Abstract
Aim
To perform an empirical, pharmacological, separation of early afterdepolarizations (EADs) and transmural gradients of repolarization in arrhythmogenesis in a genetically modified mouse heart modelling human long QT syndrome (LQT) 3.
Methods
Left ventricular endocardial and epicardial monophasic action potentials and arrhythmogenic tendency were compared in isolated wild type (WT) and Scn5a+/Δ hearts perfused with 0.1 and 1 μm propranolol and paced from the right ventricular epicardium.
Results
All spontaneously beating bradycardic Scn5a+/Δ hearts displayed EADs, triggered beats and ventricular tachycardia (VT; n = 7), events never seen in WT hearts (n = 5). Perfusion with 0.1 and 1 μm propranolol suppressed all EADs, triggered beats and episodes of VT. In contrast, triggering of VT persisted following programmed electrical stimulation in 6 of 12 (50%), one of eight (12.5%), but six of eight (75%) Scn5a+/Δ hearts perfused with 0, 0.1 and 1 μm propranolol respectively in parallel with corresponding alterations in repolarization gradients, reflected in action potential duration (ΔAPD90) values. Thus 0.1 μm propranolol reduced epicardial but not endocardial APD90 from 54.7 ± 1.6 to 44.0 ± 2.0 ms, restoring ΔAPD90 from −3.8 ± 1.6 to 3.5 ± 2.5 ms (all n = 5), close to WT values. However, 1 μm propranolol increased epicardial APD90 to 72.5 ± 1.2 ms and decreased endocardial APD90 from 50.9 ± 1.0 to 24.5 ± 0.3 ms, increasing ΔAPD90 to −48.0 ± 1.2 ms.
Conclusion
These findings empirically implicate EADs in potentially initiating spontaneous arrhythmogenic phenomena and transmural repolarization gradients in the re-entrant substrate that would sustain such activity when provoked by extrasystolic activity in murine hearts modelling human LQT3 syndrome.
Keywords: arrhythmogenesis, long QT3 syndrome, propranolol
Sudden cardiac death (SCD) attributable to ventricular arrhythmogenesis is one of the major causes of mortality in the developed world, accounting for over 300 000 deaths per year in the USA (Kannel et al. 1987, Willich et al. 1987) and up to 70 000 deaths per year in the UK (NICE, 2000). Cardiac arrhythmias are clinically associated with a condition known as long QT syndrome, which is characterized by prolonged ventricular repolarization and a tendency to ventricular tachycardia, particularly torsades de pointes (TdP), leading to syncope and SCD. The LQT3 subtype results from gain of function mutations in the Scn5a gene, affecting Na+ channel inactivation and leading to an arrhythmogenic persistent late inward Na+ current (INa) (Wang et al. 1995a,b).
The induction of ventricular arrhythmias has been attributed to the following, not necessarily exclusive electrophysiological events: (1) impaired cardiac repolarization leads to small, premature depolarizations (early afterdepolarizations, EADs), interrupting the smooth repolarization phase of the action potential (AP). If EADs reach a sufficient amplitude they may initiate a premature AP (triggered beat) which may in turn precipitate into TdP (Eckardt et al. 1998, Killeen et al. 2007a). (2) Alterations in the repolarization gradients have been attributed to heterogeneous ion channel expression through the thickness of the ventricular wall. Thus, selective action potential duration (APD) prolongation or reduction within localized regions of the ventricle can alter the dispersion of repolarization; both increases (Milberg & Eckardt 2005) and decreases (Killeen et al. 2007a) in such ventricular transmural repolarization gradients, often quantified as the epicardial-endocardial difference in APD90 (ΔAPD90), have been associated with arrhythmogenesis at the whole heart level.
Previous isolated tissue and single cell experiments have indicated that most cardiac cell types are capable of generating EADs. However, arrhythmogenesis at the whole heart level may involve a differential expression of contrasting electrophysiological characteristics in a population of intercellularly coupled cells not detectable in single cell studies. Such intercellular coupling has been previously shown to either facilitate or suppress EAD induction (Huelsing et al. 2000). Furthermore, single cell studies and studies confined to isolated tissue exclude examination of important myocardial properties essential for the study of arrhythmogenesis including re-entry mechanisms and transmural gradients of repolarization. Consequently, they have failed to clarify the relationship between EADs, transmural gradients of repolarization and arrhythmogenesis at the whole heart level. The use of an intact, working myocardium for the study of arrhythmogenesis permits the measurement of multicellular parameters involving ventricular transmural gradients of repolarization and EADs, triggered beats and arrhythmias that cannot be assessed in single cell or isolated tissue preparations. The use of an intact, beating heart model thus includes all myocardial cell types and maintains intercellular coupling and thus provides more physiologically relevant information regarding the induction and propagation of arrhythmias.
We explored for empirical means of resolving the causal relationship between EADs and abnormal transmural gradients of repolarization reflected in altered ΔAPD90 values in genetically modified murine whole hearts modelling human long QT syndrome type 3 for the first time. This study was directly prompted by a recent report describing a similarly empirical pharmacological separation of EADs from arrhythmogenic substrate in a murine model of hypokalaemia-induced arrhythmogenesis (Killeen et al. 2007b). Accordingly, it made endocardial and epicardial monophasic action potential (MAP) recordings from the left ventricle of isolated, intact Langendorff-perfused ΔKPQ Scn5a (Scn5a+/Δ) and wild type (WT) hearts, exposed to a range of propranolol concentrations. Propranolol is well known to exert potent Na+ channel blockade occurring independently of its β-adrenoceptor blocking actions, thought important in its antiarrhythmic action (Rauls & Baker 1979, Matthews & Baker 1982). Propranolol concentrations used (0.1 and 1 μm) were based on values known to cause electrophysiological effects in clinical dose–response studies (Duff et al. 1983). Indeed, they are close to previous values used in earlier studies of LQTS in animal models (Shimizu & Antzelevitch 2000).
Spontaneously beating Scn5a+/Δ hearts following AV node ablation showed EADs and episodes of ventricular tachycardia (VT) never observed in WT hearts. Perfusion of spontaneously beating Scn5a+/Δ hearts with both 0.1 and 1 μm propranolol suppressed both EADs and episodes of VT in all hearts, and had no effect in WT hearts. In contrast, programmed electrical stimulation (PES) protocols applying premature stimuli successfully provoked VT in 50% of Scn5a+/Δ hearts at baseline, events never recorded from WT hearts. The lower propranolol concentrations (0.1 μm) both abolished this VT and selectively reduced APD90, restoring ΔAPD90 close to WT control values in all Scn5a+/Δ hearts. In contrast, at higher concentrations (1 μm), PES successfully induced VT in 75% of Scn5a+/Δ hearts. This proarrhythmic effect was specifically associated with a combination of a prolonged epicardial APD90 and reduced endocardial APD90, resulting in an increased ΔAPD90 and exacerbated arrhythmogenic substrate.
We achieve an empirical pharmacological separation of EADs from arrhythmic substrate through the empirical use of propranolol, resolving the causal relationship between EADs and arrhythmic substrate in a genetically modified murine whole heart model of an inherited human arrhythmic syndrome. These results thus (1) demonstrate the importance of both EADs and arrhythmic substrate in the initiation of arrhythmias in a whole heart model of arrhythmogenecity which directly corresponds to the human clinical phenotype through genetic modification of the Scn5a gene, (2) provide independent corroboration of such important relationships seen using alternative pharmacological strategies (Killeen et al. 2007b) and (3) add to recent arguments for the utility of mouse hearts in modelling human arrhythmia syndromes (Stokoe et al. 2007). Such a causal relationship between EADs and arrhythmic substrate as demonstrated in the present study in a genetically modified mouse heart modelling human LQT3 syndrome at the very least merits further testing.
Methods
Preparation of Langendorff-perfused hearts
Techniques used for the generation of Scn5a+/Δ mice and the preparation of whole hearts for Langendorff perfusion have been previously described (Head et al. 2005). Healthy, viable hearts suitable for experimentation regained a homogeneous pink coloration and spontaneous rhythmic contraction with warming. Hearts not demonstrating these features were instantly discarded prior to experimentation, to avoid false-positive results during PES (2 hearts discarded out of a total of 25). Hearts were perfused with physiological perfusion buffer for 30 min prior to experimentation, to minimize any residual effects of endogenous catecholamine release.
Programmed electrical stimulation
Programmed electrical stimulation of the intact hearts used paired (1 mm inter-pole spacing) platinum stimulating and recording electrodes, positioned on the basal epicardial surface of the right ventricle. The pacing protocols used 2 ms square-wave stimuli with amplitudes three times excitation threshold (Grass S48 stimulator; Grass-Telefactor, Slough, UK). PES is an established method of arrhythmia provocation that has been previously used to assess arrhythmogenecity in the murine heart (Killeen et al. 2007a,b). All experimental mice were bred from a 129 genetic background, which, along with C57 mice, are less susceptible to PES-induced arrhythmias than FBV or Black Swiss animals (Maguire et al. 2003). Nevertheless, complex pacing protocols involving double/triple extra-stimuli and rapid burst pacing were avoided to reduce the risk of false-positive results (Maguire et al. 2003). PES protocols comprised a drive train of eight paced S1 beats at 125 ms basic cycle length (BCL), followed by premature S2 extrastimuli every ninth beat. S1S2 intervals first equalled the pacing interval and were then successively reduced by 1 ms with each nine beat cycle until ventricular refractoriness was reached, whereupon the S2 stimuli elicited no response. All PES experiments were performed in hearts in which AV node block had not been induced.
Monophasic action potential recordings
Monophasic action potentials were recorded from the left ventricular epicardium using a spring-loaded, Ag-Cl contact (2 mm tip diameter) MAP electrode (Linton Instruments; Harvard Apparatus, Edenbridge, UK) which was positioned manually. Left ventricular endocardial recordings were obtained using a custom-built electrode, constructed from two twisted strands of Teflon-coated (0.25 mm diameter) silver wire (99.99% purity) (Advent Research Materials Ltd, Oxford, UK), galvanically chlorided and introduced into the left ventricular cavity through a small access window created in the interventricular septum and rotated such that the tip came to rest against the free wall. The endocardial electrode was initially positioned by hand, and the contact maintained by custom-designed magnetic grips positioned on a metallic platform. All recordings were performed during steady state pacing at 125 ms to negate rate-dependent differences in APD. Signals were amplified and low-pass filtered appropriately for murine recordings (0.1–300 Hz) (Gould 2400S; Gould-Nicolet Technologies, Ilford, Essex, UK) then digitized using a 1401plus analogue-to-digital converter (Cambridge Electronic Design, Cambridge, UK). All electronically stored traces were analysed using Spike II software (Cambridge Electronic Design) according to previously documented criteria of a stable baseline and triangular MAP morphology, rapid upstroke phase and a consistent amplitude (Knollmann et al. 2001). The point of maximum positive deflection was considered to be the point of 0% repolarization, and return to baseline the point of 100% repolarization.
Pharmacological agents
The concentrations of propranolol used in the present experiment (0.1 and 1 μm) were based on values known to cause electrophysiological effects from corresponding human, dose–response studies (Duff et al. 1983), and were in keeping with previously published studies of animal models of LQTS whereby a range of concentrations have been used (0.1–3.0 μm) (Shimizu & Antzelevitch 2000). Propranolol (Sigma-Aldrich, Poole, UK) was stored at −20 °C as a 1 mm stock solution in distilled water before dilution to the final drug concentrations in physiological buffer solution.
Statistical analysis
All values were expressed as mean ± SEM and different experimental groups compared by one-way analysis of variance using spss software. P < 0.05 was considered statistically significant.
Results
Long QT syndrome type 3 (LQT3) is associated with genetic modifications of the Scn5a gene, which interfere with Na+ channel inactivation and lead to persistent, arrhythmogenic late Na+ currents (Head et al. 2005). Recent reports have described murine hearts with such a genetic modification of the cardiac Na+ channel: these show a prolonged repolarization phase, altered transmural gradients of repolarization and EADs. These yield arrhythmic preparations showing triggered activity and episodes of non-sustained VT that thereby fully recapitulate the human clinical phenotype of LQT3 (Head et al. 2005, Thomas et al. 2007a).
We accordingly used this murine model to explore the causal relationship between EADs, transmural gradients of repolarization and the initiation and maintenance of arrhythmias in an experimental model system that directly corresponds to the human clinical phenotype of LQT3. Previous clinical and experimental studies had implicated both EADs and altered transmural gradients of repolarization in the initiation of arrhythmogenesis at the whole heart level but have not yet attempted to organize these phenomena into a causal sequence (Cosio et al. 1991, (Fabritz et al. 2003a,b, Milberg & Eckardt 2005). The experiments recorded left ventricular epicardial and endocardial MAPs from Scn5a+/Δ and WT murine hearts in the absence and presence of varying concentrations of propranolol.
In keeping with earlier studies using the canine ventricular wedge preparation, we perfused all murine hearts with buffer solution for a period of 30 min in order to washout residual catecholamines that may be leaking from nerve terminals (Krishnan & Antzelevitch 1991). Propranolol has well documented nonspecific pharmacological actions, blocking Na+ channels in addition to its β-adrenoceptor blocking effects. Therefore propranolol would be expected to antagonize any residual adrenergic tone caused by spontaneous release of catecholamines from nerve endings in addition to blocking Na+ channels. Nevertheless, the role of endogenous catecholamines in isolated hearts is unclear. Indeed, the presence of endogenous catecholamines has been previously reported in the rat (Weselcouch et al. 1995) and canine (Simaan & Fawaz 1970) isolated hearts. However, depletion of endogenous catecholamines with reserpine did not affect coronary flow or cardiac function in both the rat and canine heart (Simaan & Fawaz 1970, Weselcouch et al. 1995). With this in mind we acknowledge that propranolol may antagonize residual adrenergic tone in the Langendorff-perfused murine whole heart in the present study. Nonetheless, irrespective of the mechanisms of action of propranolol in the present study, we used this agent as a pharmacological tool to empirically separate out EADs from the arrhythmogenic substrate in a murine model of human LQT3 syndrome for the first time.
Inhibition of EADs and spontaneous arrhythmogenesis in intrinsically beating Scn5a+/Δ hearts
Following isolation, cannulation and perfusion of Scn5a+/Δ and WT murine hearts, left ventricular epicardial and endocardial MAPs possessing a stable waveform morphology, amplitude and duration were achieved after a 10 min equilibration period. Following this, MAP waveforms remained highly reproducible throughout all experiments. Bradycardia is a known risk factor for spontaneous arrhythmogenesis at the whole heart level and earlier studies have reported an increased incidence of EADs and consequent ventricular arrhythmogenesis under bradycardic conditions (Fabritz et al. 2003a, Killeen et al. 2007a). Furthermore, LQT3 patients predominantly experience arrhythmic episodes at rest, under bradycardic conditions (van den Berg et al. 2001, Schwartz et al. 2001). To investigate the effects of propranolol upon EADs and spontaneous arrhythmogenesis we therefore induced AV block through crush-ablation of the AV node, in keeping with earlier methodologies (Fabritz et al. 2003a).
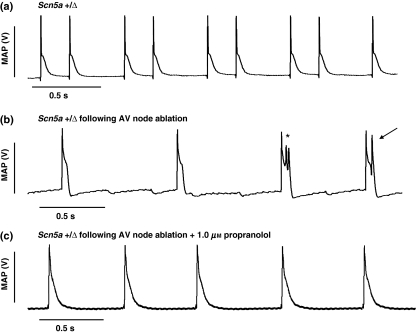
Crush ablation rendered both Scn5a+/Δ and WT hearts bradycardic (∼100 beats min−1) but nevertheless still spontaneously beating, compared to hearts in which AV block was not induced, (Fig. 1a,b). Bradycardic Scn5a+/Δ hearts spontaneously showed multiple EADs, triggered beats and episodes of non-sustained VT lasting on average 15 beats (range 7–33 beats) (n = 7) (Fig. 1b). In contrast, WT hearts never showed such events (whether EADs or polymorphic VT) following induction of AV block (n = 5).
Figure 1.
Representative monophasic action potential (MAP) recording obtained from the epicardial surface of a spontaneously-beating Scn5a+/Δ heart under physiological conditions (a). Following induction of complete atrioventricular (AV) block by crush ablation of the AV node, multiple early afterdepolarizations (*) are seen, along with an associated triggered action potential (arrow) (b). All such features are suppressed following perfusion with physiological buffer solution containing propranolol (1 μm) (c).
We then investigated the empirical effects of a range of propranolol concentrations in such spontaneously beating bradycardic Scn5a+/Δ and WT hearts to explore a correlation between EADs and spontaneous arrhythmogenesis. Perfusion with physiological buffer containing 0.1 and 1 μm propranolol suppressed not only EADs, but also triggered beats and spontaneous VT in all seven Scn5a+/Δ hearts (Fig. 1c), and had no further effects upon spontaneously beating WT hearts (n = 5). These data therefore associate EADs with spontaneous arrhythmogenesis, and their abolition with its absence in spontaneously beating, bradycardic Scn5a+/Δ murine hearts. This then led to experiments that next explored the effects of propranolol upon arrhythmogenesis provoked by extrasystolic stimuli in Scn5a+/Δ and WT murine hearts.
Paradoxical arrhythmogenic properties in Scn5a+/Δ hearts following programmed electrical stimulation
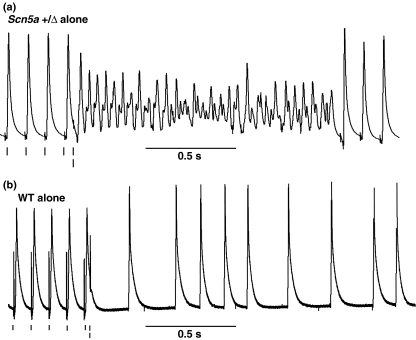
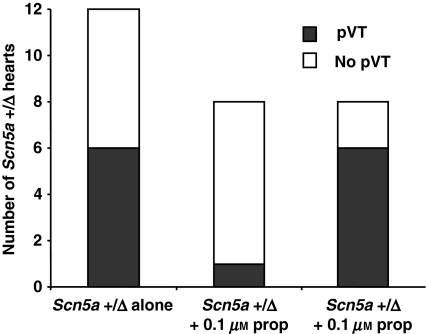
We then investigated the effects of propranolol upon provoked arrhythmogenesis using PES that incorporated extrasystolic stimuli in Scn5a+/Δ and WT murine hearts. The application of PES to Scn5a+/Δ (n = 12) and WT (n = 10) hearts following perfusion with physiological buffer solution induced VT in 6 of 12 Scn5a+/Δ hearts (Fig. 2a) but not in any WT control hearts (n = 10) (Fig. 2b). PES was then repeated in Scn5a+/Δ (n = 8) and WT (n = 8) hearts at 30 min intervals, following perfusion with physiological buffer solution containing increasing concentrations of propranolol of 0.1 and 1 μm respectively. Subsequent PES procedures induced VT in one of eight Scn5a+/Δ hearts perfused with 0.1 μm propranolol as summarized in Figure 3. However, VT was induced in six of eight Scn5a+/Δ hearts following perfusion with 1 μm propranolol, confirming our previous observations (Head et al. 2005). In contrast, PES failed to induce VT in all WT preparations at all concentrations of propranolol.
Figure 2.
Representative trace showing monophasic action potentials recorded from the epicardial surface of Scn5a+/Δ and wild type (WT) hearts during programmed electrical stimulation. Following the final paced beats of the drive train at 125 ms CL (single dash), a premature stimulus (double dash) induces non-sustained polymorphic ventricular tachycardia in the Scn5a+/Δ (a) but not WT heart (b).
Figure 3.
Numbers of Scn5a+/Δ hearts with inducible polymorphic ventricular tachycardia (pVT) during programmed electrical stimulation following perfusion with physiological buffer followed by increasing concentrations of propranolol (0, 0.1 and 1 μm).
The present PES results thus demonstrated that low concentrations of propranolol (0.1 μm) exerted an antiarrhythmic effect in Scn5a+/Δ hearts, findings that did correlate with the antiarrhythmic properties in spontaneously beating hearts described above. However, a higher concentration of propranolol (1 μm) reversed this correlation by exerting a proarrhythmic effect in Scn5a+/Δ hearts. This was in direct contrast to findings in the spontaneously beating Scn5a+/Δ hearts in which both the 0.1 and 1 μm concentrations of propranolol suppressed unprovoked arrhythmogenesis. This prompted us to explore whether these paradoxical, even if empirical, effects of propranolol in Scn5a+/Δ hearts might correlate with similar paradoxical alterations in the transmural gradient of repolarization. Accordingly, we investigated the effects of propranolol upon the transmural repolarization gradients known to exist within the left ventricle of WT (Anumonwo et al. 2001, Knollmann et al. 2001, Dilly et al. 2006) and ΔKPQ Scn5a mice (Thomas et al. 2007a). These measurements permit the quantification of the transmural gradient of repolarization, alterations which have been recently associated with arrhythmogenecity at the whole heart level (Killeen et al. 2007a,b, Thomas et al. 2007a).
The paradoxical arrhythmogenic properties correlate with opposite shifts in transmural gradients of repolarization in Scn5a+/Δ hearts
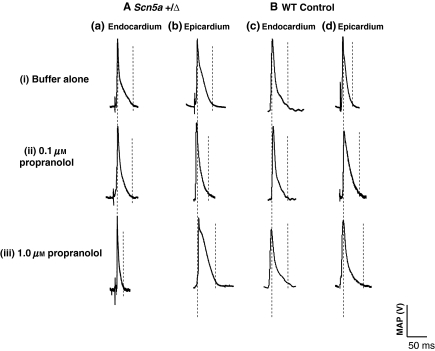
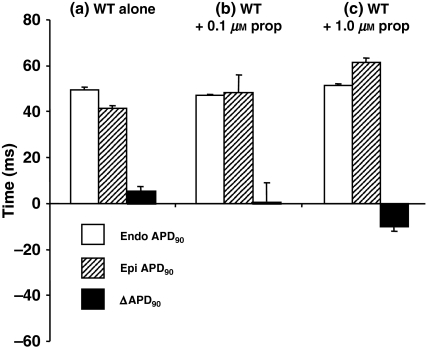
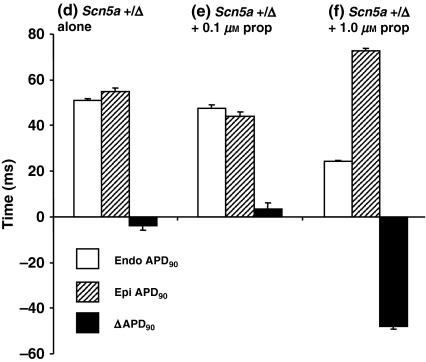
Baseline endocardial (Fig. 4a,c) and epicardial (Fig. 4b,d) APs were measured in Scn5a+/Δ (Fig. 4a) and WT (Fig. 4b) hearts from left ventricular MAPs recorded during steady state, right ventricular epicardial pacing at 125 ms BCL. In WT hearts, endocardial APD90 was significantly greater than epicardial APD90 (49.1 ± 1.2 vs. 42.9 ± 1.4 ms, respectively, n = 5, P < 0.05), resulting in a positive ΔAPD90 value of 6.2 ± 1.8 ms (Fig. 5a). In contrast, however, epicardial APD90 in Scn5a+/Δ hearts was significantly greater than the corresponding WT value (54.7 ± 1.6 ms, n = 5, P < 0.05) (Fig. 6d). Endocardial APD90 in Scn5a+/Δ hearts was calculated 50.9 ± 1.0 ms (n = 5) and was not statistically significant from the corresponding WT value (Fig. 5). This resulted in a negative ΔAPD90 value of −3.8 ± 1.6 ms in Scn5a+/Δ hearts.
Figure 4.
Representative traces of monophasic action potentials (MAPs) recorded from endocardial (a,c) and epicardial (b,d) surfaces of Scn5a+/Δ (A) and wild type (WT) (B) hearts during baseline epicardial pacing at 125 ms, following perfusion with physiological buffer solution containing increasing concentrations of propranolol (0, 0.1 and 1 μm) [(i)–(iii)]. Baseline endocardial and epicardial APD90 are indicated by vertical dotted lines.
Figure 5.
Comparison of the effect of 0, 0.1 and 1 μm propranolol on mean ± SEM endocardial APD90 (Endo), epicardial APD90 (Epi) and ΔAPD90 from monophasic action potential recordings in wild type (WT) hearts (n = 5 in each case).
Figure 6.
Comparison of the effect of 0, 0.1 and 1 μm propranolol on mean ± SEM endocardial APD90 (Endo), epicardial APD90 (Epi) and ΔAPD90 from monophasic action potential recordings in Scn5a+/Δ hearts (n = 5 in each case).
All preparations were then perfused for 30 min each with perfusion buffer containing increasing concentrations of propranolol (0.1 and 1.0 μm) [Fig. 4(ii),(iii)]. In WT hearts, propranolol (0.1 μm) significantly prolonged epicardial APD90 from 42.9 ± 1.4 ms to 48.1 ± 8.0 ms (n = 5, P < 0.05), and had no significant effect upon endocardial APD90 (49.1 ± 1.2 vs. 47.1 ± 0.3 ms, respectively, n = 5). These effects resulted in a ΔAPD90 value of 1.0 ± 8.0 ms, not significantly different to control values (Fig. 5a,b). In Scn5a+/Δ hearts, perfusion with propranolol (0.1 μm) significantly reduced epicardial APD90 from 54.7 ± 1.6 to 44.0 ± 2.0 ms (n = 5, P < 0.05), but did not significantly alter endocardial APD90 values in Scn5a+/Δ hearts (50.9 ± 1.0 ms to 47.5 ± 1.5 ms, respectively, n = 5). This effect led to an appreciable increase in ΔAPD90 from −3.8 ± 1.6 to 3.5 ± 2.5 ms in Scn5a+/Δ hearts (n = 5) (Fig. 6).
However, increased propranolol concentrations (1.0 μm) caused progressive prolongation of epicardial APD90 in WT hearts to 61.6 ± 1.8 ms (n = 5, P < 0.05). In corresponding endocardial recordings, however, perfusion of WT hearts with propranolol did not significantly affect endocardial APD90 (51.4 ± 0.7 vs. 47.5 ± 1.5 ms, respectively, n = 5). These effects of 1 μm propranolol in WT hearts thus significantly reduced ΔAPD90 from 1.0 ± 8.0 to −10.2 ± 1.9 ms (P < 0.05). In contrast, perfusion of Scn5a+/Δ hearts with 1.0 μm propranolol significantly increased epicardial APD90 to 72.5 ± 1.2 ms (n = 5, P < 0.05), and significantly shortened endocardial APD90 to 24.5 ±0.3 ms. These marked, contrasting effects of 1 μm propranolol upon epicardial and endocardial APD90, significantly altered ΔAPD90 to −48.0 ± 1.2 ms (n = 5, P < 0.05).
These findings precisely parallel the findings from the PES studies described above. Thus the baseline ΔAPD90 in Scn5a+/Δ hearts of −3.8 ± 1.6 ms corresponded to an induction of VT by PES in 6 of 12 preparations (50% incidence of arrhythmogenesis). The restoration of ΔAPD90 values close to control WT values at baseline following application of 0.1 μm propranolol was reflected in the PES studies, in which premature stimuli induced VT in only one of eight preparations (12.5% incidence of arrhythmogenesis). Finally the increased arrhythmogenic substrate of ΔAPD90 produced by prolongation of epicardial APD90 and reduction in endocardial APD90 bringing about a significantly increased arrhythmogenic substrate reflected in ΔAPD90 following perfusion of Scn5a+/Δ hearts with 1.0 μm propranolol corresponded to the increased arrhythmic substrate suggested by the induction of VT in six of eight preparations (75% incidence of arrhythmogenesis). Collectively these findings demonstrate the pharmacological separation of EADs from altered transmural gradients of repolarization in this genetically modified murine whole heart model of arrhythmogenesis for the first time through the application of propranolol at differential concentrations.
Discussion
We have previously engineered a murine whole heart model of arrhythmogenesis that directly corresponds to the human clinical phenotype of LQT3 through genetic modification of the gene encoding the cardiac Na+ channel, Scn5a (Head et al. 2005). Murine hearts heterozygous for this mutation (Scn5a+/Δ hearts) have prolonged epicardial APDs, altered transmural gradients of repolarization, and show EADs and episodes of VT (Nuyens et al. 2001, Head et al. 2005, Stokoe et al. 2007, Thomas et al. 2007a). The mechanisms responsible for cardiac arrhythmogenesis in LQT3 are not fully understood, although it is believed that heterogeneous AP prolongation both facilitates the development of EADs (Volders et al. 2000) and creates a transmural dispersion of repolarization across the ventricular wall, capable of maintaining TdP via a re-entrant mechanism (Yan et al. 2001, Restivo et al. 2004). We took advantage of this model to explore, for the first time, the causal relationship between EADs and arrhythmic substrate of transmural gradients of repolarization, through the empirical use of propranolol, in a genetically modified murine heart which fully recapitulates the arrhythmogenic human clinical phenotype.
We empirically demonstrate a paradoxical situation in which it was possible to suppress EADs and spontaneous arrhythmias on the one hand, yet exacerbate provoked arrhythmias on the other with the same empirical manoeuvre of propranolol at a concentration of 1 μm. The latter finding correlated with increased transmural gradients of repolarization, expressed as ΔAPD90, and translates into clinical findings in which LQT3 patients appear to derive less benefit from β-blocker therapy than other LQT syndrome patients (Priori et al. 2004).
First, the present study has shown, for the first time, that propranolol suppressed EADs and spontaneous arrhythmogenesis in bradycardic Scn5a+/Δ hearts. Crush-ablation of the AV node in Scn5a+/Δ hearts resulted in a bradycardic heart rate (approximately 100 beats min−1). At baseline, bradycardic Scn5a+/Δ hearts frequently displayed EADs and episodes of VT, closely correlating with earlier findings (Thomas et al. 2007a). Previous studies (Damiano & Rosen 1984, Zeng & Rudy 1995) have implicated the L-type Ca2+ channel (LTCC) as a necessary depolarizing charge carrier for EAD induction at slow stimulation rates. The mechanism for EAD induction is generally considered to involve the recovery from inactivation of LTCCs due to prolongation of APD within their critical window voltage range (January & Riddle 1989). This theory is further supported by our recent finding in which the specific LTCC blocker nifedipine suppressed EADs and arrhythmogenesis through selective inhibition of inward Ca2+ currents in Scn5a+/Δ murine hearts (Thomas et al. 2007a). Perfusion with 0.1 and 1 μm propranolol not only suppressed all EADs but also episodes of VT in spontaneously beating, bradycardic Scn5a+/Δ hearts.
Secondly, in contrast to experiments in spontaneously beating hearts to assess unprovoked arrhythmogenecity, both Scn5a+/Δ and WT hearts were subject to premature extrasystolic ventricular depolarizations using the established arrhythmia provocation protocol of PES. Under control conditions, premature stimuli successfully induced VT in 6 of 12 Scn5a+/Δ hearts (50% arrhythmia incidence) but never in WT hearts. Following subsequent perfusion with propranolol, PES only induced VT in one of eight Scn5a+/Δ hearts (12.5% arrhythmia incidence) at a concentration of 0.1 μm. However, following the increase in propranolol concentration to 1 μm, PES induced VT in six of eight Scn5a+/Δ hearts (75% arrhythmia incidence). This finding is in sharp contrast to the antiarrhythmic findings of 1 μm in spontaneously beating Scn5a+/Δ hearts.
Thirdly, the findings that a given manoeuvre can prevent both EADs and VT in spontaneously beating hearts yet induce VT in provoked hearts suggests that it may be exerting additional proarrhythmic effects that only are unmasked under arrhythmia provocation protocols. To explain differential antiarrhythmic effects in Scn5a+/Δ hearts exposed to propranolol we measured the corresponding epicardial and endocardial APD, thus permitting the subsequent quantification of transmural gradients of repolarization. Previously, alterations in ventricular transmural gradients of repolarization have been associated with arrhythmogenicity at the whole heart level in the setting of hypokalaemia (Killeen et al. 2007a,b) and in genetically modified murine hearts modelling human LQT3 (Stokoe et al. 2007, Thomas et al. 2007a) and LQT5 syndromes (Thomas et al. 2007b).
In the present study we measured transmural repolarization gradients under conditions of steady-state pacing. The quantification of murine APD and transmural repolarization gradients in the spontaneously beating heart is problematic. The intrinsic variability in heart rate as observed in spontaneously beating hearts can in turn alter APDs. It is well recognized that mammalian cardiac repolarization exhibits a cycle-length dependence, with shorter APDs recorded at rapid rates and longer APDs recorded at slow rates (Sabir et al. 2007). These effects could have important implications when determining APDs and transmural repolarization gradients. Thus APD and repolarization gradient measurements obtained from the spontaneously beating heart may not fully represent the observed experimental conditions but may instead reflect spontaneous intrinsic increases or decreases in heart rate. Therefore, in keeping with recent studies correlating murine ventricular repolarization gradients and arrhythmogenicity reported from our laboratory (Killeen et al. 2007a,b, Stokoe et al. 2007, Thomas et al. 2007a,b) and elsewhere (Fabritz et al. 2003a,b 2005, London et al. 2007) we recorded APDs and repolarization gradients in the presence of an extrinsic pacing protocol that eliminated any intrinsic variability in heart rate which may in turn alter APD and repolarization gradients.
The present experiments showed that administration of 0.1 μm propranolol to Scn5a+/Δ hearts produced selective abbreviation of epicardial APD90, as opposed to endocardial APD90, thus restoring ΔAPD90 to positive values close to those recorded from control WT hearts. This finding is in agreement with earlier reports demonstrating selective abbreviation of epicardial over endocardial APD90 in response to a variety of pharmacological agents in a range of arrhythmogenic cardiac models, restoring altered transmural gradients of repolarization and exerting an antiarrhythmic effect (Aiba et al. 2005, Thomas et al. 2007b). However, perfusion of Scn5a+/Δ hearts with 1 μm propranolol produced significant prolongation of epicardial APD90 alongside significant shortening of endocardial APD90. These opposite effects of 1 μm propranolol on epicardial and endocardial APD90 in Scn5a+/Δ hearts produced a significant increase in the arrhythmogenic substrate of ΔAPD90, resulting in a higher incidence of provoked arrhythmogenesis. Increases in transmural repolarization gradients have been previously correlated with arrhythmogenicity in a range of cardiac models (Antzelevitch et al. 1998, Aiba et al. 2005, Milberg & Eckardt 2005, Thomas et al. 2007a).
Following the administration of propranolol to WT hearts, ΔAPD90 was reduced to −10.2 ± 1.9 ms, however, neither spontaneous nor provoked arrhythmias were recorded under these conditions. Under baseline conditions, however, LQT3 murine hearts displayed a significantly less negative ΔAPD90 of −3.8 ± 1.6 ms (P < 0.05) alongside a 50% incidence of arrhythmogenesis. These data suggest that the ΔKPQ Scn5a murine model of human LQT3 syndrome is significantly more sensitive to changes in the transmural repolarization gradient which may precipitate arrhythmogenesis compared to WT hearts. To the best of our knowledge, this finding has not been previously reported in the setting of LQT3 syndrome.
These findings in the present study correlate well with earlier reports suggesting differential epicardial and endocardial effects of propranolol alongside an increase in arrhythmogenicity. Firstly, a study in the canine ventricular wedge preparation demonstrated that propranolol increased epicardial APD, yet shortened endocardial APD (Krishnan & Antzelevitch 1991). Such differential effects were also observed in canine tissue under conditions of simulated ischaemia (Lukas & Antzelevitch 1993). Electrophysiologically, endocardial and epicardial ventricular regions are primarily characterized by their relative densities of Ito: epicardial myocytes have been reported to have a significantly higher density of Ito in the murine heart (Killeen et al. 2007a). These differing densities of Ito are in part responsible for the shorter APD in epicardial compared to endocardial regions (Killeen et al. 2007a).
Blockade of Na+ channels with propranolol would be expected to reduce the amplitude of phase 0. Additionally, blockade of the Na+ window/late Na+ current by propranolol would act to shorten endocardial repolarization times. Reductions in epicardial INa and ICa, due to a direct effect of propranolol on the Na+ channel and indirect effects due to a reduction in the amplitude of phase 0, respectively, would be considerably overwhelmed by the prominent epicardial Ito component, which would open at more negative potentials. In keeping with the study in the canine ventricular wedge preparation, we documented a prolongation of epicardial APD in response to propranolol administration (Krishnan & Antzelevitch 1991).
Ito would bring the membrane potential to a more negative potential which would in turn affect ICa. Hyperpolarization would be expected to shift the membrane potential outside the activation range of ICa, which would in turn reduce ICa. However, if the Ca2+ channels are already activated, these would be expected to carry more inward Ca2+ current as hyperpolarization would increase the electrical gradient acting on the Ca2+ ions. Inactivation of Ito would reduce outward current and would give rise to a net inward current, although ICa may be small and slow in onset. This delay in the onset of ICa would in turn delay the opening of other murine delayed rectifier repolarizing components such as IKslow, causing prolongation of epicardial APD. Such a scheme of events has been previously proposed to take place in the canine myocardium and account for the contrasting effects of propranolol on epicardial and endocardial APD (Krishnan & Antzelevitch 1991).
In the canine wedge preparation administration of propranolol prolonged epicardial APD, however, when a higher concentration of propranolol was used a shortening effect upon epicardial APD was observed. Higher concentrations of propranolol reduced phase 0 amplitude to a critical level as to permit premature repolarization due to an overwhelming Ito (Krishnan & Antzelevitch 1991). Premature epicardial repolarization achieved through significant reductions in the amplitude of phase 0 is primarily responsible for the appearance of the epicardial extrasystole and the subsequent phase 2 re-entry mechanism which is thought to underlie arrhythmogenesis in the Brugada syndrome (Antzelevtich & Yan 2000). We did not increase propranolol concentrations beyond 1 μm in the present study due to the fact that plasma concentrations of propranolol beyond 1 μm are not clinically relevant and are therefore not likely to occur in the treatment of LQT3 syndrome patients. Higher concentrations of propranolol may have reduced phase 0 amplitude to an even greater extent in the LQT 3 murine heart which would lead to premature epicardial repolarization, in keeping with previous findings performed in the canine ventricular wedge model (Krishnan & Antzelevitch 1991).
We have previously correlated alterations in the transmural repolarization gradient with arrhythmogenicity in murine models of LQT3 syndrome (Thomas et al. 2007a), LQT5 syndrome (Thomas et al. 2007b) and hypokalaemia-induced arrhythmogenesis (Killeen et al. 2007a,b). However, we fully acknowledge that additional factors beyond transmural repolarization gradients may indeed account for the observed incidences of arrhythmias in LQT3 mouse hearts in the present study. In mammalian hearts, cardiac repolarization follows tightly regulated patterns, from epicardium to endocardium and from apex to base, which are considered to maintain cardiac pump function and to provide a safeguard against arrhythmias. In addition to transmural repolarization gradients, studies have suggested that apico-basal repolarization gradients also play an important role in arrhythmia induction. In a recent study, London et al. (2007) measured apex to base repolarization gradients in genetically modified murine hearts harbouring deletions of components for Ito. Increases in the apex to base repolarization gradient were associated with arrhythmogenesis. Alterations in this gradient can lead to disrupted patterns of depolarization and repolarization, which may in turn lead to the development of regions of conduction block which can facilitate arrhythmogenesis through re-entrant mechanisms (Killeen & Sabir 2007). Such alterations in depolarization and repolarization patterns in an alternative mouse model of LQT3 syndrome have been recently reported (Tian et al. 2007). We therefore cannot exclude the fact that similar mechanisms may be taking place in the present study, which may also account for the incidences of arrhythmogenesis observed in LQT3 mice. Nevertheless, these findings in the present study demonstrating alterations in the transmural repolarization gradient are in agreement with recent findings describing alterations in apico-basal repolarization patterns in a genetically modified murine model of human LQT3 syndrome (Tian et al. 2007).
Measuring cardiac repolarization through MAP recordings helps to quantify changes in AP shape and duration that may correspond to an arrhythmogenic phenotype. In the present study, continuous recording of epicardial and endocardial APs in the isolated mouse heart enabled us to monitor changes in transmural gradients of repolarization throughout the duration of experiments and to correlate these changes with an altered arrhythmic tendency. Similarly, optical mapping of the isolated heart can provide important information regarding dispersion of repolarization alongside activation and repolarization patterns in the murine ventricle at a high resolution (Baker et al. 2000). However, optical mapping studies in the intact heart are limited to recording signals from only the epicardial surface. MAPs can be recorded continuously and simultaneously from multiple sites in the isolated heart (Fabritz et al. 2003a,b, Killeen et al. 2007a). In the present study, recording from ventricular epicardial and endocardial sites served as an accurate and sensitive marker for changes in transmural repolarization gradients in the mouse heart and as a useful indicator of proarrhythmia.
At the whole heart level we have previously shown that 1 μm propranolol increases electrogram duration (EGD) and increases the dispersion of ventricular conduction curves as measured through paced electrogram fractionation analysis, when administered to Scn5a+/Δ hearts (Head et al. 2005). These latter effects were also accompanied by an increase in arrhythmogenecity (Head et al. 2005). Increases in EGD have been previously associated with increased re-entrant substrate and a higher incidence of arrhythmogenesis at the clinical (Saumarez et al. 1992) and experimental (Balasubramaniam et al. 2003) levels.
The aim of this study was to use propranolol as an empirical means of separating conditions known to contribute to arrhythmogenesis rather than to elucidate the anti and pro-arrhythmic mechanisms of action of propranolol in Scn5a+/Δ hearts. Nevertheless, it has been known for some time that the antiarrhythmic effects of propranolol may be unrelated to antagonism of the β-adrenoreceptor (Woosley et al. 1979). Additional local anaesthetic actions of propranolol, Na+ channel blockade, leading to reductions in APD have previously been observed in cardiac tissue (Davis & Temte 1968, Pruett et al. 1980, Matthews & Baker 1982). Indeed, Matthews & Baker (1982) concluded that concentrations of propranolol required to produce a 50% blockade of Na+ channels closely correlated with concentrations required to exert antiarrhythmic effects on isolated rabbit atria (Rauls & Baker 1979). Additionally, at the clinical level, Duff et al. (1983) reported that suppression of ventricular arrhythmias in 40% of patients necessitates a plasma propranolol concentration in excess of that producing substantial β-receptor blockade. At high plasma concentrations (472 ± 68 ng ml−1 ≈ 1.82 μm), additional shortening of the endocardial MAP duration and QTc interval was observed, beyond that seen at low plasma propranolol concentrations (104 ± 17 ng ml−1 ≈ 0.57 μm) (Duff et al. 1983). These findings suggest that propranolol possesses an intrinsic efficacy of potent Na+ channel blockade, irrespective of its β-adrenoceptor blocking actions.
Previous reports have attempted to examine the effects of β-adrenergic stimulation and antagonism in LQT3 syndrome (Shimizu & Antzelevitch 2000, Head et al. 2005). Administration of isoproterenol to a surrogate, pharmacological model of LQT3 syndrome in the canine ventricular wedge preparation reduces transmural repolarization gradients through preferential abbreviation of the M-cell APD and reduces the incidence of arrhythmogenesis (Shimizu & Antzelevitch 2000). Propranolol appears to antagonize this protective effect of isoproterenol and confers a higher degree of arrhythmogenesis in the wedge preparation (Shimizu & Antzelevitch 2000). These results suggest that β-adrenoceptor stimulation may be antiarrhythmic in the setting of LQT3 syndrome. However, results from our previous study suggest otherwise: perfusion of LQT3 mice with identical isoproterenol concentrations used in the above study did not exert any antiarrhythmic effects (Head et al. 2005). In keeping with earlier results in the canine wedge ventricular preparation (Shimizu & Antzelevitch 2000) and clinical findings which suggest that LQT3 patients derive less benefit from β-adrenoceptor blockade therapy than other LQTS patients (Priori et al. 2004), administration of propranolol did not exert an antiarrhythmic effect in the LQT3 murine heart (Head et al. 2005). These results are in keeping with the present study in which propranolol exerted proarrhythmic effects in LQT3 murine hearts through amplification of left ventricular transmural repolarization gradients.
In conclusion, we report, for the first time, the empirical separation of EADs from arrhythmic substrate in a genetically modified mouse model of human LQT3 syndrome. In this study we used propranolol at different concentrations as a pharmacological tool to separate EADs from arrhythmic substrate of ΔAPD90 in an intact genetically modified whole heart model of arrhythmogenesis that models human LQT3 syndrome. Regardless of the underlying mechanism, we have demonstrated that low and high concentrations of propranolol reduced arrhythmogenecity in spontaneously beating Scn5a+/Δ hearts by removing the trigger for the arrhythmia, the EAD. However, high concentrations of propranolol exacerbated transmural gradients of repolarization in Scn5a+/Δ hearts, which resulted in a proarrhythmic effect unmasked in provoked arrhythmogenesis studies in which premature stimuli, acting as surrogate EADs, imposed upon an arrhythmic substrate.
We also show, for the first time, that higher concentrations of propranolol can exert a proarrhythmic effect in a genetically modified intact murine model of LQT3 syndrome. These findings correspond with clinical observations in which β-adrenoceptor blocker therapy has proved considerably less effective in reducing cardiac events in LQT3 patients than in the other LQTS subtypes (Moss 1998, Moss et al. 2000, van den Berg et al. 2001, Schwartz et al. 2001, Priori et al. 2004).
Acknowledgments
Supported by grants from the British Heart Foundation, the Medical Research Council, the Wellcome Trust, the Helen Kirkland Fund for Cardiac Research and the Raymond and Beverly Sacker Medical Research Centre. MJK thanks the Physiological Laboratory for the award of an Avrith Studentship. We would also like to thank Simon Gray from Cambridge Electronic Design, UK, for technical help and assistance.
Conflict of interest
We report no conflict of interest.
References
- Aiba T, Shimizu W, Inagaki M, Noda T, Miyoshi S, Ding WG, et al. Cellular and ionic mechanism for drug-induced long QT syndrome and effectiveness of verapamil. J Am Coll Cardiol. 2005;45:300–307. doi: 10.1016/j.jacc.2004.09.069. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Yan GX. Cellular and ionic mechanisms responsible for the Brugada syndrome. J Electrocardiol. 2000;33(Suppl.):33–39. doi: 10.1054/jelc.2000.20321. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Shimizu W, Yan GX, Sicouri S. Cellular basis for QT dispersion. J Electrocardiol. 1998;30(Suppl.):168–175. doi: 10.1016/s0022-0736(98)80070-8. [DOI] [PubMed] [Google Scholar]
- Anumonwo JM, Tallini YN, Vetter FJ, Jalife J. Action potential characteristics and arrhythmogenic properties of the cardiac conduction system of the murine heart. Circ Res. 2001;89:329–335. doi: 10.1161/hh1601.095894. [DOI] [PubMed] [Google Scholar]
- Baker LC, London B, Choi BR, Koren G, Salama G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ Res. 2000;86:396–407. doi: 10.1161/01.res.86.4.396. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam R, Grace AA, Saumarez RC, Vandenberg JI, Huang CL. Electrogram prolongation and nifedipine-suppressible ventricular arrhythmias in mice following targeted disruption of KCNE1. J Physiol. 2003;552:535–546. doi: 10.1113/jphysiol.2003.048249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg MP, Wilde AA, Viersma TJW, Brouwer J, Haaksma J, van der Hout AH, et al. Possible bradycardic mode of death and successful pacemaker treatment in a large family with features of long QT syndrome type 3 and Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:630–636. doi: 10.1046/j.1540-8167.2001.00630.x. [DOI] [PubMed] [Google Scholar]
- Cosio FG, Goicolea A, Lopez Gil M, Kallmeyer C, Barroso JL. Suppression of Torsades de Pointes with verapamil in patients with atrio-ventricular block. Eur Heart J. 1991;12:635–638. doi: 10.1093/oxfordjournals.eurheartj.a059952. [DOI] [PubMed] [Google Scholar]
- Damiano BP, Rosen MR. Effects of pacing on triggered activity induced by early afterdepolarizations. Circulation. 1984;69:1013–1025. doi: 10.1161/01.cir.69.5.1013. [DOI] [PubMed] [Google Scholar]
- Davis LD, Temte JV. Effects of propranolol on the transmembrane potentials of ventricular muscle and Purkinje fibers of the dog. Circ Res. 1968;22:661–677. doi: 10.1161/01.res.22.5.661. [DOI] [PubMed] [Google Scholar]
- Dilly KW, Rossow CF, Votaw VS, Meabon JS, Cabarrus JL, Santana LF. Mechanisms underlying variations in excitation-contraction coupling across the mouse left ventricular free wall. J Physiol. 2006;572:227–241. doi: 10.1113/jphysiol.2005.102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff HJ, Roden DM, Brorson L, Wood AJ, Dawson AK, Primm RK, et al. Electrophysiologic actions of high plasma concentrations of propranolol in human subjects. J Am Coll Cardiol. 1983;2:1134–1140. doi: 10.1016/s0735-1097(83)80340-4. [DOI] [PubMed] [Google Scholar]
- Eckardt L, Haverkamp W, Borggrefe M, Breithardt G. Experimental models of torsade de pointes. Cardiovasc Res. 1998;39:178–193. doi: 10.1016/s0008-6363(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Fabritz L, Kirchhof P, Franz MR, Eckardt L, Monnig G, Milberg P, et al. Prolonged action potential durations, increased dispersion of repolarization, and polymorphic ventricular tachycardia in a mouse model of proarrhythmia. Basic Res Cardiol. 2003a;98:25–32. doi: 10.1007/s00395-003-0386-y. [DOI] [PubMed] [Google Scholar]
- Fabritz L, Kirchhof P, Franz MR, Nuyens D, Rossenbacker T, Ottenhof A, et al. Effect of pacing and mexiletine on dispersion of repolarisation and arrhythmias in DeltaKPQ SCN5A (long QT3) mice. Cardiovasc Res. 2003b;57:1085–1093. doi: 10.1016/s0008-6363(02)00839-8. [DOI] [PubMed] [Google Scholar]
- Fabritz L, Emmerich M, Fortmueller L, Franz MR, Breithardt G, Carmeliet P, et al. Aggravation of bradycardia by propranolol in deltaKPQ SCN5A (Long QT3) mice. Eur Heart J. 2005;26(Abstract Supplement) [Google Scholar]
- Head CE, Balasubramaniam R, Thomas G, Goddard CA, Lei M, Colledge WH. Paced electrogram fractionation analysis of arrhythmogenic tendency in KPQ Scn5a mice. J Cardiovasc Electrophysiol. 2005;16:1329–1340. doi: 10.1111/j.1540-8167.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- Huelsing DJ, Spitzer KW, Pollard AE. Electrotonic suppression of early afterdepolarizations in isolated rabbit Purkinje myocytes. Am J Physiol Heart Circ Physiol. 2000;279:H250–H259. doi: 10.1152/ajpheart.2000.279.1.H250. [DOI] [PubMed] [Google Scholar]
- January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Cupples LA, D’Agostino RB. Sudden death risk in overt coronary heart disease: the Framingham Study. Am Heart J. 1987;113:799–804. doi: 10.1016/0002-8703(87)90722-8. [DOI] [PubMed] [Google Scholar]
- Killeen MJ, Sabir IN. Repolarization gradients and arrhythmogenicity in the murine heart. J Physiol. 2007;583:419–420. doi: 10.1113/jphysiol.2007.140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen MJ, Thomas G, Gurung IS, et al. Arrhythmogenic mechanisms in the isolated perfused hypokalaemic murine heart. Acta Physiol (Oxf) 2007a;189:33–46. doi: 10.1111/j.1748-1716.2006.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen MJ, Gurung IS, Thomas G, Stokoe KS, Grace AA, Huang CL. Separation of early afterdepolarizations from arrhythmogenic substrate in the isolated perfused hypokalaemic murine heart through modifiers of calcium homeostasis. Acta Physiol (Oxf) 2007b;191:43–58. doi: 10.1111/j.1748-1716.2007.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann BC, Katchman AN, Franz MR. Monophasic action potential recordings from intact mouse heart: validation, regional heterogeneity, and relation to refractoriness. J Cardiovasc Electrophysiol. 2001;12:1286–1294. doi: 10.1046/j.1540-8167.2001.01286.x. [DOI] [PubMed] [Google Scholar]
- Krishnan SC, Antzelevitch C. Sodium channel block produces opposite electrophysiological effects in canine ventricular epicardium and endocardium. Circ Res. 1991;69:277–291. doi: 10.1161/01.res.69.2.277. [DOI] [PubMed] [Google Scholar]
- London B, Baker LC, Petkova-Kirova P, Nerbonne JM, Choi BR, Salama G. Dispersion of repolarization and refractoriness are determinats of arrhythmia phenotype in transgenic mice with long QT. J Physiol. 2007;578:115–129. doi: 10.1113/jphysiol.2006.122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas A, Antzelevitch C. Differences in the electrophysiological response of canine ventricular epicardium and endocardium to ischemia. Role of the transient outward current. Circulation. 1993;88:2903–2915. doi: 10.1161/01.cir.88.6.2903. [DOI] [PubMed] [Google Scholar]
- Maguire CT, Wakimoto H, Patel VV, Hammer PE, Gauvreau K, Berul CI. Implications of ventricular arrhythmia vulnerability during murine electrophysiology studies. Physiol Genomics. 2003;15:84–91. doi: 10.1152/physiolgenomics.00034.2003. [DOI] [PubMed] [Google Scholar]
- Matthews JC, Baker JK. Effects of propranolol and a number of its analogues on sodium channels. Biochem Pharmacol. 1982;31:1681–1685. doi: 10.1016/0006-2952(82)90668-2. [DOI] [PubMed] [Google Scholar]
- Milberg P, Eckardt L. Transmural dispersion in LQT3 and arrhythmogenesis. Cardiovasc Res. 2005;68:338–339. doi: 10.1016/j.cardiores.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Moss AJ. Management of patients with the hereditary long QT syndrome. J Cardiovasc Electrophysiol. 1998;9:668–674. doi: 10.1111/j.1540-8167.1998.tb00952.x. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, et al. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- NICE. Guidance on the Use of Implantable Cardioverter Defibrillators for Arrhythmias. London: Department of Health; 2000. [Google Scholar]
- Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, et al. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med. 2001;7:1021–1027. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- Pruett JK, Walle T, Walle UK. Propranolol effects on membrane repolarization time in isolated canine Purkinje fibers: threshold tissue content and the influence of exposure time. J Pharmacol Exp Ther. 1980;215:539–543. [PubMed] [Google Scholar]
- Rauls DO, Baker JK. Relationship of nonspecific antiarrhythmic and negative inotropic activity with physicochemical parameters of propranolol analogues. J Med Chem. 1979;22:81–86. doi: 10.1021/jm00187a018. [DOI] [PubMed] [Google Scholar]
- Restivo M, Caref EB, Kozhevnikov DO, El-Sherif N. Spatial dispersion of repolarization is a key factor in the arrhythmogenicity of long QT syndrome. J Cardiovasc Electrophysiol. 2004;15:323–331. doi: 10.1046/j.1540-8167.2004.03493.x. [DOI] [PubMed] [Google Scholar]
- Sabir IN, Fraser JA, Cass TR, Grace AA, Huang CL. A quantitative analysis of the effect of cycle length on arrhythmogenicity in hypokalaemic Langendorff-perfused murine hearts. Pflugers Arch. 2007;454:925–936. doi: 10.1007/s00424-007-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumarez RC, Camm AJ, Panagos A, Gill JS, Stewart JT, de Belder MA, et al. Ventricular fibrillation in hypertrophic cardiomyopathy is associated with increased fractionation of paced right ventricular electrograms. Circulation. 1992;86:467–474. doi: 10.1161/01.cir.86.2.467. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778–786. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]
- Simaan J, Fawaz G. The effect of endogenous catecholamines and norepinephrine administration on the performance of the isolated canine heart. Naunyn Schmiedebergs Arch Pharmakol. 1971;268:17–26. doi: 10.1007/BF00997169. [DOI] [PubMed] [Google Scholar]
- Stokoe KS, Thomas G, Goddard CA, Colledge WH, Grace AA, Huang CL. Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a+/delta murine hearts modelling long QT syndrome 3. J Physiol. 2007;578:69–84. doi: 10.1113/jphysiol.2006.117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Gurung IS, Killeen MJ, Hakim P, Goddard CA, Mahaut-Smith MP, et al. Effects of L-type Ca2+ channel antagonism on ventricular arrhythmogenesis in murine hearts containing a modification in the Scn5a gene modelling human long QT syndrome 3. J Physiol. 2007a;578:85–97. doi: 10.1113/jphysiol.2006.121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Killeen MJ, Gurung IS, Hakim P, Balasubramaniam R, Goddard CA, et al. Mechanisms of ventricular arrhythmogenesis in mice following targeted disruption of KCNE1 modelling long QT syndrome 5. J Physiol. 2007b;578:99–114. doi: 10.1113/jphysiol.2006.118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian XL, Cheng Y, Zhang T, Liao ML, Yong SL, Wang QK. Optical mapping of ventricular arrhythmias in LQTS mice with SCN5A mutation N1325S. Biochem Biophys Res Commun. 2007;352:879–883. doi: 10.1016/j.bbrc.2006.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, et al. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc Res. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, et al. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet. 1995a;4:1603–1607. doi: 10.1093/hmg/4.9.1603. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995b;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- Weselcouch EO, Baird AJ, Sleph PG, Dzwonczyk S, Murray HN, Grover GJ. Endogenous catecholamines are not necessary for ischaemic preconditioning in the isolated perfused rat heart. Cardiovasc Res. 1995;29:126–132. [PubMed] [Google Scholar]
- Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- Woosley RL, Kornhauser D, Smith R, Reele S, Higgins SB, Nies AS. Suppression of chronic ventricular arrhythmias with propranolol. Circulation. 1979;60:819–827. doi: 10.1161/01.cir.60.4.819. [DOI] [PubMed] [Google Scholar]
- Yan GX, Wu Y, Liu T, Wang J, Marinchak RA, Kowey PR. Phase 2 early afterdepolarization as a trigger of polymorphic ventricular tachycardia in acquired long-QT syndrome: direct evidence from intracellular recordings in the intact left ventricular wall. Circulation. 2001;103:2851–2856. doi: 10.1161/01.cir.103.23.2851. [DOI] [PubMed] [Google Scholar]
- Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J. 1995;68:949–964. doi: 10.1016/S0006-3495(95)80271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]