Abstract
Signal transduction through the leukocyte integrins is required for the processes of firm adhesion, activation, and chemotaxis of neutrophils during inflammatory reactions. Neutrophils isolated from knockout mice that are deficient in the expression of p59/61hck (Hck) and p58c-fgr (Fgr), members of the Src-family of protein tyrosine kinases, have been shown to be defective in adhesion mediated activation. Cells from these animals have impaired induction of respiratory burst and granule secretion following plating on surfaces that crosslink β2 and β3 integrins. To determine if the defective function of hck−/−fgr−/− neutrophils observed in vitro also results in impaired inflammatory responses in vivo, we examined responses induced by lipopolysaccharide (LPS) injection in these animals. The hck−/−fgr−/− mice showed marked resistance to the lethal effects of high-dose LPS injection despite the fact that high levels of serum tumor necrosis factor α and interleukin 1α were detected. Serum chemistry analysis revealed a marked reduction in liver and renal damage in mutant mice treated with LPS, whereas blood counts showed a marked neutrophilia that was not seen in wild-type animals. Direct examination of liver sections from mutant mice revealed reduced neutrophil migration into the tissue. These data demonstrate that defective integrin signaling in neutrophils, caused by loss of Hck and Fgr tyrosine kinase activity, results in impaired inflammation-dependent tissue injury in vivo.
Neutrophils (polymorphonuclear leukocytes, PMNs) serve as the primary effector cells in innate immune responses. During inflammatory responses chemotatic stimuli induce PMNs to bind to endothelial cells and migrate into the tissues. During this migratory process, PMNs become fully activated to release reactive oxygen intermediates and granule constituents. Adhesive interactions between PMNs and extracellular matrix proteins or counterreceptors on endothelial cells are required for activation of PMNs (1). The integrin receptors (principally β2 and β3) have been shown to be the major adhesive molecules that mediate PMN activation (2–4).
Uncontrolled neutrophil activation is a major cause of tissue damage in a number of inflammatory models including lipopolysaccharide (LPS)-induced endotoxemia, ischemia-reperfusion injury, immune complex deposition, and complement activation (5–9). In all cases, inhibition of integrin-mediated cell adhesion, through use of blocking mAbs, has been shown to limit the tissue damage. In the endotoxin model, both β2-intracellular adhesion molecule 1 (ICAM-1) and β1-vascular cell adhesion molecule 1 (VCAM-1) interactions have been shown to be involved in the steps by which PMNs migrate out of the liver microvascular and then adhere directly to hepatocytes (5, 10). The requirement for adhesion molecules in PMN migration and activation in the LPS-endotoxin model is validated by the observation that ICAM-1-deficient animals show reduced accumulation of PMNs in the liver and improved survival following high-dose LPS injection (11).
The intracellular signaling pathways that lead from integrin ligation to PMN activation depend on tyrosine kinases (12, 13). The principle tyrosine kinases that have been implicated in adhesion-dependent PMN activation are the Src-family kinases—in PMNs these are p59/61hck (Hck), p58c-fgr (Fgr), and p53/56lyn (Lyn)—as well as the p72syk kinase (14, 15). The critical role of Hck and Fgr in integrin signaling in PMNs was directly demonstrated by using hck−/−fgr−/− knockout mice. PMNs from these mice fail to undergo respiratory burst when plated on extracellular matrix proteins or ICAM-1-coated plates because of an inability to appropriately adhere to these molecules (16). More recently, it has also been found that double mutant PMNs are also deficient in degranulation following adhesion (A. Mocsai, E. Ligeti, C.A.L., and G.B., unpublished data). These defects are presumably caused by the inability of β2 and β3 integrins in hck−/−fgr−/− PMNs to signal changes in rearrangement of the actin cytoskeleton that lead to neutrophil spreading and activation. These in vitro observations would predict that hck−/−fgr−/− mice should show defects in in vivo inflammation models that are dependent on PMN adhesion. In this report, we show that hck−/−fgr−/− mice are dramatically resistant to LPS-induced liver damage and have reduced accumulation of PMNs in the liver tissue during endotoxemia. These data demonstrate that ineffective signaling from leukocyte integrins can lead to PMN functional defects in vivo.
MATERIALS AND METHODS
Mice.
The hck−/−fgr−/− double mutant mice used in these experiments were generated as described (17). All mice used were backcrossed to C57BL/6J for four generations. Animals used as wild-type controls were derived from these same backcrosses, thus controlling for genetic background effects on LPS responses. All animals were maintained at the University of California, San Francisco, Transgenic Facility under specific-pathogen free conditions. Animals were used for single experiments only and never re-used. All animals used ranged in age from 8 to 12 weeks to match for age and body weight during LPS injection experiments. Routine genotyping of animals was carried out by PCR as described (18).
Endotoxic Shock.
LPS from Eschericahia coli 0127:B7 and d-galactosamine (d-Gal) were purchased from Sigma. LPS was suspended in PBS/2% fetal calf serum (FCS) and stored as a 20 mg/ml stock. Dilutions prior to injection were into PBS/2% FCS. FCS was used to ensure solubilization of LPS by providing a source of LPS binding protein. Animals were weighed prior to injection of LPS and stock LPS was diluted to the appropriate dose for each animal. Mice were injected i.p. For coinjections of LPS and d-Gal, the d-Gal stock was diluted into PBS/2% FCS. Animals were monitored at least twice daily during endotoxic shock, and moribund animals were removed.
Blood Collections, Chemistry, Hematology, and Cytokine Determinations.
A total of 200–300 μl of blood was collected by retroorbital sinus bleeding at the indicated time points by using heparinized microcapillary pipettes. Serum was sent to Consolidated Veterinary Diagnostics (West Sacramento, CA) for determination of the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which both assess liver damage; blood urea nitrogen (BUN), which is effected by renal function; and lactate dehydrogenase (LDH), which reflects muscle, kidney, and liver damage. For hematology, retroorbital blood was collected into EDTA-containing microtubes (Becton Dickinson) and sent to Consolidated Veterinary Diagnostics laboratories for automated blood counts and differentials. Cytokine levels in serum were determined by ELISA by using commercially available kits (Endogen, Cambridge, MA). For each assay, serum was serially diluted to ensure that values obtained were within the linear range of the standards provided with each kit. Each sample was done in duplicate, and data from individual mice were averaged.
Histology.
Liver sections from LPS-treated mice were fixed in 4% paraformaldehyde at 4°C for 12–18 hr and then processed for imbedding in glyceryl methacsylate-plastic (to allow thinner sections for histochemistry). Sections were stained with hematoxylin/eosin or for chloroacetate esterase (Sigma) to facilitate visualization of neutrophils. Naphthol-AS D chloroacetate was used as the substrate to detect the nonspecific esterase present in neutrophils by using the procedure suggested by the manufacturer (Sigma). Neutrophils numbers were counted in ×40 fields chosen randomly from different sections. Histologic sections were evaluated by observers that were not aware of the genotypes of the mice from which the tissues were isolated.
RESULTS
Double Mutant hck−/−fgr−/− Mice Are Resistant to High-Dose LPS but Are Sensitive to Low-Dose LPS + d-Gal.
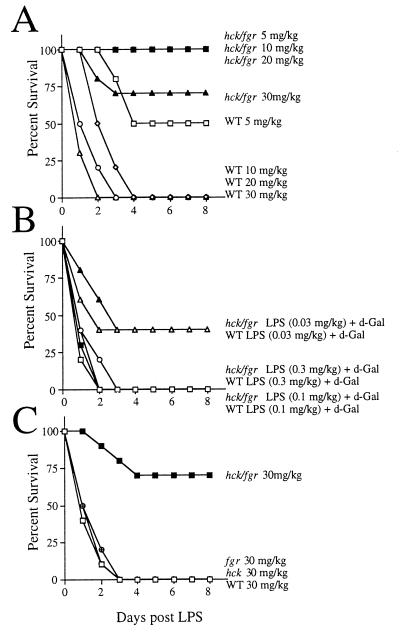
To test the physiologic significance of the previously observed defects in adhesion-dependent neutrophil function in hck−/−fgr−/− mice (ref. 16 and Mocsai et al., unpublished data), we tested for alterations in tissue damage and survival after induction of endotoxic shock. In pilot experiments it was noted that double mutant hck−/−fgr−/− mice displayed significantly improved survival following administration of high doses of LPS compared with wild-type littermates. To formally demonstrate this, we generated a large cohort of age/weight/genetic background-matched animals and conducted a complete dose response/survival curve following high-dose LPS administration. As shown in Fig. 1A, wild-type mice showed 50% mortality after injection of LPS at 5 mg/kg and 100% mortality at higher doses. In contrast, hck−/−fgr−/− animals showed only 20% mortality at the highest doses of LPS, and there were no deaths in the lower dose groups. Despite their enhanced survival after LPS injections, the hck−/−fgr−/− animals did demonstrate many features characteristic of endotoxic shock including lethargy, shivering, watery eyes, hypothermia (rodents develop hypothermia to endotoxic shock whereas humans develop fever), and a 20% body weight loss. Many of these systemic signs of endotoxemia are mediated by the high levels of pro-inflammatory cytokines that are released into the circulation in the initial phases of the response (19, 20).
Figure 1.
Double mutant hck−/−fgr−/− mice are resistant to high-dose LPS but are sensitive to low dose LPS following d-Gal sensitization. (A) Wild-type and hck−/−fgr−/− mutant mice were injected i.p. with the indicated doses of LPS, and survival was monitored over the subsequent 8 days. (B) Wild-type and double mutant mice were injected with d-Gal (20 mg) plus the indicated doses of LPS, and survival was monitored. (C) Wild-type, hck−/−, fgr−/−, and hck−/−fgr−/− mutant mice were injected with 30 mg/kg LPS, and survival was monitored. Data for all graphs are from independent experiments done separately. In A and C, 10 age and weight-matched animals were used per curve (hence A represents a total of 40 hck−/−fgr−/− mice and 40 wild-type animals); in B, 10 hck−/−fgr−/− and 10 wild-type mice were used for the 0.3 mg/kg doses; other doses used 5 animals per curve.
We also tested the responses of cohorts of wild-type and mutant mice to coinjection of low dose LPS and d-Gal. In this model, the d-Gal serves to impair liver metabolism rendering mice extremely sensitive to very low doses of LPS. The tumor necrosis factor α (TNF-α) released into the blood during low-dose endotoxemia acts as a direct hepatotoxin in d-Gal-treated mice (21–23). In contrast to the high-dose endotoxic model used above, wild-type and double mutant mice showed equivalent sensitivity to coadministration of low-dose LPS and d-Gal (Fig. 1B). These data demonstrate that double mutant mice show normal hepatoxicity to TNF-α when sensitized with d-Gal. In pilot experiments, double mutant mice were also very sensitive to coinjection of Staphylococcal enterotoxin B (SEB) and d-Gal. In this exotoxin model the SEB activates T cells, which in turn induce macrophage production of TNF-α (24). Because neither Hck nor Fgr are expressed in T cells (25), these mutations should not directly affect T cell function. Therefore, further evaluation of the responses of hck−/−fgr−/− mice to SEB-induced shock was not done.
Previous examination of adhesion-dependent respiratory burst and degranulation in in vitro experiments revealed that single mutant hck−/− or fgr−/− PMNs functioned normally (16). To determine if this were the case in the high-dose endotoxic shock model, cohorts of wild-type, single mutant, and double mutant animals were injected with LPS and examined for survival. In contrast to double mutants, we found that hck−/− and fgr−/− single mutant mice showed equivalent sensitivity to LPS compared with wild-type animals (Fig. 1C). Hence, a disruption of both kinases is required for development of LPS resistance.
Cytokine Production Is Normal in Double Mutant Mice.
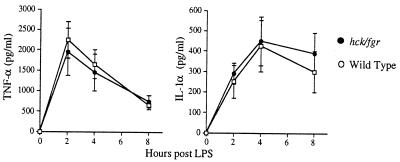
To determine if cytokine production was affected in double mutant mice, we determined the serum TNF-α and interleukin 1 (IL-1) levels in these mice at various time points following LPS administration. As shown in Fig. 2, wild-type and hck−/−fgr−/− mice displayed similar kinetics and total levels of production of these pro-inflammatory cytokines. TNF-α levels peaked at 1 hr following LPS administration, whereas IL-1α levels became maximal at 2–3 hr, in both wild-type and mutant mice, a kinetic pattern that has been well established in the high-dose LPS model (26, 27). Normal cytokine production in double mutant mice is expected because these animals showed extreme sensitivity to low-dose LPS when sensitized with d-Gal, and mutant macrophages respond normally to LPS challenge in vitro (18).
Figure 2.
Serum levels of TNF-α and IL-1 are equivalent in wild-type and mutant mice following LPS injection. Wild-type and hck−/−fgr−/− mice were injected i.p. with LPS (30 mg/kg), and serum was collected by retroorbital bleeding at the indicated time points. Serum levels of TNF-α and IL-1α were determined by ELISA. Data shown are averaged values from five mice per genotype ± SD.
Reduced Tissue Damage and Peripheral Blood Neutrophilia in hck−/−fgr−/− Mice During Endotoxic Shock.
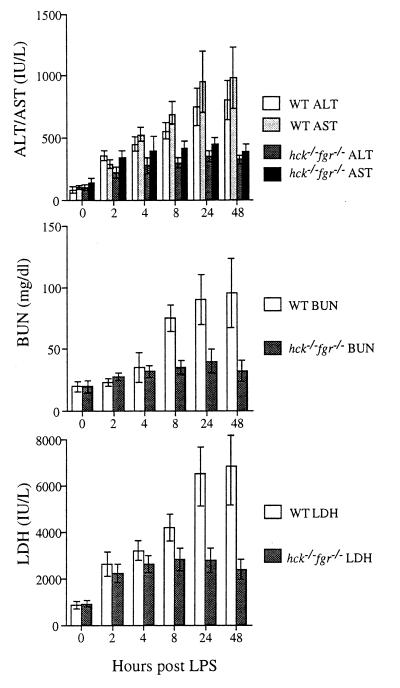
To determine if the reduced sensitivity of double mutant mice to LPS was caused by reduced tissue damage, we examined the serum levels of proteins that reflect liver necrosis, renal function, and muscle/kidney damage after LPS administration. Although wild-type mice showed progressive increases in the serum levels of AST/ALT, BUN, and LDH over the 2-day time course, the levels of these enzymes in double mutant mice showed only an initial increase then remained steady (Fig. 3). Perhaps the most dramatic change seen in wild-type mice was the increase in serum BUN, which reflects progressive renal failure during endotoxic shock. In contrast, double mutant mice did not develop this profound renal failure. Interestingly, double mutant animals did show a 20% decrease in body weight over the course of the experiment, whereas wild-type animals did not loose weight. These data demonstrate that double mutant mice had significantly reduced levels of tissue damage following LPS administration.
Figure 3.
Double mutant hck−/−fgr−/− mice develop only mild tissue damage during endotoxic shock. Serum chemistry levels assessing liver damage (AST/ALT), renal function (BUN), and muscle/kidney damage (LDH) were determined in wild-type and double mutant mice at the indicated time points following i.p. injection of LPS (30 mg/ml). A total of 15 mice per group were injected, and blood was collected by retroorbital bleeding at the indicated times; however within 24 hr 9 wild-type mice had died and by 48 hr only 2 animals remained; therefore, these two time points reflect data averaged from fewer animals. Over the course of this experiment 13 hck−/−fgr−/− mutants survived completely (2 died before 48 hr; hence the final data points reflects results averaged from remaining animals). To facilitate comparison between wild-type and mutant animals, we determined the slopes of curves between the 0 and 24-hr time points for each analyte. For all analytes the slope was greater than 3-fold higher in wild-type (wt) than hck−/−fgr−/− animals (AST: wt = 31, mutant = 8; ALT: wt = 22, mutant = 7; BUN: wt = 2.9, mutant 0.6; LDH: wt = 203, mutant = 47). Error bars are SD.
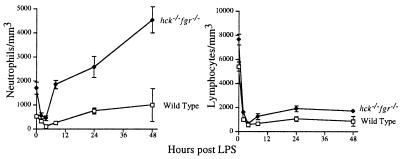
Peripheral blood counts also were determined in these same cohorts of wild-type and double mutant mice after LPS administration. As previously seen in murine high dose LPS models (11), wild-type mice developed both severe lymphopenia and neutropenia following LPS administration (Fig. 4). The drop in lymphocyte counts is presumably caused by high levels of endogenous corticosteroids produced during endotoxemia, which in turn induce massive lymphocyte apoptosis, whereas the decline in PMNs is caused by sequestration in the tissues (28–30). Double mutant mice demonstrated a similar drop in blood lymphocyte counts, and there was obvious evidence of lymphocyte apoptosis in histologic sections of the spleen (data not shown). In contrast, double mutant mice showed a transient fall in blood PMNs within 2–4 hr following LPS administration, and thereafter peripheral blood levels of PMNs rose dramatically.
Figure 4.
Double mutant mice develop peripheral neutrophilia during endotoxic shock. Total blood neutrophil and lymphocyte counts were determined in the same cohort of mice used in Fig. 3.
PMN Accumulation in the Liver During Endotoxemia Is Reduced in Double Mutant Animals.
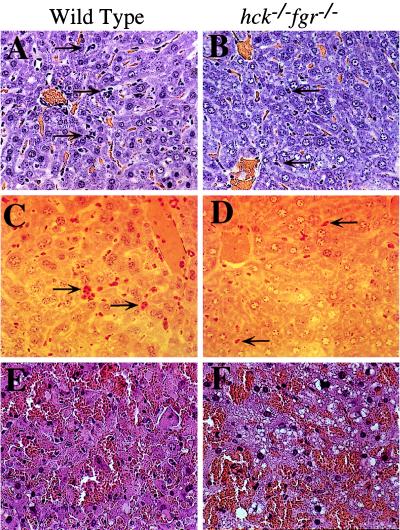
To directly assess migration of PMNs into tissues during endotoxemia, we examined histologic sections of various tissues isolated 24 hr after LPS administration. The clearest evidence of PMN accumulation was seen in liver sections (Fig. 5A). PMNs, either single cells or in clusters, were observed around venules or within the liver tissue. The severity of PMN infiltration varied from section to section; however, all sections from wild-type mice exhibited clear evidence of PMN accumulation within the liver. To confirm that the observed leukocytes were PMNs, sections were stained for chloroacetate esterase, which is present in the azurophilic granules of PMNs (31). All infiltrating leukocytes showed clear evidence of esterase activity in this histochemical stain (Fig. 5C). In contrast to wild type, livers from hck−/−fgr−/− mice had dramatically lower levels of PMN infiltration, reflected in both hematoxylin/eosin stains and histochemical stains for esterase (Fig. 5 B and D). To quantitate the relative differences between mutant and wild type, the total number of PMNs in five randomly chosen microscopic fields from three separate animals were counted. An average of 85 ± 12 PMNs per ×40 high power field were seen in wild-type livers, whereas double mutant livers showed 20 ± 10 cells. These data provide direct evidence of impaired neutrophil adhesion and infiltration to the liver parenchyma during endotoxemia.
Figure 5.
Neutrophil migration to the liver during endotoxic shock is reduced in hck−/−fgr−/− mice. (A–D) Wild-type and double mutant mice were injected i.p. with LPS (30 mg/kg) and killed 24 hr later. Liver tissues were fixed for 18 hr in 4% paraformaldehyde and mounted in GMA-plastic. Sections were stained with (A and B) hematoxylin/eosin or (C and D) chloroacetylesterase (to detect neutrophil esterases). Arrows indicate neutrophils present either as clusters or as single cells within the liver parenchyma. Photomicrographs are representative of samples from five independent animals. (E and F) Wild-type and double mutant mice were injected with d-Gal (20 mg) and LPS (0.3 mg/kg) and killed 24 hr later. Liver tissues were fixed/mounted as above and stained with hematoxylin/eosin.
The histologic findings in livers of wild-type and mutant mice treated with low-dose LPS + d-Gal were very similar (Fig. 5 E and F). In both cases, severe hemorrhagic necrosis of hepatocytes was clearly present. Robust liver infiltration by PMNs was not obvious in these samples, consistent with the notion that the primary mechanism of injury in this model is via direct toxicity of TNF-α on d-Gal sensitized hepatocytes.
DISCUSSION
Signal transduction through cell surface integrins is required for the firm adhesion of PMNs to vascular surfaces as well as for the transmigration of PMNs into the tissues. These same adhesion-mediated signals activate PMNs to induce respiratory burst and degranulation (1). PMN activation and chemotaxis is intimately linked to the ability to rearrange the actin cytoskeleton following integrin ligation at the cell surface; indeed, most of the functional consequences of integrin-mediated cell adhesion are dependent on the ability of these receptors to signal cytoskeletal rearrangements that lead to cell spreading (32). Among the signaling molecules in PMNs, the Src-family kinases Hck and Fgr have been shown to be critical in initiating cytoskeletal rearrangement leading to radical oxygen intermediates generation and degranulation (ref. 16 and Mocsai et al., unpublished data). This finding has been demonstrated by using hck−/−fgr−/− knockout mice; in vitro assays have demonstrated defective adhesion-dependent PMN spreading and activation by using cells isolated from mutant mice. In both assays, it has been found that single mutant PMNs are functionally normal; hence these kinases show functional redundancy in the integrin signaling pathway. Through the use of mAbs, it was demonstrated that signaling through both β2 and β3 integrins are affected in hck−/−fgr−/− cells. To test whether these defects would affect PMN function in vivo, we examined neutrophil responses during high-dose LPS-mediated endotoxemia. The observations that hck−/−fgr−/− mice show reduced PMN infiltration into the liver (with accumulation of cells in the peripheral blood), reduced tissue damage, and improved survival following LPS administration demonstrates that these kinases play significant roles in PMN function in vivo. The functional redundancy observed in in vitro assays was also reflected in this in vivo model as single mutant animals showed no survival advantage over wild-type animals.
The role of the neutrophil in mediating tissue damage during sepsis has recently been reviewed (27, 33). The models of PMN function during endotoxemia are discussed with respect to liver damage as this is the most extensively studied tissue that is affected by LPS administration. Within 1–3 hr following LPS administration there is a rapid drop in peripheral blood PMN counts because of sequestration of cells in the microvasculature; the mechanism of this initial drop is thought to be caused by increased viscosity of activated PMNs which restricts passage of cells through capillaries (34). Subsequent accumulation of PMNs within the liver parenchyma is due principally to cell adhesive interactions (versus the mechanical restrictions that occur initially) that are required for transmigration of PMNs into the liver. During the process of adhesion and migration, PMNs release free oxygen intermediates and proteases that damage the vascular endothelium and surrounding hepatocytes. The primary stimulus that may recruit PMNs to the liver during endotoxemia is severe ischemia caused by the transient vasoconstriction induced by production of leukotrienes. Following restoration of normal blood flow, the inflammatory response in the liver follows a well-described process seen in any reperfusion injury: local production of cytokines, up-regulation of vascular adhesion molecules (ICAM-1), and attraction of PMNs which mediate tissue damage. This process is likely occurring in kidney, muscle, and other tissues, which accounts for the widespread tissue damage during endotoxemia. The central role of the PMN in mediating the tissue damage is evidenced by the fact that neutrophil depletion protects mice from LPS-induced lethality (35).
LPS-induced tissue damage requires appropriate PMN adhesion to vascular endothelium. This result was convincingly demonstrated by the observation that mice lacking ICAM-1, the major endothelial cell ligand for leukocyte integrins, are resistant to high-dose LPS (11). ICAM-1 deficient animals have a phenotype very similar to the hck−/−fgr−/− mutants in that they produce normal levels of cytokines following LPS administration, have reduced migration of PMNs into the liver and hence reduced tissue damage, whereas manifesting a transient fall in blood PMN counts (probably because of mechanical accumulation of cells in the microvasculature) followed by a dramatic neutrophilia. The development of neutrophilia seen in both hck−/−fgr−/− double mutants and icam-1−/− animals is probably caused by increased PMN production in the bone marrow in response the elevated serum cytokine levels produced during endotoxic shock; however in both mutant mouse strains the newly formed PMNs fail to marginate along endothelial cells in the microvasculature and instead accumulate in the blood. Like hck−/−fgr−/− mice, ICAM-1 deficient animals are also very susceptible to low-dose LPS following d-Gal sensitization. However, unlike hck−/−fgr−/− animals, the icam-1−/− mutants are resistant to exotoxin (SEB). This latter difference is probably due to the fact that ICAM-1-dependent cell–cell interactions are needed for SEB-induced T cell activation; because Hck and Fgr are not expressed in T cells, the absence of these kinases should not affect T cell adhesive events. Overall, the high similarly in the LPS-resistant phenotype of icam-1−/− and hck−/−fgr−/− animals suggests that these mutations are affecting the same physiologic function (i.e., neutrophil adhesion). The ICAM-1 deficiency removes the main ligand for β2 integrins whereas the absence of Hck/Fgr affects the signaling through these integrins required for firm adhesion of PMNs to endothelial cells.
These data would predict that other immune responses in hck−/−fgr−/− animals should be impaired because of poor PMN adhesion. Indeed, the previously described susceptibility of these mice to Listeria infection (17) may be because of impaired integrin-mediated signaling, as blockade of leukocyte β2 integrins also results in susceptibility to Listeria (36). Hence, further examination of inflammatory reactions in these mice is clearly warranted. Our findings demonstrate that molecules implicated in integrin signaling are essential for inflammation-dependent tissue injury, thus suggesting that Src-family kinases are potential therapeutic targets for controlling inflammatory diseases.
Acknowledgments
We thank the University of California, San Francisco, Animal Care technical staff for assistance with the very large number of animals used in this study. This work was supported by National Institutes of Health Grants DK50267 and HL54476 to C.A.L. and by grants from the Italian Association for Cancer Research (Associazione Italiana per la Ricerca sul Cancro) and the National Research Council of Italy (Consiglio Nazionale delle Ricerche, Strategic Project: Cytokines) to G.B.
ABBREVIATIONS
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- BUN
blood urea nitrogen
- d-Gal
d-galactosamine
- ICAM-1
intracellular adhesion molecule 1
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- PMN
polymorphonuclear leukocyte
- SEB
Staphylococcal enterotoxin B
- VCAM-1
vascular cell adhesion molecule 1
- TNF-α
tumor necrosis factor α
- IL
interleukin
References
- 1.Berton G, Yan S R, Fumagalli L, Lowell C A. Int J Clin Lab Res. 1996;26:160–177. doi: 10.1007/BF02592978. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C, Srimal S, Farber C, Sanchez E, Kabbash L, Asch A, Gailit J, Wright S D. J Cell Biol. 1989;109:1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shappell S B, Toman C, Anderson D C, Taylor A A, Entman M L, Smith C W. J Immunol. 1990;144:2702–2711. [PubMed] [Google Scholar]
- 4.Zhou M, Brown E J. J Exp Med. 1993;178:1165–1174. doi: 10.1084/jem.178.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeschke H, Farhood A, Smith C W. Am J Physiol. 1991;261:1051–1056. doi: 10.1152/ajpgi.1991.261.6.G1051. [DOI] [PubMed] [Google Scholar]
- 6.Mulligan M S, Smith C W, Anderson D C, Todd R F d, Miyasaka M, Tamatani T, Issekutz T B, Ward P A. J Immunol. 1993;150:2401–2406. [PubMed] [Google Scholar]
- 7.Jaeschke H, Farhood A, Smith C W. FASEB J. 1990;4:3355–3559. [PubMed] [Google Scholar]
- 8.Seekamp A, Mulligan M S, Till G O, Smith C W, Miyasaka M, Tamatani T, Todd R F D, Ward P A. Am J Pathol. 1993;143:464–472. [PMC free article] [PubMed] [Google Scholar]
- 9.Mulligan M S, Wilson G P, Todd R F, Smith C W, Anderson D C, Varani J, Issekutz T B, Miyasaka M, Tamatani T, Myasaka M, et al. J Immunol. 1993;150:2407–2417. [PubMed] [Google Scholar]
- 10.Essani N A, Bajt M L, Farhood A, Vonderfecht S L, Jaeschke H. J Immunol. 1997;158:5941–5948. [PubMed] [Google Scholar]
- 11.Xu H, Gonzalo J A, St Pierre Y, Williams I R, Kupper T S, Cotran R S, Springer T A, Gutierrez-Ramos J C. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laudanna C, Rossi F, Berton G. Biochem Biophys Res Commun. 1993;190:935–940. doi: 10.1006/bbrc.1993.1139. [DOI] [PubMed] [Google Scholar]
- 13.Fuortes M, Jin W W, Nathan C. J Cell Biol. 1993;120:777–784. doi: 10.1083/jcb.120.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan S R, Huang M, Berton G. J Immunol. 1997;158:1902–1910. [PubMed] [Google Scholar]
- 15.Yan S R, Fumagalli L, Berton G. J Inflamm. 1995;45:297–311. [PubMed] [Google Scholar]
- 16.Lowell C A, Fumagalli L, Berton G. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowell C A, Soriano P, Varmus H E. Genes Dev. 1994;8:387–398. doi: 10.1101/gad.8.4.387. [DOI] [PubMed] [Google Scholar]
- 18.Meng F, Lowell C A. J Exp Med. 1997;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beutler B, Cerami A. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello C A, Wolff S M. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 21.Freudenberg M A, Galanos C. Infect Immun. 1991;59:2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann V, Freudenberg M A, Galanos C. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiegs G, Wolter M, Wendel A. Biochem Pharmacol. 1989;38:627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- 24.Miethke T, Wahl C, Regele D, Gaus H, Heeg K, Wagner H. Immunobiology. 1993;189:270–284. doi: 10.1016/S0171-2985(11)80362-1. [DOI] [PubMed] [Google Scholar]
- 25.Tsygankov A, Bolen J. Stem Cells. 1993;11:371–380. doi: 10.1002/stem.5530110504. [DOI] [PubMed] [Google Scholar]
- 26.Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez-Ramos J C, Bluethmann H. Immunol Today. 1997;18:329–334. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 28.Wilson M E. Rev Infect Dis. 1985;7:404–418. doi: 10.1093/clinids/7.3.404. [DOI] [PubMed] [Google Scholar]
- 29.Worthen G S, Haslett C, Smedly L A, Rees A J, Gumbay R S, Henson J E, Henson P M. Fed Proc. 1986;45:7–12. [PubMed] [Google Scholar]
- 30.Movat H Z, Cybulsky M I, Colditz I G, Chan M K, Dinarello C A. Fed Proc. 1987;46:97–104. [PubMed] [Google Scholar]
- 31.Borregaard N, Cowland J B. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 32.Yamada K M, Geiger B. Curr Opin Cell Biol. 1997;9:76–85. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- 33.Jaeschke H, Smith C W. J Leukocyte Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 34.Erzurum S C, Downey G P, Doherty D E, Schwab B d, Elson E L, Worthen G S. J Immunol. 1992;149:154–162. [PubMed] [Google Scholar]
- 35.Hewett J A, Schultze A E, VanCise S, Roth R A. Lab Invest. 1992;66:347–361. [PubMed] [Google Scholar]
- 36.Conlan J W, North R J. J Leukocyte Biol. 1992;52:130–132. doi: 10.1002/jlb.52.1.130. [DOI] [PubMed] [Google Scholar]