Abstract
In this study, the authors investigated the role of enkephalins in morphine-induced conditioned place preference, locomotor sensitization, and analgesic tolerance. Both preproenkephalin wild type (ppENK [+/+]) and knockout (ppENK [−/−]) mice showed similar preference for the morphine-paired chamber over the vehicle-paired chamber, indicating morphine induced comparable conditioned place preference in ppENK (+/+) and ppENK (−/−) mice. Sensitization developed to the motor stimulatory action of morphine after its repeated administration, but the magnitude of this response was not altered in ppENK (−/−) mice. However, as shown previously, ppENK (−/−) mice displayed blunted morphine analgesic tolerance. Taken together, the results suggest that enkephalins may be important for the development of analgesic tolerance but not for conditioned place preference or behavioral sensitization induced by morphine.
Keywords: morphine, enkephalins, analgesic tolerance, locomotor sensitization, conditioned place preference
At least three classes of opioid receptors exist, namely mu, delta, and kappa, and a distinct gene has been cloned for each of them (for review, see Minami & Satoh, 1995; Satoh & Minami, 1995). These receptors mediate the actions of exogenous and endogenous opioids. Within the endogenous opioid family, three major classes of peptides exist, namely endorphins, enkephalins, and dynorphins, derived from three precursor proteins: proopiomelanocortin, proenkephalin, and prodynorphin, respectively (for review, see Akil et al., 1984). The discovery of endomorphin-1 and endomorphin-2 has extended the endogenous opioid family (Zadina, Hackler, Ge, & Kastin, 1997).
The endogenous opioid system is involved in many physiological responses, such as modulation of pain, stress, fear, anxiety, and hedonic homeostasis. Opioids are potent analgesics and are used for the treatment of moderate to severe pain. However, their high abuse potential and the development of tolerance and dependence limit their effective use in the clinical setting. Despite being the subject of decades of research in the field of drug addiction, the neurobiological substrates mediating the rewarding and addictive properties of morphine and other opioids remain poorly understood.
An accumulating body of evidence suggests that activation of the delta opioid receptor may be involved in morphine-induced analgesic tolerance. For example, administration of naltrindole, a delta opioid receptor antagonist (Abdelhamid, Sultana, Portoghese, & Takemori, 1991), or an antisense against the delta opioid receptor (Kest, Lee, McLemore, & Inturrisi, 1996) has been shown to attenuate morphine analgesic tolerance. Moreover, chronic morphine treatment has been demonstrated not to produce analgesic tolerance in delta opioid receptor knockout mice (Zhu et al., 1999). Likewise, mice lacking preproenkephalin (ppENK) have been shown to display blunted analgesic tolerance (Nitsche et al., 2002), suggesting morphine-produced analgesic tolerance via the release of enkephalins (methionine- and/or leucine-enkephalins) or possibly the C-terminal extended forms of enkephalins (Met-Enk-Arg-Phe and Met-Enk-Arg-Gly-Leu) or other larger peptides, such as peptide E (which contains Met-Enk at the N-terminus and Leu-Enk at the C-terminus) and peptide F (which contains two Met-Enk sequences, one at each terminus) derived from ppENK.
Enkephalins may also be involved in the reinforcing effects of opioids. For instance, delta opioid receptor antagonists attenuated the reinforcing effects of heroin (Martin et al., 2000; Negus et al., 1993). Furthermore, binge heroin administration increased pallidal levels of endogenous opioid peptides, predominantly methionine- and leucine-enkephalins (Olive & Maidment, 1998b), and lesions of the pallidum attenuated heroin-induced self-administration in rats (Hubner & Koob, 1990). Moreover, local pallidal injection of naloxone induced aversion (Skoubis & Maidment, 2003), supporting the importance of pallidal enkephalinergic neurons in hedonic homeostasis. It is of interest that the hedonics of feeding are altered in ppENK knockout (KO) mice (Hayward, Pintar, & Low, 2002), raising the possibility that enkephalins may also be important in the rewarding and addictive properties of morphine. Pallidal enkephalins may also be involved in morphine-induced locomotor sensitization (Johnson & Napier, 2000). Thus, the present study was designed to determine the role of enkephalins in morphine-induced conditioned place preference (CPP) and locomotor sensitization. For comparison, we also determined the role of enkephalins in morphine analgesic tolerance.
Method
Subjects
We obtained ppENK KO mice, backcrossed for at least 10 generations to the C57BL/6J strain, and C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) and used them to generate our ppENK heterozygous (HZ) breeding colony. Male offspring (4−6 months of age) of the ppENK HZ mice were used for all experiments. Mice were housed 2−4 per cage with free access to food and water in a colony room maintained at controlled temperature and humidity on a 12-hr light–dark cycle. Mice were transferred to the testing room for behavioral testing 1 hr prior to any experimentation. All observations were made during the light cycle. All experiments were in accordance with the ethical guidelines of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences.
Drugs
Morphine sulfate obtained from Sigma Chemical (St. Louis, MO) was dissolved in saline. All doses used in this study are for the salt form of the drug and were administered subcutaneously in a volume of 10 μl/g.
Experimental Procedures
The role of enkephalins in morphine-induced CPP
First, using a CPP paradigm, we tested whether the rewarding action of morphine was altered in ppENK KO mice. To accomplish this, we used a CPP apparatus consisting of three chambers: a gray neutral chamber (NCh) as a starting point for pre- and postconditioning sessions (see below) and two other chambers designated as conditioning chambers. One of the conditioning chambers was the vehicle-paired chamber (VPCh) and the other was the drug-paired chamber (DPCh). Both conditioning chambers were accessible from the NCh via removable doors. The two conditioning chambers were distinguishable on the basis of odor (almond and citrus scents) and visual (one chamber was decorated with wallpaper of alternating 1-in. horizontal black and white stripes and the other chamber with wallpaper of alternating 1-in. vertical black and white stripes) cues. The CPP paradigm was conducted over a 10-day period and consisted of three sessions (preconditioning, conditioning, and postconditioning). On Day 1 (the preconditioning session), each mouse was placed in the NCh and was allowed to freely explore all three chambers for 15 min. On Day 2 (the first conditioning day), mice were treated with either saline or morphine (5 mg/kg) and confined to either the VPCh or the DPCh for 1 hr. All treatments were balanced between the conditioning chambers. Moreover, half of the mice received morphine in the almond-scented chamber and the other half received morphine in the citrus-scented chamber. On the following day (the second conditioning day), mice that were injected with morphine on the first conditioning day received saline and were confined to the VPCh for 1 hr. Likewise, mice that were treated with saline on the first conditioning day received morphine (5 mg/kg) on the second conditioning day and were confined to the DPCh for 1 hr. All mice were given these alternate treatments during the conditioning sessions on Days 2−9. On Day 10 (the postconditioning session), mice were placed in the NCh and allowed to freely explore all three chambers of the CPP apparatus for 15 min. The amount of time that the mice spent in each chamber during preconditioning and postconditioning sessions was recorded using a standard TV and VCR video recording system (Kocom 260× power zoom) and used for data analysis.
The role of enkephalins in morphine-induced motor stimulation and behavioral sensitization
We next determined whether the acute motor stimulatory action of morphine was altered in ppENK KO mice. Mice were habituated to motor activity chambers (black beakers with a diameter of 17 cm and height of 22 cm) for 1 hr, then injected with saline or morphine (5 mg/kg). The subsequent distance traveled (measured in centimeters) was recorded for 1 hr using a Videomex-V system (Columbus Instruments, Inc., Columbus, OH). Further, in a separate experiment using a different group of mice, we determined whether morphine-induced locomotor sensitization was altered in mice lacking enkephalins. We used a 7-day, once daily morphine treatment protocol for the induction of sensitization. On Day 1, wild type (WT) and ppENK KO mice were habituated to the testing chambers for 1 hr, then injected with saline or morphine (5 mg/kg). Their subsequent distance traveled (measured in centimeters) was recorded for 1 hr, as described above. On Days 2−7, mice were treated with saline or morphine (7.5 mg/kg) in their home cages and then challenged with morphine 7 days later (on the test day) for locomotor sensitization. On the test day, mice were habituated to the test chambers for 1 hr, then injected with morphine (5 mg/kg). Subsequent motor activity was recorded for 1 hr. For both experiments, distance traveled (in centimeters) was taken as a measure of motor activity and used for data analysis.
The role of enkephalins in analgesic tolerance to morphine
Finally, we determined whether the same 7-day morphine treatment used for the development of locomotor sensitization also induced analgesic tolerance in ppENK KO mice. On Day 1, mice were tested for baseline latency using a radiant-heat tail-flick assay (Emdie automated TF6 tail-flick apparatus; Emdie Industries, Maidens, VA). They then were injected with morphine (7.5 mg/kg) and tested 30 min later for postdrug tail-flick latency. On Days 2−6, mice received the same dose of morphine but were not tested for baseline or postdrug latency. On Day 7, mice were tested for baseline latency, then injected with morphine (7.5 mg/kg) and tested 30 min after morphine administration.
Statistical Analysis
All data are presented as means plus or minus standard errors of measurement. Tail-flick data were expressed as the percentage of maximum possible effect (% MPE) using the formula [% MPE = (tail-flick latency – baseline latency/cut-off time – baseline) × 100] and analyzed using a repeated-measure analysis of variance (ANOVA). Likewise, the repeated-measure ANOVA was used for analysis of locomotor sensitization data. A two-factor ANOVA was used to analyze the amount of time that the mice spent in the DPCh versus the VPCh during preconditioning and postconditioning sessions. The two factors were genotype (WT vs. KO) and chamber (VPCh vs. DPCh). A p < .05 was considered statistically significant.
Results
The Role of Enkephalins in Morphine-Induced CPP
First, we determined whether the rewarding effect of morphine was altered in the absence of enkephalins in a CPP paradigm. WT and ppENK KO mice were tested for baseline preference on Day 1 (see Figure 1, upper panel). A two-factor ANOVA revealed a significant effect of chamber, F(2, 30) = 5.77, p < .05, but neither a significant effect of genotype, F(1, 30) = 0.01, p > .05, nor an interaction between chamber and genotype, F(2, 30) = 1.93, p > .05. However, the amount of time that the WT and ppENK KO mice spent in the VPCh versus the DPCh was not different, p > .05, showing that the mice did not prefer the DPCh over the VPCh prior to conditioning sessions. Morphine produced CPP in both WT and ppENK KO mice, which was evident from an increase in the amount of time that the mice spent in the DPCh over the VPCh after conditioning (see Figure 1, lower panel) but not before conditioning (Figure 1, upper panel). A two-factor ANOVA revealed a significant effect of chamber preference, F(2, 30) = 30.01, p < .05, but neither a significant effect of genotype, F(1, 30) = 0.01, p > .05, nor an interaction between genotype and chambers, F(2, 30) = 0.98, p > .05, showing that morphine-induced CPP was similar for WT and ppENK KO mice ( p > .05). Thus, these results demonstrate that morphine-induced CPP was not altered in mice lacking enkephalins.
Figure 1.
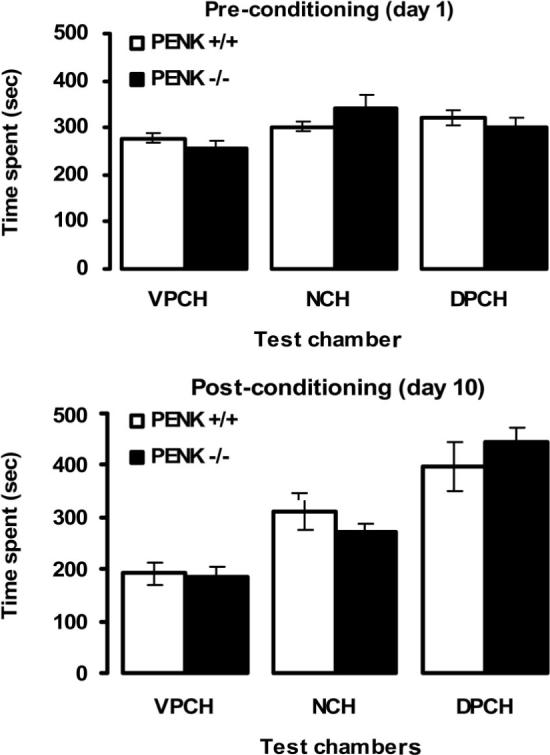
Morphine-induced conditioned place preference was not altered in preproenkephalin knockout mice. Mice were tested for baseline (preconditioning) preference on Day 1 (upper panel), received conditioning sessions on Days 2−9, and were tested for postconditioning preference on Day 10 (lower panel). Data reported are the means (± SEM, indicated by the vertical lines) of six mice per treatment per genotype. VPCH = vehicle-paired chamber; NCH = neutral chamber; DPCH = drug-paired chamber; PENK +/+ = wild type mice; PENK −/− = knockout mice.
The Role of Enkephalins in Morphine-Induced Motor Stimulation and Locomotor Sensitization
Next, we determined whether the motor stimulatory action of morphine was altered in WT and ppENK KO mice. Mice were habituated to the test chambers for 1 hr, then injected with saline or morphine. Subsequent motor activity was recorded for 1 hr. Figure 2 shows that both groups of mice had a similar increase in basal motor activity on placement in the test chambers during the habituation period. Morphine, as compared with saline, time dependently increased motor activity in both WT and ppENK KO mice (see Figure 2). A repeated-measure ANOVA revealed a significant effect of session, F(3, 72) = 3.58, p < .05, but neither a significant effect of genotype, F(1, 24) = 0.30, p > .05, nor an interaction between genotype and session, F(3, 72) = 1.42, p > .05. This showed that morphine increased motor activity, the magnitude of which was not different between WT and ppENK KO mice (p > .05).
Figure 2.

The motor stimulatory action of morphine (5 mg/kg sc) was not altered in preproenkephalin knockout mice. Motor activity was measured for 15-min sessions during habituation (1 hr prior to saline or morphine) and test (1 hr after saline or morphine) periods. Data are mean (± SEM, indicated by the vertical lines) of 6−8 mice per treatment per genotype ppENK +/+ = wild type mice; ppENK −/− = knockout mice.
We then determined whether morphine-induced locomotor sensitization was altered in mice lacking enkephalins. On Day 1, mice were habituated to the testing chambers, then injected with saline or morphine; motor activity was subsequently recorded for 1 hr (data not shown). On Days 2−7, mice received either saline or morphine (7.5 mg/kg) in their home cages and then were challenged with morphine (5 mg/kg) 7 days later. This repeated morphine treatment produced locomotor sensitization to the challenge dose of morphine given on Day 14 (see Figure 3; compare saline-pretreated mice with morphine-pretreated mice in each group). A repeated-measure ANOVA revealed a significant effect of chronic morphine treatment, F(1, 12) = 6.23, p < .05, but neither a significant effect of genotype, F(1, 12) = 0.43, p > .05, nor an interaction between treatment and genotype, F(1, 12) = 0.20, p > .05, showing that repeated morphine treatment produced locomotor sensitization, the magnitude of which was not different between WT and ppENK KO mice (p > .05). Thus, these results demonstrate that neither the acute motor stimulatory action of morphine nor the locomotor sensitization that developed after repeated morphine treatment was different between WT and ppENK KO mice.
Figure 3.
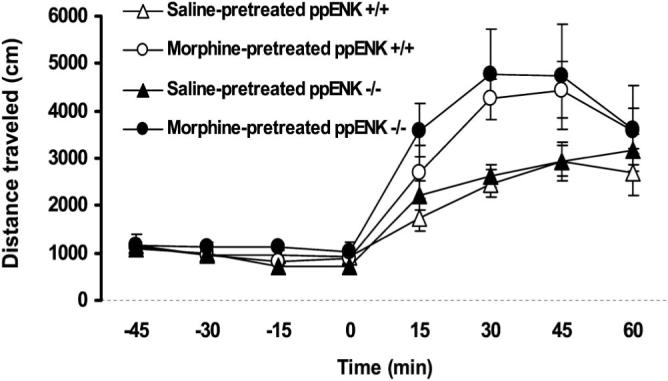
Morphine-induced locomotor sensitization was not altered in preproenkephalin knockout (ppENK KO) mice. On Day 1, wild type mice and ppENK KO mice were habituated to the test chambers for 1 hr and treated with either saline or morphine (5 mg/kg sc), and motor activity was subsequently recorded for a 1-hr period (data not shown). On Days 2−7, mice were treated with saline or morphine (7.5 mg/kg sc) and then tested for morphine-induced motor stimulation after a challenge dose of morphine (5 mg/kg sc) given on Day 14. Data represent mean (± SEM, indicated by the vertical line) distance traveled for 15-min sessions during the 1-hr habituation (prior to morphine challenge) and 1-hr test (after morphine administration) periods (n = 5−6 mice per treatment per genotype) on Day 14. ppENK +/+ = wild type mice; ppENK −/− = knockout mice.
The Role of Enkephalins in Analgesic Tolerance to Morphine
A previous study showed that ppENK KO mice displayed blunted analgesic tolerance after chronic morphine treatment (Nitsche et al., 2002). As tolerance and sensitization may share some common mechanisms, we then investigated whether the same 7-day morphine treatment used for the induction of locomotor sensitization also produced tolerance in ppENK KO mice. Figure 4 shows the development of analgesic tolerance to morphine in WT and ppENK KO mice. A repeated-measure ANOVA revealed a significant effect of repeated morphine treatment, F(1, 7) = 13.57, p < .05, and a significant effect of genotype, F(1, 7) = 8.85, p < .05, but no significant interaction between genotype and repeated morphine treatment, F(1, 7) = 3.38, p > .05. This result shows that the antinociceptive effect of morphine was significantly reduced in WT mice after repeated morphine administration (p < .05; compare Day 1 vs. Day 7), but this phenomenon was blunted in ppENK KO mice (see Figure 4; compare WT vs. KO mice on Day 7).
Figure 4.
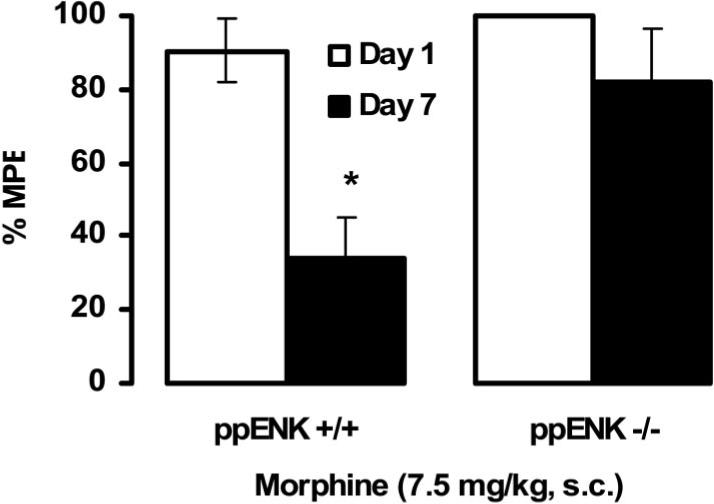
Repeated morphine administration produced tolerance to the antinociceptive effect of morphine in wild type mice, but this phenomenon was blunted in preproenkephalin knockout mice. On Day 1, mice were tested for baseline tail-flick latency, injected with morphine (7.5 mg/kg), and tested 30 min later for morphine-induced antinociception. On Days 2−6, mice were daily injected with morphine but not tested for baseline or morphine-induced antinociception. On Day 7, mice were tested for morphine-induced antinociception, as described for Day 1. Data are the mean (± SEM, indicated by the vertical lines) of 4−5 mice per genotype. ppENK +/+ = wild type mice; ppENK −/− = knockout mice; MPE = maximum possible effect. The asterisk indicates a significant difference from the all other groups (p < .05).
Discussion
The main findings of the present study are that both WT and ppENK KO mice displayed comparable morphine-induced CPP and locomotor sensitization but, as shown previously (Nitsche et al., 2002), ppENK KO mice showed blunted analgesic tolerance. These results suggest that enkephalins may be important for the development of analgesic tolerance but not CPP or locomotor sensitization induced by morphine.
Morphine has been shown to produce antinociception, reward and motor stimulation via activation of the mu opioid receptors, because these actions of the drug were absent in mice lacking mu opioid receptors (Matthes et al., 1996). The mesoaccumbens dopaminergic pathway, originating in the ventral tegmental area with projections to the nucleus accumbens, is thought to play an important role in the rewarding and addictive properties of morphine and other drugs of abuse (for review, see Koob, 1992). For example, morphine has been shown to facilitate the function of the mesolimbic dopaminergic neurons and to increase extracellular dopamine in the terminal fields of these neurons (Di Chiara & Imperato, 1988a). However, the rewarding action of opioids has also been shown to be dopamine independent, and pallidal enkephalinergic neurons may be important in this regard (Hubner & Koob, 1990).
The ventral pallidum, a basal ganglia structure with dense enkephalinergic innervations from the nucleus accumbens (Cuello & Paxinos, 1978; Kalivas, Churchill, & Klitenick, 1993; Zahm, Zaborszky, Alones, & Heimer, 1985), has been implicated in the motor stimulatory and rewarding actions of abused drugs. For example, opioids and psychostimulants have been shown to produce reward after local administration in the pallidum (Gong, Neill, & Justice, 1996; Johnson, Stellar, & Paul, 1993). Likewise, pallidal injections of opioid receptor antagonists have been shown to block morphine-induced behavioral sensitization (Johnson & Napier, 2000) and cocaine-induced CPP (Skoubis & Maidment, 2003). It is important to note that pallidal levels of endogenous opioid peptides have been shown to increase in response to systemic morphine administration (Olive, Bertolucci, Evans, & Maidment, 1995; Olive & Maidment, 1998a), raising the possibility that enkephalins may be important in the rewarding action of morphine. Thus, using a CPP paradigm, we determined whether morphine-induced CPP was altered in ppENK KO mice. Our results showed that both WT and ppENK KO mice given repeated morphine treatment in a chamber paired with visual and odor cues during conditioning sessions spent more time in the DPCh during postconditioning (Figure 1, lower panel) but not during preconditioning (Figure 1, upper panel) sessions, suggesting that morphine produced CPP. However, this preference was similar for WT and ppENK KO mice, indicating that enkephalins were not critical for the rewarding effect of morphine.
Morphine and other mu opioid receptor agonists have been shown to increase extracellular accumbal dopamine (Di Chiara & Imperato, 1988a, 1988b), an action associated with the reinforcing effects of these drugs (for review, see Koob, 1992). One consequence of the enhanced accumbal dopamine levels in rodents is an acute increase in motor activity (Wise & Bozarth, 1987), which is progressively increased with repeated treatment, a phenomenon referred to as behavioral sensitization (Kalivas & Duffy, 1993a, 1993b; Kalivas & Stewart, 1991; Post & Rose, 1976; Shuster, Yu, & Bates, 1977; Stewart & Badiani, 1993). Behavioral sensitization is thought to be important in compulsive drug-seeking behaviors as well as in relapse (see Kalivas, Sorg, & Hooks, 1993; Robinson & Berridge, 1993, 2000, 2001). Indeed, behavioral sensitization is considered a model of some aspects of drug addiction, craving in particular (for reviews, see Robinson & Berridge, 1993, 2000, 2001). Therefore, using ppENK KO mice, we determined the role of enkephalins in morphine-induced locomotor sensitization. Our results demonstrated that morphine increased motor activity, a response that was enhanced in mice pretreated with morphine (see Figure 3), indicating that sensitization developed to the motor stimulatory action of morphine. However, neither morphine-induced motor stimulation (see Figure 2) nor locomotor sensitization (see Figure 3) was altered in mice lacking ppENK, demonstrating that enkephalins may not be involved in morphine-induced locomotor sensitization.
The observation that morphine-induced CPP and behavioral sensitization were not altered in ppENK KO mice in this study was somewhat surprising, because a previous report showed that morphine analgesic tolerance was blunted in ppENK KO mice (Nitsche et al., 2002). Therefore, we investigated whether the development of tolerance to the antinociceptive effect of morphine was altered in ppENK KO mice. Consistent with the results of Nitsche et al. (2002), we found that repeated morphine treatment attenuated the antinociceptive effect of morphine in WT mice, a response that was blunted in ppENK KO mice (see Figure 4). Previous studies have shown that morphine may activate the delta opioid receptor to produce analgesic tolerance (Abdelhamid et al., 1991; Kest et al., 1996; Zhu et al., 1999). However, the blunted tolerance observed in ppENK KO mice suggests that morphine may not directly activate the delta opioid receptor to produce this phenomenon. Rather, as proposed previously (Nitsche et al., 2002), morphine probably triggers the release of enkephalins that result in delta opioid receptor activation, thereby leading to the development of analgesic tolerance in WT mice. However, because of the lack of enkephalins, analgesic tolerance was blunted in ppENK KO mice (see Figure 4). Furthermore, our results suggest that morphine induces CPP and behavioral sensitization irrespective of the presence of enkephalins, because these phenomena were not altered in ppENK KO mice (see Figures 1 and 3). An alternative explanation for the current data is that morphine administration causes the release of enkephalins in selective central nervous system regions that leads to attenuation of morphine-induced antinociception but not CPP or locomotor sensitization after repeated administration of the drug. It is interesting that intrathecal injection of leucine-enkephalin reduces the antinociceptive effect of morphine via an inverse agonist action at the δ2-opioid receptors (Rady et al., 2001). However, further studies are needed to determine whether morphine causes the release of enkephalins in mice and whether the release of enkephalins occurs in central nervous system structures implicated in the phenomenon of analgesic tolerance.
In conclusion, our results demonstrate that morphine-induced CPP and locomotor sensitization were not altered in mice lacking enkephalins but that tolerance was blunted in these mice, suggesting that enkephalins may be playing a functional role in analgesic tolerance but not in locomotor sensitization or CPP induced by morphine. The present data also support the notion that different mechanisms may be mediating analgesic tolerance, CPP, and locomotor sensitization induced by morphine.
Acknowledgments
The present study was supported in part by an intramural grant through Western University of Health Sciences and in part by National Institute on Drug Abuse-funded Grants DA017298 to Theodore C. Friedman and DA016682 to Kabirullah Lutfy. Ramkumarie Baliram and Paul Marquez were supported by Grant DA016682. We thank Charles Young for his suggestions and Hoa Lam and Jin Oh for their help with the genotyping protocols.
Contributor Information
Theodore C. Friedman, Division of Endocrinology, Molecular Medicine and Metabolism, Department of Medicine, Charles R. Drew University of Medicine and Science—University of California, Los Angeles, School of Medicine
Kabirullah Lutfy, Department of Pharmaceutical Sciences, College of Pharmacy, Western University of Health Sciences, and Division of Endocrinology, Molecular Medicine and Metabolism, Department of Medicine, Charles R. Drew University of Medicine and Science—University of California, Los Angeles, School of Medicine..
References
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. Journal of Pharmacology and Experimental Therapeutics. 1991;258:299–303. [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: Biology and function. Annual Review of Neuroscience. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Paxinos G. Evidence for a long Leu-enkephalin striopallidal pathway in rat brain. Nature. 1978 January 12;271:178–180. doi: 10.1038/271178a0. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences, USA. 1988a;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. Journal of Pharmacology and Experimental Therapeutics. 1988b;244:1067–1080. [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr. Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Research. 1996;707:64–74. doi: 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking beta-endorphin and enkephalin. Journal of Neuroscience. 2002 September 15;22:8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner CB, Koob GF. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Research. 1990 January 29;508:20–29. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Napier TC. Ventral pallidal injections of a mu antagonist block the development of behavioral sensitization to systemic morphine. Synapse. 2000;38:61–70. doi: 10.1002/1098-2396(200010)38:1<61::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stellar JR, Paul AD. Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology. 1993;32:1305–1314. doi: 10.1016/0028-3908(93)90025-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine: I. Dopamine axon terminals. Journal of Neuroscience. 1993a;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine: II. Dopamine perikarya. Journal of Neuroscience. 1993b;13:276–284. doi: 10.1523/JNEUROSCI.13-01-00276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behavioural Pharmacology. 1993;4:315–334. [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Research: Brain Research Reviews. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kest B, Lee CE, McLemore GL, Inturrisi CE. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Research Bulletin. 1996;39:185–188. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends in Pharmacological Sciences. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Cannon DG, Sizemore GM, Bian D, Porreca F, Smith JE. Antagonism of delta(2)-opioid receptors by naltrindole-5′-isothiocyanate attenuates heroin self-administration but not antinociception in rats. Journal of Pharmacology and Experimental Therapeutics. 2000;294:975–982. [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996 October 31;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Minami M, Satoh M. Molecular biology of the opioid receptors: Structures, functions and distributions. Neuroscience Research. 1995;23:121–145. doi: 10.1016/0168-0102(95)00933-k. [DOI] [PubMed] [Google Scholar]
- Negus SS, Henrikson SJ, Mattox A, Pasternak GW, Portoghese PS, Takemori AE, et al. Effects of antagonists selective for mu, delta and kappa opioid receptors on the reinforcing effects of heroin in rats. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1245–1252. [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and pre-proenkephalin knock-out mice. Journal of Neuroscience. 2002 December 15;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Bertolucci M, Evans CJ, Maidment NT. Microdialysis reveals a morphine-induced increase in pallidal opioid peptide release. Neuroreport. 1995;6:1093–1096. doi: 10.1097/00001756-199505300-00005. [DOI] [PubMed] [Google Scholar]
- Olive MF, Maidment NT. Opioid regulation of pallidal enkephalin release: Bimodal effects of locally administered mu and delta opioid agonists in freely moving rats. Journal of Pharmacology and Experimental Therapeutics. 1998a;285:1310–1316. [PubMed] [Google Scholar]
- Olive MF, Maidment NT. Repeated heroin administration increases extracellular opioid peptide-like immunoreactivity in the globus pallidus/ventral pallidum of freely moving rats. Psychopharmacology (Berlin) 1998b;139:251–254. doi: 10.1007/s002130050712. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976 April 22;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Rady JJ, Holmes BB, Tseng LF, Fujimotor JM. Inverse agonist action of Leu-enkephalin at delta(2)-opioid receptors mediates spinal antianalgesia. Journal of Pharmacology and Experimental Therapeutics. 2001;297:582–589. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research: Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Satoh M, Minami M. Molecular pharmacology of the opioid receptors. Pharmacology and Therapeutics. 1995;68:343–364. doi: 10.1016/0163-7258(95)02011-x. [DOI] [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology (Berlin) 1977;52:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Maidment NT. Blockade of ventral pallidal opioid receptors induces a conditioned place aversion and attenuates acquisition of cocaine place preference in the rat. Neuroscience. 2003;119:241–249. doi: 10.1016/s0306-4522(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behavioural Pharmacology. 1993;4:289–312. [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94:469–492. [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997 April 3;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Zaborszky L, Alones VE, Heimer L. Evidence for the coexistence of glutamate decarboxylase and Metenkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Research. 1985 January 28;325:317–321. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]


