Abstract
Nontyphoidal strains of Salmonella (NTS) are a common cause of bacteremia among African children. Cell-mediated immune responses control intracellular infection, but they do not protect against extracellular growth of NTS in the blood. We investigated whether antibody protects against NTS bacteremia in Malawian children, because we found this condition mainly occurs before 2 years of age, with relative sparing of infants younger than 4 months old. Sera from all healthy Malawian children tested aged more than 16 months contained anti-Salmonella antibody and successfully killed NTS. Killing was mediated by complement membrane attack complex and not augmented in the presence of blood leukocytes. Sera from most healthy children less than 16 months old lacked NTS-specific antibody, and sera lacking antibody did not kill NTS despite normal complement function. Addition of Salmonella-specific antibody, but not mannose-binding lectin, enabled NTS killing. All NTS strains tested had long-chain lipopolysaccharide and the rck gene, features that resist direct complement-mediated killing. Disruption of lipopolysaccharide biosynthesis enabled killing of NTS by serum lacking Salmonella-specific antibody. We conclude that Salmonella-specific antibody that overcomes the complement resistance of NTS develops by 2 years of life in Malawian children. This finding and the age-incidence of NTS bacteremia suggest that antibody protects against NTS bacteremia and support the development of vaccines against NTS that induce protective antibody.
Introduction
Nontyphoidal strains of Salmonella (NTS), principally Salmonella enterica serovars Typhimurium and Enteritidis, are a major but neglected cause of invasive disease in Africa and the commonest cause of bacteremia in Malawi and much of tropical Africa (1–3). In developed countries, NTS infection is mainly foodborne and presents as gastroenteritis, with bacteremia a rare complication often associated with immunodeficiency (4). In Africa, NTS bacteremia particularly occurs in HIV-infected adults (5) and children under 2 years of age, the majority of whom are not HIV infected (1–3). NTS bacteremia frequently occurs in the absence of gastrointestinal symptoms (1, 3), and clinical NTS isolates differ from those found in animal contacts, suggesting human-to-human spread of infection (6). The lack of specific clinical presentation of NTS bacteremia makes diagnosis difficult (1). Even where blood culture facilities and appropriate antibiotics are available, case fatality rates from NTS bacteremia are as high as 24% in children (1, 3), emphasizing the need for an effective vaccine. No vaccine against NTS is currently available for use in humans. The increasing emergence of multidrug resistance to NTS (2) and a lack of new targets for drug development (7) indicate that the absence of NTS vaccines should be urgently addressed. An understanding of the relevant protective immune mechanisms against NTS bacteremia is essential if a vaccine is to be developed in a timely manner.
Immunity against Salmonella is complex (8, 9). Salmonellae are facultative intracellular bacteria that are adapted to survive within macrophages (10). Intracellular survival is essential for virulence in mice (10), and, as for many other intracellular bacteria, cell-mediated immunity is of key importance for control of NTS infection within macrophages (11–13). The majority of studies on immunity to Salmonella have focused on cell-mediated responses. Individuals with defects in the IL-12/23–IFN-γ axis, which is required for macrophage activation, are particularly susceptible to invasive NTS disease (12, 13). However, NTS are also capable of rapid extracellular growth. We hypothesize that in NTS bacteremia in African children, cell-mediated mechanisms fail to contain the intracellular NTS infection, and antibody and complement become critical for preventing extracellular growth of NTS.
Although relatively little attention has been given recently to humoral mechanisms and the role of antibody in immunity to Salmonella, resistance to complement-mediated killing is a recognized virulence trait of Salmonella (14). This resistance seems to be conferred independently by lipopolysaccharide and certain outer membrane proteins, in particular a 17-kDa protein encoded by the resistance to complement killing (rck) gene (15). Smooth strains of Salmonella with long-chain lipopolysaccharide are less susceptible to serum bactericidal activity than are rough strains (16), whose lipopolysaccharide lacks polysaccharide side chains. Lipopolysaccharide of S. Typhimurium activates complement to a lesser degree than does lipopolysaccharide of S. Enteritidis (17).
There is evidence of a role for both bactericidal and opsonizing antibody in immunity to Salmonella (14, 18). S. Typhi polysaccharide vaccines that produce T cell–independent antibody induce protection in humans (19). Limited information on prototype NTS vaccines and their antibody responses is available in humans, but in mice, protection induced by heat-killed salmonellae correlates with anti-Salmonella antibody titer (20). Adoptive transfer studies have found that optimal protection against Salmonella in mice is conferred by antibody and T cells (21, 22). Despite rapid uptake of Salmonella by the spleen and liver during murine systemic salmonellosis, there is a chronic low-grade bacteremia that can become uncontrolled and cause death (23). Antibody against Salmonella has recently been shown to markedly reduce murine bacteremia as well as to prevent primary infection and impede hematogenous spread of NTS (24).
If systemic salmonellosis in the mouse is analogous to life-threatening NTS bacteremia in African children, targeting the immune response to control extracellular NTS growth would be expected to reduce mortality from this disease. Here we examined the potential role of antibody and complement in the control of NTS bacteremia in African children.
Results
Age distribution of NTS bacteremia among Malawian children.
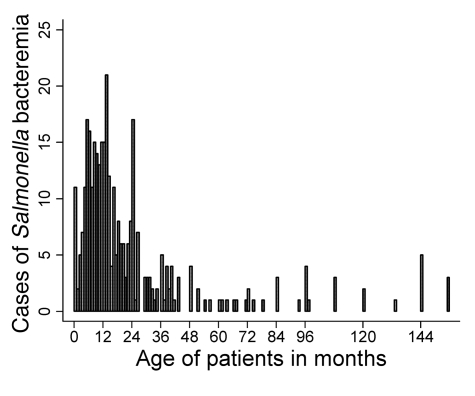
The age distribution of the 352 Malawian children admitted to Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi, with NTS bacteremia during the 1-year study period is shown in Figure 1. Ages were not available for 10 children. The median age was 13 months (interquartile range, 8 to 24 months). Of the children studied, 82% were less than 36 months old. Among those 2 years of age and younger, fewer were aged less than 4 months than would be expected per uniform distribution (actual, 9.7%; expected,16.7%; P = 0.02). S. Typhimurium was isolated from 319 (90.6%) children, and S. Enteritidis from 30 (8.5%). Only 3 isolates (<1%) were not serovar Typhimurium or Enteritidis: 1 S. Bovis-morbificans, 1 S. Sundsvall, and 1 untyped Salmonella. During the same period, S. Typhi was cultured from the blood of 7 children admitted to QECH.
Figure 1. Cases of NTS bacteremia in Malawian children.
Shown are 352 cases of consecutive blood culture–confirmed NTS bacteremia by age in children presenting to QECH between August 1, 2003, and July 31, 2004 (ages not available for 10 children).
Susceptibility of NTS to in vitro killing by control Malawian adult blood and serum.
All 8 S. Typhimurium isolates tested were killed by whole blood from each of 4 healthy Malawian adults, with an overall mean log10 kill of 1.83 at 180 minutes (individual assays ranged 1.11–2.57 log10 kill; Figure 2A). The isolates were also susceptible to killing by serum from each of the 4 adults. Their serum killed the bacteria more effectively than did whole blood, and washed blood cells failed to kill (Figure 2B). We confirmed that each sample of adult blood contained anti-Salmonella IgG and IgM by flow cytometry. Fluorescence microscopy studies showed no evidence for agglutination being responsible for the decreases in bacterial colony-forming units observed in these assays. This was confirmed by our finding of no reduction in bacterial counts when salmonellae were incubated in heat-inactivated serum for 5 minutes.
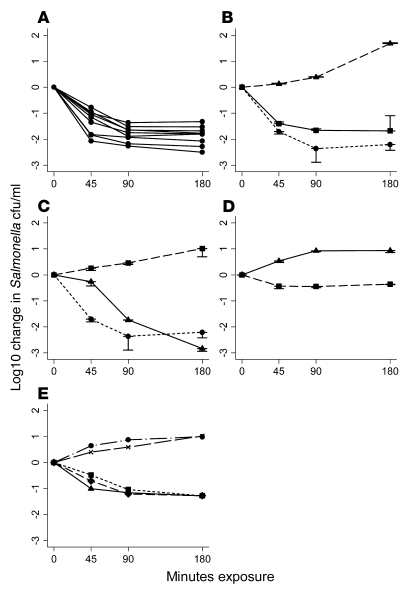
Figure 2. Killing of NTS by blood and serum from Malawian adults and by C9-deficient serum at 45, 90, and 180 minutes.
(A) Killing of 8 invasive Malawian S. Typhimurium isolates by whole blood from a healthy adult Malawian donor. (B) Killing of S. Typhimurium isolate D23580 by whole blood (squares), serum (circles), and blood cells washed in RPMI to remove antibody and complement (triangles). (C) Effect of heat inactivation of serum on ability to kill D23580: fresh serum (circles), heat-inactivated serum (squares), and heat-inactivated serum reconstitution with lyophilized complement (triangles). (D) Inability of C9-deficient serum (triangles) to kill D23580. Killing of Salmonella was enabled by addition of exogenous C9 (squares). (E) Killing of D23580 by fresh and heat-inactivated serum: 100% fresh (triangles); 60% fresh, 40% heat-inactivated (diamonds); 20% fresh, 80% heat-inactivated (squares); 10% fresh, 90% heat-inactivated (circles); and 100% heat-inactivated (x). Negative values correspond with a decrease in viable salmonellae compared with the initial concentration of 106 salmonellae/ml. In A, B, C, and E, data indicate blood and/or serum from 1 healthy adult and are representative of 4 healthy adult donors tested. Where error bars are present, data are mean ± 1 SD of 3 experiments.
In vitro killing of NTS by serum is complement dependent.
Complement inactivation by heating to 56°C for 30 minutes destroyed the bactericidal activity of serum, which was restored with lyophilized complement (Figure 2C). To prove that Salmonella killing was complement dependent, serum bactericidal assays were performed using C9-deficient serum. No bactericidal activity was observed, but Salmonella killing was induced by the addition of exogenous C9 at normal physiological concentrations (60 μg/ml; Figure 2D). We confirmed that the C9-deficient serum contained anti-Salmonella antibody. This finding not only confirmed the requirement for complement in killing of NTS by serum, but also indicated that Salmonella killing is mediated by the membrane attack complex, which is formed by the polymerization of C9 as the final event in the complement terminal pathway. Serum bactericidal assays were performed with different proportions of fresh and heat-inactivated serum and showed that Salmonella killing occurred with 20% fresh serum and 80% heat-inactivated serum, but was impaired with 10% fresh serum and 90% heat-inactivated serum (Figure 2E).
NTS-bactericidal activity of blood from Malawian children.
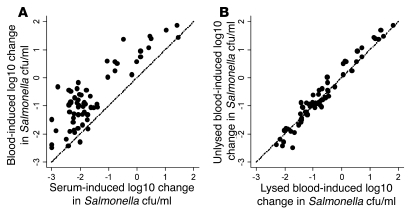
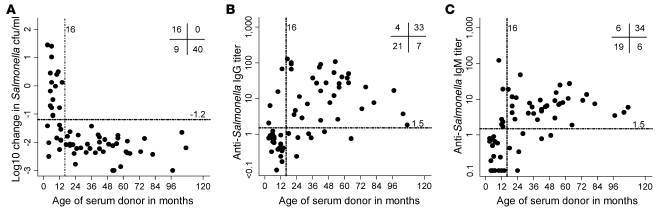
Killing of Salmonella by serum from Malawian children correlated with killing by whole blood (Spearman r = 0.66; 95% confidence interval [CI], 0.50 to 0.78), although killing by serum (mean log10 kill, 1.59) was greater than that by whole blood (mean log10 difference, 0.92; Figure 3A). Because salmonellae are capable of intracellular survival, we tested whether lysis of blood cells could affect the recovery of Salmonella in the whole-blood assay; this resulted in no increase in the number of viable salmonellae sampled after 45, 90, or 180 minutes’ incubation (Figure 3B). The ability of sera to kill S. Typhimurium isolate D23580, a typical and representative Malawian invasive NTS isolate, was dependent on donor age (Figure 4A). All sera from children older than 16 months effected a greater than 1.2 log10 kill at 180 minutes (designated “normal kill”), whereas sera from 9 of 25 (36%) children less than 16 months of age achieved this. Hemolytic complement function was normal for all sera regardless of capacity to kill Salmonella. Median total pathway (670 CH100 U/ml) and alternative pathway (100% normal) hemolytic complement activity were the same for sera with normal and impaired killing.
Figure 3. Killing of NTS by whole blood and serum from Malawian children.
In vitro killing of S. Typhimurium isolate D23580 (initial concentration 106 salmonellae/ml) at 180 minutes by (A) whole blood compared with serum and (B) whole blood with test aliquot lysed or unlysed prior to determination of viable salmonellae. Paired blood and serum are from 65 healthy Malawian children. Each point corresponds to blood and serum from 1 child. Lines of equivalence are shown.
Figure 4. Killing of NTS and anti-Salmonella IgG and IgM titers for serum from Malawian children.
(A) In vitro killing of S. Typhimurium isolate D23580 at 180 minutes (initial concentration 106 salmonellae/ml), (B) anti-Salmonella D23580 IgG levels, and (C) anti-Salmonella D23580 IgM levels for sera from 65 healthy Malawian children compared with age. Each point corresponds to serum from 1 child. Numbers at top right denote the number of points in the indicated quadrants, as divided by patient age (more or less than 16 months) and normal or impaired killing (more or less, respectively, than 1.2 log10 kill or 1.5 U Ig titer as appropriate).
Antibody and NTS killing by sera from Malawian children.
Median anti-Salmonella IgG levels were much lower in sera from children less than 16 months old than in sera from older children (0.76 versus 14.2 U; Figure 4B); the same was observed for median anti-Salmonella IgM levels (0.58 versus 6.28 U; Figure 4C). All sera with more than 1.5 U anti-Salmonella IgG effected normal killing of Salmonella, while 57% of sera with less than 1.5 U failed to kill (Figure 5A). Similarly, for anti-Salmonella IgM, all sera with more than 1.5 U anti-Salmonella IgM effected normal killing, while 64% of sera with less than 1.5 U failed to kill (Figure 5B). All sera with impaired Salmonella killing had less than 1.5 U of both specific IgG and IgM.
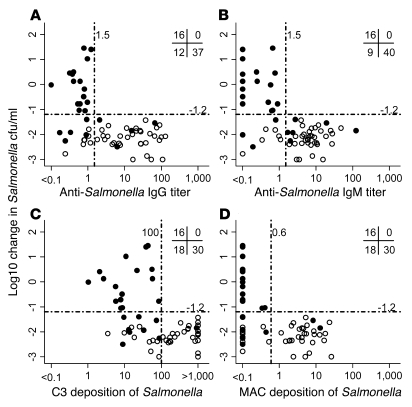
Figure 5. Killing of NTS by serum from Malawian children compared with anti-Salmonella IgG and IgM titers and complement C3 and membrane attack complex deposition in vitro killing of S. Typhimurium isolate D23580 by serum at 180 minutes.
(A and B) NTS killing compared with specific anti-Salmonella D23580 IgG (A) and IgM (B) levels. (C and D) NTS killing compared with complement C3 (C) and C5b-9 membrane attack complex (MAC; D) deposition on D23580. The initial concentration was 106 salmonellae/ml. Each point corresponds to serum from 1 child, either less than (filled circle) or more than (open circle) 16 months of age. Numbers at top right denote the number of points in the indicated quadrants, as divided by threshold for normal killing (more or less than 1.5 U Ig, 100 U C3, and 0.6 U C5b-9 membrane attack complex) and normal or impaired killing (more or less, respectively, than 1.2 log10 kill).
Four children had anti-Salmonella IgG (>1.5 U) but lacked anti-Salmonella IgM, while 8 children had anti-Salmonella IgM (>1.5 U) but not anti-Salmonella IgG. All 12 of these sera killed Salmonella normally, indicating that both specific IgG and IgM antibodies can induce bactericidal activity in the presence of complement. Above 1.5 U, the degree of Salmonella killing was not augmented by greater levels of anti-D23580 antibody. In addition, 4 of 65 sera samples (6%) had less than 1.5 U of both IgG and IgM but were still able to kill D23580 normally, suggesting that either our antibody assay was insufficiently sensitive to detect Salmonella-specific antibody in these sera or that another factor was responsible for activating complement.
Deposition of complement on NTS by sera from Malawian children.
Normal killing of Salmonella by serum occurred consistently above a threshold deposition of 100 U C3 (Figure 5C) and 0.6 U membrane attack complex (Figure 5D). The deposition of C3 and membrane attack complex correlated with levels of specific IgG (C3, Spearman r = 0.78, 95% CI, 0.67 to 0.86; membrane attack complex, Spearman r = 0.83, 95% CI, 0.73 to 0.89) and IgM (C3, Spearman r = 0.72, 95% CI, 0.57 to 0.82; membrane attack complex, Spearman r = 0.72; 95% CI, 0.58 to 0.82). The deposition of membrane attack complex correlated with C3 deposition (Spearman r = 0.79; 95% CI, 0.68 to 0.87). The apparent lack of complement deposition by some bactericidal sera is probably the consequence of the shorter serum incubation in the complement assays (20 minutes) than in the serum bactericidal assay (180 minutes).
Alternative pathway complement activation is insufficient for NTS killing.
The finding of normal total and alternative pathway hemolytic complement function and absence of Salmonella-specific antibody in the sera of children with impaired Salmonella killing strongly suggests that complement alone through alternative pathway activation is insufficient for NTS killing. To confirm this, we performed serum bactericidal assays using control adult serum (containing anti-Salmonella antibody) in which activation of the classical and mannose-binding lectin pathways of complement was blocked by addition of EGTA, at a final concentration of 10 mM, to chelate calcium ions. Calcium is required for classical and mannose-binding lectin pathway activity, but not activation of complement through the alternative pathway. MgCl2 at a final concentration of 10 mM was also added to serum to ensure sufficient magnesium ions for alternative pathway activation. Addition of EGTA combined with MgCl2 prevented the killing of NTS by control serum (Figure 6A).
Figure 6. Effect of the EGTA/MgCl2 combination, anti-Salmonella IgG, and mannose-binding lectin on killing of NTS by control adult serum and child serum lacking specific antibody to Salmonella.
In vitro killing of S. Typhimurium isolate D23580 by serum was determined at 45, 90, and 180 minutes (initial concentration, 106 salmonellae/ml). (A) Killing of Salmonella by control adult serum (squares) and inhibition of killing by addition of 10 mM EGTA and MgCl2 (triangles). (B) Absence of killing of Salmonella by child serum lacking Salmonella-specific antibody (squares). Addition of 0.2 g/l exogenous IgG containing anti-Salmonella antibody enabled killing of Salmonella in child serum lacking Salmonella-specific antibody (triangles), but not in heat-inactivated serum (circles). (C) Inability of child serum lacking Salmonella-specific antibody to kill Salmonella (squares) was unaffected by addition of 5 μg/ml exogenous mannose-binding lectin (triangles). Data are mean ± 1 SD of 3 experiments.
Antibody is required for in vitro killing of NTS.
The association of anti-Salmonella antibody with in vitro killing of NTS by sera from Malawian children strongly suggests that antibody is necessary for killing to occur. To confirm this, total IgG (12.5 g/l concentration) was isolated from control adult serum containing anti-Salmonella IgG (284 U by flow cytometric assay) using Streptococcal protein G. The purified IgG was added to child serum that lacked Salmonella-specific antibody at final concentrations of 1.0 g/l and 0.2 g/l. At both concentrations of exogenous IgG, the child serum effected normal killing of Salmonella. Addition of purified total IgG to heat-inactivated serum did not result in Salmonella killing, indicating that antibody and complement together are required for in vitro killing (Figure 6B).
Salmonella killing was also possible when child serum lacking antibody to Salmonella was supplemented with IgG in the form of Gammagard and Sandoglobulin commercial intravenous Ig preparations. We used the flow cytometric antibody assay to confirm the presence of anti-Salmonella IgG and the absence of anti-Salmonella IgM in total IgG purified from control adult serum using Streptococcal protein G and both intravenous Ig preparations. This indicates that IgG can activate complement-mediated killing of NTS in the absence of IgM. Finally, child serum lacking anti-Salmonella antibody effected normal killing of Salmonella when provided with anti-Salmonella antibody by adding heat-inactivated control adult serum at a 1:9 ratio of heat-inactivated adult serum to child serum.
Mannose-binding lectin cannot replace the role of antibody for in vitro killing of NTS.
The possibility that serum killing of NTS can take place in the absence of specific antibody through the mannose-binding lectin pathway of complement activation was investigated by adding purified mannose-binding lectin to child serum lacking Salmonella-specific antibody at final concentrations of 1 μg/ml (average serum concentration of mannose-binding lectin) and 5 μg/ml. No killing of Salmonella was observed at either concentration (Figure 6C).
Molecular characterization of invasive Malawian NTS isolates.
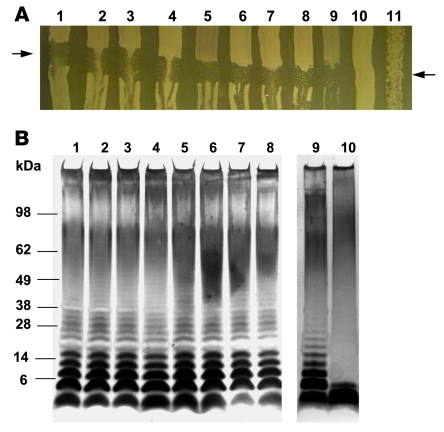
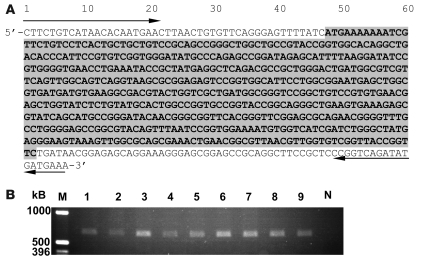
All 8 Salmonella strains studied were morphologically smooth, susceptible to P22 bacteriophage lysis (indicating intact surface lipopolysaccharide), and had long-chain lipopolysaccharide (Figure 7). PCR experiments showed amplified products of the predicted size for the rck amplicon (15) in all 8 isolates (Figure 8).
Figure 7. P22 bacteriophage sensitivity and lipopolysaccharide profiles of invasive NTS isolates from Malawi.
(A) P22 bacteriophage sensitivity of 8 Malawian clinical isolates of S. Typhimurium from bacteremic children (lanes 1–8) compared with smooth S. Typhimurium strain SL1344 (lane 9), E. coli strain C600 (lane 10), and rough S. Typhimurium strain BA85 (lane 11). P22 was streaked across LB agar between the arrows and allowed to dry before the bacterial strains were cross-streaked. The plate was left overnight at 37°C before photographing. (B) Comparison of lipopolysaccharide profiles of 8 Malawian clinical S. Typhimurium isolates from bacteremic children (lanes 1–8) with laboratory smooth (SL1344; lane 9) and rough (BA85; lane 10) strains of S. Typhimurium. Lipopolysaccharide was prepared from S. Typhimurium and separated by SDS-PAGE prior to silver staining.
Figure 8. The rck gene of invasive NTS isolates from Malawi.
(A) Partial nucleotide sequence of 120-kb plasmid of D23580 demonstrating the 555-bp sequence (gray shading) of rck (15) and oligonucleotide primer sequences used to PCR amplify across the rck gene (arrows). (B) Predicted 668 bp product, as demonstrated by 1% agarose gel, of amplification shown in A in all 8 Malawian S. Typhimurium clinical isolates (lanes 1–8) and 1 Malawian clinical S. Enteritidis isolate (lane 9). M, marker; N, negative control.
NTS lipopolysaccharide resists complement-mediated killing in the absence of specific antibody.
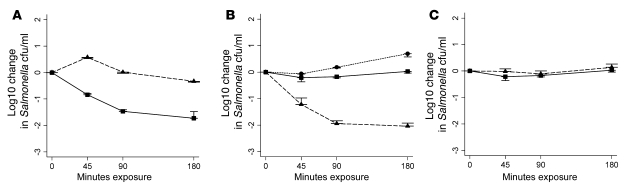
To investigate the importance of lipopolysaccharide in the resistance of invasive Malawian NTS to serum killing, serum bactericidal assays were performed using a deep rough galE mutant of D23580 with control adult serum, child serum lacking Salmonella-specific antibody, heat-inactivated adult serum, and C9-deficient adult serum. As expected, and in common with the parent wild-type strain, rough D23580 was susceptible to in vitro killing by adult serum. Whereas wild-type D23580 was resistant to killing by serum lacking Salmonella-specific antibody, the rough mutant of D23580 was susceptible. Compared with the sensitivity of the smooth parent strain to adult serum, the rough D23580 mutant was exquisitely sensitive to serum killing, with a log10 kill of 3.0 occurring within 45 minutes even when anti-Salmonella antibody was absent (Figure 9A). Rough D23580 resisted killing in heat-inactivated and C9-deficient serum, but full killing was induced in the latter by addition of exogenous C9. This finding confirms that serum killing of rough D23580 is mediated through the complement membrane attack complex. Killing still occurred in the presence of 10 mM EGTA combined with MgCl2, indicating that complement activation occurs through the alternative pathway (Figure 9B).
Figure 9. Killing of smooth wild-type NTS and a rough NTS mutant by Malawian adult and child serum, heat-inactivated serum, and C9-deficient serum.
In vitro killing by serum of smooth wild-type S. Typhimurium D23580 and a deep rough mutant of D23580 with disrupted galE gene at 45, 90, and 180 minutes (initial concentration, 106 salmonellae/ml). (A) Killing of mutant rough D23580 by adult serum (circles) and child serum lacking Salmonella-specific antibody (diamonds) and of wild-type smooth D23580 by adult serum (squares) and child serum lacking Salmonella-specific antibody (triangles). (B) Killing of rough D23580 by child serum lacking Salmonella-specific antibody (squares) was maintained in the presence of 10 mM EGTA and MgCl2 (triangles). Killing of rough D23580 did not occur in heat-inactivated serum (circles) or C9-deficient serum (diamonds), but addition of exogenous C9 to C9-deficient serum induced killing (x). Data are mean ± 1 SD of 3 experiments.
Discussion
NTS bacteremia is a common problem in African children. NTS have consistently been the commonest cause of bacteremia among children at QECH since a blood culture service for the hospital began at the Malawi-Liverpool-Wellcome Trust Research Programme in 1997. In this study we report 352 cases of pediatric NTS bacteremia in a 1-year period, emphasizing the extent of the problem.
In keeping with a previous review (1), we found that NTS bacteremia was most common among children less than 3 years of age. We also found a relative absence of NTS bacteremia among children aged 1–4 months, which is consistent with protection conferred by maternal IgG. In infants, a nadir of antibody levels occurs as maternal antibody levels wane (25), just prior to the start of the peak of NTS bacteremia cases. The frequency of cases declines in children older than 16 months. This age profile of NTS bacteremia is similar to that for invasive disease caused by pathogenic extracellular bacteria, such as meningococcus, in which antibody is known to be important for immunity (26). The peak of NTS bacteremia cases occurs at an age when a majority of Malawian children have impaired bactericidal activity against NTS and lack Salmonella-specific antibody, suggesting the importance of humoral mechanisms and antibody for immunity against NTS.
The whole-blood assay we developed provides an in vitro model of NTS bacteremia. Because serum kills NTS more effectively than whole blood, humoral factors rather than phagocytes are primarily responsible for NTS killing in blood. We showed that antibody-mediated agglutination was not responsible for an artifactual fall in viable bacterial counts, by microscopic examination and by briefly incubating salmonellae in heat-inactivated serum as proposed by Taylor (14). The possibility that a substantial proportion of viable bacteria were located intracellularly within phagocytic cells in the whole-blood assay was excluded, because there was no increase in viable counts when blood cells were lysed at each time point prior to viable count determination.
The removal of Salmonella killing by heat inactivation of complement and restoration of killing by addition of exogenous complement indicates that NTS killing is complement dependent. This was confirmed by our experiments with C9-deficient serum with and without addition of exogenous complement, the results of which indicate that the mechanism of killing is through complement membrane attack complex formation. A small number of cases of Salmonella disease has been described in association with deficiency of C2 and C4 components of complement (9, 27). As killing occurs with as little as 20% normal complement levels, complement availability is unlikely to be a significant factor in susceptibility to NTS bacteremia unless there is a genetic complement deficiency, high levels of complement consumption, or impaired production of complement.
In the present study, we have shown that in Malawian children, anti-Salmonella IgG and IgM antibody titers and bactericidal activity of serum against NTS rose with age, corresponding with a fall in cases of NTS bacteremia (Figure 10). All sera with anti-Salmonella IgG or IgM effected normal NTS killing in the presence of complement, and anti-Salmonella IgG and IgM were both absent from all sera with impaired NTS killing. These findings strongly suggest that antibody is the determinant of whether NTS killing by serum occurs. Previously, IgM has been shown to have more ability to activate complement on S. Typhimurium than IgG (28), but because we found substantial correlation between anti-Salmonella IgG and IgM levels (Spearman r = 0.73; 95% CI, 0.59 to 0.83), we were unable to determine the relative importance of these Ig isotypes from the assays performed. However, because sera of 16 of 65 Malawian children contained either anti-Salmonella IgG in the absence of anti-Salmonella IgM or anti-Salmonella IgM without anti-Salmonella IgG yet were capable of killing NTS, we conclude that either of Salmonella-specific IgG or IgM is sufficient to kill NTS in the presence of complement.
Figure 10. Age-based comparison of healthy Malawian children with cases of NTS bacteremia in Malawian children: serum bactericidal activity and serum anti-Salmonella IgG and IgM titers.
Serological data are from 65 Malawian children as shown in Figure 4 grouped by age (0–6, 6–12, 12–18, and 18–24 months; 2–3, 3–4, 4–5, and 5–10 years). Shown are mean and 95% CI serum bactericidal activity against S. Typhimurium isolate D23580 at 180 minutes (solid line) and geometric mean and 95% CI anti-D23580 IgG (short dashes) and IgM (long dashes) titers. Below, age distribution of NTS bacteremia cases is reproduced from Figure 1 for reference.
Correlation between complement deposition and antibody titer supported antibody-dependent complement-mediated killing as the mechanism of bactericidal activity. Low titers of C3 deposition were insufficient for killing, which suggests a threshold level of C3 deposition to drive stable membrane attack complex formation on NTS. Because no child studied had a history of known Salmonella infection or vaccination, it is likely that their NTS-specific antibody is usually generated in response to subclinical infection or colonization by commensal intestinal bacteria (29) with common antigenic epitopes. However, because most cases of diarrhea are managed at health centers in Blantyre and stool culturing is not routinely available, diagnosis of NTS infection is limited to bacteremic children admitted to hospitals. In Malawi and elsewhere in Africa, childhood diarrhea is a common condition, and we estimate that about 5% of cases are caused by NTS. The presence of IgM anti-Salmonella antibody suggests that ongoing subclinical infection is common in African children or that a subpopulation of B cells do not class switch and give rise to long-term IgM production.
The observation that all child sera studied had normal functional complement activity (both total and alternative pathway), regardless of whether they were able to kill Salmonella, indicates that although necessary for bactericidal activity, alternative pathway complement alone is insufficient for killing invasive Malawian NTS. Where microorganisms are susceptible to killing by complement alone, complement activation occurs via the magnesium-dependent alternative pathway. Further support for the inability of complement in isolation to kill NTS was provided by the inhibition of NTS killing in control adult serum by the combination of EGTA and MgCl2, which blocked the classical and mannose-binding lectin pathways of activation while preserving the alternative pathway.
We also confirmed the requirement for antibody in serum killing of NTS by adding total IgG isolated from control adult serum (containing Salmonella-specific IgG) to child serum lacking antibody to NTS. This serum was then able to kill NTS normally through activation of the classical complement pathway. It has previously been observed that a small amount of anti-Salmonella antibody is required to enable complement-mediated killing of Salmonella (30). The final concentration of 0.2 g/l exogenous IgG added to the child serum represented an approximately 60-fold dilution of the total IgG in the donor adult serum and equated to a final specific anti-Salmonella IgG titer of approximately 5 U, as determined by our flow cytometric assay. This is consistent with our observed minimum requirement of 1.5 U anti-Salmonella IgG to ensure normal serum bactericidal activity.
IgG provided in the form of heat-inactivated control adult serum and 2 intravenous Ig preparations from pooled plasma from donors in the developed world also enabled Salmonella antibody–deficient serum to kill NTS. The latter finding indicates that commercial intravenous Ig and the donor plasma from which it is fractionated contains antibody against invasive African NTS strains; we confirmed this with our anti-Salmonella antibody assay. This finding raises the possibility that intravenous Ig could have therapeutic potential in the management of NTS bacteremia in children lacking specific antibody to Salmonella. A clinical trial of intravenous Ig for the treatment of S. Typhimurium infection among preterm neonates in Turkey found that intravenous Ig with antibiotics reduced mortality and complications compared with antibiotics alone (31).
A third pathway of complement activation is via binding of mannose-binding lectin to bacterial surfaces. The finding of reduced levels of mannose-binding lectin in Gram-negative bacterial infections compared with Gram-positive infections suggests a role for mannose-binding lectin in such infections (32). However, addition of purified mannose-binding lectin to anti-Salmonella antibody–deficient serum failed to induce bactericidal activity against NTS, which indicates that mannose-binding lectin cannot replace antibody in activating bactericidal complement activity against NTS. This is consistent with the laboratory finding that expression of intact lipopolysaccharide on S. Typhimurium prevents binding by mannose-binding lectin (33).
The similar degree of susceptibility to in vitro killing of all 8 Malawian NTS strains examined by blood from healthy Malawian adults suggests homogeneity among these strains. This is supported by the finding that these strains shared 2 XbaI endonuclease-digest pulsed-field gel electrophoresis patterns differing by 1 band and by these patterns being common to greater than 90% invasive Malawian S. Typhimurium isolates obtained during a 1-year period in 2003 and 2004 (C.L. Msefula, unpublished observations). In contrast, previous studies of Salmonella and other Gram-negative bacteremias have found varying serum-susceptibility among clinical isolates (34, 35).
The 8 NTS isolates studied all possessed long-chain lipopolysaccharide and rck, yet all were susceptible to killing by adult serum. The lack of NTS killing and complement deposition in serum from children without anti-Salmonella antibody strongly suggests that long-chain lipopolysaccharide and rck only confer protection against complement in the absence of antibody. To test the hypothesis that lipopolysaccharide protects against complement in these invasive isolates, we generated a deep rough mutant of S. Typhimurium D23580 by disrupting the galE gene. The rough mutant was exquisitely sensitive to serum killing, with a 3.0 log10 kill in 45 minutes using anti-Salmonella antibody–deficient serum. The finding that specific antibody was not necessary for this dramatic killing strongly supports the concept that lipopolysaccharide confers protection against complement, and further support comes from the finding that this killing activity was not inhibited by the combination of EGTA and MgCl2. The 3.0 log10 kill at 45 minutes was also observed with serum containing Salmonella-specific antibody. This contrasts with the 1–2 log10 kill of wild-type D23580 at 180 minutes and indicates that although lipopolysaccharide does not fully protect against antibody-dependent complement-mediated killing, it provides some protection that may be important for hematogenous spread of NTS between macrophages (24, 36).
In the 1980s, Joiner showed that when complement membrane attack complex forms on lipopolysaccharide on the surface of salmonellae, it can be cleaved off (37) and thus fail to insert into the bacterial outer membrane (38). In addition, rck is associated with abnormal polymerization of C9 in the membrane attack complex (39). In view of our present findings, we propose that antibody overcomes the protective effects of lipopolysaccharide and rck in invasive African NTS strains by directing complement deposition to sites on the bacterial membrane where it can polymerize effectively and kill without being removed (40, 41). Through this mechanism, the adaptive immune response could protect against NTS bacteremia.
The imperative clinical application of our findings is in vaccine development. The demonstration of the need for specific anti-Salmonella antibody for activation of bactericidal complement provides a rationale for the development of vaccines that induce protective antibody against NTS. The pressing challenge is to identify the nature of the targets of bactericidal antibody; this is the focus of our ongoing work. Our studies to date suggest that over 90% of invasive Malawian S. Typhimurium isolates (serogroup B) are susceptible to a 1.2 log10 kill in control adult serum, but that S. Enteritidis strains (serogroup D) are susceptible to a lesser degree of killing (C.A. MacLennan, unpublished observations).
It will be important for vaccine development to identify appropriate antibody targets common to serovars Typhimurium and Enteritidis. In the present study, over 99% of invasive NTS isolates comprised these 2 serovars, and their dominance among NTS strains is common elsewhere in tropical Africa (3). S. Typhi accounts for only 1% of invasive Salmonella disease at QECH, and vaccines, including the antibody-inducing Vi vaccine, already exist to protect against infection with this organism. Because intramacrophage infection is an important feature of Salmonella infection and is likely to be responsible for the common finding of NTS bacteremia recrudescence among African adults infected with HIV (5), a successful vaccine against NTS should produce both antibody and T cell immunity.
The susceptibility of invasive NTS isolates to antibody-dependent complement-mediated killing is consistent with the epidemiological finding that NTS bacteremia predominantly affects children under 3 years of age and rarely occurs in immunocompetent adults. From a pathogen survival perspective, NTS may best be served by having limited serum resistance. As facultative intracellular pathogens, salmonellae — in common with mycobacteria — may establish persistent latent infection within the macrophage beds of the spleen and liver. In this scenario, occasional seeding of Salmonella into the circulation is likely, and it is to the advantage of the pathogen to ensure that death of the host does not result. Although individuals with isolated defects of the IL-12/23–IFN-γ axis are unable to control intracellular Salmonella infection, none of these has been reported to have died from Salmonella infection (13). This observation may be attributable to the presence of normal anti-Salmonella antibody in these individuals.
Characteristically, salmonellae are intracellular pathogens, and the importance of cell-mediated immunity against intracellular infection has been firmly established (8–13). Nevertheless, bacteremia is the major threat posed by NTS to African children and is likely to involve extracellular bacterial growth, which cannot be prevented by cellular immune mechanisms. Our findings indicate that antibody specific to NTS develops in most African children during the second year of life, coinciding with a decline in cases of NTS bacteremia (Figure 10). This antibody is capable of activating complement-mediated killing of NTS even at low titers (30). These findings support an important role for antibody in control of extracellular growth of NTS and prevention of NTS bacteremia in African children. They provide a rationale for the development of a vaccine that accelerates the natural process of antibody acquisition against NTS.
Methods
Study approval.
The study was approved by the College of Medicine Research and Ethics Committee, College of Medicine, University of Malawi, and by the ethics committee of the Liverpool School of Tropical Medicine. Informed consent was obtained from all participants or their parents or guardians.
Clinical records.
We retrospectively examined the records of 352 consecutive cases of blood culture–proven NTS bacteremia in children admitted to QECH between August 1, 2003, and July 31, 2004. Blood cultures were taken in sick, febrile children with suspected septicemia. The age profile of children with NTS bacteremia was determined.
Study population.
Blood samples were taken from 65 healthy Malawian children (median age, 24 months; range, 3–107 months; 2 HIV infected) attending surgical outpatient clinics at QECH and Beit Cure International Hospital (Blantyre, Malawi), so that at least 6 children were recruited into each of the following 8 age groups: 0–6 months, 6–12 months, 12–18 months, 18–24 months, 2–3 years, 3–4 years, 4–5 years, and 5–10 years. No child had a previous history of known Salmonella infection or vaccination.
Materials.
Unless otherwise stated, chemicals and reagents were obtained from Sigma-Aldrich. Gammagard and Sandoglobulin intravenous Ig preparations were from Baxter and ZLB Bioplasma, respectively. C9-deficient serum and purified C9 were a gift from B.P. Morgan (Cardiff University, United Kingdom). Purified mannose-binding lectin was a gift from R. Read (University of Sheffield, Sheffield, United Kingdom).
Salmonella killing assays.
We developed whole blood and serum bactericidal assays to investigate in vitro killing of S. Typhimurium, the commonest serovar in our bacteremia series. We first assessed the sensitivity of 8 randomly selected Malawian S. Typhimurium blood culture isolates from 2004 using control blood and serum from 4 healthy Malawian adults. Isolate D23580, from a 26-month-old female patient, was selected for further studies based on having intermediate sensitivity to serum killing compared with the other 7 isolates and having a genotype by pulse-field electrophoresis common to over 90% of S. Typhimurium isolates from Malawi tested to date (C.L. Msefula, unpublished observations).
Killing of D23580 was assessed using (a) heparinized blood (4 IU/ml sodium heparin) within 2 hours of venesection; (b) serum separated within 2 h and stored at –80°C — complement was inactivated by heating serum to 56°C for 30 minutes where required; or (c) blood-cell-suspensions, prepared by washing blood twice with RPMI-1640 medium (Invitrogen) to remove antibody and complement before resuspending in RPMI to the original blood volume. Viable salmonellae (2 × 105 in 20 μl), prepared according to the recommendations of Taylor (14), were added to 180 μl of 100% whole blood, undiluted serum, or blood-cell-suspensions to give a final Salmonella concentration of 1 × 106/ml regardless of white cell count. These mixtures were then incubated at 37°C on a rocker plate at 20 rpm. Numbers of viable salmonellae were determined after 45, 90, and 180 minutes by serial dilution of 25 μl of the mixture plated in triplicate on Luria Bertani (LB) agar.
To assess the possibility of antibody-mediated agglutination of salmonellae contributing to an apparent decline in viable bacterial numbers, aliquots were taken from serum bactericidal assays at 5, 45, 90, and 180 minutes. These were stained with acridine orange and examined by fluorescence microscopy. No antibiotics were used in any of our assays. Pilot studies showed that after 180 minutes in serum bactericidal assays and whole blood bactericidal assays, the number of salmonellae tended to increase. This is likely the result of complement degradation and/or consumption. Where required, blood cells were lysed with distilled autoclaved water for 1 minute immediately prior to determining viable bacterial numbers. Flow cytometric analysis and viable bacterial count determination showed that this method lysed all blood cells, without affecting the number of viable salmonellae recovered.
Flow cytometric assays for Salmonella-specific Igs and complement deposition; functional complement assays.
Flow cytometric assays were developed to determine Salmonella-specific IgG and IgM antibody titers and complement C3 and membrane attack complex deposition on Salmonella strain D23580. Salmonellae (1 × 107) in 5 μl PBS were incubated for 20 minutes with 45 μl of serum diluted 1:10 in PBS for antibody determination, or 45 μl undiluted serum for complement deposition (final Salmonella concentration, 2 × 108/ml). Dilution of serum for the antibody assay was necessary because a prozone effect was observed at dilutions of less than 1:5. The salmonellae were then washed twice in PBS and incubated for 15 minutes with FITC-conjugated polyclonal rabbit anti-human IgG or IgM antibody (Sigma-Aldrich), FITC-conjugated anti-C3 antibody (Dako), or a mouse monoclonal antibody against a neoepitope of C5b-9 membrane attack complex (Dako). In the C5b-9 assay, a third incubation step was performed using FITC-conjugated polyclonal rabbit anti-mouse Ig antibody (Dako). Salmonellae were given a further 2 washes in PBS and resuspended in PBS plus 1% formaldehyde.
Sample data were acquired on a FACSCalibur flow cytometer (BD Biosciences). Salmonellae (20,000 events per assay) were identified by light scatter characteristics and a rabbit polyclonal anti-Salmonella antibody (Difco). Salmonella-specific Ig and complement deposition titers were calculated as the FITC geometric mean fluorescence intensity (GMFI). Hemolytic complement function was determined by radial immunodiffusion (Binding Site).
Purification of total IgG from serum.
Total IgG was isolated from control adult serum containing anti-Salmonella IgG using Streptococcal Protein G HiTrap Columns (GE Healthcare). Briefly, columns were equilibrated with PBS, loaded with 2 ml serum, and washed with PBS. IgG was eluted with 0.1 M citrate buffer, pH 2.6, and the eluate was neutralized with 1 M Tris-HCl, pH 8.8. Eluates were dialyzed overnight against PBS and sterilized using 0.2-μm filters. Flow cytometry was used to confirm the presence of anti-Salmonella antibody in purified IgG.
Lipopolysaccharide and rck gene.
The 8 invasive Malawian S. Typhimurium isolates used in our Salmonella killing assays (see above) were investigated for the presence of long-chain lipopolysaccharide and rck. P22 bacteriophage susceptibility was assessed to indicate the presence of intact lipopolysaccharide coat. P22 and each of the 8 S. Typhimurium isolates were cross-streaked on LB agar. Lipopolysaccharide from each isolate was separated by SDS-PAGE and visualized by silver staining (42). Sequencing of the 120-kb plasmid of Salmonella strain D23580 (http://www.sanger.ac.uk/Projects/Salmonella) identified rck (15). PCR using primers 5′-TTTCATCATATCTGACCGG-3′ and 5′-CTTCTGTCATAACACAATGAAC-3′ complementary to rck-flanking sequences was used to detect rck in the remaining 7 isolates.
Generation of rough mutant of S. Typhimurium D23580 by disruption of galE gene.
The galE gene of S. Typhimurium D23580 was disrupted using the red recombinase method (43) to generate a deep rough mutant. Primer oligonucleotides 5′-AACGGCGATGCGGATGATCGATGGGATTAAATGGGGTCATAACAACGTCCTGTGTAGGCTGGAGCTGCTTCG-3′ and 5′-CTTTGTTATGCTATGGTTATTCCATACCATAGGCTTAACGGAGCGAATTCATATGAATATCCTCCTTAG-3′ were used to generate the PCR amplicon using the template pKD4. galE encodes UDP–galactose 4–epimerase and is required for the biosynthesis of long-chain polysaccharide in cell wall lipopolysaccharide in the absence of exogenous galactose (44). The mutant was grown without added galactose, and SDS-PAGE with silver staining (42) was used to confirm the absence of long-chain lipopolysaccharide.
Statistics.
The Spearman approach was used for estimation of correlation because data were not normally distributed. Correlations were estimated with 95% confidence intervals using Stata version 9 (StataCorp). Ages of cases were examined using a χ2 goodness-of-fit test. A P value less than 0.05 was considered significant.
Acknowledgments
We thank the parents, guardians, and children who participated in this study. We are grateful to Elizabeth Molyneux and Eric Borgstein and the staff at QECH and Beit Cure International Hospital for their assistance. We are indebted to the Department of Microbiology at Malawi-Liverpool-Wellcome Trust Clinical Research Programme for NTS blood culture data and isolates and the Sanger Core Sequencing Facility for the rck sequence. We thank B. Paul Morgan for providing C9-deficient serum and purified C9 and Robert Read for providing purified mannose-binding lectin. This work was supported by a Training Fellowship in Clinical Tropical Medicine from the Wellcome Trust, a grant from the Endowment Research Fund of the United Hospital of Birmingham (both to C.A. MacLennan), and a Programme Grant from the Wellcome Trust (to M.E. Molyneux).
Footnotes
Nonstandard abbreviations used: CI, confidence interval; LB, Luria Bertani; NTS, nontyphoidal strain(s) of Salmonella; QECH, Queen Elizabeth Central Hospital.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:1553–1562 (2008). doi:10.1172/JCI33998
C. Anthony Hart died following the submission of this manuscript.
References
- 1.Graham S.M., et al. Nontyphoidal Salmonella infections of children in tropical Africa. . Pediatr. Infect. Dis. J. 2000;19:1189–1196. doi: 10.1097/00006454-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Graham S.M. Salmonellosis in children in developing and developed countries and populations. Curr. Opin. Infect. Dis. 2002;15:507–512. doi: 10.1097/00001432-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Brent A.J., et al. Salmonella bacteremia in Kenyan children. . Pediatr. Infect. Dis. J. 2006;25:230–236. doi: 10.1097/01.inf.0000202066.02212.ff. [DOI] [PubMed] [Google Scholar]
- 4.Hohmann E.L. Nontyphoidal salmonellosis. Clin. Infect. Dis. 2001;32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 5.Gordon M.A., et al. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS. 2002;16:1633–1641. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 6.Kariuki S., et al. Lack of clonal relationship between non-typhi Salmonella strain types from humans and those isolated from animals living in close contacts. . FEMS Immunol. Med. Microbiol. . 2002;33:165–171. doi: 10.1111/j.1574-695X.2002.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 7.Becker D., et al. Robust Salmonella metabolism limits possibilities for new antimicrobials. . Nature. 2006;440:303–307. doi: 10.1038/nature04616. [DOI] [PubMed] [Google Scholar]
- 8.Mastroeni P. Immunity to systemic Salmonella infections. . Curr. Mol. Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- 9.Mastroeni P., et al. Resistance and susceptibility to Salmonella infections: lessons from mice and patients with immunodeficiencies. . Rev. Med. Microbiol. 2003;14:53–62. [Google Scholar]
- 10.Fields P.I., Swanson R.V., Haidaris C.G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. . Proc. Natl. Acad. Sci. U. S. A. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanden R.V., Mackaness G.B., Collins F.M.1966Mechanisms of acquired resistance in mouse typhoi d . J. Exp. Med. 124585–600. 10.1084/jem.124.4.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jouanguy E., et al. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 1999;11:346–351. doi: 10.1016/S0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 13.MacLennan C., et al. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against salmonella in humans. J. Infect. Dis. 2004;190:1755–1757. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 14.Taylor P.W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol. Rev. 1983;47:46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heffernan E.J., Harwood J., Fierer J., Guiney D. The Salmonella typhimurium virulence plasmid complement resistance gene rck is homologous to a family of virulence-related outer membrane protein genes, including pagC and ail. . J. Bacteriol. 1992;174:84–91. doi: 10.1128/jb.174.1.84-91.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muschel L.H., Larsen L.J. The sensitivity of smooth and rough gram-negative bacteria to the immune bactericidal reaction. Proc. Soc. Exp. Biol. Med. 1970;133:345–348. doi: 10.3181/00379727-133-34472. [DOI] [PubMed] [Google Scholar]
- 17.Liang-Takasaki C.J., Grossman N., Leive L. Salmonellae activate complement differentially via the alternative pathway depending on the structure of their lipopolysaccharide O-antigen. J. Immunol. . 1983;130:1867–1870. [PubMed] [Google Scholar]
- 18.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–107. doi: 10.1016/S0966-842X(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 19.Acharya I.L., et al. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. . N. Engl. J. Med. 1987;317:1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- 20.Xu H.R., Hsu H.S., Moncure C.W., King R.A. Correlation of antibody titres induced by vaccination with protection in mouse typhoid. Vaccine. 1993;11:725–729. doi: 10.1016/0264-410X(93)90256-W. [DOI] [PubMed] [Google Scholar]
- 21.Mastroeni P., Villarreal-Ramos B., Hormaeche C.E. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect. Immun. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McSorley S.J., Jenkins M.K. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. . Infect. Immun. 2000;68:3344–3348. doi: 10.1128/IAI.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins F.M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in nonvaccinated mice challenged by various routes. . J. Bacteriol. 1969;97:667–675. doi: 10.1128/jb.97.2.667-675.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham A.F., et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. . 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 25.Jolliff C.R., et al. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin. Chem. 1982;28:126–128. [PubMed] [Google Scholar]
- 26.Goldschneider I., Gotschlich E.C., Artenstein M.S. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross S.C., Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine. 1984;63:243–273. [PubMed] [Google Scholar]
- 28.Robbins J.B., Kenny K., Suter E. The isolation and biological activities of rabbit γM- and γG-anti-Salmonella typhimurium antibodies. . J. Exp. Med. 1965;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michael J.G., Whitby J.L., Landy M. Studies on natural antibodies to gram-negative bacteria. J. Exp. Med. . 1962;115:131–146. doi: 10.1084/jem.115.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glynn A.A., Milne C.M. A kinetic study of bacteriolytic and bactericidal action of human serum. Immunology. 1967;12:639–653. [PMC free article] [PubMed] [Google Scholar]
- 31.Gökalp A.S., Toksoy H.B., Türkay S., Bakici M.Z., Kaya R. Intravenous immunoglobulin in the treatment of Salmonella typhimurium infections in preterm neonates. Clin. Pediatr. (Phila.) 1994;33:349–352. doi: 10.1177/000992289403300607. [DOI] [PubMed] [Google Scholar]
- 32.Dumestre-Pérard C., Doerr E., Colomb M.G., Loos M. Involvement of complement pathways in patients with bacterial septicemia. Mol. Immunol. 2007;44:1631–1638. doi: 10.1016/j.molimm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Devyatyarova-Johnson M., et al. The lipopolysaccharide structures of Salmonella enterica serovar Typhimurium and Neisseria gonorrhoeae determine the attachment of human mannose-binding lectin to intact organisms. . Infect. Immun. 2000;68:3894–3899. doi: 10.1128/IAI.68.7.3894-3899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roantree R.J., Rantz L.A. A study of the relationship of the normal bactericidal activity of human serum to bacterial infection. J. Clin. Invest. 1960;39:72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elgefors B., Olling S. The significance of serum-sensitive bacilli in gram-negative bacteremia. Scand. J. Infect. Dis. 1978;10:203–207. doi: 10.3109/inf.1978.10.issue-3.08. [DOI] [PubMed] [Google Scholar]
- 36.Brown S.P., et al. Intracellular demography and the dynamics of Salmonella enterica infections. PLoS Biol. 2006;4:e349. doi: 10.1371/journal.pbio.0040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joiner K.A., Hammer C.H., Brown E.J., Cole R.J., Frank M.M. Studies on the mechanism of bacterial resistance to complement-mediated killing I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. . J. Exp. Med. 1982;155:797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joiner K.A., Hammer C.H., Brown E.J., Frank M.M. Studies on the mechanism of bacterial resistance to complement-mediated killing II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. . J. Exp. Med. 1982;155:809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heffernan E.J., et al. Mechanism of resistance to complement-mediated killing of bacteria encoded by the Salmonella typhimurium virulence plasmid gene rck. . J. Clin. Invest. 1992;90:953–964. doi: 10.1172/JCI115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank M.M., Joiner K., Hammer C. The function of antibody and complement in the lysis of bacteria. Rev. Infect. Dis. 1987;9:S537–S545. doi: 10.1093/clinids/9.supplement_5.s537. [DOI] [PubMed] [Google Scholar]
- 41.Joiner K.A., Tartanian A.B., Hammer C.H., Schweinle J.E. Multimeric C9 within C5b-9 deposits in unique locations in the cell wall of Salmonella typhimurium. . J. Immunol. 1989;142:4450–4457. [PubMed] [Google Scholar]
- 42.Tsai C.M., Frasch C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-X. [DOI] [PubMed] [Google Scholar]
- 43.Datsenko K.A., Wanner B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. . Proc. Natl. Acad. Sci. U. S. A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osborn M.J., Rosen S.M., Rothfield L., Horecker B.L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc. Natl. Acad. Sci. U. S. A. 1962;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]