Abstract
Residual viremia can be detected in most HIV-1-infected patients on antiretroviral therapy despite suppression of plasma RNA to <50 copies per ml, but the source and duration of this viremia is currently unknown. Therefore, we analyzed longitudinal plasma samples from 40 patients enrolled in the Abbott M97-720 trial at baseline (pretherapy) and weeks 60 to 384 by using an HIV-1 RNA assay with single-copy sensitivity. All patients were on therapy (lopinavir/ritonavir, stavudine, and lamivudine) with plasma HIV RNA <50 copies per ml by week 96 of the study and thereafter. Single-copy assay results revealed that 77% of the patient samples had detectable low-level viremia (≥1 copy per ml), and all patients had at least one sample with detectable viremia. A nonlinear mixed effects model revealed a biphasic decline in plasma RNA levels occurring over weeks 60 to 384: an initial phase of decay with a half-life of 39 weeks and a subsequent phase with no perceptible decay. The level of pretherapy viremia extrapolated for each phase of decay was significantly correlated with total baseline viremia for each patient (R2 = 0.27, P = 0.001 and R2 = 0.19, P < 0.005, respectively), supporting a biological link between the extent of overall baseline viral infection and the infection of long-lived reservoirs. These data suggest that low-level persistent viremia appears to arise from at least two cell compartments, one in which viral production decays over time and a second in which viral production remains stable for at least 7 years.
Keywords: HIV persistence, HIV therapy, HIV viremia
Reduction of HIV-1 RNA levels to <50 copies per ml is frequently achieved with combination antiretroviral therapy, but residual low-level viremia has been detected, using ultrasensitive assays, despite such therapy (1–5). The source and dynamics of this residual viremia are currently unknown. Viremia could arise from ongoing cycles of viral replication in a sanctuary site where there is suboptimal drug penetration, from long-lived productively infected cells, or from activation of virus expression from latently infected cell reservoirs (1, 4, 6–9).
Using a recently developed real-time HIV RNA assay with single-copy sensitivity (10), our prior work has shown that >80% of patients on suppressive standard protease inhibitor or nonnucleoside reverse transcriptase inhibitor-containing antiretroviral therapy for 60 weeks had measurable levels of persistent viremia (4). These therapies suppress plasma viremia to a new setpoint that correlates with baseline pretherapy plasma HIV-1 RNA levels but not with treatment regimen. Furthermore, longitudinal studies revealed no significant decline in viremia between weeks 60 and 110. Together, these results suggest that persistent viremia arises, at least in part, from long-lived cells infected before the initiation of therapy.
In the present study, we have extended the duration of observation to 7 years after the initiation of suppressive therapy to assess its long-term impact on persistent viremia. Among patients with plasma HIV-1 RNA consistently below 50 copies per ml through 7 years of treatment with lopinavir/ritonavir (LPV/r), stavudine (d4T), and lamivudine (3TC), we found, using the single-copy assay (SCA), that 77% of samples had quantifiable viremia and all patients had at least one sample with quantifiable viremia. A nonlinear decline in plasma HIV-1 RNA below 50 copies per ml beginning 1 year after initiation of suppressive therapy was also observed, suggesting additional phases of viral dynamics. These findings have important implications for the duration and source of persistent viremia, as well as the potential eradication of HIV with suppressive therapy.
Results
HIV-1 Persistence During Therapy.
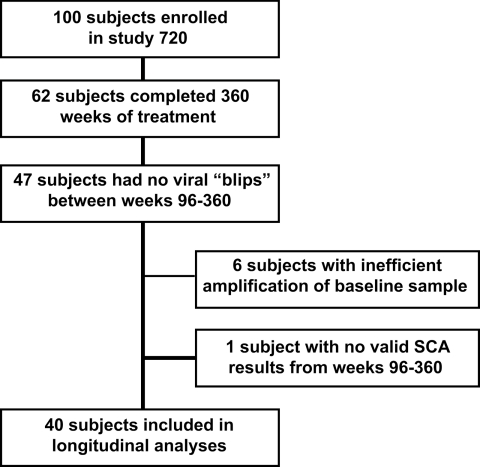
Measuring the level of persistent viremia for over 7 years in patients on suppressive therapy provided a unique opportunity to examine the dynamics of low-level viremia in patients responding to treatment. For this purpose, we analyzed plasma samples from a subset of patients enrolled in the Abbott M97-720 trial at baseline (pretherapy) and weeks 60 to 384. Of the 62 patients who completed the Abbott M97-720 trial, 47 were eligible for analysis (Fig. 1) and 41 of 47 patients had baseline SCA values comparable to Amplicor values (data not shown), indicating suitability for further testing by SCA. The remaining 6 patients with inefficient amplification of pretherapy HIV-1 RNA by SCA were excluded from further analysis. One additional patient had invalid SCA results with internal standard values unacceptably low for all samples, leaving 40 patients with 293 samples for longitudinal analyses. All patients were on therapy (LPV/r plus d4T plus 3TC) with plasma HIV RNA <50 copies per ml by week 96 of the study and thereafter. Overall, 77% of the patient samples had detectable low-level viremia ranging from 1 to 99 HIV-1 RNA copies per ml (median of 3.34 copies per ml) during the study period, and all patients had at least one sample with detectable low-level viremia.
Fig. 1.
Sample selection and number of patients in the M97-720 trial included for analysis with the single-copy assay.
Biphasic Decay of Viremia.
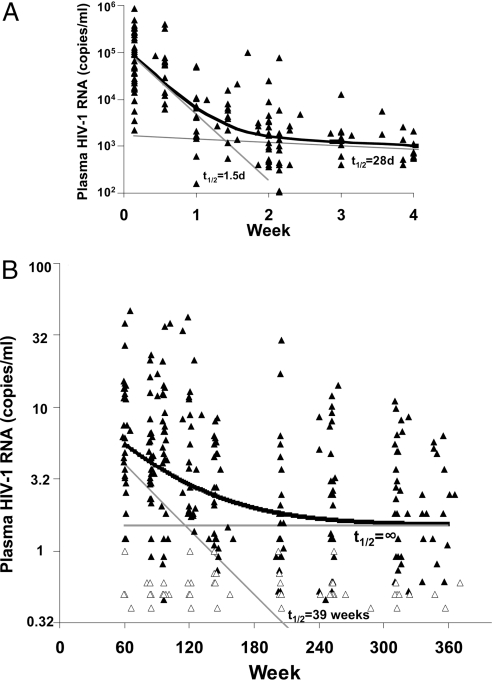
To relate our results to prior studies on HIV-1 decay after therapy, we initially examined the level of viremia in our patient cohort for the first 5 weeks after initiation of therapy (Fig. 2A). Although HIV-1 RNA determinations early in the study were too infrequent to allow in-depth modeling of the initial decay, a simplified nonlinear regression model suggested first- and second-phase decay rates comparable to those previously reported (11, 12), with half-lives of ≈1.5 and 28 days, respectively. During this 4-week period, plasma viral RNA declined from a median of ≈83,000 copies of RNA per ml at day 1 to ≈700 at 4 weeks (Fig. 2A).
Fig. 2.
Decline in persistent viremia over 7 years of treatment. (A) Initial two-phase decline in viremia after the initiation of treatment for the 720 patients included in this study, as assayed by Amplicor. (B) Individual HIV-1 RNA values determined by SCA starting at week 60 (open symbols indicate negative assay values plotted at the limit of quantification). Thick black line, fitted biphasic decay model; thin gray lines, decay rates corresponding to the compartments and half-lives shown.
We then used SCA to examine the levels of viremia below 50 copies per ml during the 60- to 384-week study interval, analyzing an average of ≈10 samples per patient. We observed a nonlinear decline in plasma HIV-1 RNA (Fig. 2B). The decline in persistent viremia appeared to be biphasic, indicating a third and fourth phase of viral decay during suppressive therapy after the first two phases described above.
Two nonlinear mixed regression models were used to represent the third and fourth phase of viral decay (see Materials and Methods, Models 1 and 2) (13, 14). Of the two models used, the data were best represented by a model showing a third phase of decay with an estimated half-life of 39 weeks (95% CI, 25 to 90 weeks, P = 0.009 for the null hypothesis of no decay), followed by a constant fourth phase i.e., a decay rate of zero and a half-life therefore defined as infinite (Fig. 2B). By extending the fitted model back to week 0, we estimated the mean baseline, or pretherapy, plasma HIV RNA contribution of the two “cellular compartments” (defined as compartments 3 and 4) to be 11.6 and 1.5 copies per ml, respectively. A sensitivity analysis that included only the subjects who achieved plasma RNA levels <50 copies per ml by week 48 and thereafter yielded similar results.
Individual data for six representative patients, shown in Fig. 3 along with empirical Bayes estimates of individual fitted decay curves, generally declined over time but demonstrated relatively high visit-to-visit variability. This variability may reflect stochastic fluctuation in the small numbers of virus-producing cells in these patients. As illustrated above, however, the data as a whole showed a clear and highly significant downward trend over the 7-year period of observation.
Fig. 3.
Individual data from six typical patients. Plasma HIV-1 RNA values (triangles) and empirical Bayes estimates of fitted individual biphasic decay curves are plotted.
Correlation with Baseline HIV-1 RNA Levels.
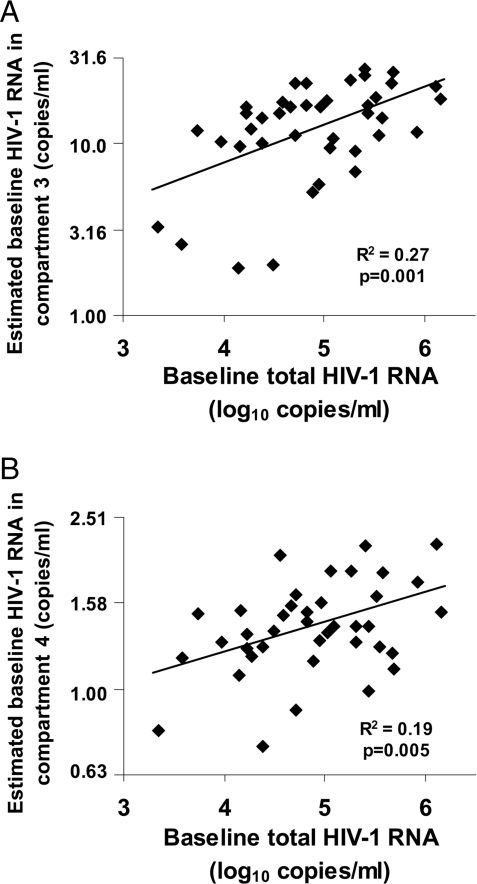
Our earlier studies revealed a highly significant association between low-level viremia at week 60 and baseline HIV RNA levels, as well as a lack of association of this viremia on therapy with treatment regimen, suggesting that persistent viremia arises from reservoirs of long-lived cells infected before the initiation of therapy (4). The model used to fit the biphasic decay in the current study provides a mechanism to estimate individual patient parameter values, allowing us to further evaluate this theory. The empirical Bayes estimates of patient-specific baseline HIV RNA levels arising from compartment 3 and compartment 4 were significantly correlated (R2 = 0.27, P = 0.001 and R2 = 0.19, P = 0.005, respectively) with pretherapy plasma viremia, supporting the hypothesis that persistent viremia on treatment results from virus production by cells that are infected before initiation of therapy (Fig. 4).
Fig. 4.
Correlation between baseline plasma HIV-1 RNA and subject-specific estimates of baseline HIV-1 RNA from each compartment. Empirical Bayes estimates of individual-patient baseline HIV-1 RNA arising from compartment 3 (A) and compartment 4 (B) were computed and found to be significantly correlated with total baseline plasma viremia.
Decline of Persistent Viremia: Combined Data from Two Independent Studies.
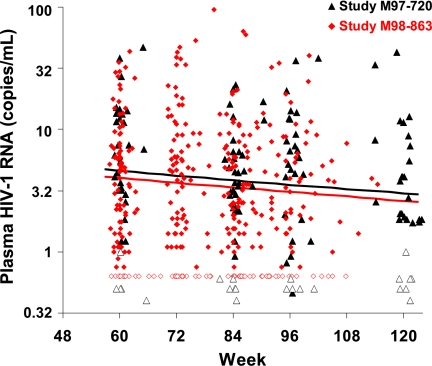
To further examine the biphasic nature of persistent viremia in patients on suppressive therapy, we conducted an analysis that included data from 157 patients in two studies who consistently had <50 copies of RNA per ml by conventional assays: the present study and a previous study (Abbott trial M98-863 of LPV/r or nelfinavir, plus d4T/3TC; 370 samples from 117 patients from weeks 60–110) (4, 15). The time period for the previous study overlaps but does not completely span the analysis time of our current study (60–384 weeks) and thus it provides insight primarily into the third phase of decay. Using a model allowing for different decay rates in each study (see Materials and Method, Model 3) (13, 14, 16), the estimated half-life of the third phase of decay was found to be consistent between the two studies: 63 weeks for the combined analysis (95% CI, 37–219 weeks) and 69 weeks for the M98-863 study (95% CI, 38–408 weeks) (P = 0.87 for the difference between studies). Results of this comparison over the time period common to both studies are shown in Fig. 5. Notably, the estimated third-phase half-life for study M97-720 based on the combined analysis was comparable to that of the primary analysis of the M97-720 data alone (39 weeks; 95% CI, 25–90 weeks). Similar to observations when study M97-720 data were analyzed alone, empirical Bayes estimates of patient-specific baseline viremia arising from compartment 3 were correlated with baseline viremia in each study (M97-720, R2 = 0.24 and P = 0.001; M98-863, R2 = 0.11 and P < 0.001).
Fig. 5.
Comparison of phase 3 decay in two different studies. Analysis of longitudinal samples from 157 patients enrolled in the M97-720 (red diamonds) and M98-863 (black triangles) studies who consistently had viral RNA levels of <50 copies per ml. A statistically significant decline in HIV-1 RNA, representing a third phase of decay, was consistent between the studies (black line, M97-720; red line, M98-863). Only values obtained between 60 and 120 weeks are shown for the M97-720 study. Levels of virus above the limit of detection are shown by filled symbols, and levels below this limit are shown by open symbols plotted at the assay limit.
Discussion
Initial therapy of HIV-1 infection with potent combinations of antiretroviral drugs results, after a short lag of 1–2 days, in two phases of viral decay: a rapid phase 1 decay with a half-life of 1–2 days followed by a gradual phase 2 decay with half-life of 2–3 weeks. Because modeling implies that the rate of decay of viremia after initiation of treatment is a function of the lifetime of productively infected cells, the two phases have been attributed to two different populations of such cells with very different life spans (11, 12, 17–20). The first phase is due largely or entirely to infected activated CD4+ T cells, and the second has been attributed to macrophages, but direct evidence for macrophage involvement is lacking. Although there are a number of reports of detectable low-level viremia after viremia becomes undetectable by standard assays (<50 copies of RNA per ml), the dynamics of subsequent phases of decline have not been well described. Potent antiretroviral therapy is effective in suppressing but not eradicating HIV-1 infection, and recent studies have revealed persistent viremia in most infected patients on antiretroviral therapy despite suppression of detectable plasma RNA for at least 2 years. The source of this persistent viremia and whether it declines over years of therapy are unknown (1, 2, 21). Our previous studies have shown that diverse antiretroviral regimens suppress viremia to a new setpoint that correlates with pretherapy viremia regardless of the treatment regimen (4), but these studies did not span a time interval sufficiently large enough to detect a significant decline in the viremia setpoint over 60–110 weeks of therapy. In the present study, we used samples obtained from a long-term trial of lopinavir/ritonavir-based antiviral therapy (22) to extend the duration of observation to up to 7 years after the initiation of suppressive therapy.
Analysis of 293 longitudinal samples from 40 patients on suppressive therapy for at least 7 years with viral RNA levels of <50 copies per ml revealed that the majority of the patient samples (77%) had measurable (≥1 copy per ml) levels of persistent viremia. This finding is consistent with earlier studies showing that viremia persists in patients on successful therapy, for up to 3 years (1, 2, 5, 21).
Individual patients in the study showed highly variable levels of viremia over time. Preliminary results suggest that the visit-to-visit variation in an individual patient exceeds the intraassay variability, implying that the observed variation represents a real biological phenomenon, most likely from stochastic fluctuation in the small numbers of virus-producing cells present at different times. A more detailed comparison of intraassay variation will be required to confirm this hypothesis.
Despite the individual fluctuation, analysis of many patients individually and all patients together revealed a nonlinear decline in plasma HIV-1 RNA below 50 copies per ml within 1 year after the initiation of suppressive therapy, suggesting additional third and fourth phases of viral decay beyond the previously described first and second phases, and, by extension, two additional classes of infected, virus-producing cells. Fitting of the levels of measurable viremia to a model with two distinct decay phases (third and fourth) did not detect a decay of viremia during the fourth phase (13, 14). Thus, the model used for the analysis of viral decay used a constant term to reflect a viral decay of zero during the fourth phase. Biologically, our model implies that low-level persistent viremia arises from two different compartments (defined as compartments 3 and 4) of infected cells, one with a half-life (39 weeks; 95% CI, 25–90 weeks) and one with a half-life longer than the 7-year treatment period.
We can only speculate as to what cells might be responsible for production of the virus detected during the latter two phases. The half-life of third-phase viremia (39–63 weeks or 9–15 months) is comparable to that previously described for latently infected CD4+ T cells (6–44 months) (23–25), implicating this cell type, which may also contribute to the fourth phase of viremia. The stability of fourth-phase viremia also raises the possibility that a very small fraction of infected cells is capable of surviving indefinitely while continuing to produce virus, perhaps by division at a rate equal to its death rate. Interestingly, recent data show that residual viremia in plasma samples from patients on suppressive therapy is genetically distinct from viruses identified in CD4+ cells, indicating that long-term persistent viremia found in plasma arises from a different cellular compartment or infected cells (26, 27). Furthermore, in many patients, much of this plasma virus belongs to a single predominant clone, suggesting that it, too, might arise from slowly dividing virus-producing cells. Further genetic analysis of the virus populations in patients on suppressive therapy should help resolve the cellular origin of persistent viremia.
Extrapolation of results from our fitted model to the time of therapy initiation indicated that the cells in compartment 3 typically account for ≈10 copies per ml of the pretherapy viremia with a half-life of ≈9 months, whereas the cells in compartment 4 typically account for ≈1.5 copies per ml of pretherapy viremia, with no detectable decay. For comparison, the median viremia in this patient group at baseline was ≈83,000 copies per ml. Thus, virus produced by the cells detected as phase 3 was ≈1/10,000 of the total pretherapy viremia, whereas phase 4 accounted for ≈6-fold less. To ensure that we did not include any patients who might have been failing therapy, we excluded from analysis 15 of the initial 62 patients who had viremia exceeding 50 copies of RNA per ml after week 96. Because some of the excluded patients may, in fact, have had well controlled viremia at a level near the 50-copy limit, we may have slightly underestimated the contributions of third- and fourth-phase virus to the pretherapy viremia. Viremia associated with the second phase of decay is typically estimated as 1% of total pretherapy viremia, with a decay half-life of 14–21 days (11, 13). These values are consistent with those estimated for the patients analyzed here: ≈1.5% and a half-life of 28 days (Fig. 2A). At these rates of decay, a typical patient with 100,000 copies of RNA per ml at baseline would have 10−6 to 10−3 copies per ml remaining after 60 weeks. Even with the slower second-phase half-life of 28 days observed in our study, such a patient would have, after 60 weeks, residual viremia from the second phase of decay of 0.03 copies per ml, >20-fold below the assay detection limit and 100-fold below the median viremia measured at week 60. Thus, the residual viremia detected at week 60 and beyond does not appear to arise from cells that correspond to the second phase of decay.
We found statistically significant correlations between patient-specific estimates of the pretreatment viremia in each compartment and total pretherapy plasma HIV RNA (even though the model used data starting >1 year after treatment initiation), supporting a biological link between the extent of overall baseline viral infection and the infection of long-lived reservoirs. One limitation of our results is imposed by the simplicity of our model, which allows for only two compartments, one of which decays and one of which remains stable over time. Either of the “compartments” we specify could represent multiple biological phenomena. Furthermore, the “stable” compartment in our model may decay but at a rate simply too slow to be detected in our study of 7 years.
Models for HIV dynamics usually arise from a series of differential equations representing complex interplay between the virus and infected and uninfected target cells (26–28). Our model is based on the simplifications proposed by Wu and Ding (13, 14), which is appropriate because many complications of modeling early HIV-1 RNA dynamics (intracellular/pharmacological delay, initial dramatic increases of CD4+ T cell counts, and decay of free virus and productively infected cells) occur before our analysis, and important parameters are likely to be stable over the 1- to 7-year observation period.
In an analysis that included data from a prior study (M98-863) and the present study (M97-720), we observed a statistically significant third phase of decay with a half-life that was consistent between studies [half-lives of 69 and 63 weeks, respectively (Fig. 5)] and consistent with that observed in the analysis of the M97-720 data alone (half-life of 39 weeks). The detection of a statistically significant decay in study M98-863 reflects a change from the original conclusions of the prior study (4), in which a slope of decline was detected but did not reach statistical significance, due in part to the comparatively short time frame analyzed and to the use of a linear instead of a biphasic decay model.
The existence of the long-lived reservoirs implied by these studies has important therapeutic consequences. Even though the amount of virus RNA in plasma after years of therapy is extremely small relative to baseline viremia, assuming that it represents infectious virus, it is more than sufficient to rekindle infection after interruption of therapy. Given the relative stability of the phase 4 viremia, it is improbable that eradication can be achieved within the lifetime of any HIV-infected patient by using antiviral drugs alone. By contrast, given that untreated HIV infection is sustained by constant cell-to-cell transmission of infectious virus, with no evidence whatsoever for any physical or genetic bottleneck after transmission, it is highly unlikely that the very small fraction of persistently infected cells plays any significant role in natural infection, pathogenesis, or transmission of HIV.
In conclusion, longitudinal analysis of persistent viremia in 40 patients revealed a nonlinear decline in plasma HIV-1 RNA below 50 copies per ml within 1 year after the initiation of suppressive therapy, suggesting additional phases of viral dynamics beyond the first- and second-phase decay periods previously described. Based on this study and a combined analysis of two studies, the third-phase half-life was estimated to be ≈9–15 months. No decay during a fourth phase was discernable, and patient-specific estimates of pretherapy viremia corresponding to the third and fourth phases of decay were correlated with total pretherapy viremia. Therefore, low-level persistent viremia appears to arise from at least two cellular compartments, one with a half-life comparable to latently infected CD4+ T cells and one or more with a half-life longer than the 7-year treatment period analyzed. The indication that different cellular compartments contribute to persistent viremia in treated patients has important implications for strategies to eradicate infection. Clearly, these compartments cannot be eliminated by standard antiretroviral therapy, and new therapeutic approaches will be needed to eliminate virus from long-lived reservoirs.
Materials and Methods
Clinical Specimens.
In the Abbott M97-720 trial, 100 antiretroviral-naive patients at 11 study sites in North America received lopinavir/ritonavir (400/100 mg twice daily) with stavudine and lamivudine twice daily for up to 7 years (22). Patients remaining on study for 7 years (384 weeks) who met both of the following criteria were included in the analysis: plasma HIV-1 RNA <400 copies per ml by standard Amplicor assay at all visits from week 96 to week 384 (every 12 weeks) and plasma HIV-1 RNA <50 copies per ml by standard Amplicor assay at all visits from week 96 to week 384 (yearly until week 300, then every 12 weeks). Forty-seven participants treated at investigational sites in North America were identified for SCA analysis. Of these, six were excluded for inefficient amplification of the baseline sample and one was excluded for invalid internal standard results for all samples analyzed (Fig. 1).
Before participation in the study, all M97-720 patients provided informed consent for viral RNA quantification. The protocols and procedures for subsequent SCA analysis of these samples by the HIV Drug Resistance Program were reviewed and approved the by National Institute of Allergy and Infectious Diseases Institutional Review Board.
HIV-1 RNA Determination.
The SCA for HIV-1 RNA detection was performed as described in ref. 10, starting with ≈3 ml of plasma, leading to a typical lower limit of quantitation for these samples of 0.63 copies per ml. For each sample, three separate aliquots of the cDNA product were assayed for HIV-1 RNA and two aliquots for the recombinant avian retrovirus internal standard RNA using real-time PCR of conserved sequences within gag as described in ref. 10. For five patients, an archived pretherapy sample was unavailable and a postbaseline value was obtained during the first 2 weeks of treatment. Six of the M97-720 patients were excluded because SCA and Amplicor assays on pretherapy samples were significantly discordant due to inefficient amplification by SCA—probably a result of polymorphism in the probe or primer sequences (A.W. and S.P., unpublished observations). In all other samples, there was a close correlation (within 3-fold) between commercial Amplicor RT-PCR and SCA values for pretherapy samples (data not shown). Further details of extraction, optimum amplification conditions, and performance characteristics, as well as quality control procedures to prevent artifactual amplification, can be found in ref. 10.
Study M97-720 used the PCR-based Amplicor HIV-1 MONITOR assay version 1.0 for plasma HIV-1 RNA quantitation, performed according to manufacturer's specifications (Roche Diagnostics).
Statistical Analysis.
For log-transformed baseline viral RNA determinations, comparisons between Amplicor HIV-1 RNA assays and SCA were conducted using linear regression and Pearson's correlation.
Based on the nonlinear trends observed in the data suggesting a biphasic decay of plasma HIV-1 RNA levels (see Results), nonlinear mixed-effects models based on those described by Wu and Ding (13, 14) were fitted to the complete dataset. Two basic models were used: the first represents two distinct phases of decay, whereas the second represents a first phase of decay followed by a constant value (i.e., a second phase with no viral decay).
An advantage of these models is their biological interpretability with respect to longitudinal plasma HIV-1 RNA data: V(t) represents the plasma HIV-1 RNA level at time t (weeks since baseline), V3 and V4 represent baseline viral load arising from each of two compartments, and parameters δ and λ are the decay rate of virus from each compartment. The compartments are labeled 3 and 4 because they represent third and fourth phases of decay that become apparent after virus from the first two phases of decay described by several reports has become negligible (11, 13, 14, 19).
Empirical Bayes estimates of individual patient baseline viral load arising from compartment 3 and compartment 4 were computed and tested for association with total baseline viral load using linear regression.
Data from the current study were compared with those obtained from a previous study (study M98-863) (4). In that study, patients treated with a lopinavir/ritonavir-based or nelfinavir-based regimen with long-term suppression by standard plasma HIV-1 RNA assays were assessed using SCA over weeks 60 to 110. Thus, the time period analyzed overlaps but does not span the time period of the current study (weeks 60–384). The following model was used to allow comparison of first-phase decay rates:
where i = 1 refers to the present study (study M97-720) and i = 2 refers to the prior study (study M98-863). Thus, δ1 and δ2 determine the first-phase decay rates for study M97-720 and study M98-863, respectively.
ACKNOWLEDGMENTS.
We thank Valerie Boltz, Balakrishna Hosmane, Mary Kearney, and Wei Shao for many helpful conversations; Florence McMillan and Kathleen Sheehan for management of logistics and databases; and the patients for participating in these studies. This work was supported in part by National Cancer Institute Contract 25XS119 (to J.W.M.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Dornadula G, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. J Am Med Assoc. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 2.Havlir DV, et al. Predictors of residual viremia in HIV-infected patients successfully treated with efavirenz and lamivudine plus either tenofovir or stavudine. J Infect Dis. 2005;191:1164–1168. doi: 10.1086/428588. [DOI] [PubMed] [Google Scholar]
- 3.Lewin SR, et al. Use of real-time PCR, molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. J Virol. 1999;73:6099–6103. doi: 10.1128/jvi.73.7.6099-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maldarelli F, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 6.Blankson JN, Persaud D, Siliciano RF. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 7.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 8.Frenkel LM, et al. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J Virol. 2003;77:5721–5730. doi: 10.1128/JVI.77.10.5721-5730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siliciano JD, Siliciano RF. A long-term latent reservoir for HIV-1: Discovery and clinical implications. J Antimicrob Chemother. 2004;54:6–9. doi: 10.1093/jac/dkh292. [DOI] [PubMed] [Google Scholar]
- 10.Palmer S, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perelson AS, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 12.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Ding AA. Population HIV-1 dynamics in vivo: Applicable models and inferential tools for virological data from AIDS clinical trials. Biometrics. 1999;55:410–418. doi: 10.1111/j.0006-341x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Ding AA, De Gruttola V. Estimation of HIV dynamic parameters. Stat Med. 1998;17:2463–2485. doi: 10.1002/(sici)1097-0258(19981115)17:21<2463::aid-sim939>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Walmsley S, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 16.Ding AA, Wu H. Relationships between antiviral treatment effects and biphasic viral decay rates in modeling HIV dynamics. Math Biosci. 1999;160:63–82. doi: 10.1016/s0025-5564(99)00021-8. [DOI] [PubMed] [Google Scholar]
- 17.Bonhoeffer S, Coffin JM, Nowak MA. Human immunodeficiency virus drug therapy and virus load. J Virol. 1997;71:3275–3278. doi: 10.1128/jvi.71.4.3275-3278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho DD, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 19.Perelson AS, Essunger P, Ho DD. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. Aids. 1997;11(Suppl A):S17–S24. [PubMed] [Google Scholar]
- 20.Polis MA, et al. Correlation between reduction in plasma HIV-1 RNA concentration 1 week after start of antiretroviral treatment and longer-term efficacy. Lancet. 2001;358:1760–1765. doi: 10.1016/s0140-6736(01)06802-7. [DOI] [PubMed] [Google Scholar]
- 21.Havlir DV, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. J Am Med Assoc. 2001;286:171–179. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- 22.Hicks C, et al. Long-term safety and durable antiretroviral activity of lopinavir/ritonavir in treatment-naive patients: 4 year follow-up study. AIDS. 2004;18:775–779. doi: 10.1097/00002030-200403260-00008. [DOI] [PubMed] [Google Scholar]
- 23.Di Mascio M, et al. In a subset of subjects on highly active antiretroviral therapy, human immunodeficiency virus type 1 RNA in plasma decays from 50 to <5 copies per milliliter, with a half-life of 6 months. J Virol. 2003;77:2271–2275. doi: 10.1128/JVI.77.3.2271-2275.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siliciano JD, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Zhang H, Siliciano JD, Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay after entry into resting CD4+ T cells. J Virol. 2005;79:2199–2210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey JR, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80:6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieffer TL, et al. Genotypic analysis of HIV-1 drug resistance at the limit of detection: Virus production without evolution in treated adults with undetectable HIV loads. J Infect Dis. 2004;189:1452–1465. doi: 10.1086/382488. [DOI] [PubMed] [Google Scholar]
- 28.Di Mascio M, Ribeiro RM, Markowitz M, Ho DD, Perelson AS. Modeling the long-term control of viremia in HIV-1 infected patients treated with antiretroviral therapy. Math Biosci. 2004;188:47–62. doi: 10.1016/j.mbs.2003.08.003. [DOI] [PubMed] [Google Scholar]