Abstract
Leishmania is unable to synthesize heme and must acquire it from exogenous source, the mechanism of which is not known. We have shown that Leishmania endocytoses hemoglobin (Hb) and subsequently degrade it probably to generate heme. To understand how internalized Hb is degraded, we have cloned and expressed Rab7 homolog from Leishmania donovani. Interestingly, Rab7 in Leishmania is found to be localized both on early and late endocytic compartment and regulates both uptake and degradation of endocytosed Hb demonstrating that Rab7 in Leishmania play a very unique role connecting both early and late events of Hb endocytosis. Our data also indicate that overexpression of Rab7:WT in Leishmania induces transport of Hb to lysosomes and rapidly degrade internalized Hb. Whereas Hb transport to lysosomes and its degradation is significantly inhibited in cells overexpressing Rab7:T21N, a GDP locked mutant of Rab7. Moreover, cells overexpressing Rab7:T21N grow at a slower rate (<50%) compared with control Leishmania. Addition of exogenous hemin recovers the growth of Rab7:T21N mutant cells almost to the control level, suggesting that intracellular heme generated by Rab7-mediated Hb degradation is required for optimal growth of the parasites. Thus, our results identify a potential target which might be exploited to suppress the growth of Leishmania.
Leishmania donovani, a pathogenic trypanosomatid, is the etiological agent of the fatal form of human disease known as visceral leishmniasis, which causes considerable morbidity and mortality of people worldwide (1). Considering the toxicity of drugs used in chemotherapy of Leishmaniasis (2) and the unavailability of appropriate vaccine, a search is on to find newer targets to suppress the parasite growth. Because Leishmania lack a complete heme biosynthetic pathway (3), the exploitation of heme dependency of parasites (4) is the key target for the drug development (5). However, how this parasite meets the requirement of heme is not known. The majority of the heme in the host is sequestered in erythrocytes in the form of Hb. Thus, endocytosis of Hb from the host cells by parasites and followed by its degradation might generate intracellular heme. Our previous results show that Leishmania endocytosed Hb through a specific receptor located in the flagellar pocket that rapidly internalizes Hb, which is subsequently degraded (6, 7). However, how Hb is transported to the degradative compartment to generate intracellular heme is not known.
It is evident from studies in mammalian cells that intracellular trafficking of endocytic ligands are specifically regulated by small GTP binding proteins of Rab family along with their effectors (8, 9). In the endocytic pathway, Rab5 is the early acting Rab and regulates transport from plasma membrane to early compartment (10, 11), whereas Rab7 mediates transport from early to late compartment (12, 13). Although it is evident that Rab like proteins are present in parasites, their role in the regulation of intracellular trafficking in parasites remains to be characterized (14). Endocytic Rabs like Rab4, Rab5, and Rab11 have been identified in Trypanosoma brucei (15, 16). Among these Rabs, Rab4 and Rab11 appear to be involved in recycling, whereas different isoforms of Rab5 regulate distinct steps of endocytosis in Trypanosoma (17, 18). Similarly, Rab5 is found to regulate cholesterol acquisition from the host cell in Toxoplasma gondii (19), and Rab4 is associated with the endocytic pathway in Dictyostelium discoideum (20). Interestingly, only 11 Rab GTPases are present in Plasmodium (21), in contrast to the ≈60 different Rabs present in mammalian cells (8, 9). Recently, we showed that Rab5 homologue in Leishmania localizes in the early endocytic compartment and plays a major role in Hb trafficking in this parasite (22). However, not much is known about the regulation of late endocytic trafficking in parasites. Thus, it will be interesting to determine the role of Rab7 in the regulation of Hb transport to the late degradative compartment in Leishmania.
Here, we report the cloning, expression, and characterization of Rab7 homologue from L. donovani. We have shown that overexpression of LdRab7 in Leishmania induces the transport of Hb to the late compartment whereas Hb targeting to the lysosomes is blocked in the cells expressing of GDP-locked mutants of LdRab7. Moreover, cells expressing GDP-locked mutant do not grow optimally as they fail to generate heme by intracellular degradation of Hb in the late compartment.
Results
Cloning and Expression of Rab7 Homolog from L. donovani.
To clone a Rab7 homologue from L. donovani, a BLAST search was performed by using mouse Rab7 sequence as a query, which identified a putative Rab7-like sequence from L. major genome showing 65% homology to mouse Rab7 sequence. Using appropriate forward and reverse primers, we amplified a 672-bp fragment from L. donovani cDNA by PCR. The PCR product was cloned, sequenced and hypothetically translated into a 223-aa sequence. The sequence analysis of the cloned protein (LdRab7) revealed the presence of highly conserved motifs of Rab proteins (23), such as the guanine nucleotide binding regions, effector loop, and C-terminal isoprenylation motif (Fig. 1). Comparison of LdRab7 sequence by CLUSTAL W multiple sequence alignment demonstrated that the cloned protein has similarities of ≈96% with Leishmania major Rab7, 76% with T. brucei Rab7, 61% with Drosophila melanogaster Rab7, 61% with Plasmodium falciparum, and 65% with mammalian Rab7 sequences.
Fig. 1.
Multiple alignment of amino acid sequence of LdRab7 with Rab7 sequences from different organisms, namely L. major (GenBank accession no. CAJ03806.1), T. cruzi (AAD32707), H. sapiens (X93499.1), M. musculus (X89650.1), R. norwegicus (BC072470.1), D. melanogaster (AM294823.1), and P. falciparum (CAB92946.2). Our sequence data of LdRab7 is in the GenBank database under accession no. EF507729. Residues implicated in guanine phosphate binding (PM1–3), GTP/GDP binding (G1–3), and isoprenylation motif (I) are marked in bold face.
To further characterize the role of LdRab7 in Hb trafficking in Leishmania, three mutants were generated by site-directed mutagenesis based on the previous knowledge of analogous mutations in mammalian Rabs (24). The LdRab7:T21N mutant was generated by substituting asparagine for threonine in the GKT/S region, whereas leucine was substituted for glutamine in the WDTAGQE region in LdRab7:Q66L mutant. LdRab7:ΔC mutant was generated by deletion of last cysteine residues from the C terminus. Subsequently, GTP binding ability and GTPase activity of Rab7 and its mutants were checked. Our results showed that LdRab7:Q66L and LdRab7:ΔC mutants bind [α-32P]-GTP comparable to LdRab7:WT protein, whereas LdRab7:T21N fails to bind GTP. Analysis of GTPase activity of these mutants revealed that LdRab7:WT hydrolyze GTP to GDP like any other Rab GTPases, whereas GTP hydrolysis is blocked in LdRab7:Q66L mutant [supporting information (SI) Appendix, Fig. 1].
Overexpression and Localization of Rab7 and Its Mutants in Leishmania.
To determine the role of Rab7 in Hb trafficking in Leishmania, cells were overexpressed with LdRab7 or its GDP or GTP locked mutants as GFP fusion protein in Leishmania, using pXG vector, and stable clones were selected in the presence of G418 antibiotic. GFP was placed in the N terminus of the Rab7 or its mutants to keep C terminus of Rab7 free for prenylation, which is required for membrane attachment and functioning of Rab (8, 9). Cells overexpressing Rab7:T21N mutant were grown in the antibiotic containing medium supplemented with hemin. The overexpression of different forms of LdRab7 as fusion proteins in Leishmania was confirmed by Western blot analysis of the cell lysates, using specific antibodies (SI Appendix, Fig. 2).
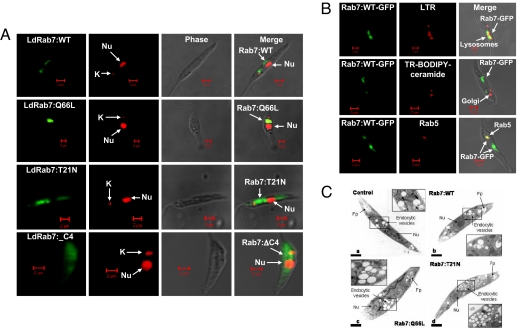
In addition, expression of different forms of LdRab7 as GFP fusion proteins also indicated their localization in Leishmania by confocal microscopy. GFP-LdRab7:WT protein was found to be predominantly localized in the perinuclear compartments with additional labeling of punctuate structures near kinetoplast (Fig. 2A). Overexpression of Rab7 as GFP fusion protein did not alter their normal localization as evident by immuno-localization of endogenous Rab7 in similar structures, using specific antibody (SI Appendix, Fig. 3). Further characterization demonstrated that LdRab7 colocalizes with the Lysotraker-Red-labeled late/lysosomal compartment (Fig. 2B). Interestingly, LdRab7 was also found to be partially localized in the Rab5 positive early endocytic compartment near kinetoplast (Fig. 2B). However, LdRab7 failed to colocalize with Texas red-conjugated BODIPY-ceramide labeled Golgi compartment (Fig. 2B). Furthermore, the expression of LdRab7:Q66L mutant labeled larger structures restricted to the perinuclear area, whereas the GFP-Rab7:T21N mutant showed diffuse cytoplasmic staining (Fig. 2A). Similarly, LdRab7:ΔC, a prenylation defective mutant was also found to be distributed through out cytoplasm (Fig. 2A).
Fig. 2.
Overexpression and localization of LdRab7 in Leishmania. (A) LdRab7 and its mutants were overexpressed in Leishmania promastigotes as described in Materials and Methods. Confocal micrographs showing the localization of LdRab7 and its mutants as GFP fusion proteins. Nucleus (nu) and kinetoplast (k) were stained with propidium iodide. (B) To characterize Rab7 positive compartment in Leishmania, lysosomes (Top) and Golgi complex (Middle) of the LdRab7-GFP overexpressed Leishmania were labeled by incubating the cells with lysotracker red (1 μM) or BODIPY-TR ceramide (5 μM), respectively, at 23°C for 30 min in serum-free M199 medium. Similarly, early endosomal compartment was visualized by anti-Rab5 antibody subsequently probed with goat anti-mouse Alexa Fluor 488-labeled second antibody after fixing the cells with formaldehyde (Lower). Yellow indicates the colocalization of Ld Rab7 with indicated compartment in one plane after after Z-stack analysis by confocal microscopy. (C) Morphology of the endocytic compartments in LdRab7 and its various mutants overexpressed Leishmania were determined by electron microscopic analysis as described in Materials and Methods.
To determine whether the expression of Rab7:WT, Rab7:Q66L, and Rab7:T21N mutants altered the morphology of the endocytic vesicles, morphological analysis was carried out by using ultrathin sections made from Leishmania promastigotes expressing different Rab7 proteins by electron microscopy (Fig. 2C). Expression of Rab7:WT (Fig. 2B) did not alter the localization and size of endocytic vesicles (130 ± 12.6 nm) compared with untransfected (Fig. 2A) control cells (120 ± 15.3 nm), whereas expression of Rab7:Q66L (Fig. 2C) generated increased number of moderately enlarged endocytic vesicles (183 ± 11.2 nm) concentrated in perinuclear region. In contrast, cells expressing Rab7:T21N (Fig. 2D) showed large number of smaller vesicles (42 ± 6.8 nm) scattered throughout the cell cytoplasm.
Role of Rab7 in Hb Trafficking in Leishmania.
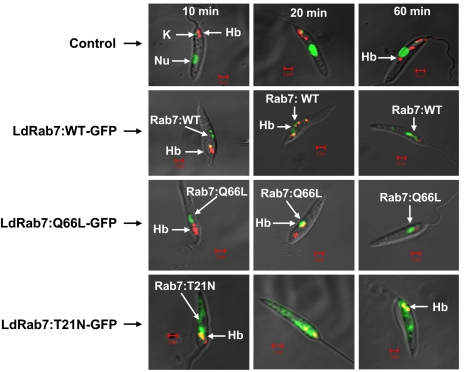
To determine the role of Rab7 in Hb transport in Leishmania promastigotes, we analyzed the trafficking of Alexa Fluor 594-conjugated Hb in the cells overexpressing Rab7:WT or its mutants along with untransfected control cells by confocal microscopy. The results presented in Fig. 3 showed that bound Hb internalized into early endosomal compartment by 10 min, which is supported by their colocalization with Rab5 (data not shown), and finally reached the Rab7 positive perinuclear late/lysosomal compartment by 60 min in control cells (Fig. 3 Top). Moreover, internalized Hb was retained in this compartment even after 60 min in control cells (data not shown). In contrast, Hb was rapidly internalized into early endocytic compartment and targeted to Rab7 positive late compartments within 20 min in cells overexpressing Rab7:WT and Rab7:Q66L mutants (Fig. 3 Middle), and almost no Hb was found in these cells after 60 min. Interestingly, most of the internalized Hb was retained in the early endosomes near the flagellar pocket even after 60 min of uptake in Rab7:T21N overexpressed cells (Fig. 3 Lower), indicating that Rab7:T21N mutant inhibits the transport of Hb to the late compartment.
Fig. 3.
To determine the kinetics of Hb transport in Rab7 and its mutants overexpressed Leishmania, cells were allowed to bind Alexa Fluor 594-conjugated Hb at 4°C and subsequently chased for indicated times at 23°C as described in Materials and Methods. Finally, cells were fixed with paraformaldehyde and visualized under confocal microscope. Results are the representative of three independent observations.
Rab7 Induce Transport of Hb to Late/Lysosomal Compartment in Leishmania.
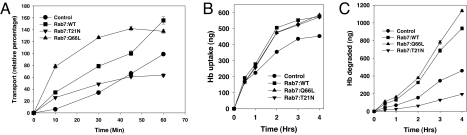
To determine the Rab7 induced transport of Hb to the lysosomal compartments, a ligand mixing assay was developed to measure the kinetics of biotinylated-Hb (BHb) transport from the early compartment to the avidin-HRP (AHRP) preloaded lysosomes in Leishmania expressing Rab7 and its mutants. The results presented in Fig. 4A show that the BHb significantly colocalized with AHRP loaded late/lysosome compartments within 30 min, and maximum fusion was observed within 60 min in the cells overexpressing Rab7:WT. The rate of transport of BHb to the lysosomes in LdRab7:WT overexpressed cells was found to be 2-fold higher than that of control cells. The kinetics of Hb transport to the late compartment in Rab7:Q66L-overexpressing cells was even higher than the Rab7: WT expressing cells, at least in early time points, probably because of faster transport of Hb to the lysosomes and their simultaneous degradation. In contrast, significant inhibition in transport of BHb was observed in the cells overexpressing Rab7:T21N mutant compared with the control cells (Fig. 4A). The apparent increase in the colocalization of the BHb with AHRP at early time points in these cells was due to the mixing of both ligands in the early compartments, because the transport of AHRP to the late compartment was also blocked in Rab7:T21N mutant overexpressing cells.
Fig. 4.
Rab7 induced the transport of Hb to the late compartment. (A) To determine the role of Rab7 in Hb transport to the lysosomes in Leishmania, B-Hb transport to avidin-HRP preloaded lysosomes was measured in Rab7, and its mutants overexpressed cells as described in Materials and Methods. At respective time points, cells were lysed and B- Hb-avidin-HRP complexes were immunoprecipated from the lysate by anti-Hb antibody. HRP activity associated with the complex was measured as relative transport to the lysosomes. Transport observed at 60 min in untransfected control cells was taken as 100%, and results are expressed as relative percentage of transport to the lysosomes from three independent experiments ± SD. One hundred percent transport in untreated control cells corresponds to 15.9 ng of HRP activity per milligram of protein. (B and C) Uptake (B) and degradation (C) of 125I-Hb in LdRab7 or its mutant overexpressed Leishmania. Uptake and degradation of 125I-Hb by LdRab7 or its mutant overexpressed Leishmania along with untransfected control cells were determined as described in Materials and Methods. At respective times, cell-associated radioactivity (B) and trichloroacetic acid-soluble noniodide radioactive products (C) released in the medium were determined. Results are expressed as nanograms of Hb per milligram of cell protein from three independent determinations ± SD.
Rab7-induced transport of Hb to the late/lysosomal compartments and its subsequent clearance from these cells prompted us to determine whether this effect is due to the enhanced degradation of the Hb in the lysosomal compartments in these cells. Thus, we measured the kinetics of uptake and degradation of 125I-Hb at 23°C in these cells. Our results demonstrated that Hb uptake reached a steady state plateau after 3 h, whereas TCA-soluble radioactivity continued to increase at a linear rate (Fig. 4 B and C), indicating simultaneous uptake and degradation of 125I-Hb by Leishmania promastigotes. Moreover, in correlation with our previous finding, we also observed ≈2.25- and 2.75-fold higher degradation of Hb in the cells overexpressing Rab7: WT and Rab7:Q66L proteins, respectively, compared with the control cells (Fig. 4C). Most importantly, degradation of 125I-Hb was severely impaired in cells expressing Rab7:T21N mutant, indicating that Rab7 plays an important role in the transport of Hb to the late compartment in Leishmania for intracellular degradation of Hb. Interestingly, our results also demonstrated that overexpression of LdRab7 stimulated a more significant uptake of Hb in Leishmania than control cells (Fig. 4B).
Rab7-Mediated Hb Degradation Is Required for the Growth of Leishmania.
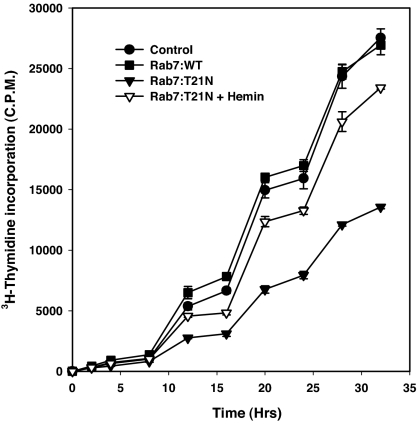
Our initial hypothesis was that internalized Hb would be targeted to the lysosome like compartment, where it would be degraded to generate heme that Leishmania cells could use for their growth and survival. To verify the above hypothesis, we measured the growth of the cells overexpressing Rab7:T21N, where Hb degradation is reduced. To measure the growth of Rab7 and its mutant overexpressed cells, cells were incubated in the presence of 3H-thymidine for different periods of times and the amount of radioactivity incorporated by cells was measured to determine the cell proliferation. Under this experimental condition, we found that untransfected control Leishmania promastigotes grow exponentially with a doubling time of ≈8 h (Fig. 5). Overexpression of Rab7:WT did not alter the growth rate of Leishmania promastigotes. In contrast, ≈50% inhibition of thymidine incorporation was observed in the cells overexpressing Rab7:T21N mutant compared with the control cells, demonstrating that Rab7:T21N mutant overexpressing cells failed to grow to their optimal level (Fig. 5). To determine whether the inhibition of the growth of Leishmania overexpressing Rab7:T21N is due to the inability of generating heme from Hb degradation, these cells were grown in the presence of exogenous hemin (10 μg/ml), a heme analogue that possibly diffuse into the cells. Interestingly, addition of hemin in the culture medium recovered the growth of Rab7:T21N mutant overexpressing Leishmania to ≈80% of the growth of control cells (Fig. 5), suggesting that heme generated from Rab7-mediated Hb degradation is required for the growth of Leishmania.
Fig. 5.
Effect of Rab7 or its mutants on the growth of Leishmania. Growth of the parasites overexpressing different mutants were measured by [3H]thymidine incorporation over different periods of times as described in Materials and Methods. At respective time points, cells were harvested and radioactivity incorporated by respective Leishmania was measured. Results are expressed as radioactivity incorporated by the cells from three independent experiments ± SD.
Discussion
Previously, we identified that endocytosis of Hb in Leishmania is mediated through receptors located in the flagellar pocket (6, 7). Because Leishmania lack a complete heme biosynthetic pathway (3) and depend on the exogenous supply of heme for their survival, this receptor system appears to play a very significant role in the biology of the parasites, probably by generating intracellular heme after degradation of internalized Hb. Our recent studies have shown that Rab5 in Leishmania is located in early endocytic compartment and promotes the homotypic fusion between early endosomes to regulate early events in Hb endocytosis in Leishmania. Interestingly, Hb-containing early endosomes were shown to fuse also with the late endosomes through a signal transduced from the cytoplasmic tail of Hb receptor (22). It appears that Leishmania has devised a unique mechanism of Hb endocytosis, so Hb is targeted to lysosomes to generate intracellular heme for their use. One of the possible signals to target Hb to the late compartment could be Rab7 homologue from Leishmania, because this GTPase is shown to regulate transport to the late compartment in mammalian cells (12, 13). Thus, we have cloned and expressed Rab7 homologue from Leishmania, LdRab7, which specifically binds GTP. Sequence comparison of the cloned protein along with Rab7 homologues from different organisms reveals that LdRab7 contains Rab7 specific effector domain (YKATIGADF) as seen in Rab7 sequence of other organisms (23). Moreover, LdRab7 also contains an insertion of 20 amino acid residues (EAANVNNGACAGAGDSAAPE) preceding the G3 domain, which are present only in the Rab7 sequences of other trypanosomatid parasites (25, 26). However, the functional relevance of this insertion sequence in trypanosomatid parasite trafficking has not been determined.
It is well documented that mutations within highly conserved regions of Rab proteins generally perturb nucleotide binding and hydrolysis and thereby interfere with membrane traffic in a dominant negative way (27–29). For example, replacing leucine for glutamine in the WDTAGQE region or substitution of asparagine for serine or threonine in the GKT/S region generates mutants that are constitutively in the GTP or GDP-bound conformations, respectively (30, 31). Subsequently, Rab:T to N mutant proteins have been shown to have a dominant negative effect on transport, whereas the Rab:Q to L mutant proteins have a stimulatory effect on fusion assays. To characterize the role of LdRab7 in Hb trafficking in Leishmania, we have generated similar mutants of LdRab7, namely Rab7:Q66L, Rab7:T21N, and Rab7:ΔC. Subsequently, Rab7 and its mutant proteins are overexpressed in Leishmania as GFP fusion protein to determine their localization. GFP-LdRab7 predominantly localizes in the perinulear late/lysosomal compartment like endogenous Rab7 in Leishmania, in contrast to the localization of Rab7 in the Golgi compartment in Trypanosoma cruzi (26). Interestingly, GFP-LdRab7 also colocalizes with Rab5 positive early endosomal compartment in Leishmania. Furthermore, LdRab7:Q66L localizes in some sort of larger endocytic vesicles as revealed by morphological analysis possibly because of enhanced fusion between early and late endosomes or the homotypic fusion of Rab7 positive late compartments. In addition, overexpression of dominant negative Rab7:T21N disintegrates endocytic vesicles and thereby possibly inhibits the transport of Hb to the late compartment.
Because the localization of the endogenous Rab7 and GFP-tagged LdRab7 are identical, Leishmania over-expressing LdRab7 or its mutants as GFP fusion protein are used to dissect out their role in Hb trafficking in Leishmania. Kinetics of the Hb trafficking in Leishmania reveal that overexpression of Rab7 or GTP-locked mutant of Rab7 induce the transport of the Hb to the late compartment compared with the untransfected control cells and most of the internalized Hb is cleared out of the cells within 60 min. This clearance of Hb in these cells is due to the rapid degradation of internalized Hb in lysosomes. In contrast, overexpression of dominant negative mutant of Rab7 in Leishmania inhibits the transport of internalized Hb to the lysosomes, and most of the Hb is localized in the early compartment even after 60 min. More than 50% inhibition of Hb degradation is observed in the cells overexpressing Rab7:T21N mutant compared with control Leishmania, further supporting this observation. Surprisingly, our results show that overexpression of Rab7 or its GTP-locked mutant also stimulates the uptake of Hb in Leishmania. Rab7 localizes in early compartment and also triggers the uptake of Hb in Leishmania, indicating that Rab7 in Leishmania is a dynamic endocytic Rab with dual functions coupled to both ends of endocytic pathway.
The intracellular amastigote form of Leishmania resides in the macrophages, which takes senescent RBC (32), and the promastigote form resides in the insect gut where RBC are available from blood meal (33, 37), indicating parasite has access to Hb from lysed RBC. In addition, Leishmania can be grown in the culture medium containing hemin (34) or Hb (35), strongly indicating the possibility of generating heme from Hb degradation by the parasites emerging from our studies. Thus, Hb endocytosis and Rab7-mediated targeting of Hb to the lysosome to generate intracellular heme might be crucial for the growth of the parasites. In this notion, it is pertinent to determine the growth of the parasites that overexpress the Rab7:T21N mutant, where Hb degradation is significantly inhibited. Thus, we have measured the proliferation of different Rab7 overexpressed Leishmania by 3H-thymidine incorporation as a parameter to monitor their growth in the culture medium. Interestingly, significant inhibition of growth of the Rab7:T21N-overexpressing Leishmania is observed compared with the normal cells, demonstrating that Rab7-mediated transport of some endocytosed nutrients to the lysosomes is essential for parasites growth. Although, overexpression of GDP-locked mutant of Rab7 in Leishmania is expected to inhibit targeting of all endocytosed ligands to lysosomes, and cell growth might be compromised because of unavailability of required product generated from lysosomal degradation of internalized ligands. Nonetheless, our results show that it is possible to restore the growth Rab7:T21N-overexpressing cells almost to the control level by the addition of exogenous hemin in the culture medium demonstrating Hb degradation is critical for the growth of the parasites. However, overexpression of Rab7:WT do not alter the growth rate of Leishmania promastigotes, even though these cells degrades ≈2-fold more Hb than untransfected control cells. This may be because cells need only optimal amount of heme for their growth or possibly because of the limitation of the other down stream factors required for functioning the LDRab7. Taken together, our results indicate that impaired growth of Rab7:T21N-overexpressing cells is due to the unavailability of heme from Hb degradation, which unequivocally proves that Leishmania acquire heme from Rab7-mediated degradation of the internalized Hb for their growth.
Our results represent the first demonstration of Leishmania acquiring heme from intracellular degradation of Hb in lysosome-like compartment through a Rab7-dependent pathway for their growth. Interestingly, our results also demonstrate that Rab7 in Leishmania has a unique function by regulating both uptake and transport of Hb to the lysosomes. Therefore, we have shown that Leishmania has devised a novel Hb endocytic pathway to generate heme from Hb degradation in lysosomes to compensate for their inability to synthesize heme. Because Hb endocytosis and degradation is a prerequisite for the growth of Leishmania, preventing Hb endocytosis or its intracellular degradation could be a target that might be exploited for inhibiting the growth of the parasite.
Materials and Methods
Materials.
N-hydroxysuccinimido-biotin (NHS-biotin) and avidin horseradish peroxidase (avidin-HRP) were purchased from Vector Laboratories. All HRP-labeled secondary antibodies were obtained from Santa Cruz Biotechnology. Alexa Fluor 594, SYTO dyes, Prolong antifade kit were obtained from Molecular Probes. pXG vector used for over-expression of Ld-Rab in Leishmania was received as a gift from S. M. Beverley (Washington University, St Louis, MO). Enhanced chemiluminescence (ECL) reagents were obtained from Amersham Biosciences. All other reagents used were of analytical grade. Unless otherwise stated, all reagents were obtained from Sigma. Antibodies against Hb and purified LdRab7 were raised in mice by the standard technique described in ref. 22. Hb was biotinylated by using NHS-biotin as described in ref. 36.
Leishmania.
L. donovani promastigotes (UR 6) were obtained from Indian Institute of Chemical Biology, Kolkata, India. Cells were routinely maintained on blood agar slants containing glucose, peptone, sodium chloride, beef heart extract, rabbit blood, and gentamycin (37). For experiments, cells were grown in liquid medium M199 (pH 7.4) supplemented with 10% FCS and gentamicin (50 μg/ml) at 23°C, and log-phase cells were harvested in phosphate-buffered (10 mM, pH 7.2) saline (0.15M).
Cloning and Expression of Rab7 from L. donovani (LdRab7).
To clone Rab7 from Leishmania, a putative Rab7-like sequence was identified from a Leishmania major genome with substantial homology with T. brucei Rab7, using BLAST. Accordingly, forward (5-′GGATCCATGTCGATGAAG-3′) and reverse (5′- GAATTCTTAGCAGCTGCAGGCGG-3′) primers were designed against start and stop codons of the putative L. major Rab7 sequences, respectively. RT-PCR was performed by using these primers to amplify the ORF of the putative Rab7 sequence, using L. donovani cDNA. mRNA isolated from Leishmania promastigotes, using an Oligotex mRNA kit (Qiagen) was used for cDNA synthesis, using Thermo Script RT-PCR kit (GibcoBRL) as per manufacturers' instructions. Subsequently, PCR was performed by using the above primers in a Perkin–Elmer thermocycler for 30 cycles as follows: denaturation for 1 min at 94°C; annealing at 65°C for 30 s, and extension at 72°C for 1 min. The PCR product was cloned into pGEM-T-easy vector and sequenced by using m13 universal primers in an automated sequencer. Finally, the PCR product was cloned into BamHI/EcoRI sites of pGEX-4T-2 vector (Amersham Biosciences) and transformed into Escherichia coli.
Expression and Purification of LdRab7:WT and Mutant Proteins.
To purify recombinant proteins, E. coli were transformed with respective constructs. Cells were grown in LB and induced with 0.2 mM IPTG for 3 h at 30°C for expression of GST respective proteins. Cells were harvested and lysed, and fusion proteins were purified from the supernatant, using reduced glutathione beads by standard procedure.
Overexpression of Rab7 and Its Mutants in Leishmania Promastigotes.
To overexpress LdRab7 or its mutants in Leishmania promastigotes as GFP fusion protein having GFP tag in the N terminus, the full-length LdRab7 or its mutants gene was subcloned into pXG-GFP+2 vector (38). For subcloning, vector was first linearized by digestion with BamHI and subsequently partially digested with EcoRI followed by ligation with BamHI-EcoRI-digested LdRab7 or its mutants. Leishmania promastigotes were then transfected with respective constructs, using standard protocol (7). Briefly, Leishmania cells were grown to the late log phase (≈1.0 × 107/ml) at 23°C in M199 medium supplemented with 10% FCS. Cells were resuspended at a density of 1.0 × 108/ml in Hepes-buffered saline [21 mM Hepes, 137 mM NaCl, 5 mM KCl, 0.7 mM NaH2PO4, and 6 mM glucose (pH 7.4)]. Cells (0.4 ml) were transferred to precooled electroporation cuvette, and appropriate constructs of chilled DNA (40 μg) were added, followed by electroporation, using a GenePulser (Bio-Rad) to facilitate DNA uptake by cells. Cells were then incubated on ice for 10 min and transferred into drug-free medium for 30 h at 23°C. Subsequently, stable clones were selected in the presence of G418 antibiotic (50 μg/ml). Overexpression of the respective protein was confirmed by Western blot analysis, using indicated antibodies, and by confocal microscopy.
Determination of the Morphology of Endocytic Compartment in the LdRab7 or Its Mutants Overexpressed Leishmania.
To determine the effect of overexpression of LdRab7 and its various mutants on endosome morphology, cells (5 × 105) overexpressing Rab7:WT, Rab7:Q66L, and Rab7:T21N were harvested from freshly grown culture, washed three times with cold PBS, and fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3). Fixed cells were washed twice with cacodylate buffer and treated with 1% OsO4 in cacodylate buffer (pH 7.3) for 2 h 4°C. Subsequently, the cells were rinsed and dehydrated in ethanol and embedded in araldite (6). Thin sections were double stained with uranyl acetate and lead citrate and examined on a Philips CM10 transmission electron microscope.
Kinetics of Intracellular Trafficking of Hb in Rab7 and Its Mutants Overexpressed Leishmania.
To determine the kinetics of intracellular trafficking of Hb, Hb was labeled with Alexa Fluor 594 (Alexa-Hb) according to the manufacturer's protocol. Freshly grown respective Leishmania cells (107 cells per ml) expressing GFP-Rab7 protein were harvested by centrifugation (2,000 × g for 5 min) and washed twice with ice-cold PBS. Cells were resuspended in 0.5 ml of ice-cold serum free M199 medium containing Alexa-Hb (120 μg/ml) and incubated for 30 min at 4°C to allow the cell surface binding. Cells were washed three times with cold PBS to remove unbound Alexa-Hb and resuspended in preworm (23°C) serum-free medium for indicated periods of times. At respective times, cells were transferred onto ice and washed twice with chilled PBS. Untransfected control cells were stained with Syto 16 (10 μM) to mark the position of nucleus and kinetoplast. Finally, cells were fixed with 3% paraformaldehyde on ice for 20 min, washed with PBS, and visualized under a Zeiss LSM 510 META confocal microscope.
Reconstitution of Endosome-Lysosme Transport to Determine the Effect of Rab7 or Its Mutant on Hb Trafficking in Leishmania.
To determine the role of LdRab7 and its mutants in Hb transport to the lysosomes in Leishmania, we have developed a ligand mixing assay to measure the transport of biotinylated-Hb (B-Hb) from early endocytic compartment to avidin-HRP-loaded late/lysosomal compartments as described in refs. 39 and 40. Briefly, Leishmania promastigotes (106 cells per ml) expressing LdRab7 or its mutants were incubated in internalization medium (IM: MEM supplemented with 20 mM Hepes and 10 mM glucose) containing avidin-HRP (500 μg/ml) for 10 min at 23°C to allow the internalization of B-Hb in early endocytic compartment. Cells were washed three times, and avidin-HRP was chased for 60 min at 23°C to label the lysosomes. Cells were then incubated with B-Hb (2 mg/ml) for 10 min at 23°C to level the early compartment. Cells were washed, and uninternalized surface-bound B-Hb was quenched by free avidin (0.1 mg/ml). Subsequently, cells were washed twice and chased for indicated times at 23°C. Cells were then solubilized in solubilization buffer (PBS containing 0.5% Triton X-100, and 250 μg/ml avidin). Finally, cell lysates containing B-Hb-avidin-HRP complexes were immunoprecipitated by using anti-Hb coated plates and the HRP activity associated with the B-Hb was measured as relative transport unit to the lysosomes. Background value was determined by the amount of HRP activity associate with B-Hb at the 0 min time point, which was found to be very low and subtracted from all values to determine specific transport.
Uptake and Degradation of 125I-Hb in LdRab7 or Its Mutant Overexpressed Leishmania.
To determine whether LdRab7 induced transport of Hb to the lysosomes enhances the degradation of internalized Hb, we have measured the uptake and degradation of 125I-Hb (6) by LdRab7 or its mutant overexpressed Leishmania along with untransfected control cells. Briefly, Hb was labeled with Na125I by iodine monochloride-catalyzed reaction, and >99% of the radioactivity was acid precipitable with specific activity of 196 cpm/ng of Hb. Cells (1 × 107) were incubated with 125I-Hb (6 μg) in 1 ml of RPMI medium 1640 containing 1 mg/ml BSA for indicated periods of times at 23°C. At respective times, cells were washed to remove the unbound radioactivity. The cell pellet was dissolved in 0.1 N NaOH, and an aliquot was used to determine the cell-associated radioactivity. To determine the degradation of internalized Hb by Leishmania, aliquots of the supernatant from the respective medium were processed for the determination of trichloroacetic acid-soluble noniodide radioactivity after extraction with chloroform. Results were expressed as nanograms of Hb per milligram of cell protein.
Effect of Rab7 or Its Mutants on the Growth of Leishmania.
To determine whether impairment of Hb degradation by overexpression of Rab7:T21N mutant inhibits the growth of Leishmania, we have measured the growth of the parasites overexpressing different mutants by [3H]-thymidine incorporation over different periods of times (41). Briefly, promastigotes from overnight grown culture were harvested, washed, and resuspended in M199 medium. Cell suspensions (106 cells) were incubated in 200 μl of sterile M199 medium containing 3H-thymidine (0.6 μCi per well) into flat-bottom tissue culture wells for different time points at 23°C. At respective time points, cells were harvested and washed with a multiwell cell harvester (Wallac). After harvesting, dry filters were processed to measure the radioactivity incorporated by respective Leishmania, using a beta plate liquid scintillation counter. The incorporation of radioactivity was directly proportional to the growth of the parasite as evident from the untransfected control cells.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by grants from the Department of Biotechnology, Government of India, and Indo-French Centre for the Promotion of Advanced Research, India (to A.M.) and fellowships from the National Institute of Immunology, Department of Biotechnology (N.P. and S.B.S.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. EF507729).
This article contains supporting information online at www.pnas.org/cgi/content/full/0800404105/DC1.
References
- 1.Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp Immun Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Berman JD. Human Leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:648–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 3.Sah JF, et al. Genetic rescue of Leishmania deficiency in porphyrin biosynthesis creates mutants suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. J Biol Chem. 2002;277:14902–14909. doi: 10.1074/jbc.M200107200. [DOI] [PubMed] [Google Scholar]
- 4.Chang KP, Trager W. Nutritional significance of symbiotic bacteria in two species of hemoflagellates. Science. 1974;183:531–532. doi: 10.1126/science.183.4124.531. [DOI] [PubMed] [Google Scholar]
- 5.Kelly JX, et al. Anti-Leishmanial drug development: exploitation of parasite heme dependency. Mol Biochem Parasitol. 2003;126:43–49. doi: 10.1016/s0166-6851(02)00248-7. [DOI] [PubMed] [Google Scholar]
- 6.Sengupta S, et al. Hb endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J Biol Chem. 1999;274:2758–2765. doi: 10.1074/jbc.274.5.2758. [DOI] [PubMed] [Google Scholar]
- 7.Krishnamurthy G, et al. Hemoglobin receptor in Leishmania is a hexokinase located in the flagellar pocket. J Biol Chem. 2005;280:5884–5891. doi: 10.1074/jbc.M411845200. [DOI] [PubMed] [Google Scholar]
- 8.Zerial M, McBride H. Rab proteins as membrane organizers. Nature Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 9.Goosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 11.Mukhopadhyay A, Barbieri AM, Funato K, Roberts R, Stahl PD. Sequential actions of rab5 and rab7 regulate endocytosis in the Xenopus oocytes. J Cell Biol. 1997a;136:1227–1237. doi: 10.1083/jcb.136.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y, Press B, Wandinger-Ness A. Rab7: An important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay A, Funato F, Stahl PD. Rab7 regulates transport from early to late endocytic compartments in Xenopus oocytes. J Biol Chem. 1997b;272:13055–13059. doi: 10.1074/jbc.272.20.13055. [DOI] [PubMed] [Google Scholar]
- 14.McConville MJ, Mullin KA, Ilgoutz SC, Teasdale RD. Secretory pathway of Trypanosomatid parasites. Microbiol Mol Biol Rev. 2002;66:122–154. doi: 10.1128/MMBR.66.1.122-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field MC, Carrington M. Intracellular membrane transport systems in Trypanosoma brucei. Traffic. 2004;5:905–913. doi: 10.1111/j.1600-0854.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 16.Overath P, Engstler M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Microbiol. 2004;53:735–744. doi: 10.1111/j.1365-2958.2004.04224.x. [DOI] [PubMed] [Google Scholar]
- 17.Morgan GW, Hall BS, Denny PW, Carrington M, Field MC. The kinetoplastida endocytic apparatus. Part I: A dynamic system for nutrition and evasion of host defences. Trends in Parasitol. 2002;18:491–496. doi: 10.1016/s1471-4922(02)02391-7. [DOI] [PubMed] [Google Scholar]
- 18.Pal A, Hall BS, Nesbeth DN, Field HI, Field MC. Differential endocytic functions of Trypanosoma brucei Rab5 isoforms reveal a glycophosphatidylinositol-specific endosomal pathway. J Biol Chem. 2002;277:9529–9539. doi: 10.1074/jbc.M110055200. [DOI] [PubMed] [Google Scholar]
- 19.Robibaro B, et al. Toxoplasma gondii Rab5 enhances cholesterol acquisition from host cells. Cell Microbiol. 2002;4:139–152. doi: 10.1046/j.1462-5822.2002.00178.x. [DOI] [PubMed] [Google Scholar]
- 20.Temesvari L, Rodriguez-Paris J, Bush J, Steck TL, Cardelli J. Characterization of lysosomal membrane proteins of Dictyostelium discioideum: A complex population of acidic integral membrane glycoprotein, rab GTP binding proteins and vacuolar ATPase subunits. J Biol Chem. 1994;269:25719–25727. [PubMed] [Google Scholar]
- 21.Quevillon E, et al. The Plasmodium falciparum family of Rab GTPases. Gene. 2003;306:13–25. doi: 10.1016/s0378-1119(03)00381-0. [DOI] [PubMed] [Google Scholar]
- 22.Singh SB, et al. Rab5-mediated endosome-endosome fusion regulates hemoglobin endocytosis in Leishmania donovani. EMBO J. 2003;22:5712–5722. doi: 10.1093/emboj/cdg557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2:3007.1–3007.7. doi: 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Press B, Feng Y, Hoflack B, Wandinger-Ness A. Mutant Rab7 causes the accumulation of cathepsin D, cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140:1075–1089. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denny PW, et al. Leishmania RAB7: characterization of terminal endocytic stages in an intracellular parasites. Mol Biochem Parasitol. 2002;123:105–113. doi: 10.1016/s0166-6851(02)00133-0. [DOI] [PubMed] [Google Scholar]
- 26.Araripe JR, et al. Trypanosoma cruzi: TcRab7 protein is localized at the Golgi apparatus in epimastigotes. Biochem Biophys Res Commun. 2004;321:397–402. doi: 10.1016/j.bbrc.2004.06.159. [DOI] [PubMed] [Google Scholar]
- 27.Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of rabl and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riederer MA, Soldati T, Shapiro AD, Lin J, Pfeffer SR. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J Cell Biol. 1994;125:573–582. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenmark H, et al. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G, Stahl PD. Structure-function relationship of the small GTPase rab5. J Biol Chem. 1993;268:24475–24480. [PubMed] [Google Scholar]
- 31.Nuoffer C, Davidson HW, Matteson J, Meinkoth J, Balch WE. A GDP-bound of rabl inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol. 1994;125:225–237. doi: 10.1083/jcb.125.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: Friends or foes? Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Wilson ME, Vorhies RW, Anderson KA, Britgan BE. Acquisition of iron from transferrin and lactoferrin by the protozoan Leishmania chagasi. Infect Immun. 1994;62:3262–3269. doi: 10.1128/iai.62.8.3262-3269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gholamhosseinian A, Vassef A. Superiority of hemoglobin to hemin for cultivation of Leishmania tropica promastigotes in serum-free media. J Protozool. 1988;35:446–449. doi: 10.1111/j.1550-7408.1988.tb04128.x. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee K, et al. Live Salmonella recruits NSF on phagosomal membrane and promotes fusion with early endosomes. J Cell Biol. 2000;148:741–753. doi: 10.1083/jcb.148.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy JC. Cultivation of various Leishmania parasites in solid medium. Ind J Med Res. 1932;20:355–367. [Google Scholar]
- 38.Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 39.Hashim S, Mukherjee K, Raje M, Basu SK, Mukhopadhyay A. Live Salmonella modulate expression of rab proteins to persist in a specialised compartment and escape transport to lysosomes. J Biol Chem. 2000;275:16281–16288. doi: 10.1074/jbc.275.21.16281. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharya M, et al. IL-6 and IL-12 specifically regulate the expression of Rab5 and Rab7 via distinct signaling pathways. EMBO J. 2006;25:2878–2888. doi: 10.1038/sj.emboj.7601170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham R, et al. Modulation of immunogenicity and antigenicity of proteins by maleylation to target scavenger receptors on macrophages. J Immunol. 1995;154:1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.