Abstract
In this article, we report the family of robust layered sulfides K2xMnxSn3-xS6 (x = 0.5–0.95) (KMS-1). These materials feature hexagonal [MnxSn3-xS6]2x− slabs of the CdI2 type and contain highly mobile K+ ions in their interlayer space that are easily exchangeable with other cations and particularly strontium. KMS-1 display outstanding preference for strontium ions in highly alkaline solutions containing extremely large excess of sodium cations as well as in acidic environment where most alternative adsorbents with oxygen ligands are nearly inactive. The implication of these results is that simple layered sulfides should be considered for the efficient remediation of certain nuclear wastes.
Keywords: chalcogenide, environmental remediation, ion exchange, layered materials, nuclear waste
Current growing interest in nuclear power as a potential solution for global energy may also raise serious environmental and health concerns due to highly radioactive nuclear waste. 90Sr is one of the major heat producers and biohazards in nuclear wastes. The removal of radioactive strontium is essential to reducing the risk of human exposure to radiation and for the considerable cost savings due to minimization of the storage space requirements for the nuclear waste (1, 2). There is a long-standing and continuous research effort to develop highly selective strontium adsorbents for application in a variety of wastes. Inorganic ion exchangers possess a number of advantages as Sr2+ adsorbents over the conventional organic ion-exchange resins, such as superior chemical, thermal, and radiation stability (3). The naturally abundant ion exchangers such as clays (3, 4) and zeolites (3) are not effective as strontium adsorbents in nuclear waste solutions with extreme pH values because of their immediate decomposition (i.e., dissolution of their aluminum). Commercial inorganic adsorbents capable for Sr2+ adsorption in highly alkaline solutions with extremely high salt concentrations [i.e., conditions present in many nuclear waste types (1)] are mainly limited to titanates and silicotitanates (3, 5, 6). These materials, however, are not effective for Sr2+ capture even at mildly acidic conditions (pH < 4–5) because protons inhibit ion exchange (7). Only doped antimony silicates represent Sr2+ ion exchangers efficient in strongly acidic environment (pH ≤ 1) (8). Finally, solvent extraction and extraction chromatography methods have proven promising for strontium decontamination of acidic and alkaline nuclear wastes (9–11).
We show here that layered sulfide compounds with ion-exchangeable interlayer cations are not inhibited by protons and can be very efficient Sr2+-ion-removers over a wide pH range. Layered chalcogenides with ion-exchange properties are relatively few (12–16) and are mainly limited to alkali intercalated early transition metal dichalcogenides AxMQ2 (A = alkali ion; M = early transition metal from groups 4,5 and 6; Q = S, Se, Te) (13–16). Such materials, however, are not suitable for practical applications because of their hydrolytic instability (16).
Here, we report that the layered K2xMnxSn3-xS6 (x = 0.5–0.95) (KMS-1) exhibit huge selectivity for Sr2+ ions in both acidic and basic environments. They are especially effective in strongly alkaline environments in the presence of an enormous excess of Na+ ions. This property is highly relevant to the problem of nuclear waste remediation and points to the class of metal sulfide compounds as a highly promising source of materials for helping to solve it.
Results and Discussion
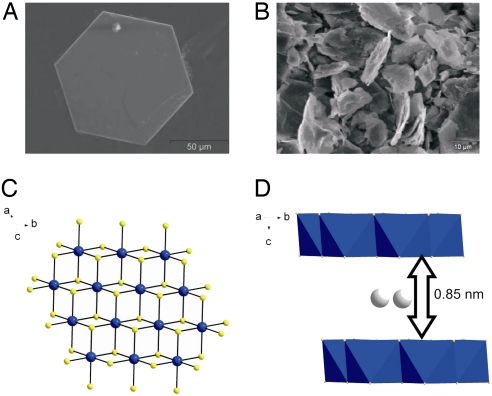
The KMS-1 materials can be easily prepared on a multigram scale and high purity with solid-state or hydrothermal synthesis techniques. They are extremely stable in atmosphere and water, while they display high thermal stability [see supporting information (SI) Figs. 5–7 (complete materials, instrumentation, and methods are provided in SI Materials and Methods, SI Figs. 5–12, and SI Table 2)]. Single-crystal data,§ obtained from hexagonal-shaped crystals (Fig. 1A) synthesized hydrothermally, revealed a layered structure of K1.9Mn0.95Sn2.05S6 (CdI2 structure type). The layer is built up by edge-sharing “Mn/Sn”S6 octahedra with Mn and Sn atoms occupying the same crystallographic position and all sulfur ligands being three-coordinated (Fig. 1C). K+ ions are found between the layers and are positionally disordered, a feature that gives them high mobility and the ability to exchange with other ions particularly with strontium (Fig. 1D).
Fig. 1.
Structure and morphology. (A) SEM image of a typical crystal of K1.9Mn0.95Sn2.05S6 (KMS-1) with a hexagonal plate-like shape obtained with a hydrothermal reaction. (B) SEM image of polycrystalline K2xMnxSn3-xS6 (x = 0.5–0.95) obtained by a solid-state reaction. (C) Part of the layer framework of K1.9Mn0.95Sn2.05S6 viewed down the c-axis. The Mn-Sn and S atoms are represented by blue and yellow balls, respectively. (D) View of the structure, with a polyhedral representation of the layers, along the c-axis.
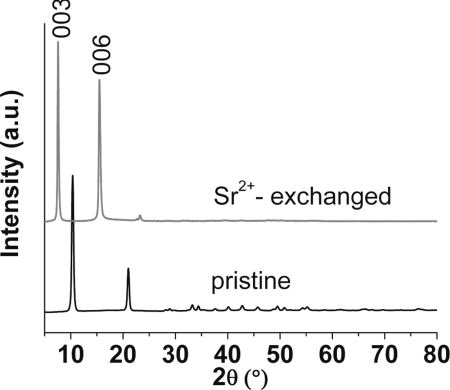
Indeed, polycrystalline samples (Fig. 1B) of KMS-1 can completely replace the K+ ions with Sr2+ within a few hours. This was confirmed with energy dispersive spectroscopy (EDS). Powder x-ray diffraction (PXRD) measurements showed that the exchanged material is isostructural with the pristine confirming a topotactic ion exchange (Fig. 2). A shift of the (003) and (006) Bragg peaks to lower 2θ values (or higher d-spacing) was observed in the diffraction patterns of Sr2+-exchanged products. Alkaline earth ions have a great tendency to be hydrated, and this results in the large c-axes for the Sr2+-exchanged materials. For example, thermal analysis data for Sr2+-exchanged samples revealed the presence of ≈5 H2O molecules per formula unit (SI Fig. 7). The expected Sr:Mn ratio in the exchanged materials should be 1 to satisfy the charge-balance requirements. Surprisingly, a Sr:Mn ratio of ≈0.5 was found because of an unusual oxidation of Mn2+ to Mn3+ occurring during the strontium exchange processes. The formation of Mn3+ is supported by x-ray photoelectron spectroscopy data (see SI Fig. 8 and 9 and SI Table 2).
Fig. 2.
X-ray powder diffraction patterns for the pristine K2xMnxSn3-xS6 (x = 0.5–0.95) and Sr2+-exchanged materials.
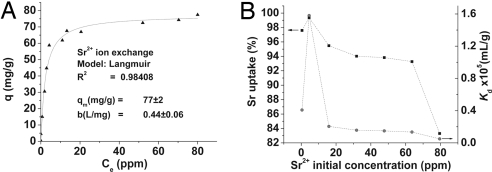
To assess the strontium removal capacity of KMS-1, we performed Sr2+ ion-exchange equilibration studies. The Sr2+ ion exchange-equilibrium data are shown in Fig. 3A. These data can be fitted with the Langmuir isotherm model expressed as
where q (mg/g) is the amount of the cation adsorbed at the equilibrium concentration Ce (ppm), qm is the maximum adsorption capacity of the adsorbent, and b (liters/mg) is the Langmuir constant related to the free energy of the adsorption.
Fig. 3.
Equilibrium ion-exchange data. (A) Sr2+-adsorption isotherms for K2xMnxSn3-xS6·2H2O (x = 0.95). The line represents the fitting of the data with the Langmuir isotherm model. (B) Plot of Sr uptake and KdSr vs. the initial concentration of Sr2+.
The maximum Sr2+ exchange capacity qm of KMS-1 (x = 0.95) was found to be 77 ± 2 mg/g (or 0.9 mmol/g), which compares well with those of the best strontium adsorbents [Sr2+ capacities = 1.0–2.0 mmol/g (7)].
The affinity of the material for Sr2+ can be expressed in terms of the distribution coefficient Kd (for definition, see Materials and Methods and ref. 17). In Fig. 3B are presented the plots of KdSr and percent strontium uptake vs. the initial strontium concentration (0.45–79.5 ppm) in the solution. It can be seen that the percent Sr2+ removal (83.3–99.4%) is high over a wide range of initial concentrations. Remarkably, the maximum KdSr value of 1.58 × 105 ml/g (Table 1) obtained lies among the highest reported in the literature for Sr2+ adsorbents (18).
Table 1.
Selected data for Sr2+ ion exchange experiments of KMS-1
| Exchanging cations | Sr2+/Na+ ratio | Conditions | Initial concentration, ppm | Final concentration, ppm | % removal | Kd, ml/g |
|---|---|---|---|---|---|---|
| Sr2+ | — | pH ∼ 3.2, V:m ∼ 971 ml/g | 4.09 | 0.15 | 96.3 | 2.49 × 104 |
| Sr2+ | — | pH ∼ 7, V:m ∼ 1,000 ml/g | 4.60 | 0.03 | 99.3 | 1.52 ×105 |
| Sr2++Na+ (0.1 M) | 1/1,887 | pH ∼ 13, V:m ∼ 971 ml/g | 4.65 | 0.01 | 99.8 | 4.50 ×105 |
| Sr2++Na+ (5 M) | 1/2.17 ×105 | pH ∼ 14, V:m ∼ 1,000 ml/g | 2.15 | 0.17 | 92.1 | 1.16 ×104 |
| Sr2++Ca2+ +Mg2++Na++Cs+ | — | pH ∼ 11, V:m ∼ 990 ml/g | 3.70 (Mg) | 0.48 (Mg) | 94.5 (Sr) | 6.64 × 103 (Mg) |
| 11.14 (Ca) | 1.17 (Ca) | 8.40 × 103 (Ca) | ||||
| 4.60 (Sr) | 0.24 (Sr) | 1.83 × 104 (Sr) | ||||
| 9.17 (Cs) | 3.17 (Cs) | 1.87 × 103 (Cs) | ||||
| 25.96 (Na) | 22.42 (Na) | 156.5 (Na) |
The % Sr removal and strontium distribution coefficient values are in boldface type.
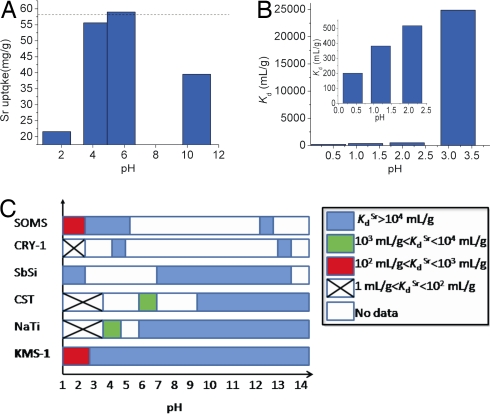
Taking into account that the pH of nuclear waste and contaminated ground water may vary from strongly acidic to extremely alkaline, we have also studied the effect of pH on Sr2+ adsorption. Sr2+ ion exchange experiments of KMS-1 with solutions of various pH (1.7–10) indicated significant Sr2+ uptake in the entire pH range tested (Fig. 4A). The capability of KMS-1 to absorb strontium from acidic solutions is particularly impressive. Specifically, the strontium adsorption at pH ∼ 4.3 did not deviate significantly from the maximum possible (≈95.9% of its maximum theoretical) and remained significant (37.2% of the maximum one) even at pH ∼ 1.7. We have also conducted ion-exchange experiments with trace concentrations of Sr2+ (4–6 ppm) at pH ∼ 0.4, 1.2, 2.1, and 3.2 (V:m ∼ 1,000 ml/g) that showed Kd values of 202, 383, 517, and 2.49 × 104 ml/g respectively (Fig. 4B and Table 1). These KdSr values revealed a remarkable strontium affinity of KMS-1 under strongly acidic conditions. For comparison, the commercial strontium adsorbent Na2Ti9O20·xH2O (NaTi) displays a Kd of 5,000 ml/g at pH ∼ 4 and 32 ml/g at pH ∼ 2 (for a more detailed comparison, see below) (19).
Fig. 4.
Comparison of KMS-1 with known sorbents. (A) Sr2+ uptake by K2xMnxSn3-xS6·2H2O (x = 0.75) as a function of pH. The dashed line indicates the maximum theoretical Sr capacity (58 mg of Sr/g) calculated for KxMnxSn3-xS6·2H2O (x = 0.75). (B) Representation of KdSr obtained with ion-exchange reactions in acidic solutions (pH ∼ 0.4–3.2). The initial Sr2+ concentration was ≈4 ppm in all reactions with the exception of the reaction at pH ∼ 0.4 with an initial strontium concentration of ≈6 ppm. (Inset) KdSr at pH 0.4, 1.2, and 2.1. (C) Diagram representing the dependence of KdSr for various materials on the pH. A Kd value greater than 10,000 ml/g is considered excellent (see ref. 19). The data for materials NaTi (sodium titanate), CST (sodium silicotitanate), CRY-1 (cryptomelane-type manganese oxide), SOMS (Sandia octahedral molecular sieves), and SbSi were obtained from the following references: NaTi, refs. 6, 8, 19, 20, and 22; CST, refs. 8 and 20; CRY-1, ref. 23; SOMS, ref. 24; and SbSi, ref. 8.
We performed competitive Sr2+-Na+ experiments (with trace concentrations of ≈1.5–5 ppm of strontium and 1.90 × 103 to 2.17 × 105-fold excess of sodium) to examine the selectivity of KMS-1 for Sr2+ over Na+ under environmentally relevant highly alkaline conditions (pH ∼ 13–14). Sodium is the most abundant cation in highly alkaline nuclear waste (1). The results (Table 1) showed quantitative (≈99.8%) removal of Sr2+ from a solution of 0.1 M NaOH (pH ∼ 13, Sr2+:Na+ = 1:1,887, reaction time of ≈24 h) with an enormous Kd of 4.50 × 105 ml/g. Even with much higher Na+ concentration (5 M) and pH ∼ 14—i.e., conditions usually present in nuclear waste—KMS-1 retained their exceptional selectivity for strontium with high KdSr values of 2.62 (Sr2+ initial concentration of ≈2.1 ppm) to 5.84 × 103 (Sr2+ initial concentration of ≈1.5 ppm) ml/g obtained in 24-h reactions. The KMS-1 continued to absorb Sr2+ even after 4 days' reaction¶ with ≈92.1% removal of Sr2+ (Sr2+ initial concentration of ≈2.1 ppm) and a KdSr of 1.16 × 104 ml/g, which is in very good comparison with those (3.70 × 103 to 2.26 × 105 ml/g) of the most efficient inorganic adsorbents (20, 21).
We also tested the effect of a mixture of competitive cations (Na+, 1.1 mM; Ca2+, 2.8 × 10−1 mM; Mg2+, 1.5 × 10−1 mM; Cs+, 6.9 × 10−2 mM) on the Sr2+ (0.02 mM) ion exchange in alkaline solutions (pH ∼ 11, V:m = 990 ml/g). The results (Table 1) revealed that the strontium affinity of KMS-1 is hardly affected by the presence of the competitive cations as is apparent by the high KdSr value (1.83 × 104 ml/g) obtained under these conditions. The selectivity order determined by the comparison of Kd values is Sr2+ > Ca2+ > Mg2+ > Cs+ ≫ Na+. This order shows the preference of the sulfide materials for the softer ions (e.g., Sr2+ vs. Ca2+)‖ and the ions with the highest charge (i.e., 2+ vs. 1+ cations).
Finally, it would be useful to compare the Sr2+ affinity and selectivity of KMS-1 with those of the state-of-the art inorganic adsorbents. In Fig. 4C, the dependence of reported KdSr values of various materials (including KMS-1) with the pH of the solution is represented. KMS-1 outperforms commercial adsorbents like sodium silicotitanate (7, 8, 20) and sodium titanate (6–8, 19, 20, 22), as well as others like CRY-1 (cryptomelane-type manganese oxide) (23), under strongly acidic conditions. The KMS-1 contain soft basic sites, the S2− ligands, which display small affinity for hard proton ions. Instead, the strontium exchange of the majority of oxygen-containing materials strongly interferes with proton ions having high affinity for the hard O2− ligands. At the same time, KMS-1 is nearly as effective as the commercial exchangers in highly alkaline environments (pH ∼ 14). Sandia octahedral molecular sieves (SOMS) also seem efficient under acidic and alkaline conditions (24), but their selectivity for strontium is dramatically reduced even with the presence of low Na+ concentrations (24). Sb silicate (SbSi) is the only inorganic adsorbent highly selective for strontium in pH as low as 1 (8), but its ability to be efficient at pH ≥ 14 (pH of many nuclear waste types) is questionable because of its appreciable dissolution at pH ≥ 13 (25).
Conclusion
The KMS-1 compounds are capable of highly selective interactions with Sr2+ or SrOH+ [this is the main form of Sr2+ at pH > 12.8 (19)] and strong discrimination against hard ions such as Na+ and H+. They possess this function despite the fact that their layers have no special features for structural preference (e.g., pore or window openings or rings of sizes specific for Sr). To the best of our knowledge, they represent the only examples ever reported of nonoxidic inorganic ion exchangers showing exceptional selectivity for strontium. The KMS-1 compounds with their soft negatively charged sulfide layers, therefore, point to a new paradigm in terms of the chemical classes that can be considered as selective adsorbents under extreme pH conditions for the effective treatment of radioactive wastes. The importance of metal sulfides in this area is poised to grow.
Materials and Methods
Polycrystalline K2xMnxSn3-xS6·yH2O (x = 0.5–0.95; y = 2–5) (KMS-1) can be synthesized by solid-state reaction of Sn (1.9 mmol), Mn (1.1 mmol), K2S (2 mmol), and S (16 mmol) at 500°C or by hydrothermal reaction of Sn (60 mmol), Mn (30 mmol), K2CO3 (30 mmol), and S (180 mmol) at 200°C. The yield for both preparation methods was found to be ≈80%. Single crystals of KMS-1, suitable for x-ray analysis, were obtained by a hydrothermal reaction of K2S (0.40 mmol), MnCl2 (0.20 mmol), Sn (0.40 mmol), and S (0.40 mmol) at 220°C. Energy dispersive spectroscopy (EDS) and inductively coupled plasma (ICP)–atomic emission (AES) analyses were used to determine the composition of KMS-1. The purity of various samples was verified by x-ray powder diffraction.
A typical ion-exchange experiment of KMS-1 with Sr2+ is as follows. In a suspension of KMS-1(0.07 mmol, 40 mg) in water (20 ml), an excess of SrCl2·6H2O (1.0 mmol) was added as a solid. The mixture was kept under magnetic stirring or constant shaking for ≈12 h. Then, the dark brown polycrystalline material was isolated by filtration, washed several times with water, acetone, and ether, and dried in air. The distribution coefficient Kd, used for the determination of the affinity and selectivity of compounds KMS-1 for Sr2+, is given by the equation Kd = (V[(C0 − Cf)/Cf])/m, where C0 and Cf are the initial and equilibrium concentration of Mn+ (ppm), V is the volume (ml) of the testing solution, and m is the amount of the ion exchanger (g) used in the experiment.
The Sr2+ uptake from solutions of various concentrations was studied by the batch method at V:m ∼ 1,000 ml/g, at room temperature and 24-h contact. The solids were then separated by centrifugation and filtration [through filter paper (Whatman No. 1)]. The Sr2+ content of the solutions was determined with ICP-AES. The data obtained were used for the determination of Sr2+ adsorption isotherms. Competitive ion-exchange experiments of KMS-1 were also carried out with the batch method at V:m ratio (1,000 ml/g), at room temperature and contact time of 1–7 days.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by National Science Foundation Grant DMR-0801855. A portion of this work was completed at the Northwestern University Integrated Molecular Structure Education and Research Center. A description of the facility and full funding disclosure can be found at http://pyrite.chem.northwestern.edu/analyticalserviceslab/asl.htm. This material is based on work supported by IEC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Crystals of K1.9Mn0.95Sn2.05S6 belong to the space group R-3m (no. 166) with a = 3.6969(5) Å, c = 25.403(5) Å, and V = 300.67(8) Å3. Other crystal data: Z = 1, Dc = 3.105 g/cm3, μ = 6.845 mm−1; total reflections, 1,007; independent reflections, 118 (Rint = 0.0483); R1 = 0.0271; wR2 = 0.0663; GOF = 1.176.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711528105/DC1.
The strontium adsorption rate at pH ∼ 14 and 5 M Na+ is remarkably fast at the beginning and decelerates as the reaction proceeds. The kinetics of ion exchange is typically controlled by film diffusion or particle diffusion (see ref. 26). In our system, in the first few minutes the sorption is probably controlled by film diffusion, where the slow step is the diffusion of Sr through the liquid diffusion layer surrounding the sorbent particles. Other factors affecting the kinetics of strontium adsorption by KMS-1 may be the structure stability and crystallinity of KMS-1. The layered structure of KMS-1 was largely retained after at least 2 h of contact of the solid with the highly alkaline solution (pH ∼ 14). Within this reaction time, the strontium adsorption was very fast with KMS-1 absorbing ≈50% and 64% of their 24-h Sr2+ uptake within 5 min and 2 h, respectively. However, the loss of the crystallinity of KMS-1 and their partial decomposition, which were observed with further increasing the reaction time (SI Fig. 12), presumably result in lowering the strontium adsorption rate. The final form of the material after 4 days of ion exchange contains almost no Sn and S, and Na-Mn in a ratio of 1:2. No Mn ions leached out from KMS-1 during the reaction. This decomposition form of KMS-1 also contains ≈1% wt of Sr, which is strongly bound because it could not be removed with copious washing with water.
According to the ion-exchange selectivity rules (see ref. 26, pp 158–159), the ion exchanger prefers the cation with the smallest hydrated sphere; i.e., that causing the least swelling. TGA (SI Fig. 11) and XRD (SI Fig. 10) analyses on strontium- and calcium-exchanged KMS-1 materials ions indicated similar hydration (≈5 H2O) of the intercalated cations and larger c-axes for the Sr2+-exchanged samples, respectively. Therefore, the preference of the material for softer ions explains its selectivity for Sr2+ over Ca2+ and not hydration-swelling effects.
References
- 1.Seever WJ. In: Handbook of Complex Environmental Remediation Problems. Blacklick OH, editor. McGraw–Hill Professional; 2001. pp. 303–341. [Google Scholar]
- 2.Morsey JR, Swanson JL. A Primer on Hanford Defense Tank Waste and Prospects for Advanced Chemical Separations. Richland, WA: Battelle Pacific Northwest Laboratories; 1991. [Google Scholar]
- 3.Braun A, et al. Application of the Ion Exchange Process for the Treatment of Radioactive Waste and Management of Spent Ion Exchangers. Vienna: International Atomic Energy Agency; 2002. Tech Rep Ser No 408. [Google Scholar]
- 4.Paulus WJ, Komarneni S, Roy R. Bulk synthesis and selective ion exchange of strontium ions in Na4Mg6Al4Si4O20F mica. Nature. 1992;357:571–573. [Google Scholar]
- 5.Anthony RG, Dosch RG, Gu D, Philip CV. Use of silicotitanates for removing cesium and strontium from defense waste. Ind Eng Chem Res. 1994;33:2702–2705. [Google Scholar]
- 6.Behrens EA, Sylvester P, Clearfield A. Assessment of a sodium nonatitanate and pharmacosiderite-type ion exchangers for strontium and cesium removal from DOE waste simulants. Environ Sci Technol. 1998;32:101–107. [Google Scholar]
- 7.Bortun AI, Bortun LN, Clearfield A. Evaluation of synthetic inorganic exchangers for cesium and strontium removal from contaminated ground water and wastewater. Solvent Extr Ion Exch. 1997;15:909–929. [Google Scholar]
- 8.Möller T, et al. Uptake of 85Sr, 134Cs and 57Co by antimony silicates doped with Ti4+, Nb5+, Mo6+ and W6+. J Mater Chem. 2001;11:1526–1532. [Google Scholar]
- 9.Wood DJ, Law JD. Evaluation of the SREX solvent extraction process for the removal of 90Sr and hazardous metals from acidic nuclear waste solutions containing high concentrations of interfering alkali metal ions. Sep Sci Technol. 1997;32:241–252. [Google Scholar]
- 10.Delmau LH, et al. Combined extraction of cesium and strontium from alkaline nitrate solutions. Solvent Extr Ion Exch. 2006;24:197–217. [Google Scholar]
- 11.Horwitz EP, Chiarizia R, Dietz ML. A novel strontium-selective extraction chromatographic resin. Solvent Extr Ion Exch. 1992;10:313–336. [Google Scholar]
- 12.Ding N, Kanatzidis MG. Permeable layers with large windows in [(CH3CH2CH2)2NH2]5In5Sb6S19·1.45H2O: High ion-exchange capacity, size discrimination, and selectivity for Cs ions. Chem Mater. 2007;19:3867–3869. [Google Scholar]
- 13.Divigalpitiya WMR, Frindt RF, Morrison SR. Inclusion systems of organic molecules in restacked single-layer molybdenum disulfide. Science. 1989;246:369–371. doi: 10.1126/science.246.4928.369. [DOI] [PubMed] [Google Scholar]
- 14.Clement RP, Davies WB, Ford KA, Green MLH, Jacobson AJ. Organometallic intercalates of the layered transition-metal dichalcogenides TaS2 and ZrS2. Inorg Chem. 1978;17:2754–2758. [Google Scholar]
- 15.Heising J, Kanatzidis MG. Exfoliated and restacked MoS2 and WS2: Ionic or neutral species? Encapsulation and ordering of hard electropositive cations. J Am Chem Soc. 1999;121:11720–11732. [Google Scholar]
- 16.Dungey KE, Curtis MD, Penner-Hahn JE. Structural characterization and thermal stability of MoS2 intercalation compounds. Chem Mater. 1998;10:2152–2161. [Google Scholar]
- 17.Fryxel GA, Lin Y, Fiskum S, Birnbaum JC, Wu H. Actinide sequestration using self-assembled monolayers on mesoporous supports. Environ Sci Technol. 2005;39:1324–1331. doi: 10.1021/es049201j. [DOI] [PubMed] [Google Scholar]
- 18.Marinin DV, Brown GN. Studies of sorbent/ion-exchange materials for the removal of radioactive strontium from liquid radioactive waste and high hardness groundwaters. Waste Manage. 2000;20:545–553. [Google Scholar]
- 19.Lehto J, Clearfield A. The ion exchange of strontium on sodium titanate Na4Ti9O20·xH2O. J Radioanal Nucl Chem Lett. 1987;118:1–13. [Google Scholar]
- 20.Behrens EA, Sylvester P, Graziano GM, Clearfield A. An assessment of inorganic ion-exchange materials for the removal of strontium from simulated Hanford tank wastes. Sep Sci Technol. 1999;34:1981–1992. [Google Scholar]
- 21.Hobbs DT, et al. Strontium and actinide separations from high level nuclear waste solutions using monosodium titanate 1. Sep Sci Technol. 2005;40:3093–3111. [Google Scholar]
- 22.Defillipi I, et al. Scale-up and testing of a novel ion exchanger for strontium. Sep Sci Technol. 1997;32:93–1113. [Google Scholar]
- 23.Dyer A, et al. Sorption behavior of radionuclides on crystalline synthetic tunnel manganese oxides. Chem Mater. 2000;12:3798–3804. [Google Scholar]
- 24.Nyman M, Tripathi A, Parise JB, Maxwell RS, Nenoff TM. Sandia octahedral molecular sieves (SOMS): Structural and property effects of charge-balancing the MIV-substituted (M = Ti, Zr) niobate framework. J Am Chem Soc. 2002;124:1704–1713. doi: 10.1021/ja017081z. [DOI] [PubMed] [Google Scholar]
- 25.Varshney KG, Sharma U, Rani S, Premadas A. Cation-exchange study on a crystalline and thermally stable phase of antimony silicate. Effect of irradiation on ion-exchange behavior and separation of cadmium(II) from zinc(II) and manganese(II) and of magnesium(II) from barium(II), calcium(II), and strontium(II). Sep Sci Technol. 1982;17:1527–1543. [Google Scholar]
- 26.Helfferich F. Ion Exchange. New York: McGraw–Hill; 1962. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.