Abstract
Rationale
Orphanin FQ/nociceptin (OFQ/N), the endogenous ligand of the opioid receptor-like (ORL-1) receptor, shows similarities to dynorphin A (1−17) in structure and functions. Dynorphin and other kappa opioid receptor agonists have been shown to block cocaine sensitization.
Objective
The present study was designed to examine the ability of OFQ/N to block cocaine-induced behavioral sensitization.
Methods
Rats were habituated to testing chambers for 1 h, injected with artificial cerebrospinal fluid (aCSF) or OFQ/N (15 nmol) followed by saline or cocaine (20 mg/kg) and locomotor activity was measured for a further 1 h. Rats were treated similarly for the next 2 days except the dose of OFQ/N was doubled on each subsequent day. Rats were then challenged with cocaine (7.5 mg/kg) in the absence of OFQ/N on day 8. The specificity of OFQ/N's action was examined in the presence of J-113397 (30 nmol), an ORL-1 receptor antagonist. The ability of OFQ/N to block the context-independent component of cocaine sensitization was also tested wherein rats were treated in their home cages on days 1−3. Finally, the effect of intra-VTA OFQ/N administration on cocaine sensitization was examined.
Results
Sensitization did not develop in rats repeatedly treated with OFQ/N, via either route of administration, prior to cocaine administration on days 1−3. The inhibitory effect of OFQ/N was not dependent on context and was blocked by pretreatment with J-113397.
Conclusion
Our results indicate that OFQ/N blocks cocaine-induced behavioral sensitization through activation of the ORL-1 receptor and that the VTA may be one of the substrates for this action of OFQ/N.
Keywords: Orphanin FQ/nociceptin, Cocaine, J-113397, Ventral tegmental area, Motor activity, Sensitization
Introduction
Repeated intermittent administration of cocaine induces a state of behavioral sensitization or reverse tolerance, a phenomenon that has been proposed as an animal model of drug craving and psychosis (for review, see Robinson and Berridge 2000). Enhanced mesoaccumbens dopaminergic neurotransmission has been associated both with psychostimulant-induced sensitization and with a number of neuropsychiatric disorders (Hockfelt et al. 1974; Robinson and Becker 1986; Robinson and Berridge 1993; Vanderschuren and Kalivas 2000). Furthermore, chronic use of psychostimulants has been shown to elicit paranoid psychosis and panic attacks (Matthysse 1973; Stevens 1973; Hockfelt et al. 1974; Post and Contel 1983; Robinson and Becker 1986; Robinson and Berridge 1993). Thus, drugs affecting the development of behavioral sensitization to cocaine could represent pharmacotherapeutic agents with potential for treatment of cocaine addiction and certain psychiatric disorders.
Behavioral sensitization is believed to be due to numerous changes that occur along the mesolimbic dopaminergic neuronal axis. In particular, changes in the dynamics of dopamine release and dopamine receptor number and function have been demonstrated (for reviews, see Woolverton and Johnson 1992; Vanderschuren and Kalivas 2000). One interesting feature of sensitization is its long-lasting aspect (Robinson and Becker 1986; Woolverton and Johnson 1992) that is reminiscent of long-term potentiation (LTP) and other N-methyl-D-aspartate (NMDA) receptor-mediated events. Indeed, extracellular levels of glutamate increase in the ventral tegmental area (VTA) after cocaine administration in sensitized rats (Kalivas and Duffy 1998) and inhibition of the NMDA receptor has been shown to block sensitization induced by psychostimulants (Karler et al. 1989, see also Vanderschuren and Kalivas 2000, for review). These data strongly suggest that hyperactivity of not only the dopaminergic but also the glutamatergic system is involved in cocaine-induced sensitization. Accordingly, an agent with dual activity to modulate both dopaminergic and glutamatergic neurotransmission might be particularly effective in blocking cocaine sensitization.
Orphanin FQ/nociceptin (OFQ/N), the endogenous agonist ligand of the opioid receptor-like (ORL-1) receptor (Meunier et al. 1995; Reinscheid et al. 1995), is a potential candidate in this regard. Thus, OFQ/N administration has been shown to attenuate basal (Murphy et al. 1996; Lutfy et al. 2001a) and drug-induced increases (Di Giannuario et al. 1999; Di Giannuario and Pieretti 2000; Lutfy et al. 2001a) in extracellular dopamine in the nucleus accumbens. Additionally, OFQ/N has been demonstrated to decrease glutamate release in the cortex (Nicol et al. 1996) and lateral amygdala (Meis and Pape 2001). The effect of OFQ/N on dopamine release and motor behaviors (Devine et al. 1996b; Murphy et al. 1996; Lutfy et al. 2001a) is similar to the action of kappa opioid receptor agonists, agents that have been shown to block cocaine sensitization (for review, see Shippenberg and Rea 1997). However, aversive responses can be observed following administration of kappa opioid receptor agonists (for review, see Herz 1998), making them unsuitable therapeutic agents. OFQ/N, on the other hand, is apparently devoid of such an effect (Devine et al. 1996a), raising the possibility that this novel peptide may block cocaine-induced behavioral sensitization without dysphoric side effects.
The VTA has been identified as a major site for mediating the development of sensitization to psychostimulants (Vezina 1993) and also as a substrate for the action of OFQ/N on dopaminergic neurotransmission (Murphy and Maidment 1999; Narayanan and Maidment 1999; Lutfy et al. 2000b). A previous report in our laboratory showed that intra-VTA OFQ/N administration failed to block development of behavioral sensitization to cocaine (Narayanan and Maidment 1999). In that study, the dose of OFQ/N injected into the VTA (30 μg/side) was held constant each day during the induction of cocaine sensitization. Since rapid tolerance develops to the action of OFQ/N (Devine et al. 1996b), the present study was designed to revisit the question by examining the effect of OFQ/N on cocaine-induced behavioral sensitization using an escalating dosing regimen of OFQ/N administered either intracerebroventricularly (ICV) or directly into the VTA. The specificity of OFQ/N's effects was determined using the ORL-1 receptor antagonist, J-113397 (Kawamoto et al. 1999). Furthermore, the effect of OFQ/N on the context-independent component of cocaine sensitization was also evaluated.
Materials and methods
Subjects
Male Sprague-Dawley rats, weighing 180−200 g obtained from Harlan (San Diego, Calif., USA), were housed two or three per cage with free access to food and water. All experiments were conducted during the light phase of a 12-h light/12-h dark cycle in accordance with the National Institutes of Health Guide for the Care and Use of Animals in Research (1996) and approved by the Institutional Animal Care-Use Committee.
Experiment 1
Initially, the effect of OFQ/N, administered ICV, was examined on cocaine-induced behavioral sensitization. For guide cannula implantation, rats were anesthetized with halothane in a mixture of oxygen and nitrous oxide (1:1) and placed in a stereotaxic frame. The skull was exposed and a 22G guide cannula (ID=0.39 mm; OD=0.71 mm; 3.5 mm long; Plastic One, Inc., Roanoke, Va., USA) was implanted 0.5 mm above the right lateral ventricle. The coordinates from bregma and skull surface, respectively, for the tip of the injection needle were AP=–0.8 mm; ML=+1.4 mm; DV=–4.0 mm according to the atlas of Paxinos and Watson (1986). The guide cannula was secured to the skull by dental cement and two metallic screws. Rats were allowed 4 days to recover from the surgery. On day 1 of sensitization paradigm, rats were habituated to cylindrical testing chambers (34 cm diameter×30 cm high made of gray plastic) for 1 h, during which time their locomotor activity was recorded as distance traveled in 15-min epochs using a Videomex-V system (Columbus Instruments, Ohio, USA). Subsequently, rats were injected with either artificial cerebrospinal fluid (aCSF) or OFQ/N (15 nmol) into the right lateral ventricle. Immediately thereafter the rats received an intraperitoneal (IP) injection of either saline (SAL) or cocaine (COC, 20 mg/kg). Total distance traveled (cm) was recorded for a further 1 h. This procedure was repeated on days 2 and 3. However, the dose of OFQ/N was doubled each day, i.e. 30 and 60 nmol on days 2 and 3, respectively. Rats did not receive any treatment for the next 4 days. On day 8, rats were placed in the testing chambers and their activity was recorded for 1 h, injected with a low dose of cocaine (7.5 mg/kg, IP) alone and locomotor activity was recorded for a further 1 h.
Experiment 2
The specificity of OFQ/N's effect on cocaine-induced behavioral sensitization was examined using J-113397, an ORL-1 receptor antagonist (Kawamoto et al. 1999). Rats were habituated to the testing chambers for 1 h and thereafter treated with vehicle [V; 20% dimethyl sulfoxide, (DMSO)] or J-113397 (J; 30 nmol, ICV). After a 15-min delay, rats were injected with aCSF or OFQ/N (15 nmol, ICV) and received cocaine (20 mg/kg, IP). Locomotor activity was measured for an additional hour. The same treatment was administered on days 2 and 3, except the dose of OFQ/N was escalated as in experiment 1. Rats were then left untreated on days 4−7 and tested on day 8 as described for experiment 1.
Experiment 3
To determine the effect of OFQ/N on context-independent component of cocaine sensitization, rats were treated with aCSF or OFQ/N (15 nmol, ICV) followed by saline or cocaine (20 mg/kg, IP) in their home cages, as opposed to the test chambers as described for experiment 1. Rats were treated similarly on days 2 and 3, except the dose of OFQ/N was escalated on days 2 and 3 as above. Rats were then tested on day 8 in the testing chambers as described for experiment 1.
Experiment 4
In this experiment, the effect of OFQ/N administered directly into the ventral tegmental area (VTA) was determined on cocaine-induced behavioral sensitization. Rats were prepared for surgery as described above, except guide cannulae (ID=0.39 mm; OD=0.71 mm; 5.0 mm long; Plastic One) were implanted bilaterally 3.7 mm above the VTA with a 10° angle. The coordinates from bregma and the skull surface, respectively, for the tip of the injection needle in the VTA were AP=–4.8 mm; ML=±2.7 mm; DV=–8.7 mm according to the atlas of Paxinos and Watson (1986). Rats were treated as described for experiment 1, except the dose of OFQ/N was 7.5, 15 and 30 nmol, on days 1, 2 and 3, respectively and injections were made bilaterally.
Histology
At the end of each experiment, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, IP) and perfused transcardially with phosphate buffered saline (PBS) followed by 50 ml phosphate buffered formalin (10%). Brains were then removed and sectioned (40 μm) using a cryostat (Leica Instruments Gmbh, Germany). Brain slices were mounted on gelatin-coated slides and stained with cresyl violet and viewed under a microscope. Based on such examination, three, one, zero and seven rats for experiments 1, 2, 3 and 4, respectively, were excluded from data analysis due to improper placement of the injection cannula.
Data analysis
Distance traveled (cm), during the 2-h recording period, was analyzed using a two-way repeated-measure analysis of variance (ANOVA) followed by the post-hoc Newman-Keuls test to reveal significant differences between the treatment groups. P<0.05 was considered statistically significant.
Drugs
Cocaine (Sigma, St Louis, Mo., USA) was dissolved in saline prior to intraperitoneal administration. OFQ/N (Phoenix Pharmaceuticals, Inc., Mountain View, Calif., USA) was dissolved in aCSF for ICV or intra-VTA injection. (±) J-113397 hydrochloride, obtained from Research Triangle Institute (Research Triangle Park, N.C., USA), was dissolved in 20% dimethyl sulfoxide (DMSO) for ICV administration. The volume of injection was 0.5 μl/side and 5 μl for intra-VTA and ICV injection, respectively. The composition of aCSF was (mM): NaCl (125); KCl (2.5); NaH2PO4 (0.9); Na2HPO4 (5); MgCl2 (1); D-glucose (2.5); CaCl2 (1.2); bovine serum albumin (0.025%).
Results
Experiment 1: effects of ICV OFQ/N administration on cocaine-induced behavioral sensitization
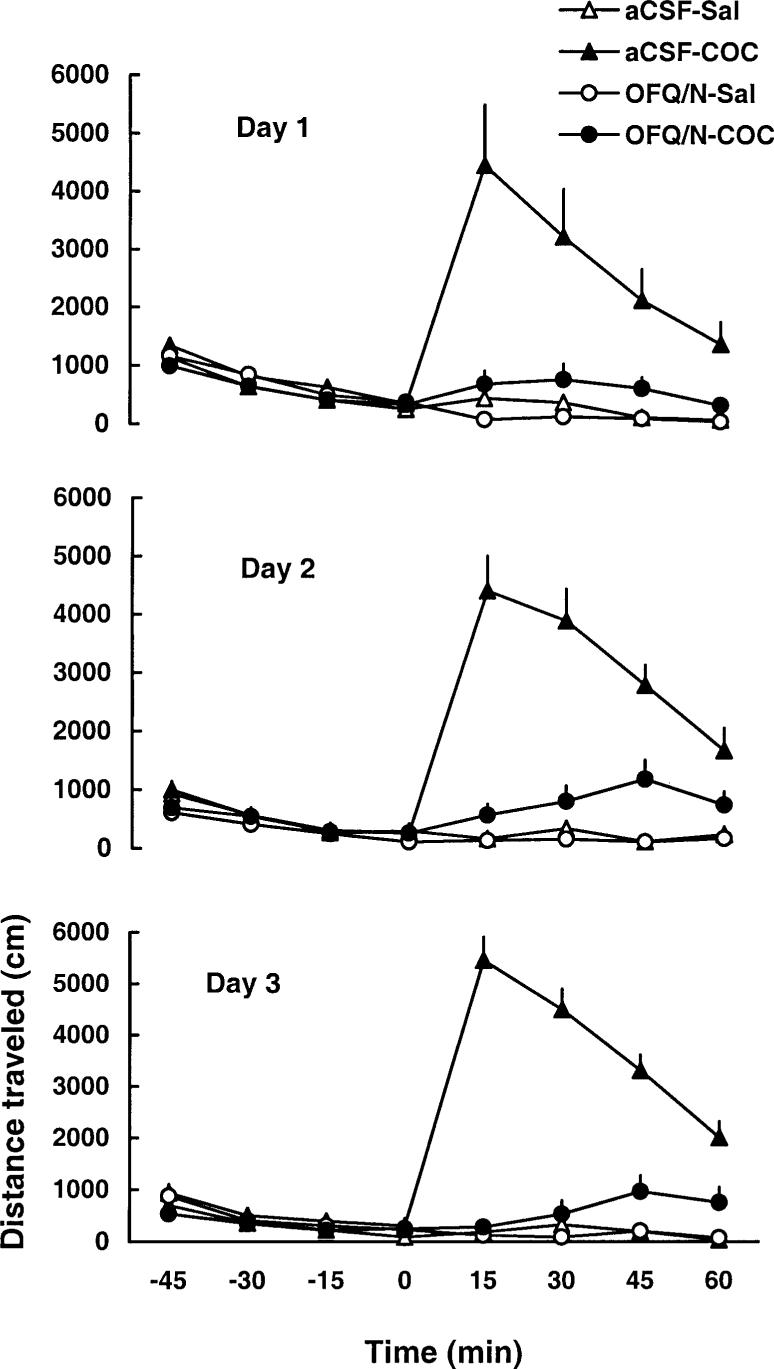
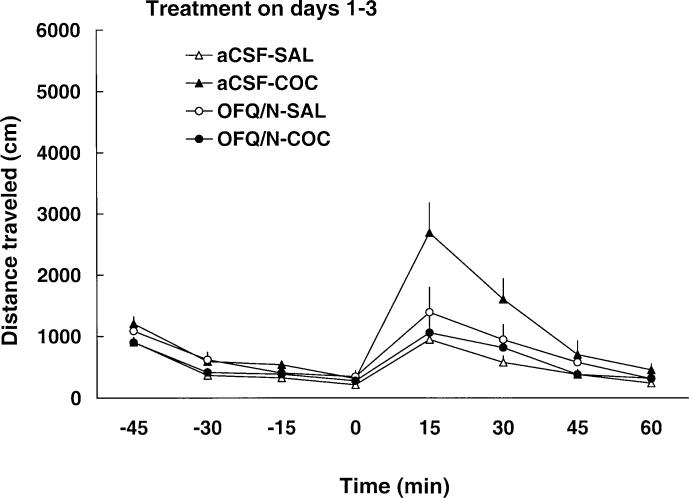
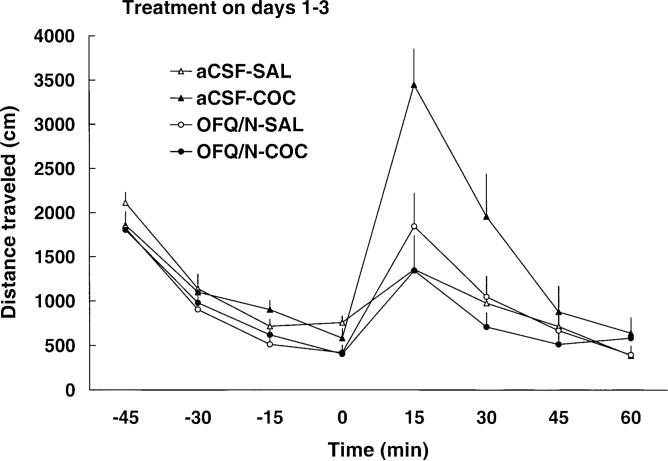
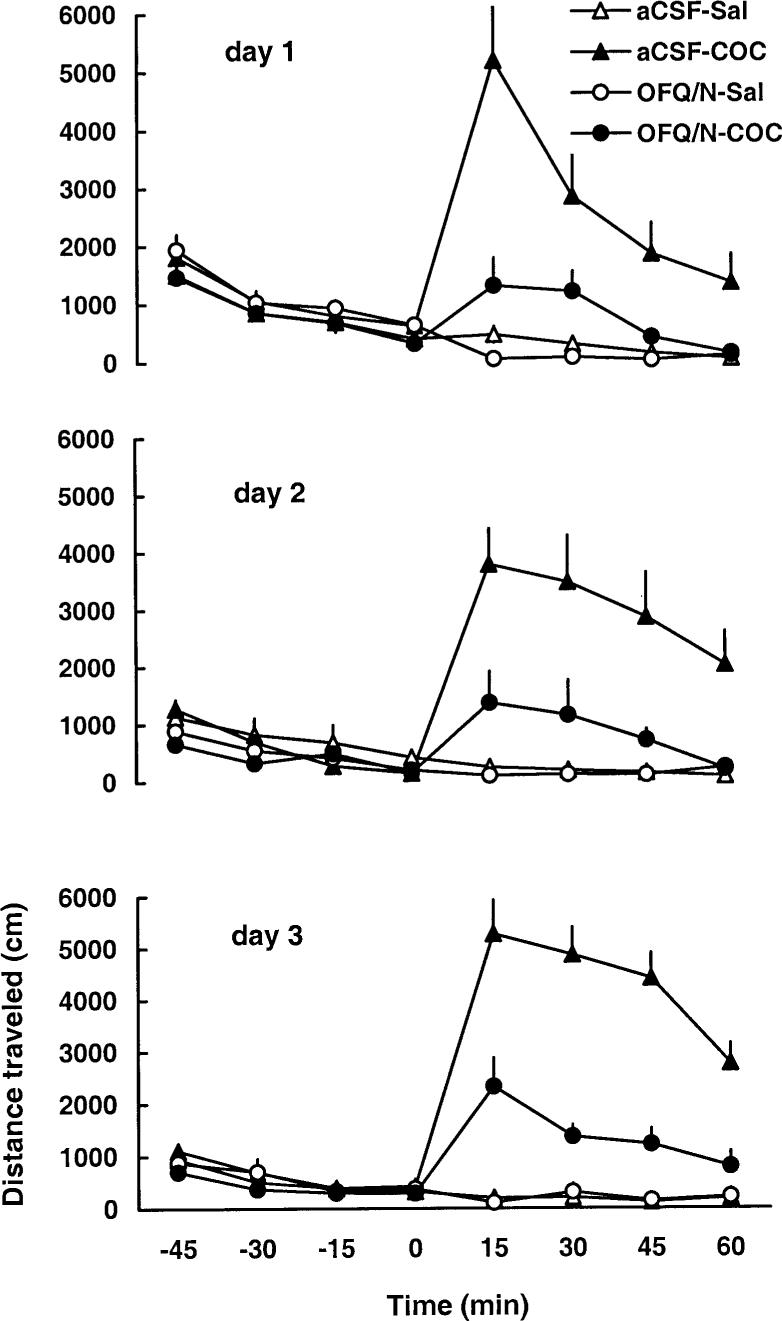
As expected, cocaine increased motor activity on day 1 and this motor stimulatory effect of the drug was attenuated by prior injection of OFQ/N (Fig. 1 and Table 1). A two-way repeated-measure ANOVA revealed a significant effect of treatment [F(3,35=12.74, P<0.05], a significant effect of time after cocaine administration [F(7,245)=7.06, P<0.0] and a significant treatment×time interaction [F(21,245)=3.99, P<0.05]. The post-hoc test showed that cocaine produced significant motor stimulation (P<0.05, compare aCSF-SAL versus aCSF-COC group) and OFQ/N administration blocked this action of cocaine (P<0.05, compare OFQ/N-COC versus aCSF-COC group). Furthermore, OFQ/N suppressed basal motor activity (Fig. 1 and Table 1, compare aCSF-SAL to OFQ/N-SAL group; P<0.05). When the dose of OFQ/N was doubled on day 2 and again on day 3, OFQ/N treatment similarly attenuated the motor stimulatory action of cocaine but showed no significant effect in saline-treated rats due to a reduced locomotor response to aCSF on these days or tolerance to the effect of OFQ/N (Fig. 1 and Table 1). Locomotor activity after a challenge dose of cocaine (7.5 mg/kg, IP), on day 8, is shown in Fig. 2 and Table 1. A two-way repeated-measure ANOVA revealed a significant effect of treatment [F(3,35)=3.71, P<0.05], a significant effect of time with regard to cocaine administration [F(7,245)=31.52, P<0.05] and a significant interaction between treatment and time [F(21,245)=2.94, P<0.05]. The post-hoc test showed that among the rats injected ICV with aCSF, the cocaine challenge produced a greater motor stimulatory effect in rats pretreated with cocaine than in animals previously administered saline (P<0.05; compare aCSF-SAL versus aCSF-COC group), indicating that sensitization developed to the motor stimulatory action of cocaine. This sensitized response was blocked in animals that received OFQ/N prior to cocaine administration on days 1−3 (P<0.05; compare OFQ/N-COC versus aCSF-COC group). OFQ/N in conjunction with saline on days 1−3 did not significantly modify the motor stimulatory action of cocaine on day 8 (P>0.05; compare aCSF-SAL versus OFQ/N-SAL group).
Fig. 1.
Intracerebroventricular administration of escalating doses of OFQ/N (15, 30, 60 nmol on day 1, 2 and 3, respectively) blocked the motor stimulatory action of cocaine on days 1−3. Rats were habituated to the testing chambers for 1 h, received an ICV injection of aCSF or OFQ/N immediately followed by saline or cocaine (20 mg/kg, IP) administration. Motor activity was then recorded for a further 1 h. Data are means±SEM of 9−11 rats/group
Table 1.
Effects of ICV or intra-VTA OFQ/N administration on basal motor activity and cocaine-induced motor stimulation. Rats were treated with aCSF or OFQ/N either ICV or directly into the VTA prior to saline or cocaine administration. The same treatment was given for 3 days, except the dose of OFQ/N was doubled on each subsequent day. Rats were left untreated until day 8, when a challenge dose of cocaine alone (7.5 mg/kg, IP) was administered. Values represent means (±SEM) of total distance traveled (cm) during the entire 1-h period after cocaine administration
| Treatment | aCSF-SAL | aCSF-COC | OFQ/N-SAL | OFQ/N-COC |
|---|---|---|---|---|
| ICV OFQ/N | ||||
| Day 1 | 974.8±186.8 | 11,134.7±2520.6 | 315.9±73.5 | 2355.2±717.6 |
| Day 2 | 848.3±215.1 | 12,739.9±1656.2 | 565.4±138.3 | 3280.9±850.9 |
| Day 3 | 739.0±125.4 | 15,274.1±1256.6 | 485.7±104.8 | 2536.0±760.2 |
| Day 8 | 2144.9±324.3 | 5449.5±1,005.3 | 3225.6±922.5 | 2314.7±621.1 |
| Intra-VTA OFQ/N | ||||
| Day 1 | 1018.4±356.8 | 11,285.9±3,152.1 | 322.3±115.9 | 3117.5±884.0 |
| Day 2 | 868.8±286.2 | 12,172.2±2,432.6 | 613.8±199.0 | 3474.8±1,231.5 |
| Day 3 | 726.7±229.9 | 17,346.5±1,756 | 793.0±436.7 | 5721.3±1,117.5 |
| Day 8 | 3323.7±426.8 | 9025.8±977.9 | 5239.3±1,149.2 | 4300.5±1,026.8 |
Fig. 2.
Repeated ICV administration of OFQ/N blocked the development of cocaine-induced behavioral sensitization. Rats were treated for 3 days, as described in the legend to Fig. 1, and left untreated until day 8. On the test day (day 8), rats were habituated for 1 h and all rats received a challenge dose of cocaine (7.5 mg/kg, IP) alone. Locomotor activity was thereafter recorded for a further 1 h. Data are means±SEM of 9−11 rats/group
Experiment 2: effects of OFQ/N administration on cocaine-induced behavioral sensitization in the presence of J-113397, an ORL-1 receptor antagonist
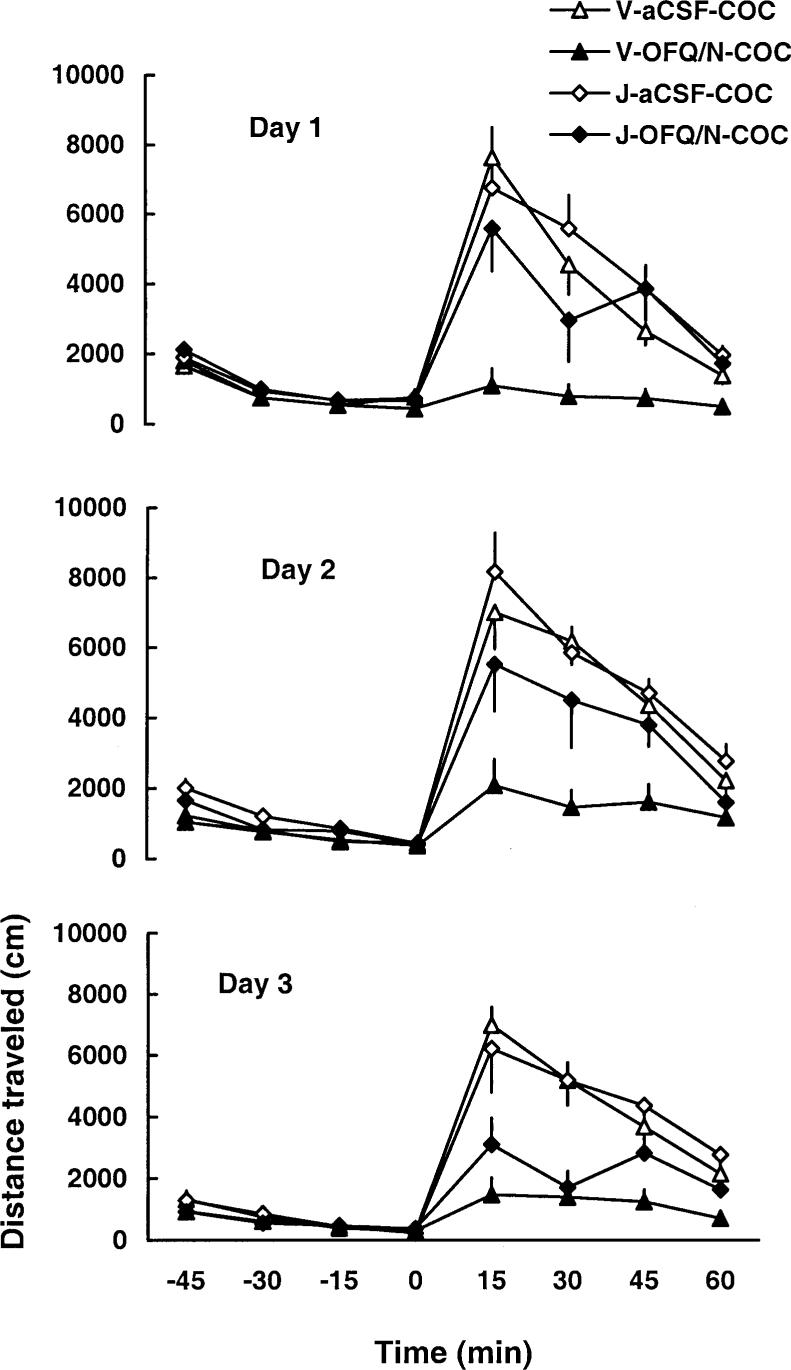
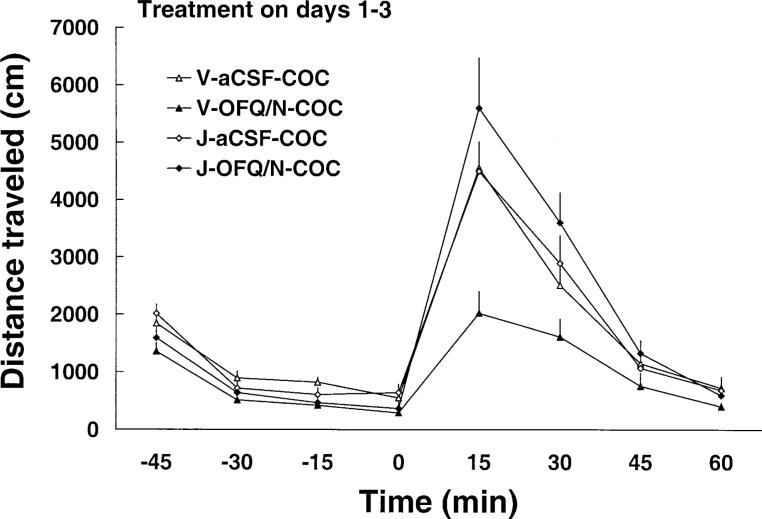
In order to show that the effect of OFQ/N is mediated through the ORL-1 receptor, the effect of OFQ/N on cocaine-induced behavioral sensitization was determined in the presence of J-113397, an ORL-1 receptor antagonist. The ORL-1 receptor antagonist completely blocked the inhibitory action of OFQ/N on day 1 (Fig. 3). A two-way repeated-measure ANOVA showed a significant effect of treatment [F(3,22)=9.71; P<0.05], a significant effect of time with regard to cocaine administration [F(7,154)=45.66; P<0.05] and a significant treatment×time interaction [F(21,154)=6.54; P<0.05]. The post-hoc test revealed that, similar to experiment 1, OFQ/N blocked the motor stimulatory effect of cocaine (P<0.05, compare V-OFQ/N-COC versus V-aCSF-COC group). However, the action of OFQ/N was significantly blocked by J-113397 (P<0.05, compare V-OFQ/N-COC versus J-OFQ/N-COC group). Despite the fact that the dose of OFQ/N was escalated on days 2 and 3, J-113397 was still able to attenuate the action of OFQ/N, the magnitude of the effect of J-113397 was diminished on day 3 (Fig. 3). The effect of a challenge dose of cocaine, administered on day 8, is illustrated in Fig. 4. A two-way repeated-measure ANOVA showed a significant effect of treatment [F(3,22)=5.93; P<0.05], a significant effect of time with regard to cocaine administration [F(7,154)=122.17; P<0.05] and a significant treatment×time interaction [F(21,154)=5.44; P<0.05]. The post-hoc analysis of the data showed that the inhibitory action of OFQ/N on cocaine-induced behavioral sensitization (P<0.05, compare V-OFQ/N-COC versus V-aCSF-COC group) was completely blocked by J-113397 (P>0.05, compare J-OFQ/N-COC versus V-aCSF-COC or J-aCSF-COC group).
Fig. 3.
The ability of OFQ/N to block cocaine-induced motor stimulation was blocked by pretreatment with J-113397, an ORL-1 receptor antagonist. Rats were treated with vehicle (V, 20% DMSO) or J-113397 (J; 30 nmol, ICV) 15 min prior to aCSF or OFQ/N (15 nmol, ICV) followed by cocaine (20 mg/kg, IP). Rats were treated similarly for 3 days, except the dose of OFQ/N was doubled on day 2 and again on day 3. Data are means±SEM of five to eight rats/group
Fig. 4.
Repeated ICV administration of OFQ/N failed to block the development of cocaine-induced behavioral sensitization in the presence of J-113397, an ORL-1 receptor antagonist. Rats were treated for 3 days, as described in the legend to Fig. 3, and left untreated until day 8. On the test day (day 8), rats were habituated for 1 h, and all rats received cocaine (7.5 mg/kg, IP). Locomotor activity was recorded for 1 h. Data are means±SEM of five to eight rats/group
Experiment 3: effects of OFQ/N on cocaine-induced behavioral sensitization when the drugs were injected in the home cage
Locomotor activity after a challenge dose of cocaine (7.5 mg/kg, IP), on day 8, is illustrated in Fig. 5. A two-way repeated-measure ANOVA revealed a significant effect of treatment [F(3,21)=4.35; P<0.05], a significant effect of time with regard to cocaine administration [F(7,147)=41.96; P<0.05] and a significant interaction between treatment and time [F(2,147)=5.93; P<0.05]. The post-hoc test revealed that repeated cocaine treatment in the home cage produced a sensitized response to the motor stimulatory action of the drug (P<0.05, compare aCSF-COC versus aCSF-SAL group). No such sensitization was observed when rats were treated with OFQ/N prior to cocaine administration on days 1−3 (P<0.05, compare OFQ/N-COC versus OFQ/N-SAL or aCSF-SAL group). Thus, OFQ/N blocked the acquisition of cocaine-induced behavioral sensitization regardless of whether the drug was given in the test environment (Fig. 2) or in the home cage (Fig. 5).
Fig. 5.
Intracerebroventricular OFQ/N administration blocked acquisition of cocaine-induced behavioral sensitization regardless of context. Rats were treated with either aCSF or OFQ/N (15 nmol, ICV) followed by saline or cocaine (20 mg/kg, IP) in their home cages. The same treatment was given for 3 days, except the dose of OFQ/N was doubled on day 2 and again on day 3. On day 8, rats were habituated to the testing chambers for 1 h, and all rats received a challenge dose of cocaine (7.5 mg/kg, IP) alone. Locomotor activity was measured for an additional 1 h. Data are means±SEM of five to eight rats/group
Experiment 4: effects of intra-VTA OFQ/N administration on cocaine-induced behavioral sensitization
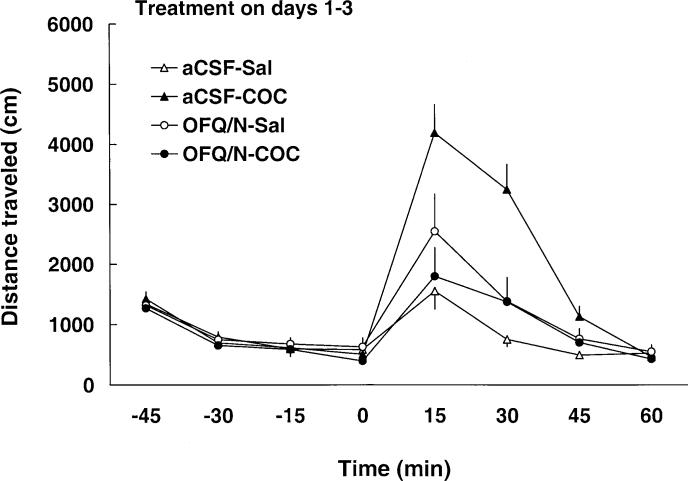
Intra-VTA OFQ/N injection had similar effects on locomotor activity on day 1 (Fig. 6 and Table 1) to those observed after administration into the right lateral ventricle (Fig. 1 and Table 1) during the induction of sensitization. A two-way repeated-measure ANOVA showed a significant effect of treatment [F(3,32)=7.53; P<0.05], a significant effect of time after cocaine administration [F(7,224)=10.54; P<0.05] and a significant treatment×time interaction [F(21,224)=6.91; P<0.05]. The post-hoc test revealed that OFQ/N attenuated the motor stimulatory effect of cocaine (P<0.05, compare aCSF-COC versus OFQ/N-COC group) and also suppressed motor activity (P<0.05, compare aCSF-SAL versus OFQ/N-SAL group). Similar to experiment 1, the inhibitory effect of OFQ/N was still evident in cocaine-, but not saline-treated, rats on days 2 and 3. Locomotor activity after a challenge dose of cocaine (7.5 mg/kg, IP) on day 8 is illustrated in Fig. 7. A two-way repeated-measure ANOVA revealed a significant effect of treatment [F(3,32)=6.51; P<0.05], a significant effect of time with regard to cocaine injection [F(7,224)=62.95; P<0.05] and a significant treatment×time interaction [F(21,224)=7.59; P<0.05]. Again, the Newman-Keuls post-hoc test revealed that the motor stimulatory action of cocaine was enhanced in rats pretreated with aCSF followed by cocaine (P<0.05; compare aCSF-SAL versus aCSF-COC), indicating that sensitization developed to the effect of cocaine in this group. Also similar to experiment 1, the sensitized response was blocked in rats that received OFQ/N prior to systemic cocaine administration on days 1−3 (P<0.05; compare aCSF-COC versus OFQ/N-COC group).
Fig. 6.
Intra-VTA administration of OFQ/N (7.5, 15, 30 nmol on day 1, 2, 3, respectively) blocked the motor stimulatory action of cocaine on days 1−3. Motor activity was recorded for 1 h (in 15-min epochs) before and after intra-VTA administration of aCSF or OFQ/N immediately followed by saline or cocaine (20 mg/kg, IP) administration. Data are means±SEM of nine rats/group
Fig. 7.
Repeated direct injection of OFQ/N into the VTA blocked the development of cocaine-induced behavioral sensitization. Rats were treated for 3 days, as described in the legend to Fig. 3, left untreated for 4 more days and challenged with cocaine on day 8. On the test day (day 8), rats were habituated to the testing chambers for 1 h, and all rats received a challenge dose of cocaine (7.5 mg/kg, IP) alone. Locomotor activity was recorded 1 h after cocaine administration. Data are means±SEM of nine rats/group
Discussion
The main finding of the present study is that escalating doses of OFQ/N administered ICV or directly into the VTA prior to systemic cocaine administration blocked the development of cocaine-induced behavioral sensitization. The action of OFQ/N was not context-dependent and was blocked by J-113397, an ORL-1 receptor antagonist.
The ability of OFQ/N to modulate dopaminergic and glutamatergic neurotransmission in the central nervous system is well established. Since dopamine and particularly glutamate systems have been implicated in cocaine-induced behavioral sensitization (for review, see Vanderschuren and Kalivas 2000), in the present investigation we determined the action of OFQ/N on this phenomenon. Consistent with our previous findings, we found that OFQ/N administration, either ICV or directly into the VTA, acutely suppressed basal motor activity and the motor stimulatory action of cocaine (Narayanan and Maidment 1999; Lutfy et al. 2001a). Similarly, we observed a trend for the motor stimulatory effect of cocaine to be enhanced when OFQ/N was administered repeatedly into the VTA (and to a lesser extent after ICV administration) in the absence of cocaine treatment (Figs 2, 5 and 7). However, our earlier report (Narayanan and Maidment 1999) showed that intra-VTA OFQ/N administration failed to block cocaine-induced behavioral sensitization. Since, in the previous study, the same dose of OFQ/N was administered each day during the induction of sensitization, we considered the possibility that tolerance may have developed to the action of OFQ/N (Devine et al. 1996b; Lutfy et al. 1999). The present study was therefore designed to re-investigate the action of OFQ/N on cocaine-induced behavioral sensitization using an escalating dosing paradigm in an attempt to counteract the development of tolerance. Using this approach, we first tested the effect of ICV OFQ/N administration on cocaine-induced behavioral sensitization. Subsequently, we determined the effect of OFQ/N administered directly into the VTA on this phenomenon because the inhibitory action of OFQ/N on motor activity and extracellular dopamine is mediated, at least in part, in the VTA (Murphy and Maidment 1999; Narayanan and Maidment 1999; Lutfy et al. 2000). In each case, the use of escalating doses produced the predicted attenuation of cocaine-induced motor stimulation on each day of the 3-day treatment period. Furthermore, the data clearly show that concomitant administration of escalating doses of OFQ/N via either route of administration blocked the development of cocaine-induced behavioral sensitization.
OFQ/N shows structural similarities to endogenous opioid peptides, particularly to dynorphin A (1−17) (Meunier et al. 1995; Reinscheid et al. 1995), administration of which also blocks sensitization to cocaine (for review, see Shippenberg and Rea 1997). Since we used relatively high doses of OFQ/N, the possibility that OFQ/N was acting through the kappa site to block cocaine-induced motor stimulation and behavioral sensitization has to be considered. Indeed, Florin and colleagues (1996) suggested that motor suppression at high doses of OFQ/N might be explained by non-specific binding of OFQ/N to kappa opioid receptors. However, we found that the action of OFQ/N on cocaine-induced motor stimulation and behavioral sensitization was blocked by J-113397, an ORL-1 receptor antagonist (Kawamoto et al. 1999), indicating that OFQ/N's actions in this regard are mediated via a specific interaction with the ORL-1 receptor.
It is possible that OFQ/N blocked the development of behavioral sensitization to cocaine simply by virtue of its ability to attenuate the acute effect of cocaine on days 1−3. However, one should bear in mind that the motor stimulatory action of cocaine on days 1−3 was not totally blocked by OFQ/N. Indeed, cocaine still produced a significant (P<0.05) motor stimulation as compared to control rats (Figs 1 and 6; compare OFQ/N-COC versus OFQ/N-SAL). However, the development of sensitization was totally blocked. Furthermore, there is some evidence showing that blockade of motor stimulatory action of cocaine by a dopamine receptor antagonist does not lead to blockade of cocaine-induced behavioral sensitization (Steketee 1998; White et al. 1998; for review, see also Vanderschuren and Kalivas 2000 and references therein). Nevertheless, since OFQ/N attenuated the motor stimulatory action of cocaine on days 1−3, it is possible that rats that received OFQ/N prior to cocaine experienced the test environment quite differently from the rats that received aCSF prior to cocaine administration on days 1−3. This is an important consideration because a component of cocaine-induced behavioral sensitization is context-dependent (for review, see Post et al. 1987). However, OFQ/N retained the ability to block the development of cocaine-induced behavioral sensitization when OFQ/N and cocaine were administered in the home cage during the induction of sensitization and challenged in a novel environment (Fig. 5). Thus, it appears that OFQ/N is acting through a novel mechanism to block acquisition of cocaine sensitization, primarily involving prevention of long-term plastic changes produced by repeated cocaine administration.
The present data highlight the VTA as a possible site of action of OFQ/N in this regard but cannot rule out other sites. Importantly, OFQ/N has been shown to block LTP in hippocampal slices (Yu et al. 1997) and impair spatial learning (Sandin et al. 1997; Redrobe et al. 2000) in rats. Furthermore, OFQ/N administration blocks morphine-induced conditioned place preference (Murphy et al. 1999; Ciccocioppo et al. 2000) and tolerance (Lutfy et al. 2001b), both of which rely on associative learning processes. Moreover, nociceptin-knockout mice display enhanced learning ability in the water-maze test (Manabe et al. 1998), water finding task (Mamiya et al. 1998) and passive avoidance task (Mamiya et al. 1999). These mice also show a greater LTP (Manabe et al. 1998) in the hippocampal CA1 region as compared to their wild-type littermates (see also Noda et al. 2000, for review). Thus, it seems possible that OFQ/N could interfere with memory processing and block cocaine-induced behavioral sensitization. Such processes are known to involve activation of the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors (for review, see Wolf 1998; Vanderschuren and Kalivas 2000). Reports of OFQ/N-induced inhibition of glutamate release in the cortex (Nicol et al. 1996) and lateral amygdala (Meis and Pape 2001) are therefore of interest in this regard.
Our data provide no further information as to the mechanisms underlying this action of OFQ/N. Repeated intermittent cocaine treatment has been shown to modify endogenous opioid peptide gene expression (Sivam 1989; Hurd et al. 1992) and opioid receptor number (Hammer et al. 1989; Unterwald et al. 1992). Furthermore, mu and delta opioid receptor antagonists have been shown to block the development of cocaine sensitization (Sala et al. 1995; Heidbreder et al. 1996). OFQ/N has been shown to oppose the action of mu and delta opioid receptor agonists in a number of physiological systems (for review, see Mogil and Pasternak 2001). It is therefore possible that OFQ/N acts in a similar manner to block the development of cocaine-induced behavioral sensitization.
In summary, OFQ/N when administered ICV or directly into the VTA, in escalating doses, prior to systemic cocaine administration, blocks the development of cocaine-induced locomotor sensitization. This action of OFQ/N was selectively mediated through the ORL-1 receptor because OFQ/N failed to exert its inhibitory effects in the presence of J-113397, an ORL-1 receptor antagonist. Given the proposed importance of such sensitized response to addictive process, OFQ/N and its receptor could represent new pharmacotherapeutic targets for the treatment of cocaine addiction.
Acknowledgements
We are grateful to Dr. Shridhar Narayanan for his critical review of the manuscript and Hoa Lam and Sherifah Farasat for technical assistance. This study was supported in part by NIDA grant DA00411 and a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (K.L.). N.T.M. and F.I.C. were supported by DA50110 and DA09045, respectively. Special thanks to Mr. and Mrs. Garen Staglin for their support of the NARSAD award.
References
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Devine DP, Reinscheid RK, Monsma FJ, Jr, Civelli O, Akil H. The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res. 1996a;727:225–229. doi: 10.1016/0006-8993(96)00476-3. [DOI] [PubMed] [Google Scholar]
- Devine DP, Taylor L, Reinscheid RK, Monsma FJ, Jr, Civelli O, Akil H. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem Res. 1996b;21:1387–1396. doi: 10.1007/BF02532380. [DOI] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S, Catalani A, Loizzo A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett. 1999;272:183–186. doi: 10.1016/s0304-3940(99)00579-0. [DOI] [PubMed] [Google Scholar]
- Florin S, Suaudeau C, Meunier J- C, Costentin J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur J Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- Hammer RP., Jr Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, Shoaib M, Shippenberg TS. Differential role of delta-opioid receptors in the development and expression of behavioral sensitization to cocaine. Eur J Pharmacol. 1996;298:207–216. doi: 10.1016/0014-2999(95)00815-2. [DOI] [PubMed] [Google Scholar]
- Herz A. Opioid reward mechanisms: a key role in drug abuse? Can J Physiol Pharmacol. 1998;76:252–258. doi: 10.1139/cjpp-76-3-252. [DOI] [PubMed] [Google Scholar]
- Hockfelt T, Ljungdahl A, Fuxe K, Johansson O. Dopamine nerve terminals in the rat limbic cortex: aspects of the dopamine hypothesis of schizophrenia. Science. 1974;184:177–179. doi: 10.1126/science.184.4133.177. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Mol Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of reverse tolerance to cocaine and amphetamine by MK-801. Life Sci. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Ozaki S, et al. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one (J-113397). J Med Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Sharza SA, Maidment NT. Tolerance develops to the inhibitory effect of orphanin FQ on morphine-induced antinociception in the rat. Neuroreport. 1999;10:103–106. doi: 10.1097/00001756-199901180-00020. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Narayanan S, Maidment NT. Orphanin FQ/nociceptin suppresses basal motor activity via a selective action on the mesoaccumbens axis; Third European Opioid Conference Abstract; 2000. T12. [Google Scholar]
- Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology. 2001a;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Hossain SM, Khaliq I, Maidment NT. Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats. Br J Pharmacol. 2001b;134:529–534. doi: 10.1038/sj.bjp.0704279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya T, Noda Y, Nishi M, Takeshima H, Nabeshima T. Enhancement of spatial attention in nociceptin/orphanin FQ receptor-knockout mice. Brain Res. 1998;783:236–240. doi: 10.1016/s0006-8993(97)01406-6. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Noda Y, Nishi M, Takeshima H, Nabeshima T. Nociceptin system plays a role in the memory retention: involvement of naloxone benzoylhydrazone binding sites. Neuroreport. 1999;10:1171–1175. doi: 10.1097/00001756-199904260-00003. [DOI] [PubMed] [Google Scholar]
- Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, Noda T, Takahashi T, Sugimoto T, Nabeshima T, Takeshima H. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- Matthysse S. Antipsychotic drug actions: a clue to the neuropathology of schizophrenia? Fed Proc. 1973;32:200–205. [PubMed] [Google Scholar]
- Meis S, Pape HC. Control of glutamate and GABA release by nociceptin/orphanin FQ in the rat lateral amygdala. J Physiol. 2001;532:701–712. doi: 10.1111/j.1469-7793.2001.0701e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Ly HT, Maidment NT. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Narayanan S, Maidment NT. Orphanin FQ and behavioral sensitization to cocaine. Pharmacol Biochem Behav. 1999;63:271–277. doi: 10.1016/s0091-3057(98)00261-5. [DOI] [PubMed] [Google Scholar]
- Nicol B, Lambert DG, Rowbotham DJ, Smart D, McKnight AT. Nociceptin induced inhibition of K+ evoked glutamate release from rat cerebrocortical slices. Br J Pharmacol. 1996;119:1081–1083. doi: 10.1111/j.1476-5381.1996.tb16007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Mamiya T, Manabe T, Nishi M, Takeshima H, Nabeshima T. Role of nociceptin systems in learning and memory. Peptides. 2000;21:1063–1069. doi: 10.1016/s0196-9781(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd edn. Academic Press; San Diego, USA: 1986. [Google Scholar]
- Post RM, Contel NR. Human and animal studies of cocaine: implications for development of behavioral pathology. In: Creese I, editor. Stimulants: neurochemical, behavioral and clinical perspectives. Raven Press; New York: 1983. pp. 169–203. [Google Scholar]
- Post RM, Weiss SR, Pert A. The role of context and conditioning in behavioral sensitization to cocaine. Psychopharmacol Bull. 1987;23:425–429. [PubMed] [Google Scholar]
- Redrobe JP, Calo G, Guerrini R, Regoli D, Quirion R. [Nphe(1)]-Nociceptin (1−13)-NH(2), a nociceptin receptor antagonist, reverses nociceptin-induced spatial memory impairments in the Morris water maze task in rats. Br J Pharmacol. 2000;131:1379–1384. doi: 10.1038/sj.bjp.0703724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Colombo M, Groppetti A, Sacco S, Gori E, Parenti M. Behavioral and biochemical evidence of opioidergic involvement in cocaine sensitization. J Pharmacol Exp Ther. 1995;274:450–457. [PubMed] [Google Scholar]
- Sandin J, Georgieva J, Schott PA, Ogren SO, Terenius L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur J Neurosci. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Rea W. Sensitization to the behavioral effects of cocaine: modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol Biochem Behav. 1997;57:449–455. doi: 10.1016/s0091-3057(96)00450-9. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- Steketee JD. Injection of SCH 23390 into the ventral tegmental area blocks the development of neurochemical but not behavioral sensitization to cocaine. Behav Pharmacol. 1998;9:69–76. [PubMed] [Google Scholar]
- Stevens JR. An anatomy of schizophrenia? Arch Gen Psychiatry. 1973;29:177–189. doi: 10.1001/archpsyc.1973.04200020023003. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Horne-King J, Kreek MJ. Chronic cocaine alters brain mu opioid receptors. Brain Res. 1992;584:314–318. doi: 10.1016/0006-8993(92)90912-s. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P. Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: an in vivo microdialysis study in the rat. Brain Res. 1993;605:332–337. doi: 10.1016/0006-8993(93)91761-g. [DOI] [PubMed] [Google Scholar]
- White FJ, Joshi A, Koeltzow TE, Hu XT. Dopamine receptor antagonists fail to prevent induction of cocaine sensitization. Neuropsychopharmacology. 1998;18:26–40. doi: 10.1016/S0893-133X(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. TIPS. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- Yu TP, Fein J, Phan T, Evans CJ, Xie CW. Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus. 1997;7:88–94. doi: 10.1002/(SICI)1098-1063(1997)7:1<88::AID-HIPO9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]