Abstract
Protein drugs have low bioavailability after oral administration, which is due in part to fast transit of the drugs or drug delivery vehicles through the gastrointestinal tract. Increasing the time that the drugs spend in the intestine after dosing would allow for greater absorption and increased bioavailability. We developed a formulation strategy that can be used to prolong intestinal retention of drug delivery vehicles without substantial alterations to current polymeric encapsulation strategies. A model drug, insulin, was encapsulated in negatively-charged poly(lactic-co-glycolic acid) (PLGA) microparticles, and the microparticles were subsequently mixed with positively-charged micromagnets, whose size will prevent them from being absorbed. Stable complexes formed through electrostatic interaction. The complexes were effectively immobilized in vitro in a model of the mouse small intestine by application of an external magnetic field. Mice that were gavaged with radio-labeled complexes and fitted with a magnetic belt retained 32.5% of the 125I-insulin in the small intestine compared with 5.4% for the control group 6 hours after administration (p=0.005). Furthermore, mice similarly gavaged with complexes encapsulating insulin (120 Units/kg) exhibited long-term glucose reduction in the groups with magnetic belts. The corresponding bioavailability of insulin was 5.11% compared with 0.87% for the control group (p=0.007).
1. Introduction
The delivery of protein drugs through oral routes is generally inefficient, as the drugs face degradation, poor absorption into the bloodstream, and fast transit through the intestine. Incremental progress has been made over the years addressing the obstacles to effective oral delivery of proteins. Protection of proteins from degradation in transit in the gastrointestinal tract is possible through polymeric encapsulation [1, 2]. However, even if the delivery vehicle reaches the small intestine, release and absorption of the protein drug remains a problem [3]. Adjuvants that increase the permeability of the intestinal mucosa to the drug can help to increase absorption [4]. Furthermore, mucoadhesive coatings of particles show promise in increasing the residence time in the small intestine [5, 6], but still face some challenges due to quick mucin turnover times [7]. Despites these advances, patients still have few clinical alternatives to the repeated injections required for management of diseases such as insulin-dependent diabetes mellitus.
In this work, we sought to devise a drug delivery strategy that builds upon these advances while addressing some of their shortcomings. Microparticles made from degradable polymers like PLGA have been used to deliver protein drugs orally [8, 9]. These microparticles effectively protect the drugs from enzymatic or acidic degradation in route to the small intestine, and their degradable nature allows subsequent release of the drugs in the small intestine to be absorbed. Previous approaches with microparticles also include using magnetic force to slow the intestinal transit of the drug delivery vehicles [10, 11]. The use of magnetic fields in medicine is FDA-approved, and its most common application is in diagnostics like MRI [12, 13]. Recently, more interest has been placed on the possibility of magnetic drug targeting [12, 14]. Co-encapsulation of nano-magnetites with insulin in liposomes or with microparticles for oral delivery has been shown to increase drug retention and absorption [10, 11]. However, nano-magnetic interactions may not be sufficiently strong for long-term retention of large quantities of drug. Furthermore, the magnetic material is small enough to be absorbed systemically, which is not ideal.
Instead of co-encapsulation of magnetic material, we chose to formulate the insulin-containing PLGA microparticles separately from the magnetic material. We obtained separate magnetic microparticles—micromagnets that were sufficiently large to prevent them from being absorbed after oral dosing. We then coupled the PLGA microparticles with the micromagnets by making use of their opposite surface charges. Positively-charged magnetic microparticles were used to facilitate electrostatic coupling with negatively-charged, drug-containing polymeric microparticles (Figure 1.A.). An externally applied magnetic field could then be used to retain the microparticle-micromagnet complexes in the intestine after oral dosing, providing an opportunity for long-term efficacy and high bioavailability of orally delivered protein drugs.
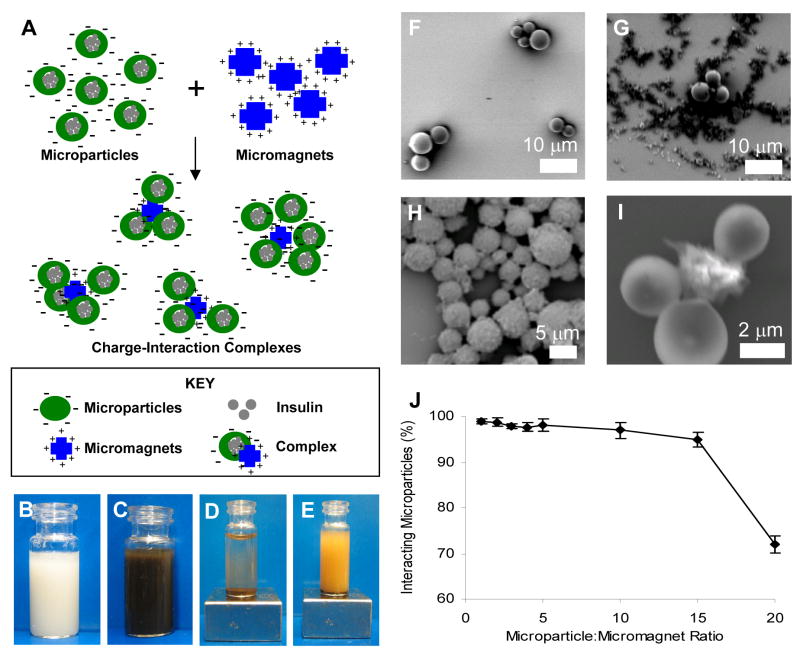
Figure 1.
(A) Scheme. Complexes formed through the interaction of negatively charged protein-containing polymer microparticles and positively charged micromagnets. (B)–(E) Particles in suspension: (B) PLGA microparticles alone; (C) Microparticle-micromagnet complexes without external magnet; (D) Complexes with external magnet applied—particles have precipitated; (E) Non-interacting negatively-charged microparticles and negatively-charged micromagnets with external magnet applied—particles remain in suspension. (F)–(I) Scanning electron microscopy (SEM) analysis for the interaction of carboxylate terminated, negatively-charged PLGA microparticles (spherical shape) (~ 4 mm) and positively-charged micromagnets (~ 1 or 6 mm) (irregular shape): (F) PLGA microparticles alone; (G) Non-interacting negatively-charged microparticles and negatively-charged micromagnets; (H) A microparticle-micromagnet complex formed by charge interaction; (I) High magnification of one complex. (J) Mixing of microparticle:micromagnet complexes at various wt:wt ratios, demonstrating that the majority of microparticles were complexed with micromagnets up to a 15:1 mass ratio.
2. Materials and Methods
2.1. Materials
Poly(lactic-co-glycolic acid) (PLGA) (50:50) with carboxylate end groups (inherent viscosity .18 dL/g in HFIP) was purchased from Lactel Absorbable Polymers (Pelham, AL, USA). Poly(vinyl alcohol) (PVA, Mw = 30 kDa), rhodamine-dextran, and HPLC grade solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Scinti-Safe scintillation cocktail was purchased from Fisher Scientific (Waltham, MA, USA), and Solvable tissue solubilizer was purchased from PerkinElmer (Waltham, MA, USA). Superparamagnetic iron oxide microparticles with amine functional groups (BioMagPlus Amine, mean diameter ~1 μm or ~6 μm) were purchased from Polysciences, Inc. (Warrington, PA, USA). Grade N40 neodymium iron boron rare earth magnets, 1 × 1 × 0.5 in., magnetized through the thickness with surface strength of 2500 gauss, were purchased from Amazing Magnets (amazingmagnets.com). Human insulin (Humulin R, 500 U/mL) was purchased from Drugstore.com (Bellevue, WA, USA). 3-[125I]IodotyrosylA14 insulin was purchased from Amersham Biosciences (Piscataway, NJ, USA). Eight week old balb/c mice were obtained from Charles River Laboratories (Wilmington, MA, USA).
2.2. Methods
2.2.1. Preparation of Drug-Loaded Polymeric Microparticles
Polymeric microparticles were prepared using the water-in-oil-in-water solvent evaporation procedure (double emulsion) to encapsulate model drugs. Briefly, the procedure was as follows: 50 mg of PLGA in dichloromethane (1 mL) was emulsified with 50 μL of the Humulin R (500 U/mL), 125I-Insulin (5–10 μCi), or Rhodamine-Dextran (20 mg/mL) using a probe sonicator at 10W for 30s. This first emulsion was transferred to a 50 mL aqueous PVA solution (1 % w/v) and homogenized at 8000 rpm for 1 minute. This second emulsion was immediately poured into a 150 mL aqueous PVA solution (0.3 % w/v) with gentle stirring. Organic solvent was removed through stirring in a fume hood at room temperature for 2.5 h. The resulting drug-containing microparticles were isolated by centrifugation at 4000 rpm and at 10 °C for 10 minutes, washed twice with double-distilled water and lyophilized.
2.2.2. Preparation of Microparticle-Micromagnet Complexes
The microparticle-micromagnet complexes were produced by simple mixing. The microparticle solution (negatively charged) was added to the micromagnet suspension (positively charged), and the resulting mixture was vigorously shaken. The complexes were washed by placing the vial on top of a magnet to pull the microparticle-micromagnet complexes to the bottom of the container. The water along with any free microparticles was poured off. The complexes were resuspended by adding water, and this procedure was repeated several times. Complexes were generated at a range of mass ratios (microparticles:micromagnets) from 1:1 to 20:1.
In order to demonstrate that the association of the microparticles and micromagnets was due to charge-interaction, microparticles (negatively charged) were separately mixed with a negatively charged micromagnet suspension (carboxylate-functionalized micromagnets). The degree of immobilization of the microparticles on the micromagnets was compared to the above mixture of oppositely charged particles.
2.2.3. Characterization of Microparticles and Microparticle/Micromagnet Complexes
Loading of Humulin R was determined by protein BCA Assay (Pierce, Rockford, IL, USA) by dissolving microparticles using a mixture of acetonitrile and water. Loading of I125-insulin was determined by dissolving microparticles in acetonitrile and water and counting radioactivity on the liquid scintillation counter (TRI-CARB Liquid Scintillation Analyzer, Model 2200CA; Packard Instrument, Downers Grove, IL, USA). The sizes of microparticles were measured on a Beckman Coulter Multisizer™-3 Coulter Counter (Beckman Coulter, Chaska, MN, USA). Zeta potentials were calculated by measuring electrophoretic mobilities on a ZetaPALS dynamic light scattering system (Brookhaven Instruments) at 25 °C. The ZetaPALS system employed BIC PALS zeta potential analysis software using the Smoluchowsky model. Morphological characterization of the particles was performed by SEM (model JSM 6060; JEOL USA, Peabody, MA, USA).
Stability in high and low pH was assayed by incubating microparticle-micromagnet complexes encapsulating 125I-Insulin in aqueous solutions adjusted to various pH. After 1 hour, the complexes were washed and remaining radioactivity was counted in the liquid scintillation counter.
2.2.4. In Vitro Retention Study
The retention of microparticles in the small intestine was modeled in flow conditions using our in vitro model of the mouse small intestine (Figure 2.A.) [11]. In the model, tubing that approximates the dimensions of small intestine of the mouse [15] (inner diameter of 1/16 in.) was perfused by a syringe pump, model NE-1600 (New Era Pump Systems, Wantagh, NY, USA). Microparticle-micromagnet complexes encapsulating 0.5 μCi of 125I-Insulin (2.5 mg of complexes, 1:1 ratio) were injected into the system. PBS was flowed through the system at approximately 0.8 mL/min, to test retention of particles in greater than physiologic flow conditions (typically 0.03 mL/min [16]). One tube was placed in the proximity of a magnet, and one tube served as the control (no applied magnetic field). The eluted liquid was collected at 5 minute intervals (about 4 mL). Scintillation cocktail (10 mL ScintiSafe) was added to the liquid, and the radioactivity was measured using the liquid scintillation counter.
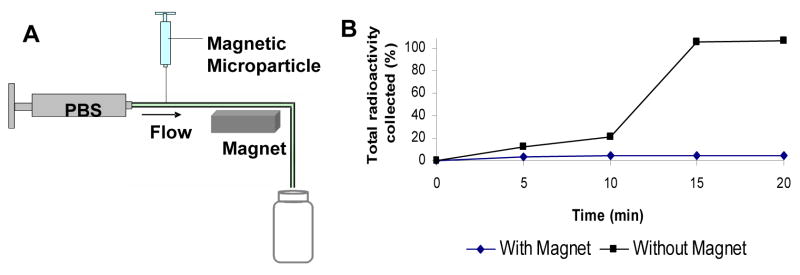
Figure 2.
(A) Schematic representation of the in vitro flow apparatus for the study of magnetic responsiveness of the microparticle-micromagnet complexes. Microparticles containing 0.5 μCi 125I radioactivity were introduced to the flow system through a syringe and eluted with PBS at a flow rate of 0.8 mL/min controlled by a syringe pump. (B) Magnetic responsiveness of the complexes: cumulative elution profile from the in vitro flow apparatus with and without the effect of the magnetic field.
2.2.5. In Vivo Retention Study
In order to determine the magnetic responsiveness of the microparticle-micromagnet complexes in vivo, we prepared complexes encapsulating rhodamine-dextran. The complexes were suspended in a 200 μL tap water solution containing 7.5 mg of the complex (2:1 ratio: 5 mg of rhodamine-dextran microparticles and 2.5 mg of micromagnets) and gavaged to mice (n=3). One group of mice was restrained 90 minutes after administration and a magnetic field was applied to the abdominal area of each mouse. The magnetic field was generated by a magnetic belt constructed using Velcro and the magnets, and it was applied to the mice for the duration of the experiment. The second group was similarly restrained in the absence of external magnetic field. Mice were euthanized after 8 hours. The small intestine was harvested from each animal and washed in PBS. The tissue was fixed for one hour in 10% formalin and washed in 30% Sucrose before being frozen in O.C.T. embedding media (Sakura Finetek, Torrance, CA, USA). Cryosections (5-micron thickness) were cut of the tissue, and images were taken at 20X under halogen and fluorescent settings (rhodamine-filtered). For each tissue, an overlay image was produced showing rhodamine-encapsulated microparticles in the small intestine wall.
For quantitative analysis, a similar experiment was performed with complexes encapsulating 125I-Insulin. Mice (n=3) were gavaged with a 200 μL suspension of the complexes and restrained after 90 min. One group was fitted with the magnetic belts, and one group served as control. After 19 h, mice were euthanized, and the small intestines were harvested and solubilized using Solvable. The solubilized tissue solutions were de-colored with hydrogen peroxide and analyzed for 125I content by liquid scintillation counting. Aliquots of the complexes were also counted to determine 100% dose.
2.2.6. Bioavailability Study
In order to test the efficacy of the system for a model drug, we formulated complexes encapsulating Humulin R. Mice were fasted overnight and gavaged with 200 μL of the complexes, which were prepared to give a dose of 120 Units of insulin/kg for each mouse. All mice (n=4 per group) were restrained 90 min. After administration of the complexes, a magnetic field was externally applied to the abdomen of the experimental group. An additional control group received 200 μL water only (no complexes or magnetic belts). The glucose level of each mouse was monitored over time using the One Touch Ultra glucose monitor (Lifescan, Milpitas, CA, USA). The bioavailability of insulin was calculated by comparing the Area- Under- Curve (AUC) for the various groups—for purposes of calculation, the glucose level was compared to fasting blood level and integrated over the time course of the study. Standards for insulin bioavailability were calculated by direct i.v. administration for another group of mice, and bioavailabilities were calculated as a percentage of the AUC for i.v. administration of the same dose.
2.2.7. Acute Toxicity Study
The toxicity of the microparticle-micromagnet complexes was acutely assayed in a separate experiment, performed similarly as the bioavailability study. Mice were fasted overnight and gavaged with 200 μL of the complexes. After 90 min. post-administration, mice were restrained with magnetic belts (n=2). These mice were euthanized after 24 hours, along with mice that had been given no treatment at all (n=2). The small intestine, liver, spleen, and kidney were collected from each mouse and fixed in 10% formalin. The tissues were further processed and fixed by standard histological techniques. Tissue sections were stained with hematoxylin and eosin (H&E). They were examined for any evidence of acute inflammation and complex toxicity.
The retention, bioavailability, and toxicity studies involving animals were performed according to the NIH’s Principles of Laboratory Animal Care and under the supervision of MIT’s Committee on Animal Care.
2.2.8. Statistical Analysis
Statistical analysis was performed using a Student’s t-test. P-values less than .05 were considered statistically significant.
3. Results
3.1. Characterization of Microparticles and Microparticle-Micromagnet Complexes
The polymeric microparticles were fabricated from PLGA with carboxylate end groups, resulting in a negatively charged surface. The diameter of the polymeric microparticles measured 3–5 microns, and the yields were in a range of 50–60% with encapsulation efficiencies of 60–80%. The double emulsion processes that involved sonication and organic solvent caused minimum degradation of insulin and minimal loss of its biological activities [17], therefore full biological activity of the encapsulated insulin was assumed to be retained. The micromagnets were chosen because they bore amine functional groups on the surface, endowing them with a positively charged surface. Upon mixing of the microparticles with the micromagnets in solution, the particles aggregated. The complexes, formed by mixing the two components together, associated due to charge interaction of the negatively-charged microparticles and the positively-charged micromagnets.
The interaction of the microparticles and micromagnets was easily seen macroscopically. After the solution of microparticles (Figure 1.B.) was mixed with the oppositely-charged micromagnets (Figure 1.C.), both the microparticles and micromagnets could be precipitated by application of the magnet. The supernatant of the resulting solution was clear (Figure 1.D.), indicating that the microparticles had associated with the micromagnets and were pulled from solution along with the magnets. When micromagnets with the same surface charge as the microparticles were used, no complex was formed and the polymeric microparticles remained in solution when the micromagnets were precipitated by the applied magnet (Figure 1.E.).
Scanning electron microscopy (SEM) analysis demonstrated that the aggregates formed were comprised of a micromagnet core surrounded by microparticles. Microparticles alone (Figure 1.F.) and mixtures of microparticles and micromagnets (Figure 1.G–I.) were imaged. Negatively-charged microparticles that were mixed with negatively-charged micromagnets (Figure 1.G.) did not show closely interacting particles. However, when the microparticles were mixed with the positively-charged micromagnets (Figure 1.H–I.), closely-associating complexes of the particles were observed.
After demonstrating that stable complexes could be formed by the mixing of oppositely charged microparticles and micromagnets, the relative amount of micromagnets necessary to complex the majority of the microparticles was titrated. For a mass ratio of up to 15:1 of microparticles to micromagnets, greater than 95% of the polymeric microparticles were incorporated into complexes (Figure 1.J.). Once coupled, the microparticles were not separable from the micromagnets under physiological conditions ranging from pH 2.5 to 8.3. After incubation of the complexes at pH 2.5 and 8.3, 88% ± 8% and 98% ± 5% of the original I125-insulin was recovered in the complexes, respectively (mean ± s.d., n=3). This recovery was comparable to complexes incubated at a pH of 7.4, which yielded 97% ± 4% (p>0.05). This pH range covers a range of environments from the acidic environment of the stomach to the slightly basic environment of the lower small intestine.
3.2. In Vitro Retention Study
The eluted fractions recovered during the in vitro flow experiment showed almost no release of insulin or insulin-containing particles for the group with an applied magnetic field (Figure 2.B.). For the control group with no applied magnet, the particles were not retained—the 125I-insulin was recovered in the eluted fractions within 15 minutes. In the first 5 minute fraction, 3.8% of the experimental magnet-applied group and 12.4% of the control 125I Insulin was collected. The difference in retention became readily apparent in the second fraction, with 4.1% of the control 125I Insulin collected compared to 21.2% of the experimental magnet-applied group. By the third fraction, all of the control 125I-Insulin was recovered, while 95.1% of the experimental 125I-Insulin remained immobilized in the tubing. The complexed microparticles that are anchored in the tubing could then release insulin to be absorbed over time.
3.3. In Vivo Retention Study
Examination of the histology sections from the small intestines of mice gavaged with microparticle-micromagnet complexes encapsulating rhodamine-dextran showed retention of the particles by application of a magnetic field. Sections from control mice that were not applied with a magnetic field did not show any fluorescence using the rhodamine filter on the fluorescent microscope (Figure 3.A.). Random sections from magnet-applied mice showed microparticles, marked by rhodamine, on the walls of the small intestine (Figure 3.B.).
Figure 3.
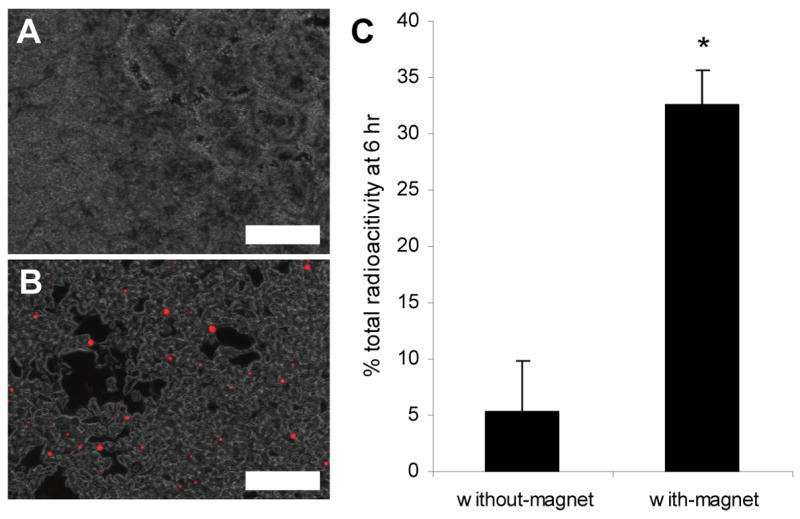
(A–B) Histology of the small intestine of mice orally dosed with the microparticle-micromagnet complexes encapsulating rhodamine-dextran in the absence (A) and presence (B) of an abdominally applied magnet after 8 h. Images are 20X overlays showing fluorescence of retained particles in small intestine (scale bar = 200 μm). (C) Recovered total radioactivity in small intestine of mice administered with microparticle-micromagnet complexes containing 1 μCi 125I-Insulin. The small intestine was analyzed for radioactive content 6 h after dosing, with the magnet-applied group retaining significantly higher quantity of the 125I-Insulin (p=0.005).
Mice that were gavaged with complexes encapsulating 125I Insulin confirmed the above result using the fluorescent complexes (Figure 3.C.). In the experimental group in which a magnetic field was applied after dosing, 32.5% ± 3.13% (mean ± s.d., n=3) of the administered 125I-Insulin was recovered in the small intestine of the mice after 6 h. In the control group, only 5.4% ± 4.41% (mean ± s.d., n=3) of the administered 125I-Insulin was recovered. This ~6-fold increase in retention was statistically significant (p=0.005).
3.4. Bioavailability Study
The results from the in vitro and in vivo retention studies suggested that the microparticle-micromagnet complexes could be effectively retained in the small intestine for many hours. As the PLGA degrades and insulin is released from the microparticles, the insulin can potentially be absorbed to enter the bloodstream to regulate glucose levels. In order to monitor the long-term efficacy of this approach for the delivery of insulin, complexes encapsulating Humulin-R were administered to fasted mice. The mice that were administered with the Humulin-containing complexes exhibited reduced glucose levels for as long as 36 hours with abdominally applied magnetic fields (Figure 4). The corresponding bioavailability of insulin was calculated to be 5.11% ± 1.24% (mean ± s.d., n=4). This value was significantly higher than the non-magnet control group, which had bioavailability = 0.82% ± 0.37% (mean ± s.d., n=4; p=0.007).
Figure 4.
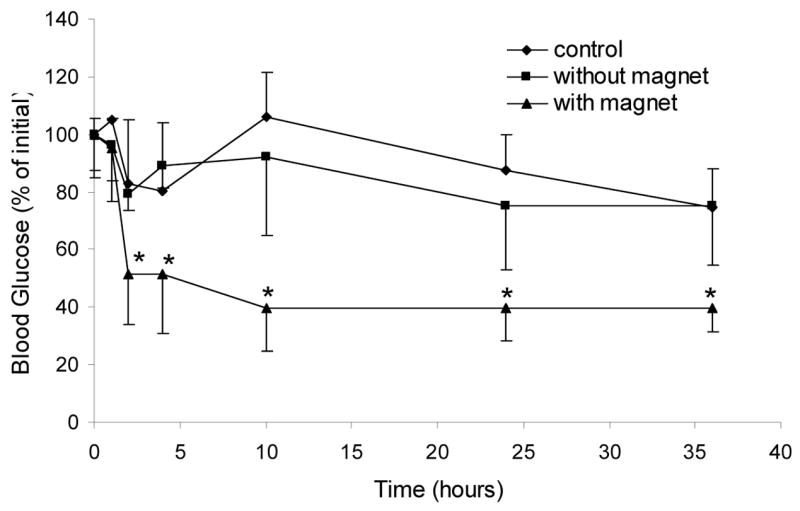
Glucose reduction assay in mice (n = 4) orally administered with microparticle-micromagnet complexes encapsulating Humulin-R (120 Units/kg) with and without applied magnetic fields. An additional group of mice were orally gavaged with tap water to serve as a control. The group with the applied magnetic field had significantly more glucose reduction compared with both controls (* p<0.05). The corresponding bioavailability for the magnet-applied group was 5.11 ± 1.24, compared with 0.82 ± 0.37 in the non-magnet-applied control group (p=0.007).
The results of the long-term efficacy study with the insulin showed that the complexes could be immobilized in the small intestine for long periods of time, allowing the insulin to be released and absorbed.
3.5. Acute Toxicity Study
As a first step in assaying the safety of this approach for the long-term delivery of insulin in the small intestine, we examined the effects of a 24 hour treatment of the mice with the complexes on several organs. Upon histological examination of experimental tissues (those from mice that had been gavaged complexes and applied with a magnetic field), no evidence of acute inflammation was observed. In particular, no tissue necrosis, edema, or leukocyte infiltration was seen. Generally, appearance of the control tissues is similar to those of the experimental tissues, and some differences appear to be artifact from processing. Representative H&E sections for each group are shown in Figure 5.
Figure 5.
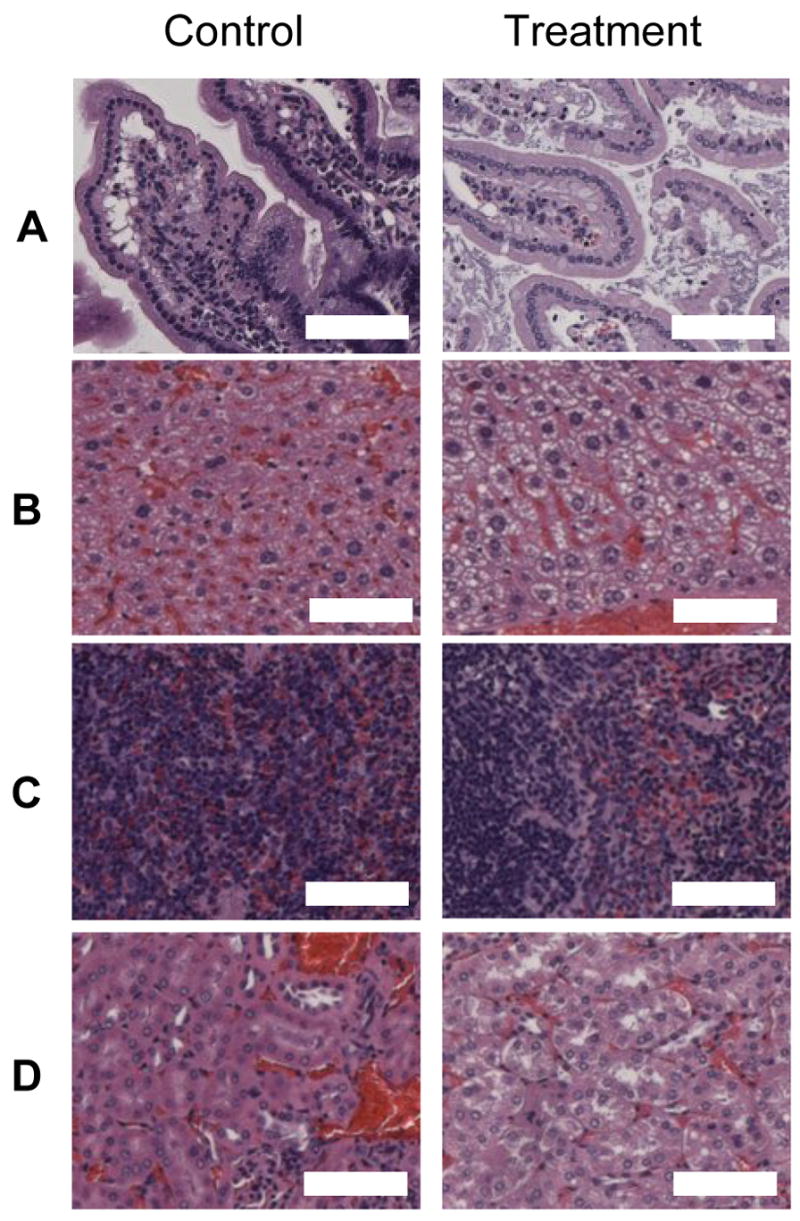
Representative tissue sections from the following organs: (A) Small Intestine, (B) Liver, (C) Spleen, (D) Kidney. The images on the left show sections from controls whereas the images on the right were taken from mice administered with magnetic particle complexes. Magnification is 50X (scale bar = 100 μm).
4. Discussion
To formulate the vehicles used in this drug delivery strategy, we encapsulated insulin as a model drug in PLGA microparticles through double emulsion—an established method of drug encapsulation. The microparticles had a negative surface charge, and they were complexed with positively-charged micromagnets due to electrostatic interaction. This method of non-covalent complexation using surface charge potentially represents a major advantage of the strategy. The strategy can be generalized to many other polymer systems and other drugs because no modification of the encapsulation procedure was required. Any drug-containing microparticles, as long as they had a surface charge, could be complexed with oppositely-charged micromagnets.
Another major advantage of a system using micron-sized magnets is that the size of the magnets should preclude them from being absorbed systemically. Previous work has demonstrated a size-dependence on the absorption of microparticles into the lymphatic system through Peyer’s Patches and intestinal epithelium, with microparticles greater than a few micron in size being largely excluded from absorption [18]. Therefore, with our system the only materials that should be available for uptake by the intestine are the polymers used in the formulation of the microparticles and the encapsulated drug. The polymer used in this study was PLGA, which is FDA-approved for use in humans and has an established safety profile.
Only small quantities of micromagnets were required to complex the vast majority of the microparticles. Beyond demonstrating that the complexes were effectively immobilized after mixing together during washing steps, the complexes maintained their strong interaction in flow conditions. While our in vitro model did not simulate peristaltic waves in the tubing that normally occur at a frequency of 8–12 per minute, we used a fast flow rate—greater than 25-times the expected physiological flow rates—in order to challenge the interaction of the complexes. The higher flow rates will correspond to some extent with higher shear stress applied to the complexes. Over the course of the flow experiment, the magnetic field immobilized the complexes with little 125I-Insulin recovered in the eluted fractions. We believe that effective immobilization of the complexes in the fast flow rate also was important in future consideration for use in human patients. The peristaltic forces involved in the human GI tract will be greater than in the mouse GI tract, and the ability of the complexes to be immobilized in relatively high flow experiments shows promise for scale-up.
The immobilization of the complexes was confirmed in vivo through using both fluorescent and radio-labeled complexes. The time-course of these experiments was relatively long: 8 and 6 hours, for fluorescent and radioactive experiments, respectively. The ability to immobilize the complexes in the small intestine is crucial for this strategy to work. The polymeric microparticles degrade over time, releasing insulin into the lumen of the intestine. The insulin directly released in the lumen of the small intestine then can be absorbed and regulate blood glucose levels [19]. The microparticles first serve to protect the insulin during transit to the small intestine and then allow the insulin to be anchored in the small intestine and be slowly released over time.
In the efficacy study, the long-term depression of the glucose levels for the experimental group compared with the control groups is highly suggestive that the insulin was continually released and absorbed over the course of the study. Insulin has a short circulating half-life, and thus insulin released into the blood will reduce glucose levels for a relatively short time. Glucose levels will usually return to normal levels within 3–4 hours after injection of insulin intra-peritoneally or intravenously [11]. The retention studies directly detecting microparticle-micromagnet complexes were shorter in duration than the efficacy study, but the continually depressed glucose levels in the experimental animals suggest long-term release of the insulin in the small intestine.
Acutely, the complexes appear to be biocompatible. Long-term study of potential toxicity of the complexes will need to be performed, as the treatment of chronic diseases like diabetes mellitus will require repeated administration of the complexes over many years. Furthermore, dose-response experiments will need to be carried out for any specific applications to insure that blood levels of the drug stay safety in the therapeutic window.
5. Conclusions
We developed a new strategy for the oral delivery of drugs by polymeric encapsulation and complexation with micromagnets. The complexes were retained long-term in the small intestine of mice, which allows for the continual release and absorption of the drug. This long-term retention of drug-containing delivery vehicles has the potential to address the relatively short transit time in the small intestine, one of the major obstacles facing oral delivery of protein drugs, in order to release and absorb the protein drugs. Insulin was used as a model drug in this study, but the system may potentially be adapted for most poorly available drugs, such as growth hormone, erythropoietin, or vancomycin. For long-term use, the strategy may best be employed for overnight delivery of drugs, where the magnetic belt would be least disruptive to patients. Furthermore, this strategy could potentially be used for other applications than oral drug delivery, such as treating local GI disease while minimizing systemic toxicity.
Acknowledgments
This research was supported by the DuPont-MIT Alliance, the NIH, the University of Illinois at Urbana-Champaign (UIUC), and the Center for Nanoscale Science and Technology at UIUC and the Siteman Center for Cancer Nanotechnology Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Delie F. Evaluation of nano- and microparticle uptake by the gastrointestinal tract. Adv Drug Deliv Rev. 1998;34:221–233. doi: 10.1016/s0169-409x(98)00041-6. [DOI] [PubMed] [Google Scholar]
- 2.Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, Chaturvedi P, et al. Biologically erodable microsphere as potential oral drug delivery system. Nature. 1997;386:410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2:289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 4.Fasano A, Uzzau S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest. 1997;99:1158–1164. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandri G, Bonferoni MC, Rossi S, Ferrari F, Gibin S, Zambito Y, et al. Nanoparticles based on N-trimethylchitosan: Evaluation of absorption properties using in vitro (Caco-2 cells) and ex vivo (excised rat jejunum) models. Eur J Pharm Biopharm. 2007;65:68–77. doi: 10.1016/j.ejpb.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Chickering DE, Jacob JS, Desai TA, Harrison M, Harris WP, Morrell CN, et al. Bioadhesive microspheres .3. An in vivo transit and bioavailability study of drug-loaded alginate and poly(fumaric-co-sebacic anhydride) microspheres. J Control Release. 1997;48:35–46. [Google Scholar]
- 7.Ponchel G, Irache JM. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv Drug Deliv Rev. 1998;34:191–219. doi: 10.1016/s0169-409x(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 8.Carino GP, Jacob JS, Mathiowitz E. Nanosphere based oral insulin delivery. J Control Release. 2000;65:261–269. doi: 10.1016/s0168-3659(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 9.Vila A, Sanchez A, Tobio M, Calvo P, Alonso MJ. Design of biodegradable particles for protein delivery. J Control Release. 2002;78:15–24. doi: 10.1016/s0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 10.Chen HM, Langer R. Magnetically-responsive polymerized liposomes as potential oral delivery vehicles. Pharm Res. 1997;14:537–540. doi: 10.1023/a:1012124205524. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JJ, Teply BA, Jeong SY, Yim CH, Ho D, Sherifi I, et al. Magnetically responsive polymeric microparticles for oral delivery of protein drugs. Pharm Res. 2006;23:557–564. doi: 10.1007/s11095-005-9444-5. [DOI] [PubMed] [Google Scholar]
- 12.Hafeli UO. Magnetically modulated therapeutic systems. Int J Pharm. 2004;277:19–24. doi: 10.1016/j.ijpharm.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 14.Arruebo M, Fernandez-Pacheco R, Ibarra MR, Santamaria J. Magnetic nanoparticles for drug delivery. Nano Today. 2007;2:22–32. [Google Scholar]
- 15.Kararli TT. Comparison of the gastrointestinal anatomy, physiology and biochemistry of humans and commonly used laboratory-animals. Biopharm Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 16.Pettersson G, Ahlman H, Kewenter J. Comparison of small intestinal transit-time between rat and guinea-pig. Acta Chir Scand. 1976;142:537–540. [PubMed] [Google Scholar]
- 17.Bilati U, Allemann E, Doelker E. Nanoprecipitation versus emulsion-based techniques for the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues. Aaps Pharmscitech. 2005;6:E594–E604. doi: 10.1208/pt060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florence AT, Hillery AM, Hussain N, Jani PU. Nanoparticles as carriers for oral peptide absorption-studies on particle uptake and fate. J Control Release. 1995;36:39–46. [Google Scholar]
- 19.Whitehead K, Shen ZC, Mitragotri S. Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. J Control Release. 2004;98:37–45. doi: 10.1016/j.jconrel.2004.04.013. [DOI] [PubMed] [Google Scholar]