Abstract
Studies assessing the function of monocyte derived dendritic cells (MD-DC) in individuals with hepatitis C virus (HCV) infection have shown conflicting results. Impaired MD-DC function in chronic HCV infection would have important implications both for understanding the pathogenesis of HCV infection and in the use of autologous MD-DC in vaccination strategies. We determined the allostimulatory capacity of MD-DC in the same patient before and after HCV infection. Next, the phenotype, cytokine production and allostimulatory function of immature and mature MD-DC in individuals with persistent HCV infection were compared directly with MD-DC from healthy individuals. Finally, we assessed the ability of MD-DC to prime autologous naïve peptide specific CD8+ T cells using HLA-A2 class-I tetramers. DCs retained the same allostimulatory capacity before and following the establishment of persistent HCV infection. The surface phenotype and the amount of interleukin (IL)-10 and IL-12(p70) produced during DC maturation did not differ between HCV-infected individuals and healthy controls. Mature DCs from HCV-infected individuals performed comparably in an allogeneic MLR compared with healthy individuals. Mature MD-DC from HCV-infected individuals stimulated the expansion of peptide specific naïve CD8+ T cells. MD-DC from HCV-infected and healthy individuals are phenotypically indistinguishable and perform comparably in functional assays.
Keywords: dendritic cells, hepatitis C virus, monocytes, ribavirin
Introduction
Hepatitis C virus (HCV) infection is a major public health issue, with 170 million people infected worldwide. The majority of individuals who are infected develop persistent infection [1], which may be associated with the development of liver fibrosis and hepatocellular carcinoma. During primary HCV infection, a vigorous multi-specific HCV-specific CD8+ and CD4+ T-cell response can be detected [2,3]. However, these responses are not maintained in individuals who develop persistent infection when HCV specific T-cell responses become weak or undetectable ex vivo [2,4,5]. This is in marked contrast to other persistent, but controlled infections such as Epstein Barr Virus (EBV) and Cytomegalovirus (CMV) where strong and focused responses remain detectable [6].
There are many possible reasons for the attenuated HCV-specific T-cell response that is observed during persistent HCV infection. These include T-cell exhaustion [7,8], viral variation and escape [9,10], lack of CD4+ T-cell help and induction of T-cell tolerance or deletion of activated HCV specific T cells in the hepatic environment [11]. However, it is also possible that antigen presenting cells infected with hepatitis C virus fail to prime an appropriate T-cell response.
In support of the latter hypothesis, it has been suggested that the stimulatory capacity of monocyte derived dendritic cells (MD-DC) from patients with persistent HCV is impaired [12–15], and that HCV structural and nonstructural proteins may inhibit DC function [16–18]. Defects in MD-DC phenotype and cytokine production [12–14] have also been described, although the nature of these defects has been inconsistent between studies. Furthermore, this finding has fuelled the functional assessment of circulating myeloid and plasmacytoid DCs in HCV infection, an area of current controversy [19–22].
These findings, if true, have important implications not only for understanding the pathogenesis of HCV infection, but also in the use of autologous MD-DC in vaccination strategies. However, the finding that MD-DC in patients with HCV are defective remains controversial. While some studies have shown phenotypic or functional defects following MD-DC maturation, these findings have not been supported in a number of human [19,23] or chimpanzee studies [24,25]. In addition, patients with HCV infection do not exhibit global immunosuppression in the absence of advanced liver disease, as might be expected if HCV infection adversely affects MD-DC function.
Therefore, in this study, we have revisited the question of whether MD-DC function normally in patients with HCV infection by assessing the phenotype, cytokine secretion and stimulatory capacity of MD-DCs from patients with persistent HCV. We have also analysed the ability of MD-DC to prime naïve antigen specific T cells in vitro using an established model of melan-A specific T-cell responsiveness. All experiments were run in parallel and compared with MD-DC derived from healthy donors. We have included only ribavirin naïve patients, as recent studies have shown that ribavirin has immunomodulatory properties both in vivo and in vitro [26–28], and can attenuate DC function in vitro [29] and in vivo [21].
Finally, as we demonstrate that there is a wide variation in the magnitude of the stimulatory response between individuals, as might be expected in an allogeneic MLR reaction, we have assessed the stimulatory capacity of MD-DC in a unique ‘at risk’ population of intravenous drug users (IVDU). This cohort allowed us to analyse the functional capacity of MD-DC in the same individuals before and following the establishment of persistent HCV infection.
Materials and methods
Participants
With established persistent infection
Informed consent was obtained from each patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the John Radcliffe Hospital. Fifteen patients with persistent HCV infection were consecutively recruited from the out-patient clinic and studied prospectively. Each patient was studied in parallel with a healthy control individual without risk factors for the acquisition of viral hepatitis. We excluded patients with a prior history of any treatment for HCV, including interferon (IFN)-alpha and / or ribavirin treatment.
In all patients, HCV RNA was detectable by RT-PCR in the serum on at least two consecutive occasions 6 months apart (Roche v2.0 Amplicor assay; Roche Diagnostics Ltd). HCV genotype was determined in those patients being considered for therapy (Inno-Lipa HCV II, Innogenetics, Belgium). Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Patient (age / sex) | ALT (10–45) | Treated | Genotype | Histology (Ishak) | MLR | Phenotype | Cytokine secretion | Melan-A priming | Control (age / sex) |
|---|---|---|---|---|---|---|---|---|---|
| HCV-1 (49 / M) | 120 | No | 3 | 6 / 6 (F) 8 / 18 (I) | + | + | + | − | HI-1 (39 / M) |
| HCV-2 (40 / M) | 24 | No | ND | 1 / 6 (F) 4 / 18 (I) | + | + | + | − | HI-2 (38 / F) |
| HCV-3 (37 / F) | 34 | No | 1 | 2 / 6 (F) 4 / 18 (I) | + | + | + | − | HI-3 (34 / F) |
| HCV-4 (47 / M) | 27 | No | 3 | 2 / 6 (F) 5 / 18 (I) | + | + | + | − | HI-4 (38 / M) |
| HCV-5 (48 / F) | 41 | No | ND | 1 / 6 (F) 2 / 18 (I) | + | − | − | − | HI-5 (36 / M) |
| HCV-6 (44 / F) | 29 | No | 1 | ND | + | + | − | − | HI-6 (26 / M) |
| HCV-7 (63 / F) | 22 | No | 5a | 2 / 6 (F) 2 / 18 (I) | + | − | − | − | HI-7 (36 / F) |
| HCV-8 (42 / M) | 61 | No | 1 | 1 / 6 (F) 3 / 18 (I) | − | − | + | − | HI-8 (37 / F) |
| HCV-9 (38 / M) | 21 | No | 2b | ND | − | − | − | + | HI-5 (36 / M) |
| HCV-10 (47 / F) | 42 | No | 1a / 1b | 0 / 6 (F) 3 / 18 (I) | − | − | − | + | HI-8 (37 / F) |
| HCV-11 (36 / F) | 49 | No | ND | 1 / 6 (F) 2 / 18 (I) | − | − | − | + | HI-9 (36 / F) |
| HCV-12 (41 / F) | 28 | No | 1 | 1 / 6 (F) 3 / 18 (I) | − | − | − | + | HI-9 (36 / F) |
| HCV-13 (41 / M) | 61 | No | 1 | 1 / 6 (F) 3 / 18(I) | − | − | − | + | HI-8 (37 / F) |
13 patients were recruited consecutively from outpatients and paired with healthy controls for subsequent assays. + / − indicates assays performed / not performed on each patient.
ALT, alanine transferase (normal range 10–45 IU / L), Histology – Ishak score; F, fibrosis; I, inflammation; MLR, mixed leukocyte reaction; ND, not done; IFN-α: 6-month course.
Patients studied before and after primary hepatitis C virus infection
Intravenous drug users (IVDU) at risk for HCV infection were identified in a prospective study of young IVDUs in Baltimore, MD, as described previously and as approved by the institutional review boards of the Johns Hopkins Schools of Medicine and Hygiene and Public Health [30]. Participants eligible for the study were anti-HCV antibody negative, between 15 and 30 years of age, and acknowledged use of injection drugs. Eligible subjects consented to have blood drawn for isolation of plasma and peripheral blood mononuclear cell (PBMC) in a protocol designed for monthly follow-up. At each visit, participants were provided counselling to reduce the risks of drug use. Acute HCV infection was identified by HCV antibody seroconversion, as described previously [5]. All participants who acquired HCV infection were offered treatment, but none of the subjects included in this paper elected to be treated during the period of follow-up reported in this study.
Allostimulatory capacity of MD-DC was determined using MD-DC from before and following clearance or persistence in seven subjects and, in one case, during the early phase of infection as well as before and after infection. HCV clearance was defined as the presence of anti-HCV with HCV RNA undetectable by the COBAS AMPLICOR qualitative assay in serum or plasma specimens from ≥2 consecutive visits obtained at least 300 days after initial detection of viremia. Persistence was defined as the persistent presence of anti-HCV with HCV RNA detectable by the qualitative or quantitative COBAS AMPLICOR assay in serum or plasma specimens obtained at least 300 days after initial viremia [30]. Of the seven subjects, two cleared infection and five remained persistently infected. All post-HCV infection MD-DC were derived from PBMC acquired at least 300 days after infection. One subject who ultimately cleared his HCV infection also had MD-DC derived from PBMC acquired 8 months from onset of viremia, while still HCV RNA positive, labelled his ‘viremic’ specimen. Five of the subjects were men, two were women, three were Caucasian, three were African-American, one was a Native American and the mean age at seroconversion was 26 years (range 22–29 years). They were all infected with genotype 1 HCV. All patients were seronegative for HIV by enzyme-linked immunosorbent assay.
Generation of immature dendritic cells
PBMC were isolated by centrifugation on Fycoll. To exclude the possibility that functional defects of MDDC in HCV infection is dependent on the method of generating MD-DC, we used two different techniques.
For the Oxford cohort with chronic HCV infection, monocytes were purified using anti-CD14 conjugated magnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). Immature DC were generated by culturing monocytes in RPMI-10% endotoxin low fecal calfserum (FCS) (Invitrogen), supplemented with pen-strep, 2 mm l-glutamine, 1% nonessential aminoacids, 1% pyruvate, 5 × 10−5 m 2-mercaptoethanol (Sigma, UK), granuolocyte-macrophage colony-stimulating factor (50 ng / mL; Leucomax, Schering-Plough, UK) and IL-4 (250 IU / L; PeproTech, UK) for 6 days. DCs were cultured in 6-well plates at a concentration of 400 000 DCs / mL medium.
For the Baltimore cohort, monocytes were purified by isolation of adherent cells after plating on a 6-well plate. Immature DCs were generated by culturing monocytes in Aim V media (Invitrogen) supplemented 2 mm l-glutamine, GMCSF (800 IU / mL; R&D Systems) and IL-4 (800 IU / mL; R&D Systems) at 37 °C under 5% CO2 for 6 days with replacement of 0.75 mL of Aim-V media with 600 IU each of GMCSF and IL-4 on days 3 and 5.
Maturation of immature dendritic cells
Tumour necrosis factor (TNF)-α was used as the maturation stimulus in many of the previous studies assessing allostimulatory capacity [13,14,18]. To exclude the possibility that the maturation defect in MD-DC previously described in HCV infection is dependent on the nature of the maturation stimulus used, we assessed two different maturation stimuli in our assays. For the assessment of MD-DC in subjects with chronic HCV infection immature DCs were stimulated with dsRNA (Poly I:C, Sigma), 50 μg / mL for 36 h to generate mature DCs. For the immature MD-DCs generated pre- and post-HCV infection, maturation was achieved by incubation with 10 ng / mL of TNF-α at 37 °C under 5% CO2 for 24 h.
Phenotypic analysis
The phenotype of mature and immature DCs was evaluated by surface staining with the following antibodies; Isotype controls IgG1-FITC and IgG2a-PE (Dako), HLA class A, B, C-RPE (clone W6 / 32; Dako), HLA-DP, DQ, DR-FITC (Pharmingen), CD-83-FITC (Pharmingen) and CD-86-FITC (Dako). 7-AAD staining (Pharmingen) was used to exclude dead cells from the analysis. Samples were analysed on a FACSCalibur (Becton Dickinson) using cell quest software.
Cytokine determination
Cytokine produced by maturing DCs was determined in cell supernatants 36 h after the addition of dsRNA in an ELISA, using antibodies specific for IL-10 and IL-12(p70) according to manufacturer’s instructions (Pharmingen).
Mixed leukocyte reaction
The stimulatory capacity of mature DCs from healthy control and HCV-infected individuals or for the same individual pre- and post-HCV infection was assessed via mixed leukocyte reaction (MLR). Following DC maturation, DCs were harvested and irradiated (30 Gy). DCs were co-cultured in increasing concentrations with allogeneic PBMC from a healthy, HCV naïve donor. The same healthy donor PBMCs were used in all experiments. After 5 days of co-culture, the proliferative response of the responder cells was evaluated by the addition of 1 μCi [3H] thymidine for 18 h. All assays were performed in triplicate.
Melan-A peptides and tetramers
Melan-A peptide26–35 (ELAGIGILTV) (Sigma-Genosys) was HPLC purified. This is an analogue of the melan-A 26–35 epitope with an improved HLA-A2 binding affinity [31]. HLA-A2.1 / melan-A peptide tetrameric complex was synthesized as previously described [32]. Briefly, modified HLA A2.1 and β2-microglobulin were synthesized and purified from a prokaryotic expression system and refolded in the presence of melan-A peptide. Refolded complexes were purified by FPLC and biotinylated, before combining with PE-labelled streptavidin at a 4:1 molar ratio to form tetramers.
T-cell priming
Mature DCs were generated as above by the addition of dsRNA. These were pulsed for 3 h with melan-A peptide, 1 μg / mL in serum free RPMI at 37 °C. DCs from the same individual pulsed with RPMI rather than peptide served as negative controls. Cells were washed twice and incubated with autologous PBMC at a 1:10 (DC:PBMC) ratio in RPMI with 10% FCS. Human rIL-2 (10 IU / mL Proleukin) was added after 5 days. Cells were expanded with 250 IU / mL IL-2 at day 9 and harvested for staining with melan-A tetramer at day 13. Precise timing was observed to allow accurate comparisons of melan-A CD8+ T-cell expansions between experiments. Cells were stained in PBS with melan-A tetramer for 25 min at 37 °C, washed and then incubated on ice with 7-AAD (Pharmingen) to exclude dead cells from the analysis in addition to CD8-FITC (Dako). Cells were analysed on a FACSCalibur (Becton Dickinson) using cell quest software.
Statistical analysis
Statistical analysis used the paired t-test. All analyses were performed using GraphPad Prism® 3.0a for Macintosh. A P-value <0.05 was considered significant.
Results
The phenotype of mature and immature dendritic cells in hepatitis C virus-infected and healthy individuals
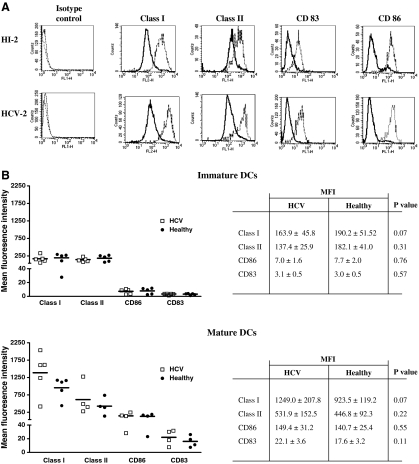
Monocyte derived dendritic cells were generated in parallel from five HCV-infected individuals and five healthy controls (Table 1). Immature MD-DC were matured with dsRNA. The expression of class-I (HLA-A, -B, -C), class-II (HLA-DR, -DQ and -DP), CD83 and CD86 before and following DC maturation was determined. As previously described [33], immature DCs expressed class-I, class-II and CD86 at intermediate levels, but not CD83. These markers were equally expressed in HCV-infected and healthy individuals (Figs. 1a,b, upper panel). Furthermore, dsRNA-induced DC maturation associated with the up-regulation of each of these markers in both HCV-infected and healthy individuals (Figs 1a,b, lower panel).
Fig. 1.
The phenotypic characteristics of immature and mature DCs in HCV infected and healthy individuals. The expression (mean fluorescence intensity–MFI) of HLA class I, HLA class II, CD 86 and CD83 on immature (bold line) and dsRNA matured (dotted line) MD-DCs from a representative HCV infected individual (HCV-2) and a healthy control individual is shown (HI-2) (A). The expression of these markers on MD-DCs is compared between healthy and HCV infected individuals on both immature (B, upper panel) and mature (B, lower panel) MD-DCs. The table inserts give the MFI ± standard error and P values for each marker.
Cytokine production by dendritic cells from healthy and hepatitis C virus-infected individuals
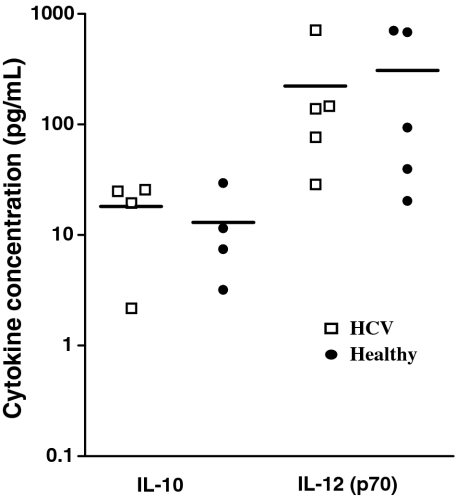
Supernatants were collected from maturing dendritic cell cultures 36 h after the addition of dsRNA and the concentration of IL-10 and IL-12(p70) was determined using an ELISA in five individuals with HCV infection and compared with five healthy controls (Table 1). While IL-10 was found at a concentration 10-fold lower than IL-12(p70) and while the amount of cytokine produced varied widely between individuals as previously described [34], there was no statistical difference in the amount of IL-10 or IL-12 produced by MD-DC from HCV-infected and healthy controls (Fig. 2).
Fig. 2.
Cytokine production by maturing DCs derived from HCV infected and healthy individuals. Culture supernatants were sampled 36 h after the addition of dsRNA to immature DCs derived from HCV and healthy individuals. IL-10 and IL-12(p70) concentrations were measured by ELISA.
The stimulatory capacity of dendritic cells from healthy and hepatitis C virus-infected individuals
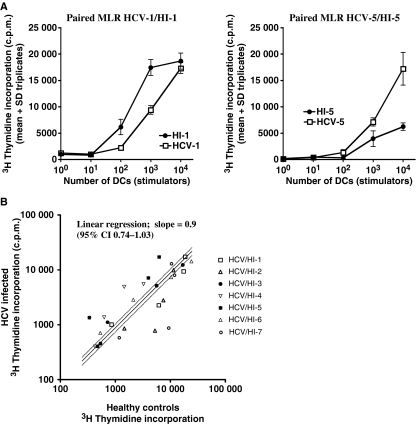
It has previously been reported that MD-DCs derived from HCV-infected patients have a reduced stimulatory capacity. We therefore assessed the stimulatory capacity of MD-DCs from seven HCV-infected treatment naïve (Table 1; HCV-1–7) patients and compared these with seven healthy (Table 1; HI-1–7) individuals in parallel experiments. The same responder (HI-10) PBMC was used in all assays. The stimulatory capacity, using 10-fold increasing concentrations of DCs (10, 102, 103 and 104) in two representative experiments from HCV-infected individuals (HCV-1, -6,) and the paired healthy controls (HI-1, -6,) is shown (Fig. 3a). Overall analysis (Fig. 3b) where the stimulatory capacity of DCs is plotted against the stimulatory capacity of the paired control at each DC concentration shows that there is no significant difference in the stimulatory capacity of DCs from HCV and healthy controls. Regression analysis slope = 0.9 (95% confidence intervals 0.74–1.03).
Fig. 3.
The stimulatory capacity of DCs derived from HCV and healthy individuals. The stimulatory capacity of DCs derived from HCV and healthy individuals was assessed in paired assays run in parallel. Following irradiation, dsRNA matured DCs at 10-fold increasing concentrations (0–104) were co-cultured with allogeneic PBMC (5 × 104). T-cell proliferation was determined on day 6 of culture following 18 h incubation with 3H thymidine (c.p.m. = counts per minute). The results are expressed as mean ± SD of wells in triplicates. The same responder PBMCs from a healthy individual were used in all experiments. Two representative experiments are shown (a). In an overall analysis (b), 3H thymidine incorporation using DCs from each HCV infected patient is plotted against 3H thymidine incorporation for the paired healthy control (HCV/HI-1–7) at each DC concentration (10, 102,103 and 104). Linear regression analysis; slope = 0.9 (95% confidence interval (CI) 0.735–1.025).
The stimulatory capacity of monocyte derived dendritic cells in at risk individuals before, during and after infection with hepatitis C virus infection
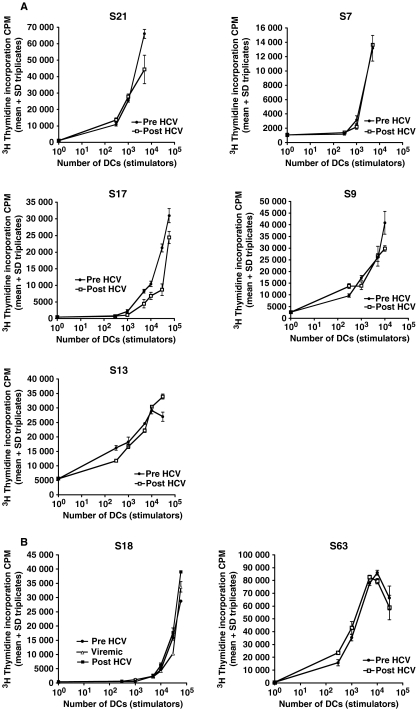
Although there was no difference in the stimulatory capacity of MD-DC in HCV-infected individuals compared with healthy controls, there was a marked variation in the allostimulatory response between individuals that did not depend on HCV infection status. To control for host variation, we assessed a cohort of patients ‘at risk’ for acquiring HCV infection, before acute infection and after the establishment of persistent HCV infection (Fig. 4a; Subjects S7, S9, S13, S17 and S21) or clearance of HCV (Fig. 4b; Subjects S18 and S63). In subject S18, who eventually cleared HCV, we also assessed the stimulatory capacity of DCs generated from PBMC acquired during early HCV infection, while still HCV RNA positive. The same responder PBMCs were used in all assays. We observed that the stimulatory capacity of MD-DC prior to HCV infection was maintained throughout and following HCV infection irrespective of the outcome of infection. Once again, wide variation in stimulatory capacity was observed between individuals, but not in the same host before and after HCV infection.
Fig. 4.
The stimulatory capacity of DCs derived from individuals before and following HCV infection. The stimulatory capacity of DCs was determined before and >300 days following HCV infection and also during acute infection in subject 18. Patients S7, S9, S13, S17 and S21 developed persistent infection (A) while patients S18 and S63 spontaneously cleared virus (B). Following irradiation, matured DCs at increasing concentrations were co-cultured with allogeneic PBMC (2 × 105). T-cell proliferation was determined on day 6 of culture following 18 h incubation with 3H Thymidine (c.p.m. = counts per minute). The results are expressed as mean ± SD of wells in triplicates. The same responder PBMCs from a healthy individual were used in all experiments.
Priming of naïve melan-A specific CD8+ T cells by dendritic cells derived from healthy and hepatitis C virus-infected individuals
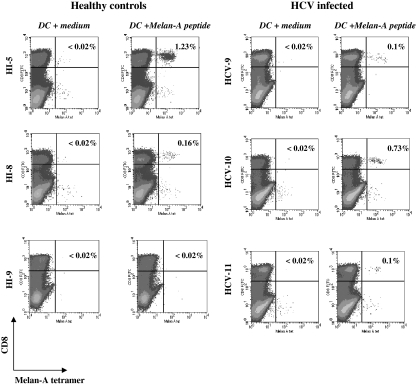
Melan-A tetramer+ cells are detectable in the peripheral blood of many healthy donors, typically at a frequency of less than 0.07% CD8+ T cells [35]. These cells are antigen inexperienced as confirmed by multiple techniques [35–37]. Consistent with these findings, nonprofessional antigen presenting cells expand melan-A specific CD8+ naïve T cells only when pulsed with very high concentrations of peptide (100 μg / mL), whereas melan-A peptide pulsed MD-DCs derived from healthy individuals induce greater expansions and require a low concentration of melan-A peptide (1 ng / mL) [38]. We assessed the ability of DCs from HCV-infected individuals, pulsed with low concentrations of melan-A peptide, to prime a naïve T-cell population. Each experiment was run in parallel with DCs obtained from healthy individuals. Three representative experiments are shown (Fig. 5). DCs from both HCV-infected and healthy individuals were able to prime naïve melan-A specific T cells. Melan-A specific T cells could be expanded in 2 / 3 healthy and 3 / 5 HCV individuals tested [not significant (P = 0.99) Fishers exact test]. The frequency of melan-A specific T cells that were primed using DCs from HCV-infected individuals (median 0.1% CD8+ T cells, range 0–0.73%) was not significantly different from the frequencies obtained using DCs from healthy individuals (median 0.16%, range 0–1.2%). In all cases, the frequencies of melan-A specific T cells co-cultured with autologous DCs that were not pulsed with melan-A peptide were <0.02% CD8+ T cells.
Fig. 5.
The efficient expansion of melan-A specific CD8+ T cells from healthy and HCV infected individuals. In three representative paired, controlled experiments, dsRNA matured MD-DCs from healthy (left hand panel; HI-5, -8, -9) and HCV infected (right hand panel; HCV-9, -10, -11) individuals were pulsed with 1 μg/mL melan-A26-35 peptide or RPMI medium, washed thoroughly and then incubated with autologous PBMC at a ratio of 1DC: 10 PBMCs. After 13 days, cultures were stained with melan-A tetramer-PE, CD 8-FITC and 7-AAD. DCs are gated on the live population. % tetramer+/CD8+ T cells is given in each FACS plot.
Discussion
In this study, we show that MD-DCs derived from HCV-infected individuals are phenotypically and functionally indistinguishable from healthy controls. Furthermore, the stimulatory capacity of MD-DC is maintained before, during early and after the establishment of persistent HCV infection within the same individual.
The observation that T–cell responses are attenuated during persistent HCV infection has prompted investigation into the effects of HCV infection on DC function, cells which are crucially involved in inducing an effective, appropriate T-cell response. However, the finding that MD-DC are functionally impaired in HCV infection is highly controversial [12–16,19,23–25]. The search for the mechanism behind this observation has also produced conflicting data. For example, it has been reported that the up-regulation of the phenotypic markers (CD86, class II and CD83) associated with DC maturation in HCV-infected individuals is normal [13] despite impaired allostimulatory capacity. However, it has also been reported that the expression of CD86 is not up-regulated [12], and that the expression of CD83 and class II are not up-regulated, while CD86 is up-regulated [14]. The assessment of IL-12(p70) production and the role of cytokines in restoring the allostimulatory defect is similarly conflicting [12,13].
Previous studies have suggested that impaired DC function is because of infection of DC with HCV. The finding in a single patient, that MD-DC are productively infected [13] means that the precursor monocytes must have also been infected. However, a previous study [39] of 10 patients failed to demonstrate HCV-negative strands that are associated with viral replication in the monocyte population in any of these individuals and HCV-negative strands were detected in the DC population in a single individual only. More recently, negative strand HCV RNA as evidence of HCV replication could be detected in blood DCs in only 3 / 24 individuals with persistent HCV infection [40]. Thus, although monocyte / DC infection may occur, it is a rare event which cannot readily account for the attenuation of T-cell responses observed in the vast majority of patients with persistent HCV infection.
Although productive infection of DCs and impaired DC function has been reported with human immunodeficiency virus [41–44] and with measles virus [45–47], both of these viruses are associated with profound clinical immune defects. It is hard to reconcile the reported general allostimulatory defect in individuals with persistent HCV infection with the clinical observation that HCV-infected individuals are not overtly immunosuppressed after over a decade of follow-up. Our observations that DCs derived from patients with HCV infection function normally are consistent with this broad clinical picture.
It is possible that the inclusion of patients taking ribavirin [13] either at the time of study or in the past [14] may have accounted at least in part for inconsistencies between studies. The pharmacokinetic properties of ribavirin include high intracellular levels with a prolonged half-life in vivo (more than 6 months) [48]. It is now clear that ribavirin has immunomodulatory properties both in vivo and in vitro [26–28], and can attenuate DC function in vitro [29] and in vivo [21].
It is also possible that study differences may be accounted for by patient cohorts with different amount of liver inflammation / fibrosis and that the alteration in DC function described is induced in part by liver pathology rather than by HCV. Notably, the majority of patients in our study had mild liver disease.
Another possible explanation for discrepancies between previous studies is that differences are reflective of host differences rather than the effects of HCV infection. Consistent with this, our results show that there is significant inter-individual variation in the stimulatory capacity of DCs using an allogenic MLR. However, the allostimulatory capacity of MD-DC from patients before and after HCV infection does not differ in the same host, showing that this variation is not a consequence of HCV infection.
Using the melan-A model to assess the ability of DCs to prime a naïve antigen specific population [38,49], we show that individuals with persistent HCV infection are able to prime a naïve T-cell response at least in vitro. We accept the limitations of this model, which may not translate to the priming environment in vivo and which does not necessarily represent the situation in primary HCV infection. However, vigorous and multi-specific T-cell responses are detected during primary infection regardless of outcome at a time when HCV viral loads are very high [2,3,50]. In keeping with our in vitro observations, in this situation, HCV-specific T-cell responses are clearly primed in vivo. However, it remains possible that a subtle defect in priming during primary infection results in an HCV-specific T-cell response that is not maintained or is not optimally functional.
The results from our study which demonstrate that DCs from HCV-infected patients function comparably with healthy controls, the lack of a common DC phenotype in previously published reports of patients with chronic HCV, and the fact that patients with a decade of HCV infection in the absence of severe liver disease are immunocompetent suggests that DC function in HCV warrants continued evaluation. Future studies that concentrate on DC function during primary infection, intra-hepatic DC function and the role of circulating myeloid and plasmacytoid DCs, will be particularly informative.
Acknowledgments
EB is an MRC clinician scientist, MS and VC were funded by CRUK, program grant C399 / A2291, PK is a Senior Wellcome clinician scientist. This work was supported by grant K08 DA11880 from the US Public Health Service to AC.
Abbreviations
- HCV
hepatitis C virus
- MD-DC
monocyte derived dentritic cell
- IVDU
intravenous drug user
- TNF
tumour necrosis factor
References
- 1.Lauer GM. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Lechner F, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner F, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Gruner NH, et al. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:1528–1536. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 5.Cox AL. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104–112. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes E. T cell failure in hepatitis C virus infection. Viral Immunol. 2002;15:285–293. doi: 10.1089/08828240260066233. [DOI] [PubMed] [Google Scholar]
- 7.Gallimore A, et al. Induction and exhaustion of LCMV-specific CTL visualised using soluble tetrameric MHC-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moskophidis D. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko T, et al. Impaired induction of CTL by antagonism of a weak agonist borne by a variant HCV epitope. Eur J Immunol. 1997;27:1782–1787. doi: 10.1002/eji.1830270728. [DOI] [PubMed] [Google Scholar]
- 10.Cox AL, et al. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741–1752. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertolino P. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol. 1998;28:221–236. doi: 10.1002/(SICI)1521-4141(199801)28:01<221::AID-IMMU221>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Kanto T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–5591. [PubMed] [Google Scholar]
- 13.Bain C. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–524. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 14.Auffermann-Gretzinger S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–3176. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 15.Szabo G. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210:237–247. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Dolganiuc A, et al. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615–5624. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, et al. Identification of hepatitis C virus core domain inducing suppression of allostimulatory capacity of dendritic cells. Arch Pharm Res. 2002;25:364–369. doi: 10.1007/BF02976640. [DOI] [PubMed] [Google Scholar]
- 18.Sarobe P, et al. Abnormal priming of CD4(+) T Cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J Virol. 2002;76:5062–5070. doi: 10.1128/JVI.76.10.5062-5070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccioli D, et al. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42:61–67. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Longman RS. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 21.Goutagny N, et al. Quantification and functional analysis of plasmacytoid dendritic cells in patients with chronic hepatitis C virus infection. J Infect Dis. 2004;189:1646–1655. doi: 10.1086/383248. [DOI] [PubMed] [Google Scholar]
- 22.Tsubouchi E. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. J Gastroenterol. 2004;39:754–762. doi: 10.1007/s00535-003-1385-3. [DOI] [PubMed] [Google Scholar]
- 23.Longman RS. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026–1029. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 24.Rollier C, et al. Chronic hepatitis C virus infection established and maintained in chimpanzees independent of dendritic cell impairment. Hepatology. 2003;38:851–858. doi: 10.1053/jhep.2003.50426. [DOI] [PubMed] [Google Scholar]
- 25.Larsson M, et al. Lack of phenotypic and functional impairment in dendritic cells from chimpanzees chronically infected with hepatitis C virus. J Virol. 2004;78:6151–6161. doi: 10.1128/JVI.78.12.6151-6161.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoofnagle JH, et al. Maintenance therapy with ribavirin in patients with chronic hepatitis C who fail to respond to combination therapy with interferon alfa and ribavirin. Hepatology. 2003;38:66–74. doi: 10.1053/jhep.2003.50258. [DOI] [PubMed] [Google Scholar]
- 27.Cramp ME. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–355. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 28.Shiffman ML. Alpha interferon combined with ribavirin potentiates proliferative suppression but not cytokine production in mitogenically stimulated human lymphocytes. Antiviral Res. 2000;48:91–99. doi: 10.1016/s0166-3542(00)00120-0. [DOI] [PubMed] [Google Scholar]
- 29.Barnes E, et al. Impact of alpha interferon and ribavirin on the function of maturing dendritic cells. Antimicrob Agents Chemother. 2004;48:3382–3389. doi: 10.1128/AAC.48.9.3382-3389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox AL, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–958. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 31.Romero P, et al. Cytolytic T lymphocyte recognition of the immunodominant HLA-A*0201-restricted Melan-A / MART-1 antigenic peptide in melanoma. J Immunol. 1997;159:2366–2374. [PubMed] [Google Scholar]
- 32.Altman J, et al. Direct visualization and phenotypic analysis of virus-specific T lymphocytes in HIV infected individuals. Science. 1996;274:94–96. [Google Scholar]
- 33.Cella M. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langenkamp A. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 35.Pittet MJ, et al. High frequencies of naive Melan-A / MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190:705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunbar PR, et al. A shift in the phenotype of melan-A-specific CTL identifies melanoma patients with an active tumor-specific immune response. J Immunol. 2000;165:6644–6652. doi: 10.4049/jimmunol.165.11.6644. [DOI] [PubMed] [Google Scholar]
- 37.Zippelius A, et al. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J Exp Med. 2002;195:485–494. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salio M, et al. Mature dendritic cells prime functionally superior melan-A-specific CD8+ lymphocytes as compared with nonprofessional APC. J Immunol. 2001;167:1188–1197. doi: 10.4049/jimmunol.167.3.1188. [DOI] [PubMed] [Google Scholar]
- 39.Mellor J. Low level or absent in vivo replication of hepatitis C virus and hepatitis G virus / GB virus C in peripheral blood mononuclear cells. J Gen Virol. 1998;79(Pt 4):705–714. doi: 10.1099/0022-1317-79-4-705. [DOI] [PubMed] [Google Scholar]
- 40.Goutagny N, et al. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J Infect Dis. 2003;187:1951–1958. doi: 10.1086/375350. [DOI] [PubMed] [Google Scholar]
- 41.Pope M, et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. issn: 0092-8674. [DOI] [PubMed] [Google Scholar]
- 42.Cameron PU. Dendritic cells exposed to HIV-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 43.Chehimi J, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 44.Chehimi J, et al. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grosjean I, et al. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaiserlian D. Infection of human dendritic cells by measles virus induces immune suppression. Adv Exp Med Biol. 1997;417:421–423. doi: 10.1007/978-1-4757-9966-8_69. [DOI] [PubMed] [Google Scholar]
- 47.Fugier-Vivier I. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lertora JJ, et al. Pharmacokinetics and long-term tolerance to ribavirin in asymptomatic patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 1991;50:442–449. doi: 10.1038/clpt.1991.162. [DOI] [PubMed] [Google Scholar]
- 49.Salio M, et al. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–1062. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 50.Gerlach J, et al. Recurrence of HCV after loss of virus specific CD4+ T cell response in acute Hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]