be larger than in Murchison (9) by well over an order of magnitude (18.85 μm/g of meteorite). Other differences were observed within the individual groups of compounds. The amino acids serine, threonine, and allothreonine and a large group of tertiary amines, although searched before in other CC, were detected for the first time only in this meteorite. Also, the lower-molecular-weight compounds (e.g., glycine, α-aminoisobutyric acid, and glycolic acid) are dominant over higher homologs, and not all amino acid abundances increase upon acid hydrolysis. In Murchison, by contrast, overall soluble compound abundances decrease linearly with increasing carbon chain length and amino acid yields increase markedly upon hydrolysis. [The complete list of the GRA 95229 individual compounds and their amounts in the meteorite are found in supporting information (SI) Tables 3–8].
Table 1.
Abundance and molecular distribution of soluble organic compounds in the GRA 95229 and Murchison meteorites
| Compound | GRA 95229 |
Murchison |
||
|---|---|---|---|---|
| nmol/g† | n‡ | nmol/g | n | |
| Amino acids | 2,015 | 50 | 600 | 100 |
| Hydroxy acids | 800 | 15 | >152 | 38 |
| Pyridine carboxylic acids | NF | / | 70 | >7 |
| Monocarboxylic acids | 908 | 23 | 3,000 | 48 |
| Dicarboxylic acids | 984 | 20 | 300 | 44 |
| Amines | 1,510 | 19 | 130 | 20 |
| Hydrocarbons | ≈200 | ≥100 | 2,000 | ≥300 |
Abundances for both meteorites are minimum numbers, many more compound are known present but cannot be positively identified. The Murchison meteorite fell in 1969 and has been extensively studied since then (1). NF, not found.
†Nanomole/gram of meteorite.
‡Number of molecular species identified.
The isotopic composition of GRA 95229 amino acids revealed both similarities and differences with other meteorites as well. It has been shown (10) that the amino acids of the Murchison meteorite likely fall in two synthetic categories: the molecular species with α-branched alkyl chains, whose high deuterium content (δD = +3,000–3,600‰) links them to extremely cold synthetic locals such as those of the interstellar medium (ISM), and the α-H species that display lower δD values (+100–2,000‰) and are believed to have formed by further processing of interstellar precursors, e.g., during the parent body aqueous phase. Murchison amino acids of the first group display variable l-ee validated by isotopic analyses, whereas compounds of the second group have shown no unequivocal ee (11). This isotopic distinction between types of amino acids was found magnified in the GRA 95229 (Table 2) with δD values for branched amino acids (≥+7,200) that are the highest ever recorded for an extraterrestrial molecules by direct analysis and fall within D/H values established spectroscopically for ISM molecules (+5,800–45,000; e.g., ref. 12).
Table 2.
δD values (‰) of individual amino acids from a GRA 95229 hydrolyzed sample
| Amino acid† | δD, ‰ |
|---|---|
| Glycine | 868 ± 150 |
| d-Alanine | 1,182 ± 63 |
| l-Alanine | 1,159 ± 55 |
| d-2-Aminobutyric acid | 2,890 ± 86 |
| 2-Aminoisobutyric acid | 7,245 ± 49 |
| dl-Isovaline | 5,807‡ |
| 4-Aminobutyric acid | 1,475 ± 30 |
| d-Glutamic acid | 1,029 ± 89 |
| l-Glutamic acid | 894 ± 81§ |
†δD = (D/Hsample − D/Hstandard/D/Hstandard) 103; terrestrial standard (mean ocean water) = 0.
‡The value is uncorrected for a coeluting procedural contaminant; mass correction based on GC-MS ion trace abundances gives δD ≥ 7,000.
§l-Glutamic and l-serine had slight ee (≤1% and 8%, respectively) that could be accounted for by traces of these compounds in procedural blanks. The l-Glutamic ee increased to 8% in hydrolyzed samples, also a procedural effect.
These analyses offer a yet unknown view of the synthetic capabilities and prebiotic potential of extraterrestrial environments. The high deuterium enrichment of some GRA 95229 amino acids places the syntheses of these compounds, or of their direct precursors, within cold cosmic environments. In the asteroidal parent body, the ensuing water chemistry must have been only mildly oxidizing and somewhat constrained by time, temperature, water/rock ratio, or a combination of the above factors to keep many of its products from further reactions or decomposition. For example, ≈50% of the amino acids in other meteorites such as Murchison are extracted in water as acid labile derivatives, whereas the abundance increase of CR2 amino acids upon hydrolysis of the extract is lower or absent (SI Table 3). Similarly, it is possible that hydroxyamino acids like serine, threonine, and allothreonine as well as the abundant suite of tertiary amines of this CR2 (SI Table 7) had not been observed in other meteorites because of their further parent body reactions. The low level of hydrocarbons also seems to support a distinct formation for these compounds (more compositional details are given in the SI Text) and the idea (13) that their larger amounts in other meteorites may have been freed from the IOM by extended parent body processes. Overall, the GRA 95229 analyses show an organic suite of long cosmic lineage that is mildly processed in solar environments and rich in water-soluble small organic molecules, many of which were preserved from further reactions during their solar residence, to a possible advantage in prebiotic chemical evolution.
Enantiomeric Excesses in Diastereomer Amino Acids: A Case for Precursor Aldehydes' Molecular Asymmetry.
In the GRA 95229 meteorite, the l-isovaline ee [2-amino-2-methylbutyric acid, CH3-CH2-C(NH2)(CH3)COOH] was 3% (SI Fig. 3), i.e., in the lower range of those (0−15%) determined for the Murchison and Murray meteorites (14). The unexpected, and welcomed, difference in comparison with other meteorites was to find that the GRA 95229 chiral protein amino acids, alanine, threonine, proline, valine, leucine, and aspartic acid, were racemic (as noted in Table 2, slight excesses of l-glutamic and l-serine were accounted for by blanks as procedural effects). These findings indicate an almost complete lack of terrestrial contaminants in the meteorite and the indigenous nature of d- and l-alanine was further confirmed by their equal δD values.
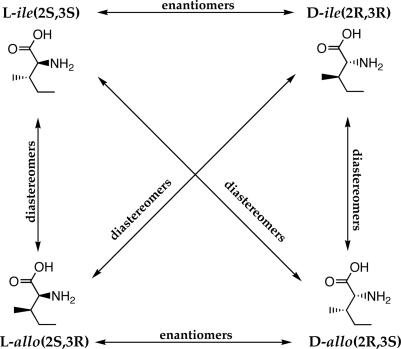
The apparent overall pristine nature revealed by this meteorite's molecular and isotopic analyses has allowed a novel reassessment of other meteoritic amino acids with protein counterparts, such as isoleucine. This compound [2-amino-3-methylpentanoic acid, CH3-CH2-CH(CH3)-CH(NH2)-COOH] contains two chiral centers, at C2 and C3, and can be present as four stereoisomers, depending on the possible distribution of the substituents at these centers. Because the molecular species having matching configurations at C2 and C3 (2S,3S and 2R,3R) are not the same as those where they differ (2S,3R and 2R,3S), these stereoisomers make up two compounds, of two enantiomers each called diastereomers [the pairs of d- and l-isoleucine (ile), and d- and l-alloisoleucine (allo); Scheme 1]. Only l-ile is found in terrestrial proteins but all four diastereomers are found in meteorites.
Scheme 1.
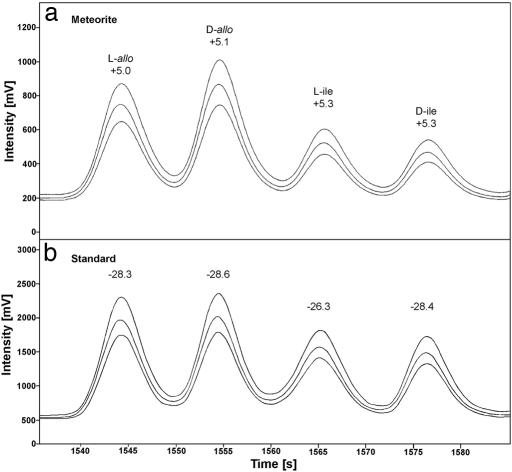
These compounds had shown in the Murchison and Murray meteorites an odd distribution of d-allo and l-ile ee (S.P. and J. R. Cronin, unpublished results); however, an unequivocal interpretation of the finding was prevented by the possibility of terrestrial contamination. l-ile is 1 of the 20 terrestrial protein amino acids, and its racemization in water at C2 leads to d-allo¶; for example, d-allo is not found in proteins but is produced with time by their decay, and its presence in fossils has been used in geo-chronology (e.g., ref. 15). We have now searched for the allo/ile diastereomer amino acids in the GRA 95229 meteorite and found a chiral distribution similar to Murchison's, with d-allo ee of 12.1% (±0.6) and l-ile ee of 14.0% (±0.8) (Fig. 1). We believe these ee are indigenous and are the result of asymmetry in the diastereomers' precursor molecules.
Fig. 1.
Chiral gas chromatographic separation of the CR2 95229 alloisolleucine/leucine diastereomers (mass spectrometric detection, single ion traces).
As mentioned above, it has been proposed that the less deuterated amino acids of meteorites could have formed in the asteroidal parent body by the reaction of deuterated precursor molecules and lighter water. A possible reaction is the addition of HCN to ketones and aldehydes in the presence of ammonia (the cyanohydrin reaction; Scheme 2) (16); all are abundant components of interstellar ices and considered likely constituents of primitive asteroids. The occurrence of this reaction in meteorites was corroborated by the finding of its by-products, the imino acids, in the Murchison meteorite (17).
Scheme 2.
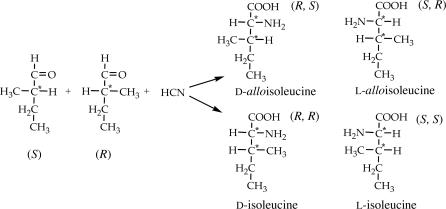
Although an asymmetric carbon (C*) is formed, this type of synthesis is nonstereospecific because the HCN addition to the carbonyl would be random and give equal amounts of d- and l-enantiomers (possibly accounting for the lack of ee in the α-H meteoritic amino acids that formed this way). In the case of longer-chain aldehydes and ketones, however, they also could carry an asymmetric carbon to the synthesis and lead to more complex chiral distribution of the products. For example, the cyanohydrin reaction of a chiral five-carbon (R)(S)-2-methylbutyraldehyde (Scheme 3) would form the four amino acid diastereomers described above, i.e., the two sets of ile and allo enantiomers.
Scheme 3.
With a racemic aldehyde reactant, the enantiomers of each diastereomeric set will also be racemic, but, had an ee been present in the aldehyde [e.g., of the (S) configuration], those amino acids that carried this S-portion of the molecule through their synthesis will be more abundant than their respective enantiomers. In the above example, this would be the (RS)-allo and (SS)-ile compounds, or, in the formalism used for amino acids, d-allo and l-ile. On this basis, therefore, the finding we report of such distribution in the diastereomer GRA CR2 amino acids would signify an S-asymmetry of their precursor aldehydes. To confirm this premise, we measured the carbon isotopic composition of the four diastereomers in the CR2 meteorite. We found (Fig. 2) that the δ13C values of both pairs of enantiomers fell within statistical error of each other, i.e., showed no indications of interference from the lower values of terrestrial biomolecules, validating the compounds' indigeneity to the meteorite as well as the indigenous nature of their ee.
Fig. 2.
Gas chromatography carbon isotope ratio mass spectroscopy of a GRA 95229 unhydrolyzed water extract showing the elution of isoleucine and alloisoleucine enantiomers (a), an alloisoleucine/isoleucine standard (b), and their δ13C values. m/z traces 44, 45, and 46, on a Cirasil-Dex column. Prior GC-MS analyses of the sample showed that elutions of the four meteoritic diasteromers were free of interference. A more extended chromatogram is shown in SI Fig. 4.
These chiral and isotopic findings, as well as the rationale of a proven water phase chemistry in the asteroidal parent body, combine to give convincing evidence for the molecular asymmetry of this precursor C5 aldehyde in the meteorite. The data also imply that such asymmetry may reside in other aldehydes, i.e., in a class of extraterrestrial compounds that have been detected in a variety of cosmic environments, including in the interstellar medium (ref. 18 and references therein). Free aldehydes were not searched for in the GRA 95229, given the scarcity of the sample, but were described previously in the Murchison and Murray meteorites, as a series of C1–C5 species (19). Because these meteorites show in their mineralogy clear signs of having experienced an asteroidal aqueous phase (20), the detection of meteoritic aldehydes would indicate that these compounds were not entirely processed in parent body reactions such as the cyanohydrin synthesis. That ee are present in the GRA 95229 isoleucine diastereomers adds to those previous insights, showing that aqueous processes also did not lead to aldehydes' extensive racemization. The presence of aldehydes in meteorites and their wide distribution in extrasolar environments further suggest that these molecules may have been the primary recipients of prebiotic asymmetric effect(s).
The finding of additional ee in the GRA 95229 meteorite presents anew the challenge of interpreting what such effects might have been and how they were implemented. One hypothesis offered for the origin of meteoritic amino acid ee was the possible differential (asymmetric) photolysis of amino acid enantiomers by UV circularly polarized light (CPL) in the presolar cloud (2, 21). However, the proposal has been weakened by the inability so far to detect UV CPL, even from strong astronomical sources (22), and by models showing that UV irradiation would easily lead to destruction of both amino acid enantiomers before ee of the level found in meteorites were achieved (14, 23). Aldehydes and ketones, on the other hand, have absorption spectra at lower frequencies than amino acids, e.g., λmax of 270–320 (24), and it is possible that these compounds could have used less destructive CPL for their asymmetric syntheses. That the known ee-carrying amino acids as well as their ee are in lower abundance in this CR2 meteorite, but ee appear larger in a precursor aldehyde, would seem to indicate that abiotic organic pools in chemical evolution were diverse and differed in both their composition and their exposure to asymmetric effects, e.g., the energies of asymmetric radiation.
A larger diversity of nonracemic molecules in such pools may have profound prebiotic significance. Chiral asymmetry is an essential molecular tool for the interaction of extant biomolecules and it is through homochirality that life ensures the critical functions of its polymers (25). It is reasonable, therefore, to suggest that molecular asymmetry might have endowed prebiotic chemistry with inductive traits toward molecular organization and evolution. How the molecular evolution that preceded life was implemented on the early Earth is not known; at the present time, prebiotic chemical or geochemical models and the organic samples obtained from meteorites offer the only basis for hypotheses. For example, a possible involvement of nonracemic amino acids in early molecular evolution was suggested by the findings that these compounds could transfer their asymmetry to C4 sugars by acting as asymmetric catalysts in the aldol condensation of glycolaldehyde (4). Dipeptides, whose formation is arguably a small evolutionary step away from amino acids, were shown to magnify that asymmetric effect to ee of up to 80% (26). Although aldehydes do not appear to have some of the catalytic advantages of nonracemic 2-methyl amino acids, e.g., their resistance to racemization in water or acid-base properties, it is not difficult to imagine a role in molecular evolution for aldehydes endowed with ee, because these compounds are known to react readily with prebiotically relevant molecules, such as alcohols, sugars, and amino-containing compounds.
As a natural sample, CC have offered a unique a glimpse of the abiotic evolutionary processes that preceded the origin of terrestrial life. The 95229 CR2 meteorite, thanks to its exceptionally pristine nature, has broadened the view of the prebiotic organic material that would have been available to the early Earth as well as of the selective processes that could have made them amenable to the onset of life. In particular, that amino acid precursor molecules of wide cosmic distribution came to be present, survived, and evolved in the solar system carrying ee supports the idea that biomolecular traits such as chiral asymmetry could have been seeded in abiotic chemistry ahead of life.
Materials and Methods
Three interior fragments of the meteorite GRA 95229 were used for the analyses. Fragments A and B weighing 600 and 856 mg, respectively, were powdered in an agate mortar. The powders were extracted with 4 ml of triple-distilled water in evacuated vials, at 100°C, for 20 h, with intermittent sonication. The extracts were separated from the powder by centrifugation followed by four 1-ml water rinses. The pH of the A and B total extracts were measured at 7.25 and 7.5, respectively. The A powder was dried in desiccator over NaOH pellets and further extracted with 5 ml of dichloromethane (DCM)/methanol (MeOH) solution (9:1, vol/vol), under vacuum, at 100°C for 24 h. The extract was decanted and cleared of sulfur with copper filings. A smaller 60-mg fragment, C, was extracted with water as above, and the extract was processed for isolation of the amines.
Isolation of Water-Soluble Compounds.
The water extracts of A and B were concentrated on a rotary evaporator. Sample A was separated in two portions, a and b, containing 1/3 and 2/3 of the extract, respectively. Sample Aa was dried completely and subsequently hydrolyzed with 1 ml of 6 M HCl at 100°C for 24 h. Samples Aa, Ab, and B were frozen and subsequently processed at different times. Each was thawed, concentrated to ≈3 ml, applied with rinses to a 4- × 2.5-cm cation exchange column (Bio-Rad AG-50, ×4), and the column was eluted with 80 ml of water followed by 30 ml of 2 M ammonium hydroxide. Water eluates (pH 3.1) provided acidic or neutral compounds, whereas the basic eluates gave amino acids and basic compounds. The basic eluates of Aa and B were dried, redissolved in citrate buffer (pH 3.3, 0.2 M Na+), applied to a reversed-phase semiprep C18 column (Supelcosil-LC-18, 25 cm × 10 mm), and eluted with the same citrate buffer at a flow rate of 45 ml/h to separate the amino acids on the basis of their chain length (ref. 27 and references therein); each fraction was again desalted by cation exchange, as described above.
Molecular Analyses.
All samples were analyzed by GC-MS either directly (hydrocarbons and monocarboxylic acids) or after derivatization to acquire the desired volatility (amino acids, amines, and dicarboxylic acids). GC-MS analyses were performed on a Hewlett-Packard HP 5880 and an HP 5970.
Amino acids.
Samples containing amino acids were dried and esterified with isopropanol, and the isopropyl esters reacted with trifluoroacetic anhydride (TFAA) following customary procedures (e.g., ref. 28). The resulting N-trifluoroacetyl (TFA)-O-isopropyl amino acid derivatives were dried and redissolved in DCM for GC-MS analyses. Two capillary columns were used for cross-reference of enantiomeric excesses. A Chirasil-L-Val column of 50 m × 0.25 mm and 0.16-μm film thickness (Alltech) used a helium flow of 0.8 ml min−1 and oven temperature program of 70°C initial, for 5 min, 2°C min−1 to 100°C, 4°C min−1 to 200°C, and 45 min at the final temperature. The other column (Chrompack, Cirasil-Dex-CB), of 25 m × 0.25 mm and 0.25-μm film thickness, used a helium flow of 0.6 ml min−1 and oven temperature program as follows: 65°C initial, for 7 min, 2°C min−1 to 85°C, 5°C min−1 to 200°C, and 90 min at the final temperature.
Monocarboxylic acids.
For monocarboxylic acids, the Ab water eluate was made basic with three drops of 0.3 M NaOH solution (to pH ≈ 11.5) and concentrated to ≈0.5 ml. The extract, plus three 100-μl rinses, were transferred to a small vial with ultratorr fitting, made acidic with concentrated phosphoric acid (to pH ≈ 1.5), and cryogenically transferred on a vacuum line. The transferred solution, plus a drop of phosphoric acid, was extracted three times with 3-ml aliquots of DCM. The DCM extract was concentrated under helium flow and analyzed by GC-MS on a Chrompack column (as for amino acids; the temperature program was as follows: 35°C initial temperature, 5-min hold, to 60°C at 2°C min−1, and then to 200°C at 4°C min−1).
Dicarboxylic acids and hydroxy acids.
These acids were obtained from the Ab residue after monocarboxylics' transfer by extraction with ether. The ether extracts were concentrated in air and derivatized with isobutanol-TFAA. The derivatives were analyzed by GC-MS on a Chrompack column with the same temperature program as that for amino acids.
Amines.
Amines were analyzed directly from the water extract of sample C. The extract was made acidic (pH ≈ 2) with a few drops of 0.5 M HCl, concentrated to ≈0.5 ml, transferred to a small vial with ultratorr fitting, made basic with a drop of 0.3 M NaOH (pH ≈12), and transferred cryogenically to another vial, to which a few drops of HCl were added upon disconnecting from the vacuum. The content was dried on rotary evaporator, derivatized with TFAA, and analyzed by GC-MS on the Chrompack column described above with the following temperature program: 34°C initial temperature, to 43°C at 3°C min−1, 10-min hold, to 200°C at 4°C min−1.
Isotopic Measurements.
The δD values of individual amino acids from the GRA 95229 hydrolyzed extract represent repeat or triple analyses of the derivatized first fraction of the C18 fractionated amino acid sample. It contains C2–C5 amino acids and all dicarboxylic amino acids. Where clear separation permitted it, as for alanine, both d- and l-enantiomer values are given. When peak separations were not complete (e.g., for isovaline) or there was the doubt of coelution, DL values or those of only one enantiomer are listed.
The δD values were obtained by gas chromatography thermal conversion-isotope ratio mass spectroscopy (GCTC-IRMS) (10). The amino acids were separated by GC on Chirasil-Val, employing the same temperature conditions as those for molecular analyses, conveyed through a high-temperature pyrolysis reactor kept at 1,440°C, and quantitatively pyrolyzed to hydrogen gas and carbon monoxide or graphite. A tank of ultra-high-purity hydrogen gas with known δD values (δ DVSMOW = −349.76‰, predetermined against commercial reference hydrogen with δDVSMOW = −218.10‰, ZTECH Corp.) was used as isotope standard during the measurements. The δD of the isopropanol derivative moiety (−119.46‰) was obtained from the GCTC-IRMS-determined δD of an isopropyl succinate standard, by subtraction of the succinate (sodium salt) moiety δD previously obtained by elemental analysis. Individual mass balance corrections for derivatized amino acids were given by the equation: δDaa = [δDder.aa − frip(−119.46)]/fraa, where frip and fraa are the derivatized amino acid (der.aa) fractional hydrogen abundances of isopropanol (ip) alkyl portion and amino acid (aa), respectively. The amino group hydrogen left after derivatization was given the same value as the isopropanol, on the basis of its easy exchange under the derivatization conditions used.
δ13C values for alloisoleucine and isoleucine enantiomers were obtained by GC combustion isotope ratio MS of a single derivatized fraction from the C18 fractionated sample B that contained six-carbon amino acids (e.g., ref. 29). The isopropanol and TFA δ13C values needed for derivatization correction (−29.2 and −32.0, respectively) were obtained by the direct analysis of isopropanol and derivatized ammonia. The mass balance equation for six-carbon amino acids is as follows: δ13Caa = [δ13Cder aa − frip(−29.2) − frTFA(−32.0)]/fraa, where the carbon fractional abundance contributed by the six-carbon amino acid, isopropanol, and TFA are 0.545, 0.273, and 0.182, respectively.
Materials.
Water and 6 M HCl were triple and double distilled, respectively, by using a quartz sub-boiling apparatus. Isopropanol and acetyl chloride for acidification of the alcohol were obtained from Alltech. The remaining material was purchased from Sigma-Aldrich: Ammonium hydroxide (28% in water) was double distilled; sodium hydroxide was 99.99% pure (semiconductor grade), and phosphoric acid (85%+ in water) was 99,9999% pure; TFA, DCM, isobutanol, and ether were distilled after purchase.
Supplementary Material
ACKNOWLEDGMENTS.
S.P. is grateful to Laurence Garvie for his foresight in saving a GRA 95229 sample for soluble organic analyses, as well as to Cecilia Satterwhite and Kevin Righter, who provided the second meteorite sample for isotopic analyses. S.P. is indebted to John Cronin for the many discussions on the diastereomer amino acids of meteorites that go back 10 years. S.P. thanks David Lindstrom and the National Aeronautics and Space Administration Cosmochemistry and Origins Programs for indispensable support. We thank Jack Szostak and two anonymous reviewers for their helpful comments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709909105/DC1.
Diastereomers allow for a special case of racemization, called epimerization, when only one chiral carbon racemizes. This is the case for ile and other 2-H amino acids in water at C2; this carbon, which is weakly acidic by its proximity to the electron-withdrawing carboxyl group, easily loses/reacquires a proton, leading to racemization. Epimerization leads not to a molecule's enantiomer but to its diasteromer of opposite configuration (e.g., from l-ile to d-allo).
References
- 1.Pizzarello S, Cooper GW, Flynn GJ. Meteorites and the Early Solar System II. Tucson, AZ: Univ of Arizona Press; 2006. pp. 625–651. [Google Scholar]
- 2.Cronin JR, Pizzarello S. Science. 1997;275:951–955. doi: 10.1126/science.275.5302.951. [DOI] [PubMed] [Google Scholar]
- 3.Engel M, Macko SA. Nature. 1997;389:265–268. doi: 10.1038/38460. [DOI] [PubMed] [Google Scholar]
- 4.Pizzarello S, Weber AW. Science. 2004;303:1151. doi: 10.1126/science.1093057. [DOI] [PubMed] [Google Scholar]
- 5.Pizzarello S. Acc Chem Res. 2006;39:231–237. doi: 10.1021/ar050049f. [DOI] [PubMed] [Google Scholar]
- 6.Zolensky M, Barrett R, Browning L. Geochim Cosmochim Acta. 1993;57:3123–3148. [Google Scholar]
- 7.Cody G, Alexander CMOD. Geochim Cosmochim Acta. 2005;69:1085–1097. [Google Scholar]
- 8.Alexander CMOD, Fogel M, Yabuta H, Cody G. Geochim Cosmochim Acta. 2007;71:4380–4403. [Google Scholar]
- 9.Pizzarello S, Feng X, Epstein S, Cronin JR. Geochim Cosmochim Acta. 1994;58:5579–5587. doi: 10.1016/0016-7037(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 10.Pizzarello S, Huang Y. Geochim Cosmochim Acta. 2005;69:599–605. [Google Scholar]
- 11.Pizzarello S, Cronin JR. Nature. 1998;394:236. doi: 10.1038/28306. [DOI] [PubMed] [Google Scholar]
- 12.Roueff E, Gerin M. Space Science Rev. 2003;106:61–72. [Google Scholar]
- 13.Sephton MA, Pillinger CT, Gilmour I. Planet Space Sci. 1999;47:181–187. [Google Scholar]
- 14.Pizzarello S, Zolensky M, Turk KA. Geochim Cosmochim Acta. 2003;67:1589–1595. [Google Scholar]
- 15.Hare PE. Org Geochem. New York: Springer; 1969. pp. 438–463. [Google Scholar]
- 16.Peltzer ET, Bada JL. Nature. 1978;272:443–444. [Google Scholar]
- 17.Pizzarello S, Cooper GW. Meteorit Planet Sci. 2001;36:897–909. [Google Scholar]
- 18.Hollis JM, Jewell PR, Lovas FJ, Remijan A, Møllendal H, Green H. Astrophys J Lett. 2004;610:L21–L24. [Google Scholar]
- 19.Jungclaus GA, Yuen GU, Moore CB, Lawless JG. Meteoritics. 1976;11:231–237. [Google Scholar]
- 20.Zolensky M, McSween HY. Meteorites and the Early Solar System. Tucson, AZ: Univ of Arizona Press; 1988. pp. 114–143. [Google Scholar]
- 21.Rubenstein E, Bonner WA, Noyes HP, Brown GS. Nature. 1983;306:118. [Google Scholar]
- 22.Bayley J. Origin Life Evol Biosphere. 2001;31:167–183. doi: 10.1023/a:1006751425919. [DOI] [PubMed] [Google Scholar]
- 23.Balavoine G, Moradpour A, Kagan HB. J Am Chem Soc. 1974;96:5152–5158. [Google Scholar]
- 24.Barth G, Bunnenberg E, Djerassy C, Elder D, Records R. Symp Faraday Soc. 1970;115:49–60. [Google Scholar]
- 25.Cronin JR, Reisse J. Lectures in Astrobiology. Vol 1. Berlin: Springer; 2005. pp. 473–514. [Google Scholar]
- 26.Weber AW, Pizzarello S. Proc Natl Acad Sci USA. 2006;103:12715–12717. doi: 10.1073/pnas.0602320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizzarello S, Cronin JR. Geochim Cosmochim Acta. 2000;64:329–338. doi: 10.1016/s0016-7037(99)00280-x. [DOI] [PubMed] [Google Scholar]
- 28.Pizzarello S, Huang Y. Meteorit Planet Sci. 2002;37:687–696. [Google Scholar]
- 29.Pizzarello S, Huang Y, Fuller M. Geochim Cosmochim Acta. 2004;68:4963–4969. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.