Abstract
Dispersal is a ubiquitous trait in living organisms. Evolutionary theory postulates that the loss or death of propagules during dispersal episodes (cost of dispersal) should select against dispersal. The cost of dispersal is expected to be a strong selective force in fragmented habitats. We analyzed patchy populations of the weed Crepis sancta occupying small patches on sidewalks, around trees planted within the city of Montpellier (South of France), to investigate the recent evolutionary consequences of the cost of dispersal. C. sancta produces both dispersing and nondispersing seeds. First, we showed that, in urban patches, dispersing seeds have a 55% lower chance of settling in their patch compared with nondispersing seeds and, thus, fall on a concrete matrix unsuitable for germination. Second, we showed that the proportion of nondispersing seeds in urban patches measured in a common environment is significantly higher than in surrounding, unfragmented populations. Third, by using a quantitative genetic model, we estimated that the pattern is consistent with short-term evolution that occurs over ≈5–12 generations of selection, which is generated by a high cost of dispersal in urban populations. This study shows that a high cost of dispersal after recent fragmentation causes rapid evolution toward lower dispersal.
Keywords: fragmentation, short-term evolution, human-altered habitat
Dispersal has evolved in almost all living organisms and is thus considered as a central life-history trait (1, 2). The evolution of dispersal is usually understood as the result of a cost–benefit process. On the one hand, three main factors selecting for dispersal have been identified (3): reduction of the competition among kin (4, 5); the temporal heterogeneity of the environment, such as local population extinction (6, 7); and last, the avoidance of inbreeding depression when mating occurs between related individuals (8). However, various costs of dispersal have been postulated in theoretical models. For instance, dispersal structures can be costly for organisms [e.g., fleshy fruits dispersed by animals (9)]. More generally, dispersing organisms may pay a high cost of dispersal because they may get lost during the displacement. This phenomenon is encapsulated under the term “cost of dispersal.” Various theoretical models have been studied, including various selective factors (3). These theoretical models conclude that increasing the cost of dispersal selects for lower dispersal. Although this cost may appear as obvious in natural populations, the strength of this selection pressure on dispersal traits is almost unknown in the wild because of the difficulties of measuring it in natural systems. When dispersal is passive (wind or water transport) and habitat choice is random, the probability of settling in a suitable site is positively dependent on the frequency of suitable sites in the landscape. Many empirical studies have reported a reduction in dispersal structures in organisms that live on islands, such as plants (10) or insects (however, see ref. 11 for a critical review of flightlessness on islands). This pattern is often interpreted as a result of a higher cost of dispersal on islands because of a higher chance of falling into the sea. More recently, Cody and Overton (12) reported a pattern of reduction in dispersal structures in several species of Asteraceae over a few generations.
An unequivocal demonstration for the selective role of the cost of dispersal under natural selection requires showing (i) that dispersing propagules suffer an actual cost relative to nondispersing propagules (cost of dispersal), (ii) that there is substantial genetic variance on traits related to dispersal in natural populations (i.e., substantial heritability), and (iii) that populations exhibit lower dispersal in a “high-cost-of-dispersal” environment.
We used the recent colonization of the weed Crepis sancta (Asteraceae) in an urban, fragmented environment to quantify the cost of dispersal and the reduction of a dispersal-related trait. By increasing the isolation among remnant suitable sites for plants, fragmentation is expected to modify dispersal processes (13). Urban environments represent a matrix of several square kilometers that are mostly unsuitable for plants because of asphalt and buildings with few isolated habitats. It constitutes a simple and manageable ecosystem in which to study evolution because urban environments are recent habitats where adaptations may be revealed (14). C. sancta is a widespread allogamous weed occupying wasteland, vineyards, and roadsides (15) and the urban environment (16) in southern France. In urban environments, C. sancta occupies small, unmanaged sites [a few meters square (m2) maximum in the unsuitable matrix]. We specifically focused on the highly fragmented habitat composed of patches around trees on the sidewalk in the city of Montpellier (South of France), which C. sancta has colonized down to the city center. These patches are small (≈1 m2), widely distributed (several thousand in the city), and regularly spaced, from 5 to 10 m from each other depending on the street. The date of building of each sidewalk is documented, which provides a time scale for evaluating contemporary evolution. These habitats, representing <1% of the total city area, are frequently disturbed by human presence, which prevents perennials from settling in the patches. Annual weeds have colonized these habitats either by seed dispersal or soil transport, and 96 common weeds have been listed in these patches within Montpellier (unpublished data). In these patches, the number of plants varies from 0 to 40 plants for C. sancta, and patches are linked by pollen transfer by bees (16). The model C. sancta is especially suitable because each individual produces both dispersing and nondispersing seeds, which corresponds to two distinct types of fruit. Such a trait is widespread in the Asteraceae family and found in >50 genera (17). In such species, individual investment in dispersal can be estimated as the ratio (hereafter, R-ratio):
In C. sancta, this R-ratio has been shown to be heritable [h2 = 0.25 estimated on a population close to Montpellier (18)]. Because suitable habitats are very sparse, we hypothesized that seeds leaving their patches in urban environments have very little chance to reach a suitable habitat, which imposes a high cost of dispersal. Local adaptation in urban patches should therefore favor reduction of dispersal.
To test this hypothesis, we first quantified the cost of dispersal in urban populations by using artificial patches mimicking urban patches. Second, we measured population differentiation for dispersal ability (R-ratio) in a greenhouse and showed a reduced dispersal rate in urban patchy populations. Last, we used quantitative genetic modeling tools to validate the scenario of short-term reduction of dispersal caused by the cost of dispersal in urban patchy populations.
Results and Discussion
Cost of Dispersal in Urban Patches.
In this experiment, we were interested in measuring the probability of a dispersing seed staying in the patch, Pstay, relative to a nondispersing seed, by measuring the R-ratio on the mother plants (before dispersal) and the R-ratio on the patch surface (after dispersal). Starting with a population with an estimated R-ratio of 0.15 (0.008) before dispersal, we estimated that the R-ratio within a patch after dispersal was 0.28 (0.012) and showed that the R-ratio significantly increased (χ12 = 38.18; P < 0.001). From the significant shift of the R-ratio during dispersal, we deduced that a dispersing seed in an urban environment has a low probability of settling in the patch that is Pstay = 0.45. This result is in total agreement with Imbert (19), who found experimentally that 78.4% of nondispersing seeds of C. sancta fall within a 0.5-m radius, whereas only 30% of dispersing seeds fall within a 0.5-m radius. Given that suitable habitats are scarce in urban environments (<1% of the total area), this experiment demonstrates that the cost of dispersal is high in urban fragmented populations, compared with large unfragmented populations.
Among-Population Differentiation for Dispersal Ability.
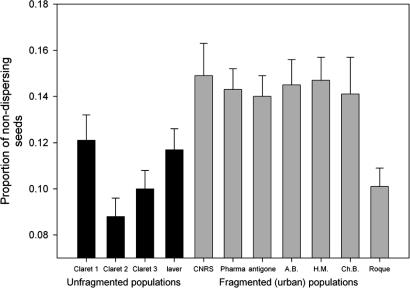
In this experiment, we estimated the among-population differentiation for dispersal ability by contrasting continuous and fragmented populations. Plants from various populations were grown in a common greenhouse. After fructification, the R-ratio per individual plant was measured. Statistical analysis of the R-ratio was performed by using a generalized linear model (20). We found a significant effect of fragmentation [F(1, 9) = 6.72, P < 0.05], which reveals population differentiation in dispersal (Fig. 1). All urban, fragmented populations exhibit a higher R-ratio (i.e., lower dispersal ability), except one population at the periphery of the city (Roque). Urban fragmented populations had higher R-ratios than unfragmented populations and the difference was ≈4.5%. Population was also significant (P < 0.01). We checked that inflorescence size (diameter of the capitula at flowering maturity) was not correlated to the R-ratio (P > 0.05), which dismisses the possibility of indirect selection on the R-ratio because of direct selection by the pollinator. Overall, the pattern is consistent with lower dispersal ability in urban fragmented populations compared with unfragmented populations. Interestingly, the urban unfragmented population (laver) is similar to countryside populations.
Fig. 1.
Mean proportion estimates (and standard error) of nondispersing seed (R-ratio) in unfragmented and fragmented “patchy” populations measured in a common environment in a greenhouse(see Materials and Methods).
The pattern observed is qualitatively consistent with the reduction of seed dispersal because of a high cost of dispersal in an urban environment.
Quantitative Response to Selection and Validation of the Evolutionary Scenario.
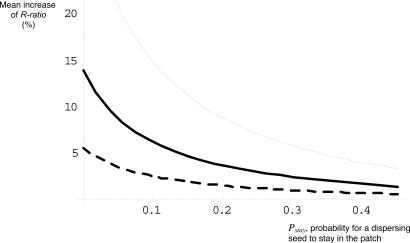
To quantify the expected response to selection and validate this scenario of reduced dispersal in an urban environment, we modeled the selection processes by using quantitative genetic tools. The Breeder's equation, R = h2·S predicts the response to selection (R) as a function of selection applied (S) and heritability of the traits, h2 (21) (see Materials and Methods). The selection applied is derived from experiment 1 (cost of dispersal). The narrow sense heritability for the R-ratio was considered as 0.25 (18). Fig. 2 summarizes the expected response to selection as a function of the probability for dispersing seeds to stay in the patch, Pstay, for various numbers of generations. Considering the Pstay estimates from experiment 1, we deduced that the pattern of reduced dispersal observed in urban patches (R-ratio increase ≈4.5%) is consistent with 12 years of selection. Note that the number of 12 generations provides an upper limit for the pattern observed, because our estimate of Pstay is probably overestimated (see Materials and Methods). In any case, the number of generations required for selection to operate is always lower than the estimated age of the populations (deduced from the date of building of the sidewalks; see Table 1). Our results are thus quantitatively consistent with contemporary evolution in urban patches.
Fig. 2.
Increase of the ratio of nondispersing seeds (R-ratio) in urban patches as a function of the probability of staying within the patch, Pstay (see text). Results are obtained for 12 generations of selection (thin line), 5 generation of selection (thick line), and 2 generations of selection (dotted line).
Table 1.
Description of populations sampled
| Populations | Description | Year of sample (year of study) |
|---|---|---|
| Unfragmented | ||
| Claret 1 | Large populations in wasteland (countryside, 30 km from Montpellier). | 2004 (2005) |
| Claret 2 | Large populations in a vineyard (countryside, 30 km from Montpellier). | 2004 (2005) |
| Claret 3 | Large populations in a vineyard (countryside, 30 km from Montpellier). | 2005 (2006) |
| Laver | Large populations in an urban vineyard within Montpellier. | 2005 (2006) |
| Fragmented | ||
| B. (built in 1987) | Urban patchy populations on the sidewalk along Auguste Broussonnet Street (Montpellier). | 2004 (2005) |
| H.M. (built in 1972) | Urban patchy populations on the sidewalk along Henri Marès Street (Montpellier). | 2004 (2005) |
| Ch.B (built in 1982) | Urban patchy populations near Chemin des Barques (Montpellier). | 2004 (2005) |
| Roque (built in 1992) | Urban patchy populations on the sidewalk along La Roqueturière Street (periphery of Montpellier). | 2004 (2005) |
| Antigone (built in 1982) | Urban patchy populations on the sidewalk along Jacques Cartier Street (Montpellier). | 2005 (2006) |
| CNRS (no information) | Urban patchy populations near CNRS buildings (periphery of Montpellier). | 2005 (2006) |
| Pharma (built in 1995) | Urban patchy populations on the sidewalk along Voie Domitienne Street (Montpellier). | 2005 (2006) |
The date of building provides an upper estimate for the age of the populations.
General Discussion
Taken together, our results provide the first quantitative study on the evolution of lower dispersal under natural selection. High cost of dispersal because of extreme fragmentation of habitats has been demonstrated and is sufficient to explain the evolution of dispersal in such habitats. Thanks to substantial inheritance of dispersal and strong selection, we predicted that the response to selection should be rapid, which was confirmed by the pattern observed in the city.
Our study of an urban metapopulation fills two gaps concerning experimental works on the evolution of plant dispersal (e.g., ref. 12). First, dispersal ability was not estimated in the field but in a common greenhouse, which allows us to interpret population differentiation as the result of directional genetic selection in fragmented patches. Second, our estimates of selection pressures coupled with heritability measurement allows us to quantify the response to selection and the number of generations required for the pattern observed. The cost of dispersal is often assumed in theoretical models (4, 5, 8); we showed here that such a cost actually occurs and leads to a rapid selection against dispersal.
Our study demonstrates that, faced with changes in land use (deforestation, urbanization, and agriculture) that cause fragmentation and the loss of habitats in human-altered ecosystems, species may respond quickly by reducing dispersal among remnant habitats. We may hypothesize that selection against dispersal in a fragmented landscape will reduce gene flow among populations, and thus exacerbate the isolation created by fragmentation, which could endanger the population persistence of plant species.
Materials and Methods
Studied Species and Population Networks.
The species C. sancta produces nondispersing fruits without a pappus at the periphery of the capitulum (≈10–15 per head) and dispersing fruits with a pappus at the center of the capitulum (≈80–100 per head). Both fruit types contain exactly one seed each. Similarly, seeds are either dispersing seeds or nondispersing seeds. Neither of these types of seeds exhibit dormancy (22). Peripheral fruits (nondispersing) are heavy (0.27 ± 0.02 mg) and have a high rate of falling [0.23 ± 0.01 m·sec−1 (22)] because of the absence of a pappus. Central fruits are light (0.1 ± 0.01 mg) and have a low rate of falling [1.48 ± 0.08 m·sec−1 (22)].
Continuous and unfragmented populations of C. sancta are large populations with several thousand plants, whereas urban populations are formed of numerous patches (≈1 m2) from 5 to 7 m distant from each other. Urban populations from Montpellier for which the date of building was known were randomly sampled during the springs of 2004 and 2005. Each year, fragmented and unfragmented populations were chosen haphazardly. Seeds from six populations were sampled in 2004 (claret 1, claret 2, A.B., H.M., Ch.B., and Roque) and were grown in 2005. Seeds from five populations [Claret 3, Laver, Centre National de la Recherche Scientifique (CNRS), Pharma, and Antigone] were sampled in 2005 and were grown in 2006. We included one continuous population within the city where dispersal was expected to be similar to the countryside populations (see Table 1 for the full description).
Cost of Dispersal in an Urban Environment and Selection Differential Estimate.
Three artificial patches (wood board) mimicking urban patches were built at the Centre d'Ecologie Fonctionnelle et Evolutive (CEFE)-CNRS to estimate the probability of dispersing seeds staying in the patch, Pstay, relative to nondispersing seeds, because of wind dispersal. Seven plants per artificial patch were placed on the patch surface. Plants were sampled in the CNRS population. Outdoor pots were wedged in holes in calm weather. The patch surface (wood) was coated with glue to catch fruits falling into the patch after dispersal. The comparison of the R-ratio on mother plants (before dispersal) and the R-ratio glued to the patch (after dispersal) allows estimation of the probability of a dispersing seeds staying in the patch in urban fragmented populations, Pstay. The sticky surface of the artificial patches does not allow secondary movement of dispersing fruits after falling on the ground. Thus, our estimate provides an upper limit for Pstay, the probability of settling in the patch compared with urban conditions, which is a conservative measure.
We performed two independent measures per patch for the ratio after dispersal during spring 2006 for a total of >5,000 fruits counted on patch surfaces. We used a binomial model (logit link) (20) to estimate the proportion of nondispersing seeds after dispersal and compared it with the proportion before dispersal by using capitula chosen on 30 random plants in the CNRS population. Slight overdispersion was corrected by using Pearson correction (20).
Population Differentiation for Dispersal Capacity in a Common Environment.
Plants were grown during the winters of 2005 and 2006 in a common greenhouse at CEFE-CNRS to reveal genetic differentiation in dispersal. Thirty plants per population (originating from dispersing seeds only) were cultivated. Because of mortality before reproduction, the design was slightly unbalanced. Positions for plants from different populations were randomized in the greenhouse. Nutrient conditions and competition are the major factors affecting capitula architecture (23). To avoid among-year environmental heterogeneity, the same greenhouse was used both years. The cultivation conditions were kept constant from 2005 to 2006, using one-liter pots (same soil, same temperature) with daily watering after planting the seedlings. When the plants were flowering, random outcross pollination within population was performed in the greenhouse by Bombus terrestris by using commercial hives. After fructification, two capitula per plant were wrapped with adhesive tape to avoid dispersal. When fruits were mature, capitula were harvested and fruits were counted. Fruits from two capitula per plant were counted for a total of >700 capitula. Statistical analyses were performed by using the GLM of SAS (20). Data were arcsin (√) transformed in the statistical analysis (24). Population type (fragmented vs. unfragmented) was considered as a fixed factor and was tested against population factor (nested in population type factor) considered as a random factor. Type III sum of square was used.
Response to Selection by Using Quantitative Genetics Tools.
We consider “countryside-type” population distribution (Gaussian) for the R-ratio (μ = 0.1, σ2 = 0.01). We first assume that colonization of urban patches occurs by dispersing seeds only. After colonization, local selection in patchy populations during several generations leads to the loss of dispersing seeds. Given that suitable habitats in the city represent <1% of the total area, we assume that a dispersing seed leaving a patch is lost. The relative fitness of a phenotype R within a patch is w(R) = (R + (1 − R)Pstay)/w̄, where Pstay is the probability of a dispersing seed staying within the patch and w̄ is the mean fitness. The selection differential, S, is the difference between population mean after selection (μs) and before selection (μ). The response to selection for short-term selection is given by the breeder's equation Δ̄R = S h2 (21), where h2 is the heritability (Mathematica file available on request). The narrow sense heritability for the R-ratio obtained from a diallel cross is 0.25 (18).
ACKNOWLEDGMENTS.
We thank D. J. Schoen, P.-A. Crochet, K. Donohue, and J. Auld for their very helpful comments on the manuscript and two anonymous reviewers and Dr. James H. Brown for providing helpful comments to clarify the text. This work was supported by “Action Concertée Incitative jeunes chercheurs” (Ministère de la Recherche) (to P.-O.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Levin SA, Muller-Landau HC, Nathan R, Chave J. The ecology and evolution of seed dispersal: a theoretical perspective. Annu Rev Ecol Syst. 2003;34:575–604. [Google Scholar]
- 2.Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. Dispersal. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 3.Gandon S, Michalakis Y. In: Dispersal. Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. Oxford: Oxford Univ Press; 2001. pp. 155–167. [Google Scholar]
- 4.Hamilton WD, May RM. Dispersal in stable habitats. Nature. 1977;269:578–581. [Google Scholar]
- 5.Motro U. Optimal rates of dispersal. I. Haploid populations. Theor Popul Biol. 1982;21:349–411. [Google Scholar]
- 6.Comins HN, Hamilton WD, May RM. Evolutionarily stable strategies. J Theor Biol. 1980;82:205–230. doi: 10.1016/0022-5193(80)90099-5. [DOI] [PubMed] [Google Scholar]
- 7.van Valen L. Group selection and the evolution of dispersal. Evolution (Lawrence, Kans) 1971;25:591–598. doi: 10.1111/j.1558-5646.1971.tb01919.x. [DOI] [PubMed] [Google Scholar]
- 8.Bengtsson BO. Avoiding inbreeding: at what cost? J Theor Biol. 1978;73:439–444. doi: 10.1016/0022-5193(78)90151-0. [DOI] [PubMed] [Google Scholar]
- 9.Herrera CM. Plant-vertebrate seed dispersal systems in the Mediterranean: Ecological, evolutionary and historical determinants. Annu Rev Ecol Syst. 1995;26:705–727. [Google Scholar]
- 10.Carlquist S. Evolution. Vol. 20. Kans: Lawrence; 1966. The biota of long-distance dispersal. II. Loss of dispersability in the Pacific Compositae. pp. 30–48. [DOI] [PubMed] [Google Scholar]
- 11.Roff DA. The evolution of flightlessness in insects. Ecol Monogr. 1990;60:389–421. [Google Scholar]
- 12.Cody ML, Overton JM. Short-term evolution of reduced dispersal in island plant populations. J Ecol. 1996;84:53–61. [Google Scholar]
- 13.Cordeiro NJ, Howe HF. Forest fragmentation severs mutualism between seed dispersers and an endemic African tree. Proc Natl Acad Sci USA. 2003;100:14052–14056. doi: 10.1073/pnas.2331023100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shocat E, Warren PS, Faeth SH, McIntyre NE, Hope D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol. 2006;21(4):186–191. doi: 10.1016/j.tree.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Cheptou PO, Lepart J, Escarre J. Mating system variation along a successional gradient in the allogamous and colonizing plant Crepis sancta (Asteraceae). J Evol Biol. 2002;15:753–762. [Google Scholar]
- 16.Cheptou PO, Avendano LGA. Pollination processes and the Allee effect in highly fragmented populations: consequences for the mating system in urban environments. New Phytol. 2006;172:774–783. doi: 10.1111/j.1469-8137.2006.01880.x. [DOI] [PubMed] [Google Scholar]
- 17.Imbert E. Ecological consequences and ontogeny of seed heteromorphism. Perspect Plant Ecol Evol Syst. 2002;5:13–36. [Google Scholar]
- 18.Imbert E. Capitulum characters in the seed heteromorphic species Crepis sancta (Asteraceae): Variance partitioning and inference for the evolution of dispersal rate. Heredity. 2001;86:78–86. doi: 10.1046/j.1365-2540.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- 19.Imbert E. The effects of achene dimorphism on the dispersal in time and in space in Crepis sancta (Asteraceae). Can J Bot. 1999;77:508–513. [Google Scholar]
- 20.SAS II. 4th Ed. Cary, NC: SAS Institute Inc.; 1989. SAS/STAT User's Guide. Version 6. [Google Scholar]
- 21.Falconer DS. Introduction to Quantitative Genetics. Essex, England: Longman Scientific & Technical; 1981. [Google Scholar]
- 22.Imbert E, Escarre J, Lepart J. Achene dimorphism and among-population variations in some biological traits in Crepis sancta (Asteraceae). Int J Plant Sci. 1996;157:309–315. [Google Scholar]
- 23.Imbert E, Ronce O. Phenotypic plasticity for dispersal ability in the seed heteromorphic Crepis sancta (Asteraceae). Oikos. 2001;93:126–134. [Google Scholar]
- 24.Zar JH. Biostatistical Analysis. London: Prentice-Hall; 1996. [Google Scholar]