Abstract
The nucleoporin Nup358 resides on the cytoplasmic face of the interphase nuclear pore complex (NPC). During metaphase, its recruitment to kinetochores is important for correct microtubule-kinetochore attachment. Here, we report that a fraction of endogenous Nup358 interacts with interphase microtubules through its N-terminal region (BPN). Cells overexpressing the microtubule targeting domain of Nup358 displayed dramatic alteration in the microtubule organization including increased microtubule bundling and stability. Ectopic expression of BPN and full-length Nup358 exhibited significantly higher levels of acetylated microtubules that were resistant to nocodazole, a microtubule depolymerizing agent. Furthermore, RNAi mediated depletion of Nup358 affected polarized stabilization of microtubules during directed cell migration, confirming the in vivo role of Nup358 in regulating interphase microtubules.
Keywords: Ran, Nup358, RanBP2, nucleoporin, microtubule
1. Introduction
Nup358 (also called RanBP2) is a large mammalian nucleoporin that possesses multiple domains, including a cyclophilin homology domain, a domain that functions as a ligase for ubiquitin-like modifiers of the SUMO family, domains that bind the GTP- and GDP-bound forms of the Ran GTPase, a leucine-rich region (LRR) and a region that contains numerous motifs for binding to nuclear transport receptors [1-4]. Nup358 localizes to metaphase spindles and kinetochores in a microtubule-dependent manner [5], where it is important for microtubule-kinetochore interactions [6-8]. Ran controls mitotic microtubule dynamics and spindle assembly by regulating the binding and release of spindle assembly factors from Ran-GTP binding proteins that act as nuclear transport receptors during interphase [6;9;10].
To better understand the role of Nup358 in controlling the microtubule cytoskeleton, we examined its recruitment to microtubules throughout the cell cycle. We found that a pool of Nup358 associated with interphase microtubules, and that this association is mediated through its N-terminal region (BPN). Overexpression of BPN markedly altered interphase microtubule organization. Our findings suggest an unexpected role for Nup358 in regulating interphase microtubule assembly, and indicate that its interaction with the microtubule cytoskeleton is not limited strictly to mitosis.
2. Materials and Methods
2.1. Cell culture
COS-7 and CHO-K1 cells were cultured in DMEM or Ham's F12 medium supplemented with 10% FBS at 37°C in a humidified incubator with 5% CO2, respectively.
2.2. Nup358 RNA interference (RNAi)
RNAi was performed according to Elbashir et al. [11]. Cells were transfected with annealed double-stranded RNA oligonucleotides (Qiagen) by using Oligofectamine (Invitrogen) at a final concentration of 100 nM. The target gene sequences used for generating the oligonucleotides were as follows: control (non-silencing) siRNA, 5'-AATTCTCCGAACGTGTCACGT-3' (Qiagen); RanBP2 (425-445 nt), 5'-AAGGTGAAGATGGATGGAATA-3'. For Nup358 RNAi, CHO-K1 cells were transfected with annealed siRNAs for 24 hr, washed with PBS, and allowed to grow in DMEM containing 10% FBS and antibiotics for the indicated times.
2.3. Plasmid constructs and transfection
The details for generating GFP tagged version of full length human Nup358 will be available upon request. In brief, pBlueScript-human Nup358 (a gift from Takeharu Nishimoto, Japan), was used for cloning the open reading frame (ORF) into pEGFP-C2 (Clontech) at NotI (engineered site) and XhoI sites to generate pEGFP-full-length Nup358. Internal ApaI sites present in the Nup358 ORF were used to subclone BPN (1-900 amino acids), BPM (901-2219 amino acids) and BPC (2220-3224 amino acids) into appropriate pEGFP-C vectors (Clontech) to produce the corresponding Nup358 fragments with GFP fused at their N termini. For deletion analysis of BPN, specific primers were used to amplify regions corresponding to 1-200, 1-400, 1600, 200-900, 400-900 and 600-900 amino acids, with pEGFP-BPN as the template. The PCR products were cloned at KpnI and SmaI sites of pEGFP-C2. Transfections were performed with effectene reagent (Qiagen) as per manufacturer's instruction.
2.4. Antibodies and Immunofluorescence Microscopy
Affinity purified Rabbit polyclonal antibodies against Nup358 have been described earlier [7]. Immunofluorescence studies were performed as described [5], except that the cells were directly fixed with either ice cold methanol or methanol: acetone (1:1) with 1mM EGTA and quickly washed with 0.1% triton X-100 in phosphate-buffered saline (PBS) or tris-buffered saline (TBS) before primary and secondary antibody incubations. Images were acquired with a Zeiss LSM 510 system or Zeiss Axiovert 200M and processed in Adobe Photoshop. The antibodies used for immunofluorescence, their sources and dilutions are as follows; Rabbit polyclonal anti-Nup358 (1:1000), Mouse anti-α-tubulin (Sigma-Aldrich, 1:2000), Mouse anti-acetyl-α-tubulin (Sigma-Aldrich, 1:5000), mAb414 antibodies (Covance, 1:1000), Rabbit anti-Glu-tubulin (Chemicon, 1:500).
2.5. Cell migration assay
CHO-K1 cells were subjected to RNAi for 24 hours and then serum-starved with 0.2% FBS for 12 hours. Confluent monolayer cells were then wounded by scratching with P20 pipette tips and provided with growth medium containing 10% FBS for 36 hours. Phase contrast images were obtained to assess the extent of wound closure. For immunofluorescent analysis of the cells moving into the wound, fixation and staining were performed 6 hours after wounding.
3. Results and Discussion
3.1. Nup358 associates with interphase microtubules through its N-terminal region
While the bulk of Nup358 is associated with NPCs during interphase, immunostaining with anti-Nup358 antibodies consistently shows staining of Nup358 within the cytoplasm of mammalian cells (Fig 1). Close examination revealed that Nup358 exists as small puncta distributed throughout the cytoplasm, and particularly enriched at cell extensions (Fig 1A). To examine whether cytoplasmic Nup358 associates with microtubules in interphase cells we immunostained CHO-K1 cells for endogenous Nup358 and tubulin (Fig. 1A). Remarkably, the endogenous Nup358 puncta often colocalized with microtubules, particularly at cell extensions (Fig. 1A, arrow). At lower exposure, the nuclear pore localization is distinctly evident (Fig 1A, arrow head). Nup358 antibody that was neutralized with the antigen used for immunization showed no staining of either puncta or nuclear pores, indicating the specificity of the antibody used (data not shown). Moreover, RNA interference (RNAi)-mediated knockdown of Nup358 significantly reduced the nuclear membrane and cytoplasmic staining of endogenous Nup358 (Fig. 1B). Together, these results suggest that in addition to its well characterized nuclear pore localization, Nup358 exists in a cytoplasmic pool that interacts with interphase microtubules in vivo.
Fig. 1. Nup358 associates with interphase microtubules.
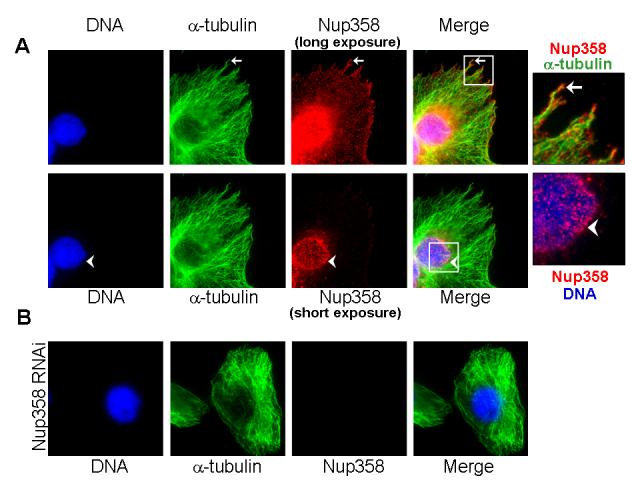
(A) CHO-K1 cells were fixed and immunostained for endogenous Nup358 (red) and microtubules (green) using specific antibodies. Arrow indicates cellular extension where Nup358 partially colocalizes with microtubules. The lower panel shows a lower exposure of Nup358 staining, revealing that the bulk of Nup358 is associated with nuclear pores.
(B) Cells depleted of Nup358 by RNAi were fixed and stained for endogenous Nup358 (red) and microtubules (green), under conditions identical to those used in Fig. 1A. Image shows a long exposure for anti-Nup358 staining, comparable to the upper panels in Figure 1A. Note that both NPC-associated and cytoplasmic staining of Nup358 are dramatically reduced.
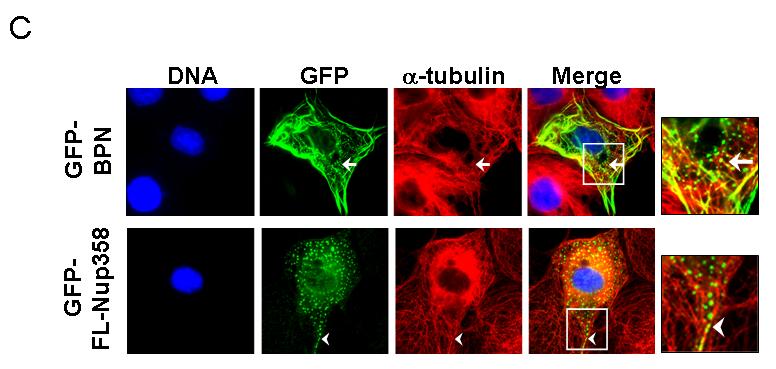
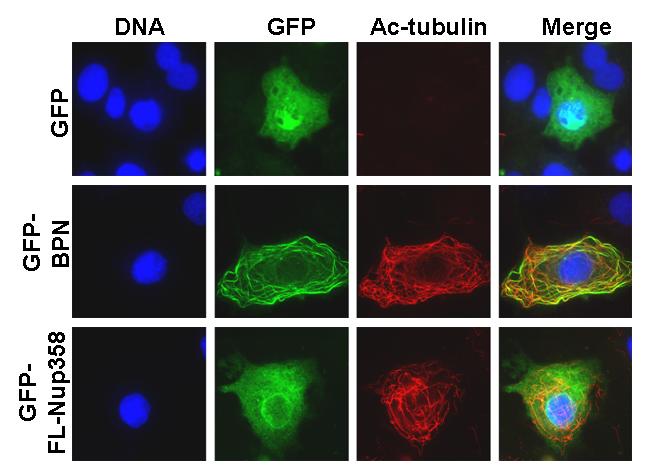
To characterize the region of Nup358 required for microtubule association, different green fluorescent protein (GFP)-tagged Nup358 fragments were transiently expressed in COS-7 cells and monitored for their localization. Ectopic expression of an N-terminal fragment that includes the LRR (residues 1-900, here called BPN; Fig. 2A) resulted in its localization to filamentous cytoplasmic structures, which upon costaining with anti-tubulin antibodies confirmed to be interphase microtubules (Fig. 2B, insert, arrow). By contrast, GFP alone showed no colocalization with microtubules, nor did GFP fusions to the middle (residues 901-2219; GFP-BPM) or C-terminal regions (residues 2220-3224; GFP-BPC) of Nup358 (data not shown). In addition to microtubule decoration, in some cells GFP-BPN could also be seen as vesicle-like puncta (Fig. 2C) possibly suggesting that BPN could exist in either of these two populations. However, the GFP-BPN puncta were often juxtaposed to microtubules (Fig 2C, arrow). Interestingly, ectopically expressed GFP-tagged full length Nup358 (GFP-FL-Nup358) appeared within similar cytoplasmic puncta in most cells. Costaining with tubulin showed a remarkable correspondence between cytoplasmic GFP-FL-Nup358 puncta and microtubules (Fig 2C, arrow head). It is noticeable that endogenous Nup358 shows punctate cytoplasmic staining that is similar to the ectopically expressed GFP-FL-Nup358 and GFP-BPN, except that the endogenous Nup358 puncta are smaller (Fig 1, Fig 2C). We speculate that the difference in size between ectopically expressed and endogenous Nup358 puncta is due to the difference in the levels of Nup358. Collectively, the results suggest that Nup358 associates with microtubules through its N-terminal region.
Fig. 2. The N-terminal region targets Nup358 to interphase microtubules.
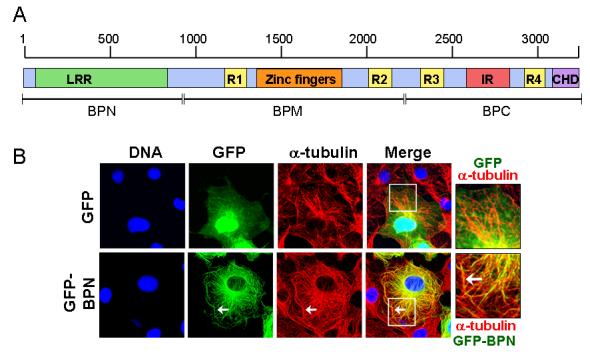
(A) Schematic representation of Nup358 showing different domains. LRR, Leucine-rich region; R, RanBP1-like Ran-GTP binding domain; IR, internal repeats; CHD, Cyclophilin homology domain. The region shown as BPN corresponds to the N-terminal 900 amino acids.
(B) GFP-BPN colocalizes with interphase microtubules. COS-7 cells were transfected with GFP or GFP-BPN and 48 hours later were fixed as described in the Materials and Methods section. The cells were stained for microtubules with anti-α-tubulin antibody (red). DNA was stained with Hoechst (blue). Arrow indicates the colocalization of GFP-BPN with microtubules. Note that GFP is distributed in a disperse fashion, and shows no concentration at microtubules.
(C) GFP-BPN and GFP-FL-Nup358 shows punctate staining in the cytoplasm that partially colocalizes with microtubules. Cells overexpressing GFP-BPN or GFP-full length Nup358 (GFP-FL-Nup358, green) were fixed and stained for tubulin (red). Arrows and arrow heads indicate punctate staining of GFP-BPN and GFP-FL-Nup358, respectively.
3.2. Expression of BPN alters microtubule organization
We wished to investigate whether the microtubule targeting region of Nup358 (BPN) has any significant effect on the microtubule organization. In normal (untransfected) cells, microtubule arrays are anchored at the centrosome and organized radially throughout the cytoplasm (Fig 3A, arrow head). By contrast, in GFP-BPN overexpressing cells the microtubules arrays were less focused at the centrosome and were often present as thick bundles around the nucleus (Fig 3A, arrow), suggesting that GFP-BPN might alter microtubule dynamics and thereby its organization. Consistent with this idea, staining with antibodies against acetylated α-tubulin or detyrosinated α-tubulin (Glu-tubulin), two markers for stable microtubules[12-14], showed greatly enhanced levels of both modifications in GFP-BPN expressing cells (Fig 3B, C), whereas untransfected cells on the same coverslip showed very few stable microtubules.
Fig. 3. Ectopic expression of BPN alters microtubule organization and dynamics.
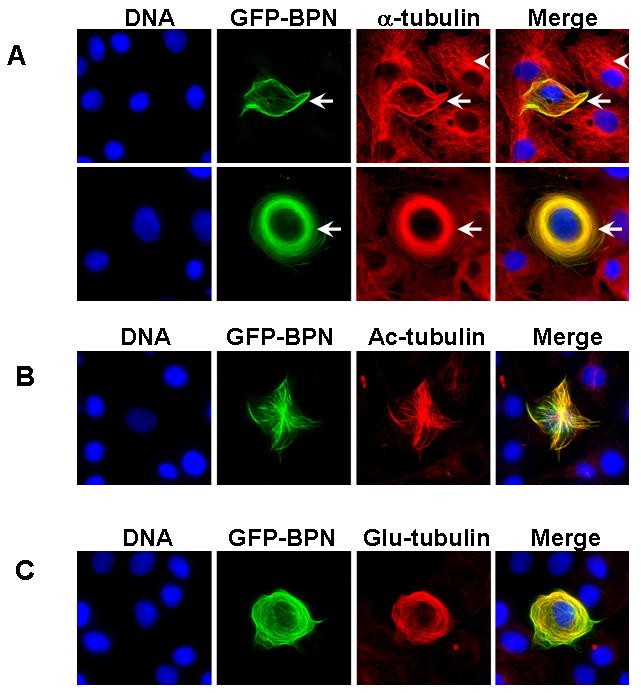
(A) COS-7 cells transfected with GFP-BPN (green) were fixed and stained for microtubules (red) using α-tubulin antibodies. Note that the microtubule organization is dramatically affected in GFP-BPN transfected cells (arrow) as compared to untransfected cells (arrow head). DNA (blue) was visualized by Hoechst staining.
(B) GFP-BPN transfected cells (green) were fixed and stained for acetylated microtubules (red) using anti-acetyl-α-tubulin antibodies. Note that the GFP-BPN decorating microtubules are highly acetylated.
(C) GFP-BPN transfected cells (green) were stained for detyrosinated microtubules (red) using anti-Glu-tubulin antibodies. Note that the GFP-BPN expressing cells have significantly higher levels of Glu-tubulin containing microtubules.
3.3. BPN harbours overlapping sequences that mediate nuclear pore and microtubule targeting
At lower levels, BPN showed localization to the nuclear pore complex (NPC) as confirmed by costaining with mAb414, a monoclonal antibody that recognizes FxFG-containing nucleoporins (Fig. 4A), suggesting that this fragment also contains the NPC targeting domain of Nup358. To delineate the regions of BPN involved in microtubule and nuclear pore association, fusions of GFP to different fragments of BPN were expressed in mammalian cells (Fig. 4B). The region corresponding to residues 600-900 of full length Nup358 was sufficient for targeting to the nuclear pore. However, microtubule association was not observed among any of the fragments, suggesting that microtubule targeting requires sequences throughout BPN. We conclude that BPN harbours overlapping sequences that mediate nuclear pore and microtubule association, but that the requirements for these localization patterns are not identical.
Fig. 4. BPN contains overlapping sequences that mediate Nup358 targeting to nuclear pores and microtubules.
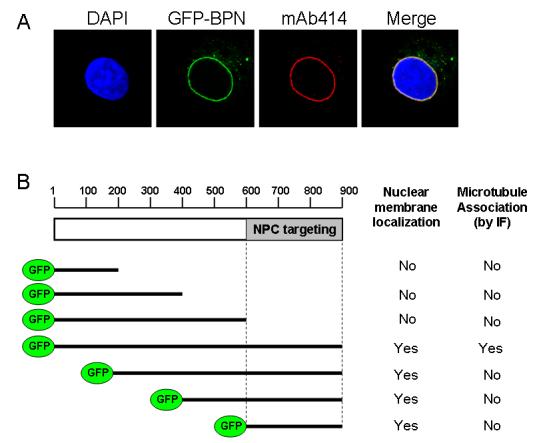
(A) Cells expressing low levels of GFP-BPN (green) were fixed and stained for mAb414 antibodies (red), which recognize FxFG containing nucleoporins. DNA was stained with Hoechst (blue).
(B) The region corresponding to residues 600-900 of full length Nup358 is sufficient for targeting to the nuclear pore. COS-7 cells were transiently transfected with the indicated constructs, fixed and analyzed for nuclear membrane localization as well as microtubule association by immunofluorescence (IF).
3.4. Expression of GFP-BPN and GFP-FL-Nup358 increases microtubule stability
To test the hypothesis that overexpression of BPN or FL-Nup358 altered the stability of microtubules, cells overexpressing the GFP-BPN or GFP-FL-Nup358 were subjected to nocodazole treatment (10μM, 30 minutes) and later fixed and stained for acetylated α-tubulin. Nocodazole-resistant stable microtubules were greatly increased in cells overexpressing GFP-BPN or GFP-FL-Nup358 as compared to cells with GFP expression (Fig. 5). Significant populations of stable, acetylated microtubules were more consistently found in BPN overexpressing cells (68%, n=200) as compared to GFP-FL-Nup358 (42%, n=100) and GFP (17%, n=200) expressing cells. Together, these results suggest that Nup358 has a microtubule stabilizing function, and the GFP-BPN fragment acts in a dominant or unregulated manner as far as microtubule binding and stabilization are concerned.
Fig. 5. Ectopic expression of GFP-BPN and GFP-FL-Nup358 increases microtubule stability.
GFP, GFP-BPN or GFP-FL-Nup358 (green) overexpressing cells were subjected to nocodazole treatment (10μM, 30 minutes) and later fixed and stained for stable microtubules (red) using anti-acetyl-α-tubulin antibodies. DNA (blue) was visualized by Hoechst staining.
3.5 Nup358 RNAi leads to impaired stabilization of microtubules and directional cell migration
In migrating cells, polarized stable microtubules are formed, which are posttranslationally modified and can be stained by specific antibodies [11;15]. To assess whether Nup358 plays any role in regulating microtubule dynamics in vivo, CHO-K1 cells were subjected to RNAi treatment and wound healing assay was performed as described in the Materials and methods section. Six hours after wounding, cells were fixed and analyzed for microtubule stability using Glu-tubulin antibodies. Nup358 depleted cells at the boarder displayed significantly reduced levels of Glu-microtubules as compared to the control RNAi cells (Fig. 6A), indicating that Nup358 plays a role in the stabilization of microtubules during directional cell migration. Consistent with the role of polarized microtubules in cell motility, Nup358 depleted cells had defective cell migration as compared to control RNAi cells (Fig. 6B). Together, these results suggest an in vivo role for Nup358 in regulating the interphase microtubule dynamics during cell migration.
Fig. 6. Nup358 RNAi affects microtubule stability and directional cell migration.
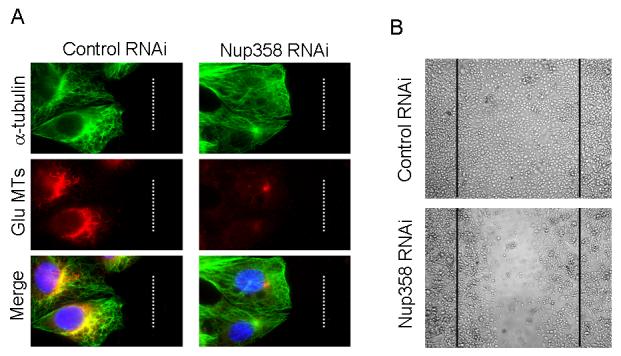
CHO-K1 cells were treated with control and Nup358 siRNA and were subjected to wound induced cell migration assay as described in Materials and Methods section.
(A) Cells were fixed and stained for total microtubules (green) and detyrosinated microtubules (red) using mouse anti-α-tubulin and rabbit anti-Glu-tubulin antibodies, respectively. DNA was stained with Hoechst (blue).
(B) The phase contrast micrograph shows the extent of cell migration into the wound.
In conclusion, previous studies have shown that Nup358 binds to mitotic spindles and kinetochores, and defined a role for Nup358 during mitosis in mediating proper microtubule-kinetochore interactions [5-8]. Our findings suggest that Nup358 also interacts with interphase microtubules through its N-terminal region (Figs 1, 2) and modulates their stability in vivo (Fig. 3, 5 & 6). It is attractive to speculate that such regulation could be mechanistically related to Nup358's function at mitotic kinetochores [6-8]. In this case, it will be of considerable interest to determine how the Ran GTPase may be involved in Nup358's control of interphase microtubule dynamics. Since the concentration of Ran-GTP is low in interphase cytosol [16;17], complexes of Ran-GTP with either RanBDs of Nup358 or with Ran-binding nuclear transport receptors should be commensurately scarce. It would thus be expected that relatively small increases in the absolute concentration of Ran-GTP could have a substantial effect in this context. In any case, our results highlight an unanticipated role for Nup358 in regulating cytoplasmic events during interphase, and open up new avenues to explore additional roles for nucleoporins in different cellular processes, including those requiring a close coordination between the nuclear and cytoplasmic events.
Acknowledgments
We thank Drs. T. Nishimoto, E. Coutavas and N.R. Yaseen for providing Nup358 clones; members of Dasso lab and Joseph lab for helpful discussions. This work was supported by the Intramural Research Program of the NICHD/NIH to M.D. and by NCCS intramural funding to J.J.
Abbreviations
- Nup358
Nuclear pore protein 358
- RanBP2
Ran binding protein 2
- BPN
N-terminal region of RanBP2
- GFP
green fluorescent protein
- EGTA
Ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetra acetic acid
- DMEM
Dulbecco's Modified Eagle's Medium
- NPC
nuclear pore complex
- Ran-GTP
Guanosine triphosphate bound form of Ran
- LRR
Leucine rich region
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell. 2002;108:109–120. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Matunis MJ, Kraemer D, Blobel G, Coutavas E. Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J Biol. Chem. 1995;270:14209–14213. doi: 10.1074/jbc.270.23.14209. [DOI] [PubMed] [Google Scholar]
- 3.Yaseen NR, Blobel G. Two distinct classes of Ran-binding sites on the nucleoporin Nup-358. Proc. Natl. Acad. Sci U. S. A. 1999;96:5516–5521. doi: 10.1073/pnas.96.10.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama N, Hayashi N, Seki T, Pante N, Ohba T, Nishii K, Kuma K, Hayashida T, Miyata T, Aebi U. A giant nucleopore protein that binds Ran/TC4. Nature. 1995;376:184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]
- 5.Joseph J, Tan SH, Karpova TS, McNally JG, Dasso M. SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J. Cell Biol. 2002;156:595–602. doi: 10.1083/jcb.200110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnaoutov A, Azuma Y, Ribbeck K, Joseph J, Boyarchuk Y, Karpova T, McNally J, Dasso M. Crm1 is a mitotic effector of Ran-GTP in somatic cells. Nat. Cell Biol. 2005;7:626–632. doi: 10.1038/ncb1263. [DOI] [PubMed] [Google Scholar]
- 7.Joseph J, Liu ST, Jablonski SA, Yen TJ, Dasso M. The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr. Biol. 2004;14:611–617. doi: 10.1016/j.cub.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Salina D, Enarson P, Rattner JB, Burke B. Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J. Cell Biol. 2003;162:991–1001. doi: 10.1083/jcb.200304080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harel A, Chan RC, Lachish-Zalait A, Zimmerman E, Elbaum M, Forbes DJ. Importin beta negatively regulates nuclear membrane fusion and nuclear pore complex assembly. Mol. Biol. Cell. 2003;14:4387–4396. doi: 10.1091/mbc.E03-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph J. Ran at a glance. J Cell Sci. 2006;119:3481–3484. doi: 10.1242/jcs.03071. [DOI] [PubMed] [Google Scholar]
- 11.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 12.Kreis TE. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987;6:2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster DR, Borisy GG. Microtubules are acetylated in domains that turn over slowly. J Cell Sci. 1989;92:57–65. doi: 10.1242/jcs.92.1.57. [DOI] [PubMed] [Google Scholar]
- 15.Bulinski JC, Gundersen GG. Stabilization of post-translational modification of microtubules during cellular morphogenesis. Bioessays. 1991;13:285–293. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- 16.Kalab P, Weis K, Heald R. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science. 2002;295:2452–2456. doi: 10.1126/science.1068798. [DOI] [PubMed] [Google Scholar]
- 17.Smith AE, Slepchenko BM, Schaff JC, Loew LM, Macara IG. Systems analysis of Ran transport. Science. 2002;295:488–491. doi: 10.1126/science.1064732. [DOI] [PubMed] [Google Scholar]


