Abstract
Structure and function of the brain are use-dependent variables based on “synapse plasticity”. Since synapses are driven by chemical transmitters, synaptic functions are liable to be modified by extrinsic chemicals displaying affinities for synaptic receptors or modulators. Caffeine is a widely used chemical substance that can invade synapses, and has several biochemical and metabolic actions on synaptic activities. This review focuses on the actions of caffeine on changes in structure and function in the region of the hippocampal formation and neocortex, which exhibit high synapse plasticity. At the synapse level, various synaptic receptors and channel activities are modulated by caffeine via mobilization of intracellular calcium, inhibition of phosphodiesterase, antagonism of adenosine receptors and GABA receptors. These actions of caffeine enable neurons to induce plastic changes in the properties of synaptic activities, such as synaptic transmission efficiency and morphology. At the network level, caffeine has the ability to activate cortical neural oscillators that deliver repetitive N-methyl-D-aspartate receptor-dependent signals to surrounding areas, causing strengthening of long-range inter-cortical communications. Caffeine might thus allow reorganization of cortical network functions via synaptic mobilizations.
1. INTRODUCTION
The brain is a complex system for information processing. The intellective device requires harmonic and coherent action of the component neuron network units, resulting in consistent and intensive operation of the network systems [23,76]. One prominent property of the brain is that structure and function, such as neural wiring and signal communicating efficiency, remain use-dependent and developmentally variable, allowing the brain to acquire the ability to process various modes of information in accordance with changing circumstances [3,9,13,78]. Mechanisms at the synapse level in local dimensions provide this brain variability. Use-dependent induction of synaptic changes is called “synapse plasticity” [11,19,43,52,53,54,59,75]. In general, induction of the synapse plasticity requires repetitive synaptic experiences. Ionotropic or metabotropic receptor activities elicited by synaptic transmission play important roles in the generation of use-dependent synapse plasticity. Production of the electro-motive forces that drive the network systems is triggered at the synapse level. Synapto-motive forces are generated by presynaptic chemical-transmitter release and postsynaptic receptor activities. Interestingly, various natural and synthetic chemicals in the external environment display affinities for synapse receptors and modulators. When these chemicals invade the synaptic cleft and chemical actions are exerted, synaptic functions are liable to be modified.
Among the natural chemicals in the external environment, caffeine is one of the most well-known chemicals able to invade the synaptic cleft. Caffeine displays affinities for several kinds of receptors embedded in the synaptic membranes and internal calcium store, and also has an affinity for cytoplasmic phosphodiesterases (PDEs), enabling caffeine to modify synaptic activities [31,32,66]. Caffeine thus displays various biochemical and metabolic actions at the synapse level. In general, plastic changes in synaptic transmission efficiency and synaptic architecture are induced according to synaptic activities via various kinds of modulation system [6,16,48]. If local synaptic changes are induced systematically and extensively, local changes may develop into network changes. The chemical activity of caffeine might therefore provide the potential for reorganization of brain function from synapse to wide-ranging networks.
Among the various areas of the brain, the hippocampal formation and neocortex exhibit a high susceptibility to the induction of synapse plasticity [11,13,53,75]. The present review focuses attention on these cortical regions, and explores the action of caffeine on plastic changes in structure and function from synapse to cortical network levels.
2. BASIC NEUROPHARMACOLOGICAL EFFECTS OF CAFFEINE
2.1. Effects of Caffeine on Adenosine A1 and A2A Receptors
Purines such as adenosine triphosphate (ATP) and adenosine play central roles in energy metabolism for all cells, and purinergic receptors are located on the cell surface and hence bind purines in the extracellular space [14,31,34]. Interestingly, xanthines such as caffeine block adenosine receptors, but not ATP receptors [20,31]. Adenosine receptors are coupled with G-protein, and can be divided into subtypes A1, A2A, A2B and A3 [20,22,24,31,32,34,69]. Among these subtypes, caffeine blocks A1 receptors that inhibit adenylyl cyclase (AC), in addition to A2A receptors that activate AC [22,24,31,32,34]. In neurons, A1 and A2A receptors are expressed at presynaptic terminals. A1 receptors negatively influence transmitter release from presynaptic terminals, whereas A2A receptors positively influence transmitter release [32]. While A1 receptors are widely distributed throughout the brain, A1 receptors are expressed at the highest level in the hippocampus and neocortex, where glutamate is used as an excitatory transmitter. Conversely, A2A receptors are not distributed widely, but are distributed locally at the highest level in the striatum and nucleus accumbens [22,24,32]. A2A receptors are expressed in dopamine-rich regions, and are co-expressed with dopamine D2 receptors [28,29,35,49,80]. A2A receptors are thus dominantly linked with the dopaminergic system, whereas A1 receptors in the hippocampus and neocortex are dominantly linked with the glutamatergic system. In addition, in hippocampal CA3 neurons, A1 receptor-selective blockade induces bursting activities, but not A2A receptor-selective blockade [82]. Caffeine is therefore considered to act predominantly on A1 receptors in the cortical regions, and positively influence presynaptic transmitter release via blockade of A1 receptors.
2.2. Effects of Caffeine on PDEs
The cyclic AMP (cAMP) cascade is one of the most important intracellular signaling pathways, playing a key role in the expression and modulation of neural function in the central nervous system (CNS) [8]. Activation of membrane receptors coupled to a specific G protein, Gs, such as β-adrenergic receptors or specific metabotropic glutamate (mGlu) receptors, initiates the operation of membrane-bound AC and production of cAMP as a second messenger. Protein phosphorylation or gene expression is finally induced by way of cAMP-dependent protein kinase (PKA) or cAMP response element-binding (CREB) proteins [18,55,85]. These cAMP cascades are negatively controlled by PDEs that breakdown cAMP and turn off the cAMP signaling pathways [4,30,79]. Caffeine depresses PDE activity, and intracellular cAMP is accumulated, resulting in the enhancement of cAMP signaling pathways [5,15,32].
2.3. Effects of Caffeine on Ryanodine Receptors
Calcium signaling pathways play an important role in regulating various brain functions [7]. In particular, increases in cytoplasmic calcium triggers down-stream of the intracellular calcium-dependent cascades. There are extra- and intracellular sources of calcium.Neurons include endoplasmic reticulum (ER) to store high concentrations of calcium. Calcium-release channels called ryanodine receptors are expressed in the membrane of the ER. When extracellular calcium enters the endoplasm through voltage- or receptor-operated calcium channels, ryanodine receptor channels are opened by the binding of calcium with the ryanodine receptors, and calcium is then released from the calcium store into the cytoplasm as a calcium-induced calcium release(CICR)[7,26].Concentrations of endoplasmic calcium are thus amplified, and intracellular calcium signaling pathways are activated in a feed-forward manner. Caffeine permeating into the cell through the cell membrane combines with ryanodine receptors. This results in activation of the ryanodine receptors, reducing the threshold of the CICR and resulting in intense facilitation of CICR [25,32,36,61]. In rat hippocampal CA3 neurons, caffeine promotes epileptic discharges via enhancement of the CICR [56], and caffeine enhances action potential-triggered CICR in rat hippocampal CA1 neurons [71]. Amplification of intracellular calcium is thus positively controlled by caffeine through ryanodine receptors.
2.4. Effects of Caffeine on GABA Receptors
Neuron network activities are based on excitatory and inhibitory synaptic activities. The GABAergic network plays important roles in the stabilization of overall network activities. A recent study revealed that caffeine can modulate the GABAergic system. In ganglion cells of the turtle retina, caffeine depresses the activities of GABA-A receptors. This depression is mediated by caffeine facilitating CICR [1]. Similarly, in dentate gyrus cells of the hippocampus, CICR elicited by caffeine depresses the activities of GABA-A receptors[21]. In neonatal hippocampal neurons, mobilization of Ca2+ from caffeine-ryanodine-sensitive stores facilitates GABA release, while caffeine simultaneously depresses the activities of postsynaptic GABA-A receptors [72]. Caffeine thus affects GABA-A receptor activities by way of facilitating CICR. Conversely, although the mechanisms remain unclear, Ca2+-independent inhibition of GABA-A receptor activities by caffeine occurs in hippocampal neurons [81].
3. NEUROPHARMACOLOGICAL EFFECTS OF CAFFEINE AT CORTICAL SYNAPSES
At the synapses, local synaptic potentials are generated bysynaptic inputs. As local synaptic potentials are summated spatio-temporally, neurons have the ability to integrate various input signals. In addition, synapses can display changes in the efficiency of synaptic transmissions and induce morphological changes according to activity. The basic targets of caffeine mentioned above are concentrated at the synapses (Fig. 1). Synapses are therefore considered to represent the dominant targets of caffeine.
Fig. (1).
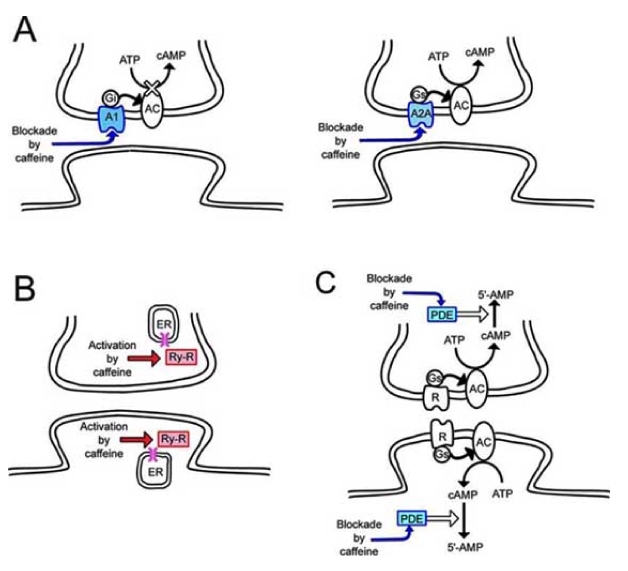
Basic actions of caffeine at synapses.
3.1. Effects of Caffeine on Presynaptic Sites
Release of excitatory transmitter is more strongly inhibited by adenosine than release of inhibitory neurotransmitters [33]. Blockade of adenosine receptors by caffeine can thus occasionally generate overactivity at excitatory synapses [2,47]. In hippocampal CA3 in guinea pigs, blockade of A1 receptors by caffeine generates paroxysmal depolarizing shifts, and the underlying mechanisms may be increased by intracellular cAMP and Ca2+ influxes [62,63]. In hippocampal CA1 neurons, caffeine enhances excitatory postsynaptic potentials (EPSPs), which are mediated by antagonism of presynaptic adenosine receptors [37].
In both glutamatergic and cholinergic neurons, caffeine affects presynaptic sites. In rat hippocampal neurons, caffeine enhances acetylcholine (Ach) release from presy-naptic terminals via blockade of A1 receptors [17].
Changes in the probability of transmitter release induced by caffeine have been investigated by focusing miniature excitatory postsynaptic currents (EPSCs) [74]. The study proposed that in rat barrel cortex, caffeine enhances glutamate release from presynaptic terminals via calcium release from ryanodine-sensitive internal Ca2+ stores.
3.2. Effects of Caffeine on Postsynaptic Sites
Postsynaptic activities can be divided into two categories: direct synaptic transmission, and indirect synaptic transmission. Direct synaptic transmission is mediated by ligand-gated ionic channel-coupled receptors. Electro-motive force at the synapse is produced by ligand-gated ionic channel-coupled receptors. In general, two types of ionotropic glutamate receptors produce EPSPs: N-methyl-D-aspartate (NMDA) receptors, and non-NMDA receptors. NMDA receptors are postsynaptic activity-dependent calcium permeable channels, and play a central role in the induction of synapse plasticity, as mentioned below.
In rat hippocampal CA3 neurons, caffeine enhances both NMDA and non-NMDA receptor activities, inducing high-frequency oscillations [39]. In rat visual and parietal cortices, caffeine also enhances both NMDA and non-NMDA receptor activities, inducing α-range oscillations [89,90,92,93]. Caffeine-enhanced synaptic activities are triggered by activation of both receptors, in turn causing enhancement of the receptor activities themselves. Since adequate repetitive synaptic inputs are required for caffeine-dependent enhancement of synaptic activities, this enhancement is considered use-dependent.
Indirect synaptic transmission is mediated by G-protein-coupled metabotropic receptors. Expressed at both pre- and postsynaptic sites, mGlu receptors are involved in modulation of synaptic activities by activation of a second messenger system. Generation of a transient increase in intracellular Ca2+ to switch on the Ca2+-signaling second messenger pathway is one of the principal roles of mGlu receptors [68]. In hippocampal CA1 neurons, the Ca2+ transient induced by postsynaptic mGlu receptor activation is blocked by caffeine [10]. Caffeine thus acts on mGlu receptor activities at postsynaptic sites.
GABA receptors are concerned with generation of inhibitory postsynaptic potentials, so GABA receptors negatively influence synaptic activities. In hippocampal dentate gyrus neurons, elevation of intracellular calcium levels by caffeine depresses postsynaptic ionotropic GABA-A receptor activities, showing that depression of GABA-A receptors by caffeine is dependent on intracellular calcium elevation [21, 72]. In contrast, caffeine depresses GABA-A receptor activities in hippocampal CA3 neurons, which are independent of intracellular calcium elevations [81].
3.3. Effects of Caffeine on Long-Term Potentiation and Long-Term Depression
At many cortical synapses, repetitive synaptic activities can produce long-term changes in synaptic efficiency [11,12, 59]. According to the patterns of temporal coincidence, location and intensity of pre- and potsynaptic activities, synaptic efficiency is potentiated or depressed over the long term, and termed long-term potentiation (LTP) or long term depression (LTD) respectively. Various kinds of LTP, LTD and associated mechanisms have been investigated and summarized in many reviews [43,48,52,53,54,59, 75]. Considering the mechanisms of LTP and LTD, whether LTP and LTD are NMDA receptor-dependent represents a central issue, since induction of activity-dependent synapse plasticity is deeply affected by NMDA receptor activities [16,43,50,73,75]. Activities of postsynaptic NMDA receptors are blocked by extracellular Mg2+, and reduction of this Mg2+ block requires postsynaptic depolarization, allowing NMDA receptors to function as important detectors of coincident pre- and postsynaptic activities [60,67]. The coincidence between pre- and postsynaptic activities is deeply involved in the induction of synapse plasticity [40].
In general, LTP in hippocampal CA1 neurons requires both postsynaptic NMDA receptors and increased levels of intracellular Ca2+ by way of NMDA receptors. In contrast, caffeine induces another form of LTP in hippocampal CA1 neurons. Caffeine-dependent LTP requires neither postsynaptic NMDA receptors nor increased intracellular Ca2+ by way of NMDA receptors, but does require the interaction of caffeine with P1 adenosine receptors, P2 prinoreceptors and ryanodine receptors, indicating that caffeine-dependent CA1 LTP is caused by increases in presynaptic transmitter release [57,58]. Another presynaptically induced caffeine-dependent LTP in hippocampal CA1 has been reported. In rat hippocampal CA1 neurons, caffeine promotes forskolin induced LTP, where adenosine A1 receptor antagonism underlies the effects of caffeine [51]. Caffeine thus increases susceptibility to the induction of cAMP-dependent LTP, via enhancement of presynaptic cAMP accumulation. Actions of caffeine at presynaptic sites may be sufficient to induce such cAMP-dependent NMDA receptor-independent LTP.
As for LTD, postsynaptically induced caffeine-dependent LTD has been reported. Caffeine-dependent LTD in hippocampal CA neurons is postsynaptically induced in a stimulation frequency-dependent manner. LTD requires both NMDA receptor activities and calcium release from internal calcium store [65].
3.4. Effects of Caffeine on Morphological Changes in Synapses
The morphology of dendritic spines exerts a substantial effect on important aspects of synaptic activities, such as synaptic transmission and integration of synaptic information [41,73,77,87,95,96]. In dendritic spines, calcium dynamics play an important role in the expression of those synaptic functions, by way of various calcium-dependent biochemical processes. Particularly in hippocampal CA1 neurons, individual spines play an important role in detecting temporal coincidence between pre- and postsynaptic activities via NMDA receptors [96].
In cultured hippocampal neurons, application of caffeine causes a transient rise in intracellular calcium levels via ryanodine receptors in dendrites and spines, resulting in increased size of excitatory dendritic spines and changes in spine shapes [44,45]. Calcium from internal stores elicited by caffeine can thus modify dendritic spine shape [38]. Dynamics of intracellular calcium increases differently between short- and long-neck dendritic spines, suggesting that control of spatio-temporal calcium increases is provided by the shape of dendritic spines [84]. Since changes in spine structure contribute to changes in brain function [64,95], caffeine might modulate brain function via increases in intracellular Ca2+ level [38].
4. NEUROPHARMACOLOGICAL EFFECTS OF CAFFEINE ON CORTICO-CORTICAL SIGNAL INTERACTIONS
Local synaptic changes may in turn induce reorganization of cortical network function. However, this might require strong and long-range synchronization of synaptic activities or firing between neuron clusters [27,46,70,76]. In this respect, a strong relationship may exist between neural oscillations and synapse plasticity [27,83]. To understand the mechanisms of neural oscillation, several network oscillation models have been proposed [27]. Theoretically, synchronization as a non-local event is convenient for induction of synapse plasticity between long-range discrete cortical areas. Even if synchronization is a local event, however, synapse plasticity is inducible between long-range discrete areas, on the condition that the propagating system is strong and stable.
Recently, a protocol for inducing synchronized membrane potential oscillation at a frequency of 8-10 Hz in the visual cortex has been developed, by applying caffeine to rat brain slices [90,92,93]. The start of oscillation requires a trigger input, and oscillation comprises several propagating wavelets. Oscillation induction requires low-frequency activation of input fibers in conjunction with caffeine application, suggesting that use-dependent mechanisms underlie oscillation induction. Notably, induction of oscillation requires both NMDA receptor activation and the release of intracellular calcium from the internal calcium store, suggesting that functional coupling between NMDA and ryanodine receptors underlies caffeine-dependent oscillation [92,93]. In the absence of caffeine, the strength of functional coupling between NMDA and ryanodine receptors in hippocampal neurons depends on the magnitude of NMDA receptor activation [42]. In the presence of caffeine, caffeine activates ryanodine receptors and potentiates presynaptic glutamate release, resulting in an increased likelihood of functional coupling between NMDA and ryanodine receptors.
Strictly speaking, caffeine-dependent oscillation comprises initial propagating components and subsequent oscillatory components. These subsequent oscillatory components emerge from the local area in the visual cortex, showing that the oscillator is localized. Although synchrony is a local event, the neural oscillator delivers NMDA receptor-dependent signals to the surrounding areas [90,94]. These signal deliveries finally cause strengthening of non-NMDA receptor-dependent inter-cortical functional connections between long-range discrete areas [94]. The oscillators are separately located in the medial and lateral secondary visual cortices. Horizontal connections in layer II/III between the primary and secondary visual cortices are strengthened after repetitive NMDA receptor-dependent signal delivery originating from the oscillators.
Another study revealed that the oscillator is also located outside the visual cortex. The retrosplenial cortex is located at a critical position between the visual cortex and hippo-campal formation. In the area of the retrosplenial cortex, the oscillator is present in the retrosplenial granular a cortex (RSGa). Activation of oscillators in both the secondary visual cortex and RSGa under application of caffeine finally opens functional connections from primary visual cortex to the postsubiculum [91]. Hence, in the presence of caffeine, an oscillator with local synchronization can induce spatially wide-ranging synapse plasticity from the visual cortex to the hippocampal formation.
These studies resulted in the “oscillator-dependent plasticity hypothesis” [90,91,94]. This hypothesis is illustrated in Fig. 2, showing the induction of plastic changes in the visual cortex in the presence of caffeine. Caffeine, in combination with low-frequency electrical stimulation, promotes the voltage oscillator delivering NMDA receptor-dependent signals at a frequency of 8-10 Hz from the secondary visual cortex to surrounding cortical areas. This induces opening and strengthening of non-NMDA receptor-dependent signal pathways. Repetitive activities of an NMDA receptor-dependent voltage oscillator thus induce use-dependent network plasticity in the cortical regions.
Fig. (2).
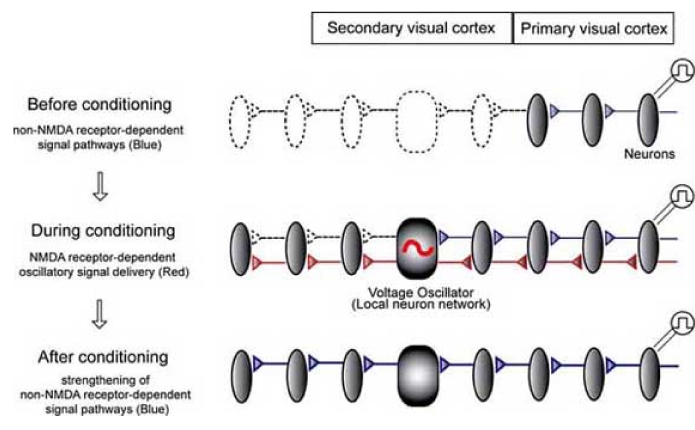
Oscillator-dependent plasticity hypothesis.
The same mechanism is present between the gustatory insular cortex and somatosensory parietal cortex. In these areas, the oscillator that delivers NMDA receptor-dependent signals is located in the parietal cortex, and is driven under application of caffeine by repetitive low-frequency stimulation.Oscillatory NMDA receptor-dependent signal delivery causes strengthening of functional connections between the insular and parietal cortices [88,89].
Theoretical investigation has demonstrated that oscillation-dependent mechanisms underlie the establishment of working memory. The study showed that NMDA receptor-mediated synaptic transmission at a frequency of 8 Hz is required to sustain persistent network activities of the prefrontal cortex [86]. Results collected by experimental and theoretical studies thus suggest that NMDA receptor-mediated α-range signal delivery plays a critical role in the generation and stabilization of functional networks via plastic changes from synapses to networks.
5. CONCLUSION
Caffeine displays various general pharmacological actions: 1) blockade of presynaptic A1 and A2A receptors, resulting in modulation of transmitter release; 2) activation of internal ryanodine receptors, resulting in reduction of CICR threshold; 3) blockade of PDEs, resulting in intracellular cAMP accumulation; and 4) blockade of GABA-A receptors, resulting in depression of inhibitory synaptic activities. In the brain, these general actions of caffeine are liable to take place at the synapses, as the targets of caffeine and its effects are concentrated at the synapses. Particularly in the regions of the hippocampal formation and neocortex, where use-dependent synapse plasticity is liable to be established, caffeine is able to enhance synaptic NMDA receptor activities and intracellular calcium signaling pathways, through which plastic changes in synaptic morphology and transmission efficiency are induced. In cortical neuron networks, the actions of caffeine in combination with adequate input fiber activation produce opening and strengthening of long-rage inter-cortical signal communications via activation of cortical neural oscillators that deliver NMDA receptor-dependent signals to surrounding areas. The actions of caffeine at a synapse level thus cause plastic changes at the cortical network level.
Most of the experimental evidence has been collected from basic studies using peculiar conditions in vitro. However, these basic studies have elicited the potential of caffeine, and the evidence indicates that caffeine exerts profound actions from synapse to neuron networks in the cortical regions. Caffeine might thus provide the potential for use-dependent reorganization of brain function.
ACKNOWLEDGEMENTS
We wish to thank Drs. N. Onoda and N. Segami for providing valuable advice on the manuscript. This work was supported by grants from the Ministry of Health, Labor and Welfare of Japan (Comprehensive Research on Aging and Health, H-17-Chouju-018).
REFERENCES
- 1.Akopian A, Gabriel R, Witkovsky P. Calcium released from intracellular stores inhibits GABAA-mediated currents in ganglion cells of the turtle retina. J Neurophysiol. 1998;80:115–1115. doi: 10.1152/jn.1998.80.3.1105. [DOI] [PubMed] [Google Scholar]
- 2.Ault B, Olney MA, Joyner JL, Boyer CE, Notrica MA, Soroko FE, Wang CM. Pro-convalsant actions of theophyline and caffeine in the hippocampus: implications for the management of temporal lobe epilepsy. Brain Res. 1987;426:93–102. doi: 10.1016/0006-8993(87)90428-8. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Ann Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 4.Beavo JA. Cyclic nucreotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 5.Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- 6.Berardi N, Pizzorusso T, RattoG M, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 7.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 8.Bhalla US, Iyengar R. Emergent properties of networks of biological signaling pathways. Science. 1999;283:381–387. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- 9.Bi G, Poo M. Synaptic modification by correlated activity: Hebb’s postulate revisited. Ann Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Bianch R, Young SR, Wong RKS. Group I mGluR activation causes voltage-dependent and –independent Ca2+ rise in hippocampal pyramidal cells. J Neurophysiol. 1999;81:2903–2913. doi: 10.1152/jn.1999.81.6.2903. [DOI] [PubMed] [Google Scholar]
- 11.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 12.Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate of anesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buonomano DV, Merzenich MM. Cortical plasticity: From Synapse to maps. Ann Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G. Overview. Purinergic mechanisms. Annals NY Acad Sci. 1990;603:1–17. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- 15.Butcher RW, Sutherland EW. Adenosine-3’, 5’-phosphate in biological materials. J Biol Chem. 1962;273:1244–1250. [PubMed] [Google Scholar]
- 16.Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- 17.Carter AJ, O’Connor WT, Carter MJ, Ungerstedt U. Caffeine enhances acetylcholine release in the hippocampus in vitro by a selective interaction with adenosine A1 receptors. J Pharmacol Exp Therapeut. 1995;273:637–642. [PubMed] [Google Scholar]
- 18.Cho K, Brown MW, Bashir ZI. Mechanisms and physiological roles of enhancement of mGlu5 receptor function by group II mGlu receptor activation in rat perirhinal cortex. J Physiol. 2002;540:895–906. doi: 10.1113/jphysiol.2001.013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Compte A, Brunel N, Goldman-Rakic , Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- 20.Daly JW, Butts-Lamb P, Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell. Mol. Neurobiol. 1983;3:69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Koninck Y, Mody I. The effects of rising intracellular calcium on synaptic GABAA receptor-channels. Neuropharmacology. 1996;35:1365–1374. doi: 10.1016/s0028-3908(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 22.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Ann Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 24.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Ann Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 25.Edwards JA, Cline HT. Light-induced calcium influx into retinal axons is regulated by presynaptic nicotinic acetylcholine receptor activity in vivo. J Neurophysiol. 1999;81:895–907. doi: 10.1152/jn.1999.81.2.895. [DOI] [PubMed] [Google Scholar]
- 26.Ehrlich BE. Functional properties of intracellular calcium-release channels. Curr Opin Neurobiol. 1995;5:304–349. doi: 10.1016/0959-4388(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 27.Ermentrout GB, Kleinfeld D. Traveling electrical waves in cortex: insight from phase dynamics and speculation on a computational role. Neuron. 2001;29:33–44. doi: 10.1016/s0896-6273(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 28.Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor integrations as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 29.Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 30.Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucl Acid Res Mol Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 31.Fredholm BB, Abbracchio MP, Burnstock G, Daly GW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacological Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 32.Fredholm BB, Bättig K, Holmén J, Nehlig A, Zuvartau EE. Actions of caffeine in the brain woth special reference to factors that contribure to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 33.Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release ? . Trends Pharmacol Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- 34.Fredholm BB, Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucreosides. Biochem Pharmacol. 1980;29:1635–1643. doi: 10.1016/0006-2952(80)90117-3. [DOI] [PubMed] [Google Scholar]
- 35.Fuxe K, Ferre S, Zoli M, Agnati LF. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Brain Res Rev. 1998;26:258–273. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 36.Garaschuk O, Yarri Y, Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J Physiol. 1997;502:13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene RW, Hass HL, Hermann A. Effects of caffeine on hippocampal pyramidal cells in vitro. Br J Pharmacol. 1985;85:163–169. doi: 10.1111/j.1476-5381.1985.tb08843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris K. Calcium from internal stores modifies dendritic spine shape. Proc Natl Acad Sci USA. 1999;96:12213–12215. doi: 10.1073/pnas.96.22.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He SJ, Xioa C, Ruan DY. Caffeine-dependent stimulus-triggered oscillations in the CA3 region of hippocampal slices from rats chronically exposed to lead. Exp. Neurol. 2004;190:525–534. doi: 10.1016/j.expneurol.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Hebb DO. The Organization of behavior. New York: Wiley; 1949. [Google Scholar]
- 41.Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 42.Isokawa M. N-methyl-D-aspartic acid-induced and Ca-dependent neuronal swelling and its retardation by grain-derived neurotrophic factor in the epileptic hippocampus. Neuroscience. 2005;131:801–812. doi: 10.1016/j.neuroscience.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 44.Korkotian E, Segal M. Fast confocal imaging of calcium released from stores in dendritic spines. Eur J Neurosci. 1998;10:2076–2084. doi: 10.1046/j.1460-9568.1998.00219.x. [DOI] [PubMed] [Google Scholar]
- 45.Korkotian E, Segal M. Release of calcium from stores alters the morphology of dendritic spines in cultured hippocampal neurons. Proc Natl Acad Sci USA. 1999;96:12068–12072. doi: 10.1073/pnas.96.21.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.König P, Engel AK, Singer W. Relation between oscillatory activity and long-range synchronization in cat visual cortex. Proc Natl Acad Sci USA. 1995;92:290–294. doi: 10.1073/pnas.92.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koryntova H, Kubova H, Tutka P, Mares P. Changes of cortical epileptic after discharges under the influence of convulsant drugs. Brain Res Bulletin. 2002;58:49–54. doi: 10.1016/s0361-9230(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 48.Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 49.Le Moine C, Svenningsson P, Fredholm BB, Bloch B. Dopamine-adenosine interactions in the striatum and the globus pallidus: inhibition of striatopallidal neurons through either D2 or A2A receptors enhances D1 receptor-mediated effects on c-fos expression. . J Neurosci. 1997;17:8038–8048. doi: 10.1523/JNEUROSCI.17-20-08038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linden DJ, Conner JA. Long-term synaptic depression. Ann Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 51.Lu KT, Wu SP, Gean PW. Promotion of forskolin-induced long-term potentiation of synaptic transmission by caffeine in area CA1 of the rat hippocampus. Chin J Physiol. 1999;42:249–253. [PubMed] [Google Scholar]
- 52.Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 53.Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 54.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Ann Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 55.Mao L, Wang JQ. Glutamate cascade to cAMP response element-binding protein phospholylation in cultured striatal neurons through calcium-coupled group I metabotropic glutamate receptors. Mol Pharmacol. 2002;62:473–484. doi: 10.1124/mol.62.3.473. [DOI] [PubMed] [Google Scholar]
- 56.Margineanu DG, Klitgaard H. Caffeine-induced epileptiform field potentials in rat hippocampal slices: a pharmacological characterization. Neuropharmacology. 2004;47:926–934. doi: 10.1016/j.neuropharm.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 57.Martín ED, Bun o W. Caffeine-mediated presynaptic long-term potentiation in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 2003;89:3029–3038. doi: 10.1152/jn.00601.2002. [DOI] [PubMed] [Google Scholar]
- 58.Martín ED, Buno W. Stabilization effects of extracellular ATP on synaptic efficiency and plasticity in hippocampal pyramidal neurons. Eur J Neurosci. 2005;21:936–944. doi: 10.1111/j.1460-9568.2005.03925.x. [DOI] [PubMed] [Google Scholar]
- 59.Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: An elevation of the hypothesis. Ann Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 60.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 61.McPherson PS, Kim YK, Valdivia H, Knudson CM, Takekura H, Franzini-Armstrong C, Coronado R, Campbell KP. The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron. 1991;7:17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- 62.Moraidis I, Bingmann D. Epileptogenic actions of xanthines in relation to their affinities for adenosine A1 receptors in CA3 neurons of hippocampal slices guinea pig. Brain Res. 1994;640:140–145. doi: 10.1016/0006-8993(94)91868-6. [DOI] [PubMed] [Google Scholar]
- 63.Moraidis I, Bingmann D, Lehmenkuhler A, Speckmann EJ. Caffeine-induced epileptic discharges in CA3 neurons of hippocampal slices of guinea pig. Neurosci Lett. 1991;129:51–54. doi: 10.1016/0304-3940(91)90718-9. [DOI] [PubMed] [Google Scholar]
- 64.Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakano M, Yamada S, Udagawa R, Kato N. Frequency-dependent requirement for calcium store-operated mechanisms in induction of homosynaptic long-term depression at hippocampus CA1 synapses. Eur J Neurosci. 2004;19:2881–2887. doi: 10.1111/j.0953-816X.2004.03390.x. [DOI] [PubMed] [Google Scholar]
- 66.Nehlig A, Daval J-L, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 67.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 68.Rae MG, Martin DJ, Collingridge GL, Irving AJ. Role of Ca2+ stores in metabotropic L-Glitamate receptor-mediated supralinear Ca2+ signaling in rat hippcampal neurons. J Neurosci. 2000;20:8628–8636. doi: 10.1523/JNEUROSCI.20-23-08628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribeiro JA, Sebastiao AM, de Mendonca A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2002;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- 70.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sandler VM, Barbara JG. Calcium-induced calcium release contributes to action potential-evoked calcium transients in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:4325–4336. doi: 10.1523/JNEUROSCI.19-11-04325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Savic′ N, Sciancalepore M. Intracellular calcium stores modulate miniature GABA-mediated synaptic currents in neonatal rat hippocampal neurons. Eur J Neurosci. 1998;10:3379–3386. doi: 10.1046/j.1460-9568.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- 73.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after NMDA receptor activation. Science. 1999;248:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 74.Simukus C R, Stricker C. The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex. J Physiol. 2002;545:521–535. doi: 10.1113/jphysiol.2002.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 76.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Ann Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 77.Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:51–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 78.Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- 79.Sutor B, Mantell K, Bacher B. Evidence for the activity of five adenosine-3’, 5’-monophosphate-degrading phosphodies-terase isozymes in the adult rat neocortex. Neurosci Lett. 1998;252:57–60. doi: 10.1016/s0304-3940(98)00551-5. [DOI] [PubMed] [Google Scholar]
- 80.Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB. Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience. 1997;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- 81.Taketo M, Matsuda H, Yoshida T. Calcium-independent inhibition of GABAA current by caffeine in hippocampal slices. Brain Res. 2004;1016:229–239. doi: 10.1016/j.brainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Thu mmler S, Dunwiddie TV. Adenosine receptor antagonists induce persistent bursting in the rat hippocampal CA3 region via an NMDA receptor-dependent mechanism. J Neurophysiol. 2000;83:1787–1795. doi: 10.1152/jn.2000.83.4.1787. [DOI] [PubMed] [Google Scholar]
- 83.Traub RD, Bibbig A, LeBeau F E N, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Ann Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- 84.Volfovsky N, Parnas H, Segal M, Korkotian E. Geometry of dendritic spines affects calcium dynamics in hippocampal neurons: theory and experiments. J Neurophysiol. 1999;82:450–462. doi: 10.1152/jn.1999.82.1.450. [DOI] [PubMed] [Google Scholar]
- 85.Wang SJ, Cheng LL, Gean PW. Cross-modulation of synaptic plasticity by beta-adrenergic and 5-HT1A receptors in the rat basolateral amygdala. J. Neurosci. 1999;19:570–577. doi: 10.1523/JNEUROSCI.19-02-00570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang XJ. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J Neurosci. 1999;19:9587–9603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whiteford KL, Dijkhuizen P, Polleux F, Gosh A. Molecular control of cortical dendrite development. Ann Rev Neurosci. 2002;25:127–149. doi: 10.1146/annurev.neuro.25.112701.142932. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimura H, Kato N, Honjo M, Sato J, Sugai T, Segami N, Onoda N. To-and-fro voltage signal propagation between the insular gustatory and parietal oral somatosensory areas in rat cortex slices. Brain Res. 2004;1015:114–121. doi: 10.1016/j.brainres.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 89.Yoshimura H, Kato N, Sugai T, Segami N, Onoda N. Age-dependent appearance of an insulo-parietal cortical signal propagation that elicits a synchronized population oscillation in the parietal cortex in rats. Brain Res Development Brain Res. 2003;143:245–251. doi: 10.1016/s0165-3806(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 90.Yoshimura H, Kato N, Sugai T, Segami N, Onoda N. Age-dependent emergence of oscillatory signal flow between the primary and secondary visual cortices in rat brain slices. Brain Res. 2003;990:172–181. doi: 10.1016/j.brainres.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 91.Yoshimura H, Sugai T, Honjo M, Segami N, Onoda N. NMDA receptor-dependent oscillatory signal outputs from the retrosplenial cortex triggered by a non-NMDA receptor-dependent signal input from the visual cortex. Brain Res. 2005;1045:12–21. doi: 10.1016/j.brainres.2005.02.084. [DOI] [PubMed] [Google Scholar]
- 92.Yoshimura H, Sugai T, Onoda N, Segami N, Kato N. Synchronized population oscillation of excitatory synaptic potentials dependent on calcium-induced calcium release in rat neocortex layer II/III neurons. Brain Res. 2001;915:94–100. doi: 10.1016/s0006-8993(01)02832-3. [DOI] [PubMed] [Google Scholar]
- 93.Yoshimura H, Sugai T, Onoda N, Segami N, Kato N. Age-dependent occurrence of synchronized population oscillation suggestive of a developing functional coupling between NMDA and ryanodine receptors in the neocortex. Brain Res Development Brain Res. 2002;136:63–68. doi: 10.1016/s0165-3806(02)00352-8. [DOI] [PubMed] [Google Scholar]
- 94.Yoshimura H, Sugai T, Segami N, Onoda N. Strengthening of non-NMDA receptor-dependent horizontal pathways between primary and lateral secondary visual cortices after NMDA receptor-dependent oscillatory neural activities. Brain Res. 2005;1036:60–69. doi: 10.1016/j.brainres.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 95.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Ann Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 96.Yuste R, Majewska A, Cash SS, Denk W. Mechanisms of calcium influx into hippocampal spines: heterogeneity among spines, coincidence detection by NMDA receptors, and optical quantal analysis. J Neurosci. 1999;19:1976–1987. doi: 10.1523/JNEUROSCI.19-06-01976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


