Abstract
Long-term alcohol exposure gives rise to development of physical dependence on alcohol in consequence of changes in certain neurotransmitter functions. Accumulating evidence suggests that the glutamatergic neurotransmitter system, especially the N-methyl-D-aspartate (NMDA) type of glutamate receptors is a particularly important site of ethanol’s action, since ethanol is a potent inhibitor of the NMDA receptors (NMDARs) and prolonged ethanol exposition leads to a compensatory “upregulation” of NMDAR mediated functions supposedly contributing to the occurrence of ethanol tolerance, dependence as well as the acute and delayed signs of ethanol withdrawal.
Recently, expression of different types of NMDAR subunits was found altered after long-term ethanol exposure. Especially, the expression of the NR2B and certain splice variant forms of the NR1 subunits were increased in primary neuronal cultures treated intermittently with ethanol. Since NMDA ion channels with such an altered subunit composition have increased permeability for calcium ions, increased agonist sensitivity, and relatively slow closing kinetics, the abovementioned alterations may underlie the enhanced NMDAR activation observed after long-term ethanol exposure. In accordance with these changes, the inhibitory potential of NR2B subunit-selective NMDAR antagonists is also increased, demonstrating excellent potency against alcohol withdrawal-induced in vitro cytotoxicity. Although in vivo data are few with these compounds, according to the effectiveness of the classic NMDAR antagonists in attenuation, not only the physical symptoms, but also some affective and motivational components of alcohol withdrawal, novel NR2B subunit selective NMDAR antagonists may offer a preferable alternative in the pharmacotherapy of alcohol dependence.
Key Words: Alcohol, dependence, withdrawal, NMDA receptor, NR2B subunit selective antagonist, pharmacotherapy
INTRODUCTION
According to the recent developments in drug abuse research across the areas of molecular genetics, cell biology, animal behaviour, and human brain imaging studies, the widely held elderly view about drug abuse and addiction —i.e. that the problem of drug abuse would go away if only the abuser or addict could change his behaviour — tends to be unmaintainable. Recent advances in our understanding of the neurobiology of drug abuse highlight the importance of the interpretation of drug addiction as a complex brain disease caused by alterations in crucial neurotransmitter systems, and thus give rise to the opportunity of pharmacological interventions [78].
Drug dependence (American Psychiatric Association, 1994) [6], also termed as drug addiction, is a chronically deteriorating disorder characterised by the desire to seek for and take the drug, leading to the loss of control in limiting intake because of the emergence of psychical and/or physical dependence, i.e. a negative emotional and/or a disturbed physiological state, when access to the drug is withdrawn. These behavioural and/or physiological abnormalities develop gradually and progressively during a course of repeated exposure to a drug of abuse. According to the recent view, drug dependence can be considered as a form of drug-induced neural plasticity. Repeated exposure to a drug of abuse alters the amounts, and even the types of genes expressed in specific brain regions. The altered expression of genes then mediates altered functions of individual neurons as well as the neural circuits within which the neurons operate. Finally, such changes in the neural circuit underlie the abnormalities seen in a drug dependent person (Fig. 1) [104, 159, 161, 92, 120, 157, 160].
Fig. (1).
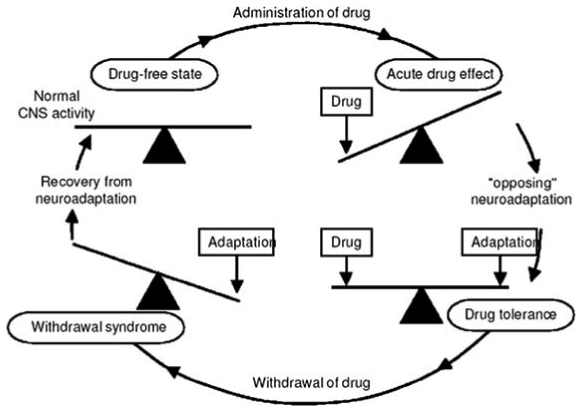
Drug dependence as neuroadaptation.
The concept is that the administration of a drug acutely “unbalances” the chemistry of the brain. In order to overcome this effect, the brain institutes a homeostatic mechanism, i.e. an “opposing neuroadaptation” that balances the effect of the drug on brain chemistry. While the drug is present in the brain, the system remains in relative balance (i.e. there is evidence of drug tolerance). However, rapid removal of the drug now exposes the adaptation because it is no longer “balanced” by the drug. The resulting functional disturbance is the cause of the drug withdrawal syndrome. In Himmelsbach’s theory, this will continue until the adaptation can be removed and the chemistry of the brain returns to its normal balancing act. Collier’s modification of this hypothesis was to propose that, since drugs act on receptors in the brain, it was logical to suppose that a primary mechanism for neuroadaptation to drugs would be to regulate the numbers of those receptors. This type of adaptation would reduce the effects of the drug, but would also cause alterations when the drug left the brain because the natural transmitters inside the brain also use the same receptors. This modified unitary, hypothesis remains implicitly accepted by neuropharmacologists today, but we are beginning to recognize that it represents a gross oversimplification of the complex cellular mechanisms for drug dependence.
From: John Littleton. (2001) Receptor regulation as a unitary mechanism for drug tolerance and physical dependence—not quite as simple as it seemed! Addiction 96, 87–101.
Among the numerous types of drug addictions, alcohol use disorders represent a substantial public health problem all over the world. In 2000, 2 billion alcohol users were estimated by the World Health Organisation (WHO), compared with 1.3 billion smokers and 185 million users of other psychoactive drugs. According to the data released by the WHO in 2003, the global prevalence of alcohol use disorders was 1.7%, and these disorders accounted for 1.4% of the total world disease burden. Data released by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) in August 2004 showed that whereas in 1991–1992, the total prevalence of 12-month alcohol abuse and dependence, according to the “Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition” (DSM-IV), was 7.41% in the USA representing 13.8 million adult Americans, this prevalence rose to 8.46% representing 17.6 million Americans in 2001–2002. Given the harmful effects of alcohol use disorder on the afflicted individuals and society as a whole, alcohol use disorders continue to represent one of the world’s major health problems, with large direct health costs (psychiatric and physical) as well as massive indirect costs to society in terms of crime, loss of earnings and productivity, and social damage [72, 134].
In alcohol dependent individuals a complex set of signs, i.e. alcohol withdrawal syndrome (AWS) occurs after alcohol cessation. The symptoms of the withdrawal syndrome include sweating, tremor, hypertension, anxiety, agitation and sympathetic hyperactivity responsible for tachycardia within the first hours following the last alcohol intake. Later on epileptic seizures and delirium tremens characterized by auditory and visual hallucinations, confusion and disorientation, clouding of consciousness and pronounced autonomic hyperactivity may also occur. This set of symptoms can even lead to death from respiratory and cardiovascular collapse. Even minor symptoms are disabling enough to lead the alcohol dependent individual to resume alcohol consumption at the early stages of withdrawal. The severity of alcohol withdrawal syndrome is therefore a major risk factor for early relapse [216].
Until recently, little could be done to help problem drinkers’control over alcohol consumption. Thanks, however, to discoveries on the neurochemistry of alcohol’s effects and on the complex pathophysiology of withdrawal symptoms, some biology-based treatments are now available and even more help is on the way. The research is leading to an increased understanding of how various neurotransmitter systems in the brain contribute to the development of alcohol dependence, thus a wider range of treatment options may arise for individuals with alcohol problems [11]. This review focuses on the role of a special type of glutamate receptors, the N-methyl-D-aspartate receptors in the development of alcohol dependence and in possible therapeutic approaches.
ETHANOL AND NMDA RECEPTORS
Although, the exact mechanism by which ethanol exerts its effect is still a matter of debate, a major step in medication development occurred in recent years when scientists discovered evidence that alcohol acts on several chemical systems in the brain to create its alluring effects. Besides its well-known effect on the release of neurotrans-mitters especially dopamine (DA), resulting in increased DA levels in the mesolimbic system including the nucleus accumbens, it is now clear that ethanol also alters the function of a number of neurotransmitter receptors (e.g. γ- amino butyric acid A (GABAA), glycine, glutamate, norepine-phrine, DA, serotonin, acetylcholine and opiate receptors), as wellastransporters adenosine, norepinephrine, DA, serotonin transporters). Particularly, ion channels including the L-type voltage-gated Ca2+ channels (VGCC) [127] and the ligand-gated channels of the main amino acid neurotransmitter systemsofthebrain– the inhibitory GABA and the excitatory glutamate – seem to be highly sensitive to the acute effect of ethanol at relevant (5 – 100 mM) concentrations [26, 63, 70, 118, 123].
In the past years, there has been increasing evidence that acute ethanol facilitates GABAergic transmission by enhancing chloride conductance through the GABAA receptors, and inhibits glutamatergic function by decreasing cationic conductance through the ionotropic (i.e. receptors containing a ligand-gated ion channel) type of glutamate receptors. Conversely, long-term ethanol exposure appears to create inverse changes in the functions of these systems leading to decreased GABAergic and increased glutamatergic functions bringing about the development of tolerance and/or dependence as well as withdrawal symptoms when alcohol use is cut off [120]. According to the recent results on cell and animal studies, the glutamatergic system is considered as an especially important factor in the mediation of the addictive effect of alcohol [48]. Among the family of ionotropic glutamate receptors including the α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA), the kainate, and the NMDAreceptors, the AMPA and particularly the NMDA type of receptors represent the highest affinity targets for ethanol in the CNS [70, 82, 110, 122].
Structure and Function of NMDA Receptors
NMDA receptors (NMDARs), like other ion-channel receptors, appear to be multimeric transmembrane proteins, composed of different types of subunits. The ubiquitously expressed NR1 subunits exist in eight distinct isoforms (depending on the inclusion or exclusion of the N1, C1, and C2 or C2’ cassettes) because of three independent sites of alternative splicing. Four different subtypes of NR2 (A, B, C and D) and two subtypes of NR3 (A, B) subunits are also identified [47, 84, 141]. Although, the precise subunit composition and stoichiometry of native NMDARs are difficult to determine, NMDARs are believed to exist as tetrameric complexes consisting of at least one NR1 and one NR2 subunits [114, 139, 140, 141, 172]. The subunits are most probably arranged as dimer of dimers with an NR1-NR1-NR2-NR2 orientation in the channel [189]. Each subunit has four hydrophobic regions, although only three of them form membrane-spanning domains (TM1, TM3, and TM4). The fourth one (M2) makes a hairpin bend within the membrane and participates in the formation of the ion channel [13, 45] (Fig. 2).
Fig. (2).
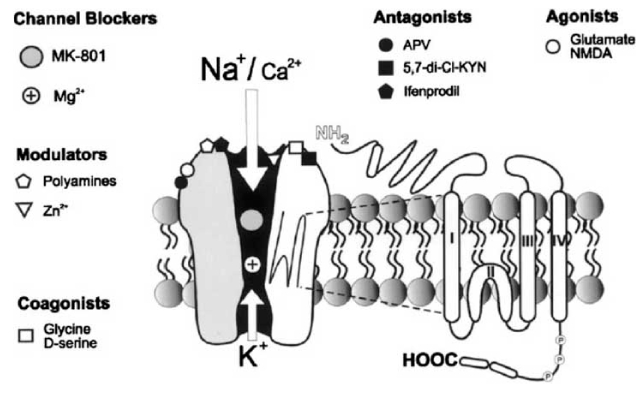
Schematic diagram of NMDA receptor ion channel.
Diagram representing NMDA receptor ion channel with its various regulatory sites. The receptor is activated by agonists such as glutamate or NMDA. APV is a competitive antagonist, 5,7-di-Cl-KYN binds to a strychnine insensitive glycine site, ifenprodil is a polyamine site antagonist. The open NMDA channel is blocked by Mg2+ and by uncompetitive antagonists such as MK-801. Glycine and D-serine act as coagonists. Additionally, polyamines and Zn2+ ions modulate the NMDA receptor. There are phosphorylation sites (P) that modulate responses of the receptor to agonists and may play a role in synaptic plasticity. Each subunit is believed to have four regions (I, II, III, and IV) within the cell membrane
From: Bisaga, A. and Popik, P. (2000) In search of a new pharmacological treatment for drug and alcohol addiction: N-methyl-D-aspartate (NMDA) antagonists. Drug Alcohol Depend. 59, 1–15.
The involvement of NMDARs in diverse processes like excitatory synaptic transmission [205], synaptic plasticity [127], neurotrophic and neurotoxic functions [102, 163, 185] rests upon their unique features, i.e. i) their high permeability to Ca2+ ions, ii) their relatively slow activation/deactivation kinetics, and iii) their voltage-sensitive blockage by extra-cellular Mg2+ ions. Glutamate, the native agonist of the NMDARs, can open the ion-channel only if the plasma membrane became depolarised and the Mg2+ blockage was displaced. Thus, NMDARs act as coincidence perceptive elements, which become active only when electrical and chemical signals are present concurrently.
Besides glutamate, NMDARs are sensitive to several other endogenous modulators including their co-agonist glycine [135] and D-serine [144]. Endogenous polyamines, spermine and spermidine also facilitate [115, 180], whereas extracellular Zn2+ ions [37] and protons [202, 206] suppress NMDAR activation. NMDARs interact with various intracellular scaffolding, anchoring, and signalling molecules associated with the postsynaptic density (Fig. 2, see review of [121]). The sensitivity of NMDARs to different ligands, its permeation, and block by divalent ions, kinetic properties, and interaction with intracellular proteins highly depend on their subunit composition [21, 39, 91]. Diheteromeric NMDARs composed of NR1/NR2A or NR1/NR2B subunits generate ‘high-conductance’, Mg2+ sensitive channels permeable also to Ca2+ ions. On the contrary, receptors containing NR2C or NR2D subunits give rise to ‘low-conductance’ channels with a lower sensitivity to Mg2+ ions and permeability principally to Na+ ions. The NR3 subunits are thought to be regulatory in nature, since they do not form functional channels with NR1 subunits but co-assemble with NR1/NR2 complexes forming ‘low-conductance’ channels [168].
Ethanol is a Potent Inhibitor of NMDA Receptors
Biochemical, electrophysiological and behavioural evidences show that ethanol - in clinically relevant concentrations (25 – 100 mM) - is a potent and selective inhibitor of NMDA receptors [50, 81,122,124]. Several studies involving recombinant receptors have demonstrated that receptors containing different types of NR2 subunits have differential sensitivity to the inhibitory effect of ethanol [170, 192]. According to the earlier results, the ability of ethanol to depress NMDA evoked currents paralleled with the neuroprotective action of ifenprodil in rat cultured cortical neurons [125]. Similar results were obtained when NMDA-induced release of (3H)-norepinephrine was measured in slices from cerebral cortex of the rat [58]. Since ifenprodil is known as an NR2B subunit selective NMDAR antagonist [213], it was assumed that ethanol acts on the same subunit. Indeed, studies performed on recombinant NMDARs showed that heteromers containing either NR2A or NR2B subunits are preferentially sensitive to ethanol inhibition vs. hetero-mers containing NR2C or NR2D subunits [24, 38, 112, 131, 136, 215, 219]. Moreover, NMDARs with NR1/NR2B subunit combination were more susceptible to the effect of ethanol compared to those composed of NR1/NR2A subunits [7, 14, 15, 192]. The co-expression of NR3 or an NR3-GFP fusion protein with NR1/NR2 (A-D) subunits did not alter the inhibitory effects of ethanol [193].
Data regarding the site of effect of ethanol on NMDARs are controversial. Earlier it was thought that ethanol binds to a hydrophobic pocket distinct from other modulatory binding sites of the NMDARs [166, 167]. Recently it was suggested that this pocket is associated with the third transmembrane domain (TM3) of the NR1 subunit [181]. While mutation of Phe639 to Ala in this region of the NR1 subunit expressed in either oocytes or HEK-293 cells significantly decreased the inhibitory effect of ethanol, the substitution of this residue for Trp resulted in receptors that were slightly more sensitive to ethanol inhibition than the wild-type receptors. These observations suggest that the 639 position of the NR1 subunit is an important determinant of ethanol sensitivity [2].
Action of ethanol on NMDARs may also be mediated by changes in the phosphorylation status of the receptor subunits. According to Alvestad et al. [5], phosphorylation of tyrosine side chains in the NR2A and/or NR2B subunits was significantly reduced following in situ exposure of hippocampal slices to 100 mM ethanol. Addition of a phosphotyrosine phosphatase inhibitor – bpV(phen) – in the recording medium prior to and during ethanol exposure significantly reduced the inhibitory effect of ethanol on NMDAR mediated excitatory postsynaptic potentials [5, 54]. These data suggest a possible mechanism by which ethanol may inhibit NMDAR functions via activation of a tyrosine phosphatase and phosphatase-mediated dephosphorylation of NMDAR subunits may play a role in mediating the inhibitory effect of ethanol.
Effect of Long-Term Ethanol Exposure on NMDAR Functions
Data from studies on neuroadaptation following long-term ethanol exposure indicate a significant role of NMDARs in the development of alcohol dependence, in the expression of alcohol withdrawal syndrome as well as in withdrawal associated neuronal damage. Initial in vivo studies showed that seizures evoked by withdrawal of ethanol in alcohol dependent animals were attenuated by NMDAR antagonists and exacerbated by administration of NMDA at doses that are not convulsant in control animals. According to these observations, it has been hypothesised that when alcohol intake is cut off, an enhanced NMDAR mediated neurotransmission underlies the observed neuronal hyperactivity [67, 69].
Indeed, several papers reported that chronic ethanol exposure leads to a selective enhancement of NMDAR function in cultured hippocampal [204, 173] and cortical neurons [31, 83, 148, 191]. For instance, while the amount of non-viable cells in hippocampal brain slice explants was significantly reduced in the presence of ethanol, cytotoxic effect of NMDA was significantly higher in ethanol-exposed samples after 24h withdrawal. Correspondingly, when cultures of rat cortical cells were treated with ethanol, the morphology of neurons was not altered, whereas obvious signs of neuronal damage and increased release of lactate dehydrogenase (LDH) were observed after 24 hours of withdrawal [148]. Interestingly, neurotoxic effect of ethanol withdrawal was observed only in those cultures, which were pre-treated with ethanol repeatedly, once daily at least for three consecutive days (Fig. 3A) [149]. Furthermore, alcohol-withdrawal induced LDH-release was not observed when ethanol was continuously present (Fig. 3B). In addition, whereas the effect of the GABAA receptor agonist muscimol was insignificant, NMDAR antagonists (MK-801 and ifenprodil) effectively reduced the neurotoxic effect of withdrawal [149]. Similarly, NMDA responses were found to be increased in cortical cultures treated with ethanol repeatedly for 3 days (Fig. 4) [149, 150]. The 3-day repeated ethanol exposure paradigm used in these experiments is similar to the in vitro neuronal model described by Hu and Ticku [81] in which chronic but intermittent ethanol treatment (CIE) was used (12h ethanol followed by 12h withdrawal). The CIE exposure also produced enhanced NMDA mediated increase in intracellular calcium levels showing increased NMDA receptor functions. These data are consistent with the previous observations that acute administration of ethanol has a small neuroprotective effect and following long-term ethanol exposure and withdrawal neurons became more sensitive to NMDA [75, 173, 204]. These observations suggest that neuronal cells pre-treated with ethanol required further ethanol for survival i.e. became dependent on ethanol. These observations support the conception that NMDARs may play a crucial role in the development of in vitro ethanol dependence and alcohol-withdrawal evoked neurotoxicity. This in vitro test system, when ethanol treatment is interrupted and the cycle of treatment and withdrawal is repeated several times, can be used as an in vitro model for studying the development of ethanol dependence and withdrawal symptoms.
Fig. (3).
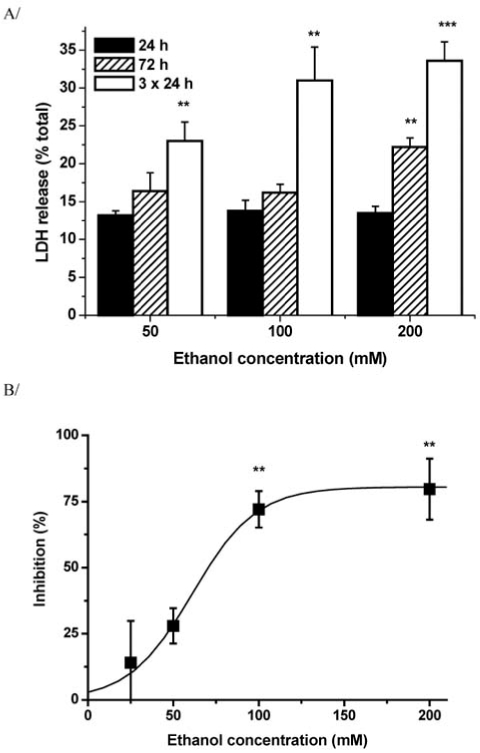
Toxic effect of 24 h ethanol-withdrawal and its inhibition by re-addition of ethanol in ethanol pre-treated primary cortical cultures.
A) LDH activity of the culture medium expressed as percentage of total activity was measured in cultures pre-treated with different concentrations of ethanol once for 24 or 72 hours as well as daily for 3 successive days.
B) Inhibition of alcohol-withdrawal induced cytotoxicity by readdition of ethanol in primary cultures of rat cortical neurones pretreated with 100 mM ethanol daily for 3 successive days.
(*: p<0.05, **: p<0.01, ***: p<0.001 as compared to the respective control not treated with ethanol)
From: Nagy J., László L. (2002) Increased sensitivity to NMDA is involved in alcohol-withdrawal induced cytotoxicity observed in primary cultures of cortical neurones chronically pre-treated with ethanol. Neurochem. Int., 40, 585–591.
Fig. (4).
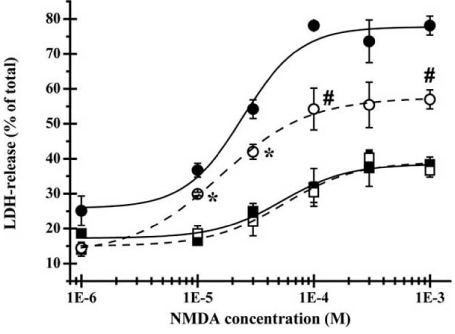
Altered excitotoxic effect of NMDA after ethanol pretreatment.
Effect of acute and chronic ethanol treatment on NMDA induced cytotoxicity. Control cortical cultures (squares) and cortical cultures pre-treated with 100mM ethanol repeatedly, once daily for 3 days (circles) were exposed to 300 μM NMDA for 15 min in the presence (open symbols) or absence (closed symbols) of 100 mM ethanol. LDH-release, expressed as percentage of total LDH content, was measured 24 h after NMDA wash out.
(*, P<0.01 compared to NMDA induced LDH-release in control cultures; #, P<0.01 compared to LDH-release in absence of ethanol).
The CIE treatment is a widely used experimental paradigm also in animal studies and is a validated model for human alcohol withdrawal syndrome. In this kind of experiments rats are exposed to intermittent episodes of intoxicating doses of ethanol and withdrawal leading to a kindling-like state of behavioural excitability. It was observed that after repeated ethanol withdrawal experience reduced GABAA receptor function and increased NMDA receptor activity become exaggerated and these changes are suggested to have a role in the development of alcohol dependence, i.e. in manifestation of hyperexcitability when alcohol is withdrawn [10, 25, 138].
Effect of Long-Term Ethanol Exposure on the Structure of NMDARs
According to earlier reports, it was observed in both in vivo and in vitro studies that chronic ethanol treatment leads to an increase in the density of NMDARs leading to facilitated receptor functions [62, 64, 88, 89, 90, 147, 215]. However, up-regulation of NMDAR expression has not been found in all studies after chronic ethanol exposition [55, 186]. Consistent with the recently emerging view, the increased NMDAR function is presumably due to a differential up-regulation of the various NMDAR subunits. This conception is supported by several papers presenting evidence for altered NMDAR subunit composition after chronic ethanol treatment.
Notwithstanding, there is a disagreement in respect of the expression of the different NR subunits and NR1 splice variant forms. On one hand, some authors reported no changes in subunit expression at all [30], and others found changes solely in the expression of the NR1 [60] or NR2A [46] subunit in consequence of long-term ethanol exposure. On the other hand, there are papers concluding that besides several other types of subunits and certain NR1 splice variants, the expression of the NR2B subunit is increased. Lots of in vitro studies showed increased NR2B subunit mRNA levels, with no change in NR1 and/or NR2A subunit transcription in cultured cortical neurons following chronic ethanol administration [73, 89, 143]. On the contrary, the levels of NR2B as well as NR2A and NR1 subunit proteins were found increased in the cortex and hippocampus of rats or mice [61, 62, 79, 96, 154]. Furthermore, in cultured cerebellar granule cells, the ‘developmental switch’ of the NR2B subunit for NR2A was found delayed resulting in higher NR2B and lower NR2A subunit levels [194].
Similarly to these later observations, in primary cultures of cortical as well as hippocampal neurons from rats, the maximal inhibitory effect of ethanol as well as some NR2B subunit selective NMDAR antagonists on NMDA evoked cytosolic calcium elevations was significantly increased after ethanol pre-treatment [150]. However, the efficiency of the non-subunit selective NMDAR antagonist channel blocker MK-801 and the glycine site specific 5,7-DCK was not changed. Accordingly, increased expression of the NR2B subunits could be detected applying a flow cytometry based immunocytochemical method. Whereas, in situ immuno-cytochemical detection of the NR2B subunits could generate only qualitative data, the combination of immunocyto-chemistry with flow cytometry made an opportunity for a quantitative analysis of the expression. This quantitative analysis showed that the NR2B specific immuno-labelling was increased in a subpopulation of the cells in ethanol pre-treated compared to control cultures. According to similar analysis, the expression of the panNR1, NR2A, NR2C, and NR2D subunits was not changed after ethanol pre-treatment in rat cortical or hippocampal cultures (Fig. 5A). In further studies, when the expression of the NR1 splice variants was investigated, similarly to the NR2B subunit, the expression of the C1 and C2’ cassette containing splice variants was found to be increased in ethanol pre-treated hippocampal cultures (Fig. 5B) [150].
Fig. (5).
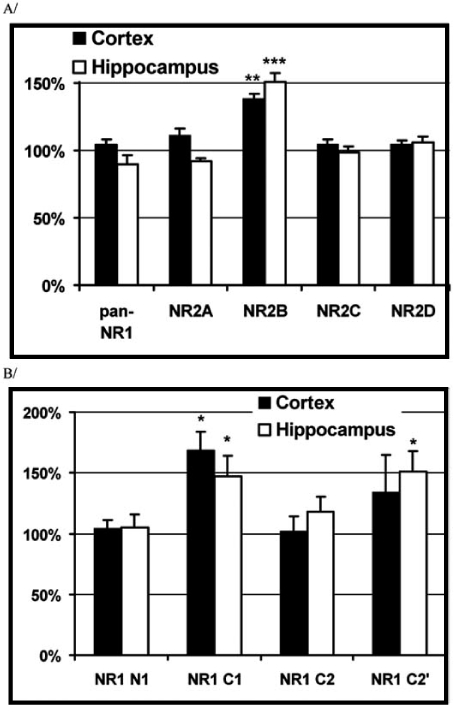
Effect of chronic ethanol pre-treatment on the expression of different NMDA receptor subunits and NR1 splice cassettes.
Primary cortical and hippocampal cultures were treated with 100 mM ethanol daily for 3 days. Fixed samples were incubated in the presence of different NR2 and NR1 splice variant specific primary antibodies (Novus Biologicals). The binding of the primary antibodies was visualised via FITC-conjugated secondary antibodies (Sigma). Intensity of the subunit specific fluorescent labelling was analysed using a FACScan flow cytometer. The arithmetic mean FITC-fluorescence intensities were calculated from fluorescence histograms. The mean fluorescence values from samples incubated with the given NMDA receptor subunit specific antibody (NR staining) were corrected with the fluorescence of samples stained with an isotype specific control antibody (background). Each column represents the percentage of corrected fluorescence values obtained from ethanol pre-treated vs. control cultures.
(Each value represents mean + S.E. (bars); *: p<0.05, **: p<0.01, ***: p<0.001 compared to the control, paired t-test).
Data from: Nagy, J., Kolok, S., Dezso?, P., Boros, A. Szombathelyi, Z. (2003) Differential alterations in the expression of NMDA receptor subunits following chronic ethanol treatment in primary cultures of rat cortical and hippocampal neurones. Neurochem. Int., 42(1), 35-43.
Correspondingly, in vivo studies on rats also showed that after chronic ethanol ingestion the NMDA receptor function was enhanced in the lateral/basolateral amygdala. The increase in the NMDA receptor current density was associated with an increase in ifenprodil inhibition and a decrease in apparent calcium-dependent current inactivation. Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) measurements demonstrated that the NR1 subunit mRNA expression, but not the NR2 or NR3 subunit transcription, was enhanced [60, 214].
The molecular mechanisms underlying these changes in subunit expression is one of the main questions in the near future. First results concerning the regulation of subunit composition by Ravindran and Ticku showed that the methylation status of the NR2B gene is altered following chronic ethanol treatment in mouse cortical neurons [177]. Theyfound that demethylation this gene could be responsible for up-regulation of the NR2B subunit expression.
Consequences of Changes in Structure of NMDARs
The increased expression of the NR2B subunits accompanying with elevated levels of the C1 and C2’ cassette containing splice variant forms of the NR1 subunits may underlie the enhanced NMDAR function. This idea is supported by the following observations: i) the deactivation time of NMDARs composed of NR1/NR2B subunits is longer than those built up of NR1/NR2A subunits [140], ii) the deactivation rate is four times faster for receptors composed of NR1 subunit containing the N1 cassette than those lacking this cassette [40, 187], and iii) NMDARs assembled of NR1 splice variants containing C1 and/or C2 cassettes may form functionally more active ion channels [175].
The phosphorylation states of the NMDARs are also altered after long-term ethanol exposure. It is known that Src family of protein tyrosine kinases, specifically c-Src and Fyn kinases potentiate the NMDA-activated currents in in vitro recombinant systems [8, 32, 101, 188] as well as in spinal neurons [211]. The up-regulation of NMDAR function by Src and Fyn accompanies with reduced sensitivity of NMDARs to ethanol [188]. Furthermore, phosphorylation status of NR1 and NR2B subunits was increased in the hippocampus of ethanol treated control but not in Fyn-deficient mice [95, 137]. This observation is in good agreement with that of Yaka et al. [221, 222], who found that the scaffolding protein RACK1 that binds Fyn kinase to the NR2B subunit dissociates from the complex due to ethanol exposure, consequently facilitating Fyn-mediated phosphorylation of the NR2B subunit leading to enhanced channel activity counteracting the inhibitory actions of ethanol. Reduced phosphorylation state of NR2 subunits –achieved by knocking out the Fyn kinase gene – increases ethanol sensitivity of NMDARs [7, 137]. In addition, transgenic mice over-expressing the Fyn tyrosine kinase and withdrawn from alcohol failed to show any increase of anxiety-like behaviour or reduction of exploratory activity like it was observed in case of their wild-type littermates [201]. This apparent lack of alcohol withdrawal-induced behavioural effects was associated with increased Fyn kinase activity and tyrosine phosphorylation of several proteins including the NR2B subunit.
Concerning the NR1 subunit, truncation (NR1858stop) [7] or phosphorylation of Ser897 of this subunit decreased the ability of ethanol to inhibit NMDAR function. In addition, the reduced sensitivity of NMDARs to ethanol was linked up with the dopamine D1 receptor activation via dopamine and cAMP-regulated phosphoprotein-32 kD (DARPP-32) phosphorylation [126]. Activation of D1 receptors prevents the dephosphorylation of the NR1 subunit via a cascade that involves phosphorylation of PKA, which in turn phosphorylates dopamine and cAMP-regulated phosphoprotein-32 kDa (DARPP-32), which then inhibits the activation of PP1 phosphatase acting on the NR1 subunit [195]. Via this cascade, D1 receptor promotion of drug reinforcement, as might arise from prior exposure to drugs of abuse, reduces the sensitivity of NMDARs to blockade by ethanol [126] and may increase the motivational effects of ethanol [179].
Not only are the subunit composition and phospho-rylation states of the NMDARs altered after long-term ethanol exposure but the localization of certain subunits. According to Carpenter-Hyland et al. [27], the co-localization of NR1 clusters with the presynaptic marker protein synapsin was increased in rat hippocampal neurons exposed to 50 mM ethanol for 4 days. This was accompanied by significant increases in the size and density of these synapsin-associated clusters with no change observed in non synapsin-associated NR1 clusters. Similar effects were observedwithNR2Bclustering afterchronicethanol exposure. The increase in synaptic NMDA receptor clustering was prevented by addition of a protein kinase A inhibitor or by co-exposure to a low concentration of NMDA and was reversed when ethanol was removed from the cultures. On the contrary, no changes were observed in the synaptic content, cluster size, or density of AMPA receptors after ethanol exposure. Electrophysiological measurements on ethanol-treated neurons revealed a similar enhancement in synaptic NMDA currents with no change in AMPA-mediated events.
Taken together, changes in subunit expression, phosphorylation states and synaptic clustering of NMDAR subunits due to long-term ethanol exposure may lead to the enhancement of NMDA responses. These changes may also explain the occurrence of acute ethanol tolerance leading to reinforcement of ethanol consumption and may underlie the development of physical dependence on ethanol and the increased sensitivity of neurons to excitotoxic insults.
Consequences of Increased NMDAR Function
Presumably in consequence of increased function of NMDARs, enhanced release of glutamate was observed after chronic ethanol exposure both in in vitro as well as in vivo experiments. Besides several other factors (e.g. functional deficits of GABA receptors and increased VGCC function [77, 212]), the NMDARs are major contributors to the increased glutamate release during alcohol withdrawal since in the brain of ethanol-dependent rats, the extracellular concentration of glutamate shows a transient, NMDAR mediated increase after cessation of ethanol intake and these changes are time-locked to the behavioural signs of ethanol withdrawal [44, 53, 183]. This enhanced glutamate release may contribute to the further shift towards the excitatory dominance in the CNS after ethanol withdrawal [184]. Furthermore, up-regulation of the NMDARs can enhance the activity of the noradrenergic system as well [51, 52], that may account for the vegetative instability seen in serious states of alcohol withdrawal, especially in delirium tremens [208, 209].
Increased calcium influx through NMDA receptors tightly coupled to calcium uptake into mitochondria causes the production of reactive oxygen species that interfere with the function of mitochondria. Primary inhibition of the mitochondrial respiratory chain can also indirectly induce further NMDA receptor stimulation. When the inhibitory action of ethanol on NMDA receptors is removed during withdrawal, the potential of neuronal injury is markedly increased through this system. Vulnerability of neurons is more pronounced when withdrawal kindling i.e. increased and/or prolonged withdrawal signs after repeated episodes of withdrawal, occurs [74].
Recently, it has been hypothesized that the same neuronal system including the mesolimbic dopaminergic pathway mediates the reinforcement for alcohol and other addictive drugs like opiates or cocaine [103, 158]. Indeed, ethanol has been reported to stimulate dopamine (DA) release in the nucleus accumbens [103] and electrophysiological studies have demonstrated a concomitant ethanol-induced increase in the activity of ventral tegmental dopaminergic neurons [22, 23]. On the other hand, the observations that the NMDAR antagonist MK-801 increased burst firing of dopaminergic neurons [224] and could stimulate the release of DA from dopaminergic terminal areas suggest that glutamate - acting through NMDARs - exerts a tonic inhibitory action on DA release in the nucleus accumbens [83, 109]. According to the model of Fadda and Rossetti [53], blockade of the NMDARs by acute ethanol treatment disinhibits dopaminergic neurons via GABAergic interneurons possessing NMDARs. Withdrawal of alcohol, similarly to withdrawal of opiates or cocaine, has been found associated with decreased DA release in the limbic forebrain areas [182] due to decreased firing rate of dopaminergic neurons [49]. Thus, reduced dopaminergic functions seen after ethanol withdrawal may arise because of enhanced NMDA responses induced by chronic ethanol exposure.
All the above-discussed findings suggest the possibility that increased NMDA mediated neurotransmission may constitute the basis of both the motor signs (e.g. tremor, seizures etc.) and the affective or emotional disturbances (e.g. craving, dysphoria) associated with alcohol withdrawal. Bearing in mind that besides the glutamatergic system other transmitter mechanisms are also involved in the adaptive changes induced by chronic ethanol treatment and that in vitro experiments allow only limited conclusions to deduce for a whole organism, it is clear that alterations in NMDAR function may play a critical role in these processes leading to the development of alcohol dependence and withdrawal symptoms. According to this view of the pathomechanism of alcohol withdrawal syndrome, the NMDAR may be a possible target and NMDAR antagonists may be useful agents for the treatment of both the physical and the psychical signs of alcohol withdrawal.
NMDAR ANTAGONISTS IN PHARMACOTHERAPY FOR ALCOHOL WITHDRAWAL
Current pharmacotherapies for alcohol dependence, disulfiram and naltrexone, aiming at alleviating symptoms of acute abstinence and minimising the risk of relapse show limited efficacy in large multicenter studies [65, 111]. Also, agents, which appear to target the glutamatergic system, are emerging as an additional therapeutic option [70, 85, 87, 110, 129, 130, 218]. In the early 90s, it was already hypothesized that NMDAR antagonists can block alcohol withdrawal induced seizures in ethanol dependent animals. Since then, extensive literature on animal experimental and preliminary clinical data suggest that NMDAR antagonists are promising candidates for the treatment of alcohol withdrawal symptoms, inasmuch as these compounds may attenuate not only the physical but also the affective and motivational components of AWS. However, the field of alcoholism research is in the relatively early phases of determining the extent to which glutamatergic agents might reduce alcohol consumption and relapse.
Acamprosate
The American Food and Drug Administration (FDA) recently granted the approval of a novel anti-alcohol medication Campral (acamprosate calcium) to maintain abstinence in patients with alcohol dependence. In Europe, more than 4 million people have been treated with this agent since 1989, when it became commercially available in France. According to several human studies, acamprosate has a consistent effect on prolonging abstinence and reducing the rate of relapse, in conjunction with an equally consistent absence of effect on self-reported craving, suggesting that it can be used as a relapse-prevention medication. In previous animal studies, it was observed that acamprosate dose-dependently reduced alcohol consumption and hypermotility during ethanol withdrawal with no effects on food and water intake and without any effects generalize to those of ethanol. In addition, acamprosate did not substantially alter the discriminative stimulus properties of ethanol, pentobarbital, or amphetamine [196, 197, 198].
The exact mechanism of action of acamprosate, originally developed as a GABA analogue, was intensively investigated in the past years (for a review see [217]). Since acamprosate is chemically similar to GABA, early studies indicated that acamprosate interacts with the GABAergic system [16] to affect behaviours related to ethanol consumption. However, this interaction of acamprosate with the GABA receptors does not appear to be comparable to the effects induced by either benzodiazepines or barbiturates since acamprosate cannot be substituted for GABA agonists in a drug-discrimination procedure [68]. In addition, it would not bind to recombinant or native GABAA receptors in transfected HEK 293 cells or enhance chloride currents in these receptors [226]. The observation of Dachour et al. [41, 42, 43] i.e. that acamprosate can lessen the increase in extracellular glutamate level in microdialysates from nucleus accumbens during ethanol withdrawal was an important finding for the therapeutic use of this agent.
According to other studies it was suggested that acamprosate has an inhibitory effect on the native or recombinant NMDA-receptors as well as on voltage-sensitive Ca2+ channels [1, 176, 199]. As acamprosate reversed the potentiating effects of spermine, it was thought that acamprosate may act at the polyamine site of the NMDA receptor [171]. Indeed, acamprosate effectively reduced both the enhanced glutamate-induced calcium entry and neuro-toxicity in ethanol pre-treated primary cultures of organotypic hippocampal [133] or neocortical neurons from rats in a concentration-dependent manner (Fig. 6A)[4, 153]. However, its protective effects against glutamate-induced neurotoxicity were observed only in ethanol-withdrawn cultures. Furthermore, although acamprosate significantly reduced the calcium entry caused by glutamate or K+ in control and ethanol-exposed cultures, the neuroprotective effects of the drug did not correlate with its effects on calcium entry, making it unlikely that acamprosate directly affects NMDA receptors via the glutamate binding site or the receptor-operated calcium channel [4]. This idea was confirmed by further studies which found that acamprosate has no direct effect on the NMDARs [4, 132, 153, 171, 196]. Al Qatari et al. [3] argued that acamprosate may have an excitatory or inhibitory effect on NMDA receptors depending upon the experimental conditions indicating that acamprosate, at least partly, acts as a ‘partial agonist’ at the NMDA receptor.
Fig. (6).
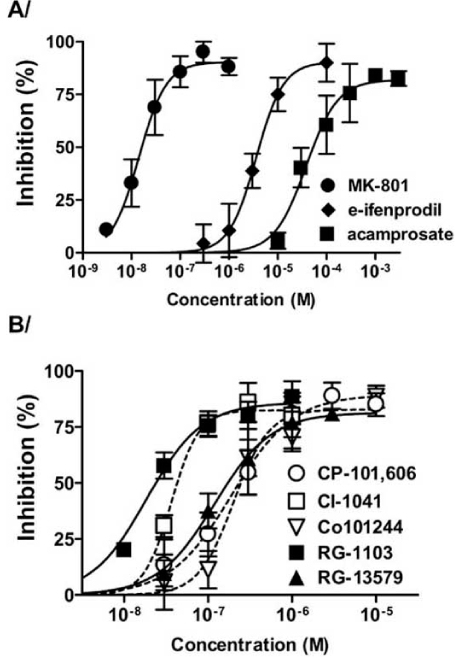
Inhibitory effect of NMDAR antagonists on ethanolwithdrawal-induced neurotoxicity.
Neuronal cell death caused by 24-hour ethanol-withdrawal in primary cultures of rat cortical neurones pre-treated with 100 mM ethanol for 3 consecutive days was quantified by measuring LDHrelease. Different concentrations of MK-801, erythro-ifenprodil and acamprosate (panel A) or some known (open symbols, dashed lines) and novel (filled symbols, straight lines) NR2B SSNAs (panel B) were present during the withdrawal period. Each point represents the percentage of inhibition (mean ± S.E. (error bars)).
From: Nagy, J., Horváth, C., Farkas, S., Kolok, S., Szombathelyi, Z. (2004) NR2B subunit selective NMDA antagonists inhibit neurotoxic effect of alcohol-withdrawal in primary cultures of rat cortical neurones. Neurochem. Int., 44(1), 17-23.
According to the observations of Harris et al. acamprosate displaced [3H]glutamate but did not compete with NMDA for [3H]glutamate binding sites in membrane preparations of cortices, cerebellums, and hippocampi of rats. Furthermore acamprosate displayed total competition with trans-ACPD (1-aminocyclopentane-trans-1,3-dicarboxylicacid)an agonist at both group I and group II metabotropic glutamate receptors and similarly to SIB-1893, a non-competitive antagonist at the mGluR5 receptor, it was neuroprotective against trans-ACPD induced neurotoxicity that likely results from mGluR mediated potentiation of NMDARs [76]. Also, in the CA1 region of ethanol pre-treated organotypic hippocampal slices, where neurotoxicity was observed after a 24-hr withdrawal, acamprosate, as well as SIB-1893, MK-801, and staurosporine were all neuroprotective. In this ethanol pre-treated slice culture preparations the polypeptide levels of mGluR5 receptors were found to be increased [77], similarly as the NR1 and NR2B subunits of NMDARs in other neuronal cultures after long-term ethanol exposure [150, 176]. Considering these observations, acamprosate may act on the mGluR5 receptors reducing its positive feedback control over the NMDARs [217]. Although the exact mechanism of action of acamprosate is still a matter of debate, the glutamatergic hypothesis may help to explain many of the effects of acamprosate in human alcohol dependence, especially in the acquisition of cue-elicited drinking behaviours [17, 41, 80, 86, 116, 117, 199].
Competitive and Channel Blocking NMDAR Antagonists
So far, the classic competitive and channel blocking NMDAR antagonists were tested and proved useful in in vitro or animal models of alcoholism. Early experiments showed that competitive NMDA receptor antagonists acting at the glutamate binding site (e.g. CGP 39551, D-CPP-ene) decreased handling-induced hyperactivity after withdrawal from chronic ethanol treatment in mice [113, 119, 178] and reduced alcohol deprivation effect (i.e. an overshoot in alcohol consumption shown by animals subjected to forced abstinence from regular drinking when ethanol is again available [105]) in rats [210]. These compounds increased the threshold for population spikes in hippocampal slices from the same animals. NMDAR antagonists acting within theionchannel (e.g. ketamine, MK-801 and ADCI) were also shown to suppress withdrawal-induced seizures effectively in both rats and mice [56, 71, 142]. Unfortunately, preclinical studies indicated that most of these compounds produce psychotomimetic or sedative effects, ataxia, muscle relaxation, neuronal damages in the cingulate cortex as well as motor and learning impairment. These serious side effects impeded their introduction to the human therapy [20, 28, 100, 145, 164]. However, due to immense therapeutic promise of NMDA antagonists in acute and/or chronic neurodegenerative and psychiatric disorders efforts have been made to develop compounds lacking these side effects. More encouraging approaches were performed with low affinity channel blockers like memantine or with NMDA antagonists acting at the glycine binding site (L-701,324) having more tolerable side effect profiles.
Novel channel blockers like memantine (1-amino-3,5-dimethyl-adamantane) and its analogue neramexane (MRZ 2/579, 1-amino-1,3,3,5,5-pentamethyl-cyclohexane hydrochloride) have improved side effect profile probably due to their moderate potency and rapid, strongly voltage-dependent blocking kinetics [165]. These compounds greatly inhibited alcohol consumption without affecting water or food intake during relapse in long-term voluntarily alcohol-drinking rats [85, 87, 169, 190]. In addition, neramexane as well as memantine effectively suppressed ethanol withdrawal induced seizures in alcohol dependent rats [12]. According to recent results by Kotlinska et al. chronic administration of neramexane inhibits the development of ethanol dependence, reflected as a decrease in ethanol withdrawal-associated audiogenic seizures as well as the acquisition and expression of ethanol-induced place preference [108].
Glycine-B Site NMDAR Antagonists
Glycine-B site antagonists were also shown to attenuate the expression of alcohol withdrawal symptoms [45]. A member of this type of NMDAR antagonists, L-701,324 (7-chloro-4-hydroxy-3-3-phenoxyphenyl-2-1H–quinolone) produced a dose-dependent inhibition of audiogenic seizures associated with alcohol withdrawal [106, 107] and potently blocked the acquisition of ethanol-induced conditioned place preference [11] and reduced alcohol consumption during alcohol deprivation [210]. Preliminary problems with glycine site antagonists included poor systemic availability has now been overcome with agents like GV196771A ((E)-4,6-Dichloro-3-(2-oxo-1-phenylpyrrolidin-3-ylidenemethyl)-1H-indole-2-carboxylic acid sodium salt) or SM-31900 (3(S)-(2-(4- (Amino methyl) -2- (1(R)-carboxyethoxy)phenylamino) -2- oxoethyl) -7- chloro -1,3,4,5- tetrahydrobenzo(c,d)indole -2- carboxylic acid hydrochloride) which are not only of very high affinity, but also have improved pharmacokinetic and physicochemical properties, such as good brain permeation and solubility [94]. Although these compounds were not tested in animal models related to alcoholism, the facts that SM-31900 has a potent anticonvulsant activity [97], GV196771A can inhibit the development of morphine tolerance [174] and both compounds are devoid of behavioural side effects (hyperactivity, motor dysfunction) make these compounds promising candidates also for the treatment of alcoholism.
NR2B Subunit Selective NMDAR Antagonists
In recent years, novel non-competitive NMDAR antagonists inhibiting the NR2B subunit containing NMDARs have emerged and received considerable attention. Although, this type of compounds was initially thought to interact with the polyamine site, recent experiments using chimeric NR2A/NR2B subunits revealed that the major determinant of the inhibitory effect of ifenprodil – the initial chemical lead of the NR2B subunit selective antagonists – localizes to a distinct site of the NR2B subunit [66, 34]. The NR2B subunit selective antagonists showed potency in animal models of neurodegeneration [99], Parkinson disease [155, 156, 200, 220], and hyperalgesia [19, 29, 36, 57]. It was also realized that this type of compounds lacks the serious side effects of the classic NMDAR antagonists’ [162]. Although, like other un-competitive NMDAR antagonists they may have some adverse effect on learning and memory, it was proved that they have a wider separation between doses that are effective in seizure or stroke models and those that disrupt learning and memory. The limited information on the novel NR2B subunit selective antagonists (Table 1) such as CP-101,606 (traxoprodil) [33, 98], Ro25-6981 [59], Co-101244 [225], CI-1041 [35] and RG-1103 [18] also suggests that these drugs are better tolerated and are largely devoid of adverse CNS effects at antinociceptive doses, at least with respect to psychotomimetic, ataxic and sedative effects [19, 29, 35, 57, 146, 203].
Table 1.
Some NR2B Subunit Selective NMDA Antagonists
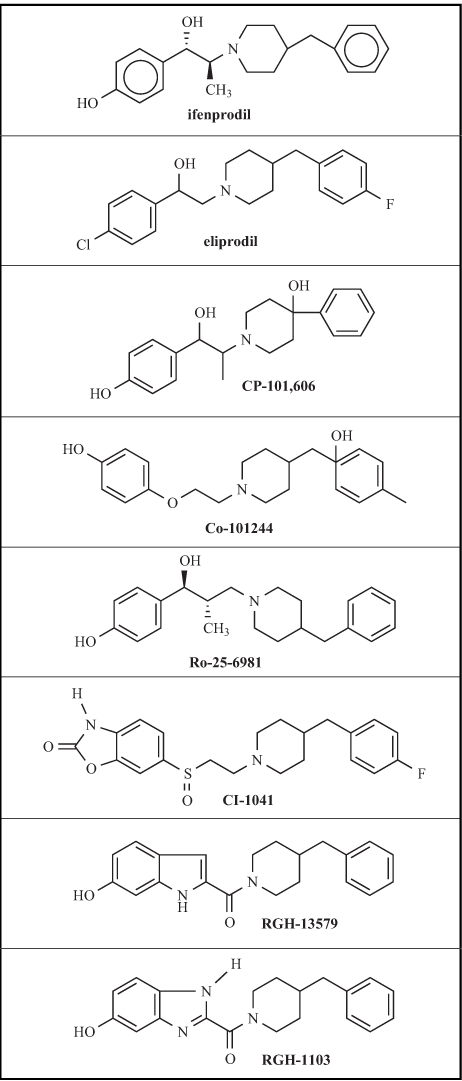 |
Previously, ifenprodil and its analogue eliprodil were found effective in animal models of alcohol dependence. According to Malinowska et al. [128], ifenprodil potently reduced the severity of withdrawal-induced seizures. Oral administration of increasing doses of eliprodil produced a dose-dependent and almost complete inhibition of ethanol withdrawal produced audiogenic seizures in alcohol dependent Sprague-Dawley rats [106]. This effect of eliprodil was achieved at doses that by themselves did not alter the basal locomotor activity of untreated control animals. Similar results were observed with ifenprodil in alcohol dependent male C57BL/6J mice [171]. The expression of spontaneous ethanol withdrawal signs (piloerection, jerk, tremor) occurring 6 – 12 hours after the discontinuation of ethanol treatment was suppressed by ifenprodil. According to Kotlinska and Liljequist [107], eliprodil effectively reversed the reduction in extracellular DA level during ethanol withdrawal but only partially and dose-independently substituted ethanol indicating that it has no discriminative stimulus properties similar to those produced by ethanol. Furthermore, ifenprodil dose-dependently reduced the expression of an alcohol deprivation effect as well [210].
With the novel NR2B subunit selective NMDAR antagonists so far only in vitro experiments were reported. In primary cultures of cortical neurons from rats pre-treated with ethanol intermittently for 3 days, CP-101,606, Co-101244 and CI-1041 as well as some of the novel indole-2-carboxamide derivative NR2B subunit selective antagonists (RGH-13579 and RGH-1103 [18]) potently and dose-dependently reduced the withdrawal-evoked LDH release (Fig. 6B). One of the novel compounds (RGH-1103) was as effective as MK-801, the most potent but not subunit selective NMDAR antagonist. The inhibitory potencies of the NR2B subunit selective antagonists for withdrawal-induced toxicity was in good linear relation with their effectiveness for inhibition of NMDA induced cytosolic calcium elevation (Table 2) [153].
Table 2.
Inhibitory Effect of NMDA Antagonists on Cytosolic Calcium Rises and Ethanol Withdrawal Induced Neurotoxicity
| Compound | NMDA evoked Ca2+ elevation | withdrawal induced toxicity | ||
|---|---|---|---|---|
| I max (%) | IC50 (μM) | I max (%) | IC50 (μM) | |
| MK-801 | 100 ± 5 | 0.037 ± 0.002 | 90 ± 5 | 0.020 ± 0.004 |
| CP-101,606 | 58 ± 2 | 0.041 ± 0.005 | 89 ± 9 | 0.201 ± 0.061 |
| Co-101244 | 67 ± 2 | 0.024 ± 0.003 | 101 ± 5 | 0.206 ± 0.045 |
| CI-1041 | 70 ± 3 | 0.007 ± 0.001 | 82 ± 6 | 0.037 ± 0.008 |
| RGH-13579 | 77 ± 3 | 0.018 ± 0.004 | 81 ± 18 | 0.137 ± 0.067 |
| RGH-1103 | 74 ± 2 | 0.002 ± 0.0004 | 87 ± 6 | 0.019 ± 0.005 |
| Acamprosate | −1.2 ± 2.9 | > 300 | 82 ± 8 | 40 ± 13 |
Compounds were tested for their inhibitory effect on cytosolic calcium elevation evoked by 40 μM NMDA and on ethanol-withdrawal produced neurotoxicity in primary cultures of rat cortical neurons.
From: József Nagy, Csilla Horváth, Sándor Farkas, Sándor Kolok, Zsolt Szombathelyi. (2004) NR2B subunit selective NMDA antagonists inhibit neurotoxic effect of alcohol-withdrawal in primary cultures of rat cortical neurones. Neurochem. Int., 44(1), 17–23.
SUMMARY
According to the recently emerged glutamatergic theory of the pathomechanism of alcohol withdrawal syndrome, increased NMDA receptor function may play a central role in the development of alcohol dependence and manifestation of the withdrawal symptoms. Despite the challenging complexity of ethanol’s action, there is now a convergence of evidence to indicate that i) the capacity of ethanol to block NMDARs is an important component of the human behavioural and intoxicating effects of ethanol, ii) ethanol tolerance and dependence are associated with alterations in NMDAR function that promote heavy drinking by reducing the negative consequences of ethanol intoxication, iii) physical ethanol dependence is associated with upregulation of certain NMDAR subunits and iv) acute ethanol withdrawal is associated with increased glutamatergic activity [98]. Along with the long-term ethanol exposure evoked structural changes in NMDARs, an increased expression of the C1 and C2’ cassette containing splice variant forms of the NR1 as well as the NR2B subunits contributes to the elevated function of these receptors [140, 151, 152]. Although, only few of the novel NMDAR antagonists have been examined in animal models of alcoholism, they were found effective with encouraging in vitro and – according to the available preliminary data, in vivo efficiency. Since “classic” NMDAR antagonists reduce hyperactivity, seizures, and neuronal cell loss as well as restore normal brain levels of glutamate and DA associated with ethanol withdrawal, NMDAR antagonists may have a role in the pharmacotherapy of withdrawal symptoms as well as in the prevention of relapse and maintenance of abstinence. To prove that the novel NMDAR antagonists, including the NR2B subunit selective ones are positively useful in the pharmacotherapy for alcoholism is a major challenge for the forthcoming years.
REFERENCES
- 1.Allgaier C, Franke H, Sobottka H, Scheibler P. Acamprosate inhibits Ca2+ influx mediated by NMDA receptors and voltage-sensitive Ca2+ channels in cultured rat mesencephalic neurones. Naunyn Schmiedeber. Arch Pharmacol. 2000;362:440–443. doi: 10.1007/s002100000285. [DOI] [PubMed] [Google Scholar]
- 2.Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 3.alQatari M, Bouchenafa O, Littleton J. Mechanism of action of acamprosate. Part II. Ethanol dependence modifies effects of acamprosate on NMDA receptor binding in membranes from rat cerebral cortex. Alcohol Clin Exp Res. 1998;22:810–814. [PubMed] [Google Scholar]
- 4.alQatari M, Khan S, Harris B, Littleton J. Acamprosate is neuroprotective against glutamate-induced excitotoxicity when enhanced by ethanol withdrawal in neocortical cultures of fetal rat brain. Alcohol Clin Exp Res. 2001;25:1276–1283. doi: 10.1097/00000374-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephospho-rylation and ethanol inhibition of N-Methyl-D-aspartate receptor function. J Biol Chem. 2003;278:11020–11025. doi: 10.1074/jbc.M210167200. [DOI] [PubMed] [Google Scholar]
- 6.AmericanPsychiatric Association Fourth Edition. Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 7.Anders DL, Blevins T, Sutton G, Chandler LJ, Woodward JJ. Effects of c-Src tyrosine kinase on ethanol sensitivity of recombinant NMDA receptors expressed in HEK 293 cells. Alcohol Clin Exp Res. 1999;23:357–362. [PubMed] [Google Scholar]
- 8.Anders DL, Blevins T, Smothers CT, Woodward JJ. Reduced ethanol inhibition of N-methyl-D-aspartate receptors by deletion of the NR1 C0 domain or overexpression of alpha-actinin-2 proteins. J Biol Chem. 2000;275:5019–5024. doi: 10.1074/jbc.275.20.15019. [DOI] [PubMed] [Google Scholar]
- 9.Barnes CA ALCOHOLISM. SOCIETY FOR NEURO-SCIENCE Brain Briefings, (www.sfn.org/briefings). 2005
- 10.Becker HC, Veatch LM, Diaz-Granados JL. Repeated ethanol withdrawal experience selectively alters sensitivity to different chemoconvulsant drugs in mice. Psychopharmacology (Berl) 1998;139:145–153. doi: 10.1007/s002130050699. [DOI] [PubMed] [Google Scholar]
- 11.Biala G, Kotlinska J. Blockade of the acquisition of ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol. 1999;34(2):175–182. doi: 10.1093/alcalc/34.2.175. [DOI] [PubMed] [Google Scholar]
- 12.Bienkowski P, Krzascik P, Koros E, Kostowski W, Scinska A, Danysz W. Effects of a novel uncompetitive NMDA receptor antagonist, MRZ 2/579 on ethanol self-administration and ethanol withdrawal seizures in the rat. Eur J Pharmacol. 2001;413:81–89. doi: 10.1016/s0014-2999(01)00743-9. [DOI] [PubMed] [Google Scholar]
- 13.Bisaga A, Popik P. In search of a new pharmacological treatment for drug and alcohol addiction: N-methyl-D-aspartate (NMDA) antagonists. Drug Alcohol Depend. 2000;59:1–15. doi: 10.1016/s0376-8716(99)00107-6. [DOI] [PubMed] [Google Scholar]
- 14.Blevins T, Mirshahi T, Woodward J. Increased agonist and antagonist sensitivity of N-methyl-D-aspartate stimulated calcium flux in cultured neurons following chronic ethanol exposure. Neurosci Lett. 1995;200:214–218. doi: 10.1016/0304-3940(95)12086-j. [DOI] [PubMed] [Google Scholar]
- 15.Blevins T, Mirshahi T, Chandler LJ, Woodward JJ. Effects of acute and chronic ethanol exposure on heteromeric N-methyl-D-aspartate receptors expressed in HEK 293 cells. J Neurochem. 1997;69:2345–2354. doi: 10.1046/j.1471-4159.1997.69062345.x. [DOI] [PubMed] [Google Scholar]
- 16.Boismare F, Daoust M, Moore N, Saligaut C, Lhuintre JP, Chretien P, Durlach J. A homotaurine derivative reduces the voluntary intake of ethanol by rats: are cerebral GABA receptors involved? Pharmac Biochem Behav. 1984;21:787–789. doi: 10.1016/s0091-3057(84)80020-9. [DOI] [PubMed] [Google Scholar]
- 17.Bolo N, Nedelec JF, Muzet M, De-Witte P, Dahchour A, Durbin P, Macher JP. Central effects of acamprosate: part 2. Acamprosate modifies the brain in-vivo proton magnetic resonance spectrum in healthy young male volunteers. Psychiatry Res. 1998;82:115–127. doi: 10.1016/s0925-4927(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 18.Borza I, Kolok S, Gere A, Agai-Csongor E, Agai B, Tarkanyi G, Horvath C, Barta-Szalai G, Bozo E, Kiss C, Bielik A, Nagy J, Farkas S, Domany G. Indole-2-carboxamides as novel NR2B selective NMDA receptor antagonists. Bioorg Med Chem Lett. 2003;13:3859–3861. doi: 10.1016/s0960-894x(03)00708-x. [DOI] [PubMed] [Google Scholar]
- 19.Boyce S, Wyatt A, Webb JK, O’Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38:611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 20.Breese GR, Knapp DJ, Moy SS. Integrative role for serotonergic and glutamatergic receptor mechanisms in the action of NMDA antagonists: potential relationships to antipsychotic drug actions on NMDA antagonist responsiveness. Neurosci Biobehav Rev. 2002;26:441–455. doi: 10.1016/s0149-7634(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 21.Brimecombe JC, Boeckman FA, Aizenman E. Functional consequences of NR2 subunit composition in single recombinant N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA. 1997;94:11019–11024. doi: 10.1073/pnas.94.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- 23.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- 24.Buller AL, Larson HC, Morrisett RA, Monaghan DT. Glycine modulates ethanol inhibition of heteromeric N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol. 1995;48:717–723. [PubMed] [Google Scholar]
- 25.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 26.Calton JL, Wilson,,W.A., Moore SD. Reduction of voltage-dependent currents by ethanol contributes to inhibition of NMDA receptor-mediated excitatory synaptic transmission. Brain Res. 1999;816:142–148. doi: 10.1016/s0006-8993(98)01144-5. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic Ethanol Induces Synaptic But Not Extrasynaptic Targeting of NMDA Receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter AJ. Many agents that antagonize the NMDA receptor-channel complex in vivo also cause disturbances of motor coordination. J Pharmacol Exp Ther. 1994;269:573–580. [PubMed] [Google Scholar]
- 29.Carter RB, Wilent W, Huber M, Xu Z, Vanover KE, Woodward RM. Anti-allodynic and anti-hyperalgesic effects of the nr2b subunit-selective nmda antagonist ci-1041 in rat models of inflammation and neuropathy. Soc Neurosci Abstr. 2000;26:6173. [Google Scholar]
- 30.Cebere A, Cebers G, Liljequist S. Enhancement of NMDA-induced functional responses without concomitant NMDA receptor changes following chronic ethanol exposure in cerebellar granule cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:623–632. doi: 10.1007/s002109900133. [DOI] [PubMed] [Google Scholar]
- 31.Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363–370. [PubMed] [Google Scholar]
- 32.Chen C, Leonard JP. Protein tyrosine kinase-mediated potentiation of currents from cloned NMDA receptors. J Neurochem. 1996;67:194–200. doi: 10.1046/j.1471-4159.1996.67010194.x. [DOI] [PubMed] [Google Scholar]
- 33.Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, Ducat MF, Dumont ML, Fox CB, Mena EE, et al. (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J Med Chem. 1995;38:3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- 34.Chenard BL, Menniti FS. Antagonists selective for NMDA receptors containing the NR2B subunit. Curr Pharm Des. 1999;5:381–404. [PubMed] [Google Scholar]
- 35.Chizh BA, Headley PM, Tzschentke TM. NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol Sci. 2001;22:636–642. doi: 10.1016/s0165-6147(00)01863-0. [DOI] [PubMed] [Google Scholar]
- 36.Chizh BA. Novel approaches to targeting glutamate receptors for the treatment of chronic pain: Rev article Amino Acids. 2002;23:169–176. doi: 10.1007/s00726-001-0124-4. [DOI] [PubMed] [Google Scholar]
- 37.Christine CW, Choi DW. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990;10:108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu B, Anantharam V, Treistman SN. Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J Neurochem. 1995;65:140–148. doi: 10.1046/j.1471-4159.1995.65010140.x. [DOI] [PubMed] [Google Scholar]
- 39.Costa ET, Savage DD, Valenzuela CF. Acute effects of ethanol on kainate receptors in cultured hippocampal neurons. Alcohol Clin Exp Res. 2000;24:706–715. [PubMed] [Google Scholar]
- 40.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 41.Dahchour A, De Witte P, Bolo N, Nedelec JF, Muzet M, Durbin P, Macher JP. Central effects of acamprosate: part 1. Acamprosate blocks the glutamate increase in the nucleus accumbens microdialysate in ethanol withdrawn rats. Psychiatry Res. 1998;82:107–114. doi: 10.1016/s0925-4927(98)00016-x. [DOI] [PubMed] [Google Scholar]
- 42.Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- 43.Dahchour A, De Witte P. Ethanol and amino acids in the central nervous system: assessment of the pharmacological actions of acamprosate. Pro Neurobiol. 2000;60:343–362. doi: 10.1016/s0301-0082(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 44.Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- 45.Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacological Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- 46.Darstein MB, Landwehrmeyer GB, Feuerstein TJ. Changes in NMDA receptor subunit gene expression in the rat brain following withdrawal from forced long-term ethanol intake. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:206–213. doi: 10.1007/s002109900180. [DOI] [PubMed] [Google Scholar]
- 47.Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- 48.Di Chiara G, Tanda G, Cadoni C, Aquas E, Bassareo V, Carboni E. Homologies and differences in the action of drugs of abuse and a conventional reinforcer (food) on dopamine transmission: an interpretative framework of the mechanism of drug dependenc. Adv Pharmacol. 1998;42:983–987. doi: 10.1016/s1054-3589(08)60911-4. [DOI] [PubMed] [Google Scholar]
- 49.Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci USA. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dildy JE, Leslie SW. Ethanol inhibits NMDA-induced increases in free intracellular Ca2+ in dissociated brain cells. Brain Res. 1989;499:383–387. doi: 10.1016/0006-8993(89)90789-0. [DOI] [PubMed] [Google Scholar]
- 51.Engberg G, Hajos M. Ethanol attenuates the response of locus coeruleus neurons to excitatory amino acid agonists in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:222–226. doi: 10.1007/BF00165740. [DOI] [PubMed] [Google Scholar]
- 52.Engberg G, Hajos M. Alcohol withdrawal reaction as a result of adaptive changes of excitatory amino acid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:437–441. doi: 10.1007/BF00171087. [DOI] [PubMed] [Google Scholar]
- 53.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 54.Ferrani-Kile K, Randall PK, Leslie SW. Acute ethanol affects phosphorylation state of the NMDA receptor complex: implication of tyrosine phosphatases and protein kinase A. Brain Res Mol Brain Res. 2003;115:78–86. doi: 10.1016/s0169-328x(03)00186-4. [DOI] [PubMed] [Google Scholar]
- 55.Ferreira VM, Frausto S, Browning MD, Savage DD, Morato GS, Valenzuela CF. Ionotropic glutamate receptor subunit expression in the rat hippocampus: lack of an effect of a long-term ethanol exposure paradigm. Alcohol Clin Exp Res. 2001;25:1536–1541. [PubMed] [Google Scholar]
- 56.Fidecka S, Langwinski R. Interaction between ketamine and ethanol in rats and mice. Pol J Pharmacol Pharm. 1989;41:23–32. [PubMed] [Google Scholar]
- 57.Fillhard JA, Kinsora JJ, Meltzer LT. The effects of CI-1041 in two tests of analgesia: acetic acid-induced writing test and formalin foot pad test. Soc Neurosci Abstr. 2000;26:617–4. [Google Scholar]
- 58.Fink K, Göthert M. Both ethanol and ifenprodil inhibit NMDA-evoked release of various neurotransmitters at different, yet proportional potency: potential relation to NMDA receptor subunit composition. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:312–319. doi: 10.1007/BF00171062. [DOI] [PubMed] [Google Scholar]
- 59.Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- 60.Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- 61.Follesa P, Ticku MK. NMDA receptor upregulation: molecular studies in cultured mouse cortical neurons after chronic antagonist exposure. J Neurosci. 1996;16:2172–2178. doi: 10.1523/JNEUROSCI.16-07-02172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Follesa P, Ticku MK. Chronic ethanol-mediated up-regulation of the N-methyl-D-aspartate receptor polypeptide subunits in mouse cortical neurons in culture. J Biol Chem. 1996;271:13297–13299. doi: 10.1074/jbc.271.23.13297. [DOI] [PubMed] [Google Scholar]
- 63.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367(6464):607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 64.Freund G, Anderson KJ. Glutamate receptors in the frontal cortex of alcoholics. Alcohol Clin Exp Res. 1996;20:1165–1172. doi: 10.1111/j.1530-0277.1996.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 65.Fuller RK, Branchey L, Brightwell DR, Derman RM, Emrick CD, Iber FL, James KE, Lacoursiere RB, Lee KK, Lowenstam I. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA. 1986;256:1449–1455. [PubMed] [Google Scholar]
- 66.Gallagher MJ, Huang H, Pritchett DB, Lynch DR. Interactions between Ifenprodil and the NR2B Subunit of the N-Methyl-D-aspartate Receptor. J Biol Chem. 1996;271:9603–9611. doi: 10.1074/jbc.271.16.9603. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez LP, Veatch LM, Ticku MK, Becker HC. Alcohol withdrawal kindling: mechanisms and implications for treatment. Alcohol Clin Exp Res. 2001;25:197S–201S. doi: 10.1097/00000374-200105051-00032. [DOI] [PubMed] [Google Scholar]
- 68.Grant KA, Woolverton WL. Reinforcing and discriminative stimulus effects of Ca-acetyl homotaurine in animals. Pharmac Biochem Behav. 1989;32:607–611. doi: 10.1016/0091-3057(89)90005-1. [DOI] [PubMed] [Google Scholar]
- 69.Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- 70.Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- 71.Grant KA, Colombo G, Grant J, Rogawski MA. Dizocilpine-like discriminative stimulus effects of low-affinity uncompetitive NMDA antagonists. Neuropharmacology. 1996;35:1709–1719. doi: 10.1016/s0028-3908(96)00147-5. [DOI] [PubMed] [Google Scholar]
- 72.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Hardy PA, Chen W, Wilce PA. Chronic ethanol exposure and withdrawal influence NMDA receptor subunit and splice variant mRNA expression in the rat cerebral cortex. Brain Res. 1999;819:33–39. doi: 10.1016/s0006-8993(98)01340-7. [DOI] [PubMed] [Google Scholar]
- 74.Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Harris RA, Valenzuela CF, Brozowski S, Chuang L, Hadingham K, Whiting PJ. Adaptation of gamma-aminobutyric acid type A receptors to alcohol exposure: studies with stably transfected cells. J Pharmacol Exp Ther. 1998;284:180–188. [PubMed] [Google Scholar]
- 76.Harris BR, Prendergast MA, Gibson DA, Rogers DT, Blanchard JA, Holley RC, Fu MC, Hart SR, Pedigo NW, Littleton JM. Acamprosate inhibits the binding and neurotoxic effects of trans-ACPD, suggesting a novel site of action at metabotropic glutamate receptors. Alcohol Clin Exp Res. 2002;26:1779–1793. doi: 10.1097/01.ALC.0000042011.99580.98. [DOI] [PubMed] [Google Scholar]
- 77.Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart SR, Scotland RL, Foster TC, Pedigo NW, Littleton JM. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2003;27:1724–1735. doi: 10.1097/01.ALC.0000093601.33119.E3. [DOI] [PubMed] [Google Scholar]
- 78.Henderson G, Morton J, Little H. Drug abuse: from gene through cell to behaviour. Curr Opin Pharmacol. 2005;5:1–3. [Google Scholar]
- 79.Henniger MS, Wotjak CT, Holter SM. Long-term voluntary ethanol drinking increases expression of NMDA receptor 2B subunits in rat frontal cortex. Eur J Pharmacol. 2003;470:33–36. doi: 10.1016/s0014-2999(03)01787-4. [DOI] [PubMed] [Google Scholar]
- 80.Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- 81.Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem. 1989;52:61937–61940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- 82.Hoffman PL, Rabe CS, Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol and the NMDA receptor. Alcohol. 1990;7:229–231. doi: 10.1016/0741-8329(90)90010-a. [DOI] [PubMed] [Google Scholar]
- 83.Hoffman PL, Tabakoff B. Alcohol dependence: a commentary on mechanisms. Alcohol Alcohol. 1996;31:333–340. doi: 10.1093/oxfordjournals.alcalc.a008159. [DOI] [PubMed] [Google Scholar]
- 84.Hollmann M. In: Structure of ionotropic glutamate receptors. Jonas P, Monyer H, editors. Ionotropic Glutamate Receptors in the CNS. Springer; 1999. pp. 1–98. [Google Scholar]
- 85.Hölter SM, Danysz W, Spanagel R. Evidence for alcohol anti-craving properties of memantine. Eur J Pharmacol. 1996;314:R1–R2. doi: 10.1016/s0014-2999(96)00670-x. [DOI] [PubMed] [Google Scholar]
- 86.Hölter, SM., Landgraf R, Zieglgansberger W, Spanagel R. Time course of acamprosate action on operant ethanol self-administration after ethanol deprivation. Alcohol Clin Exp Res. 1997;21:862–868. [PubMed] [Google Scholar]
- 87.Hölter SM, Danysz W, Spanagel R. Novel Uncompetitive N-Methyl-D-Aspartate (NMDA)-Receptor Antagonist MRZ 2/579 Suppresses Ethanol Intake in Long-Term Ethanol-Experienced Rats and Generalizes to Ethanol Cue in Drug Discrimination Procedure. J Pharmacol Exp Ther. 2000;292:545–552. [PubMed] [Google Scholar]
- 88.Hougbol SR, Ebert B, Ulrichsen J. Upregulation of glutamate receptor subtypes during alcohol withdrawal in rats. Alcohol Alcohol. 40:89–95. doi: 10.1093/alcalc/agh117. [DOI] [PubMed] [Google Scholar]
- 89.Hu XJ, Follesa P, Ticku MK. Chronic ethanol treatment produces a selective upregulation of the NMDA receptor subunit gene expression in mammalian cultured cortical neurons. Brain Res Mol Brain Res. 1996;36:211–218. doi: 10.1016/0169-328x(95)00223-f. [DOI] [PubMed] [Google Scholar]
- 90.Hu XJ, Ticku MK. Functional characterization of a kindling-like model of ethanol withdrawal in cortical cultured neurons after chronic intermittent ethanol exposure. Brain Res. 1997;767:228–234. doi: 10.1016/s0006-8993(97)00581-7. [DOI] [PubMed] [Google Scholar]
- 91.Husi H, Grant SG. Isolation of 2000-kDa complexes of N-methyl-D-aspartate receptor and postsynaptic density 95 from mouse brain. J Neurochem. 2001;77:281–291. doi: 10.1046/j.1471-4159.2001.t01-1-00248.x. [DOI] [PubMed] [Google Scholar]
- 92.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion andits persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 93.Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- 94.Jansen M, Dannhardt G. Antagonists and agonists at the glycine site of the NMDA receptor for therapeutic interventions. Eur J Med Chem. 2003;38:661–670. doi: 10.1016/s0223-5234(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 95.Kalluri HS, Ticku MK. Effect of ethanol on phosphorylation of the NMDAR2B subunit in mouse cortical neurons. Brain Res Mol Brain Res. 1999;68:159–168. doi: 10.1016/s0169-328x(99)00057-1. [DOI] [PubMed] [Google Scholar]
- 96.Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- 97.Katayama S, Ae N, Nagata R. Synthesis of tricyclic indole-2-carboxylic [correction of caboxylic] acids as potent NMDA-glycine antagonists. J Org Chem. 2001;66:3474–3483. doi: 10.1021/jo010002h. [DOI] [PubMed] [Google Scholar]
- 98.Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5:1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- 99.Kew JN, Trube G, Kemp JA. State-dependent NMDA receptor antagonism by Ro 8-4304, a novel NR2B selective, non-competitive, voltage-independent antagonist. Br J Pharmacol. 1998;123:463–472. doi: 10.1038/sj.bjp.0701634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koek W, Colpaert FC. Selective blockade of N-methyl-D-aspartate (NMDA)-induced convulsions by NMDA antagonists and putative glycine antagonists: relationship with phencyclidine-like behavioral effects. J Pharmacol Exp Ther. 1990;252:349–357. [PubMed] [Google Scholar]
- 101.Kohr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol. 1996;492(Pt 2):445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- 103.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 104.Koob GF, Sanna PP, Bloom FE. Neurosci Addiction Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 105.Koob GF. Animal models in craving research. Animal models of craving for ethanol. Addiction. 2000;95(Suppl 2):S73–S81. doi: 10.1080/09652140050111663. [DOI] [PubMed] [Google Scholar]
- 106.Kotlinska J, Liljequist S. Oral administration of glycine and polyamine receptor antagonists blocks ethanol withdrawal seizures. Psychopharmacology (Berl) 1996;127:238–244. [PubMed] [Google Scholar]
- 107.Kotlinska J, Liljequist S. The NMDA/glycine receptor antagonist, L-701,324, produces discriminative stimuli similar to those of ethanol. Eur J Pharmacol. 1997;332:1–8. doi: 10.1016/s0014-2999(97)01069-8. [DOI] [PubMed] [Google Scholar]
- 108.Kotlinska J, Biala G, Rafalski P, Bochenski M, Danysz W. Effect of neramexane on ethanol dependence and reinforcement. Eur J Pharmacol. 2004;503:95–98. doi: 10.1016/j.ejphar.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 109.Kretschmer BD. Modulation of the mesolimbic dopamine system by glutamate: role of NMDA receptors. J Neurochem. 1999;73:839–848. doi: 10.1046/j.1471-4159.1999.0730839.x. [DOI] [PubMed] [Google Scholar]
- 110.Krystal JH, Tabakoff B. Davis KL, Charney DS, Coyle JT, Nemeroff C, editors. Ethanol abuse, dependence, and withdrawal: neurobiology and clinical implications Psychopharmacology: A Fifth Generation of Progress. Philadelphia: Lippincott Williams and Wilkins. 2002:1425–443. [Google Scholar]
- 111.Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 112.Kuner T, Schoepfer R, Korpi ER. Ethanol inhibits glutamate-induced currents in heteromeric NMDA receptor subtypes. Neuroreport. 1993;5:297–300. doi: 10.1097/00001756-199312000-00029. [DOI] [PubMed] [Google Scholar]
- 113.Lamblin F, Deuceuninck D, DeWitte P. Modulation of alcohol preference by NMDA antagonists in male rats. Alcohol Alcohol. 1993;28:639–647. [PubMed] [Google Scholar]
- 114.Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lerma J. Spermine regulates N-methyl-D-aspartate receptor desensitization. Neuron. 1992;8:343–352. doi: 10.1016/0896-6273(92)90300-3. [DOI] [PubMed] [Google Scholar]
- 116.Lhuintre JP, Daoust M, Moore ND, Chretien P, Saligaut C, Tran G, Boismare F, Hillemand B. Ability of calcium bis acetyl homotaurine, a GABA agonist, to prevent relapse in weaned alcoholics. Lancet. 1985;1:1014–1016. doi: 10.1016/s0140-6736(85)91615-0. [DOI] [PubMed] [Google Scholar]
- 117.Lhuintre JP, Moore N, Tran G, Steru L, Langrenon S, Daoust M, Parot P, Ladure P, Libert C, Boismare F, et al. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol Alcohol. 1990;25:613–622. doi: 10.1093/oxfordjournals.alcalc.a045057. [DOI] [PubMed] [Google Scholar]
- 118.Li C, Peoples RW, Weight FF. Alcohol action on a neuronal membrane receptor: evidence for a direct interaction with the receptor protein. Proc Natl Acad Sci USA. 1994;91:8200–8204. doi: 10.1073/pnas.91.17.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liljequist S. The competitive NMDA receptor antagonist, CGP 39551, inhibits ethanol withdrawal seizures. Eur J Pharmacol. 1991;192:197–198. doi: 10.1016/0014-2999(91)90092-5. [DOI] [PubMed] [Google Scholar]
- 120.Littleton J. Receptor regulation as a unitary mechanism for drug tolerance and physical dependence—not quite as simple as it seemed! Addiction. 2001;96:87–101. doi: 10.1046/j.1360-0443.2001.961877.x. [DOI] [PubMed] [Google Scholar]
- 121.Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- 122.Lovinger DM, White G. and Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 123.Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci. 1990;10:41372–41379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lovinger DM. Excitotoxicity and alcohol-related brain damage. Alcohol Clin Exp Res. 1993;17:19–27. doi: 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 125.Lovinger DM. Developmental decrease in ethanol inhibition of N-methyl-D-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J Pharmacol Exp Ther. 1995;274:164–172. [PubMed] [Google Scholar]
- 126.Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, Fienberg AA, Leslie SW, Gonzales RA, Morrisett RA. DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat Neurosci. 2002;5:641–648. doi: 10.1038/nn877. [DOI] [PubMed] [Google Scholar]
- 127.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 128.Malinowska B, Napiorkowska-Pawlak D, Pawlak R, Buczko W, Gothert M. Ifenprodil influences changes in mouse behaviour related to acute and chronic ethanol administration. Eur J Pharmacol. 1999;377:13–19. doi: 10.1016/s0014-2999(99)00393-3. [DOI] [PubMed] [Google Scholar]
- 129.Mason BJ. Treatment of alcohol-dependent outpatients with acamprosate: a clinical review. J Clin Psychiatry. 2001;62(Suppl 20):42–48. [PubMed] [Google Scholar]
- 130.Mason JN, Eshleman AJ, Belknap JK, Crabbe JC, Loftis JM, Macey TA, Janowsky A. NMDA receptor subunit mRNA and protein expression in ethanol-withdrawal seizure-prone and -resistant mice. Alcohol Clin Exp Res. 2001;25:651–660. [PubMed] [Google Scholar]
- 131.Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol Pharmacol. 1994;45:324–329. [PubMed] [Google Scholar]
- 132.Mayer S, Harris B, Gibson DA, Blanchard J, Prendergast MA, Holley RC, Littleton J. Acamprosate has no effect on NMDA-induced toxicity but reduces toxicity induced by spermidine or by changing the medium in organotypic hippocampal slice cultures from rat. Alcohol Clin Exp Res. 2002;26:655–662. [PubMed] [Google Scholar]
- 133.Mayer S, Harris BR, Gibson DA, Blanchard JA, Prendergast MA, Holley RC, Littleton J. Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcohol Clin Exp Res. 2002;26:1468–1478. doi: 10.1097/01.ALC.0000033261.14548.D2. [DOI] [PubMed] [Google Scholar]
- 134.Melichar JK, Daglish MRC, Nutt DJ. Addiction and withdrawal — current views. Curr Opin Pharmacol. 2001;1:84–90. doi: 10.1016/s1471-4892(01)00011-x. [DOI] [PubMed] [Google Scholar]
- 135.Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- 136.Mirshahi T, Woodward J. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NR1 Mg(2+)-insensitive mutants. J Neuropharmacol. 1995;34:347–355. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- 137.Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- 138.Mhatre MC, Gonzalez LP. Increased Ro15-4513-induced seizures following multiple ethanol withdrawals. Pharmacol Biochem Behav. 1999;63:93–99. doi: 10.1016/s0091-3057(98)00257-3. [DOI] [PubMed] [Google Scholar]
- 139.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 140.Monyer H, Sprengler R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 141.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 142.Morrisett RA, Rezvani AH, Overstreet D, Janowsky DS, Wilson WA, Swartzwelder HS. MK-801 potently inhibits alcohol withdrawal seizures in rats. Eur J Pharmacol. 1990;176:103–105. doi: 10.1016/0014-2999(90)90138-v. [DOI] [PubMed] [Google Scholar]
- 143.Morrow AL, Devaud LL, Bucci D, Smith FD. GABAA and NMDA receptor subunit mRNA expression in ethanol dependent rats. Alcohol Alcohol. 1994;2:89–95. [PubMed] [Google Scholar]
- 144.Mothet JP, Parent AT, Wolosker H, Brady RO, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Murata S, Kawasaki K. Common and uncommon behavioural effects of antagonists for different modulatory sites in the NMDA receptor/channel complex. Eur J Pharmacol. 1993;239:9–15. doi: 10.1016/0014-2999(93)90969-o. [DOI] [PubMed] [Google Scholar]
- 146.Murray F, Kennedy J, Hutson PH, Elliot J, Huscroft I, Mohnen K, Russell MG, Grimwood S. Modulation of [3H]MK-801 binding to NMDA receptors in vivo and in vitro. Eur J Pharmacol. 2000;397:263–270. doi: 10.1016/s0014-2999(00)00263-6. [DOI] [PubMed] [Google Scholar]
- 147.Naassila M, Daoust M. Effect of prenatal and postnatal ethanol exposure on the developmental profile of mRNAs encoding NMDA receptor subunits in rat hippocampus. J Neurochem. 2002;80:850–860. doi: 10.1046/j.0022-3042.2002.00755.x. [DOI] [PubMed] [Google Scholar]
- 148.Nagy J, Müller F, László L. Cytotoxic effect of alcohol-withdrawal on primary cultures of cortical neurones. Drug Alcohol Depend. 2001;61:155–162. doi: 10.1016/s0376-8716(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 149.Nagy J, László L. Increased sensitivity to NMDA is involved in alcohol-withdrawal induced cytotoxicity observed in primary cultures of cortical neurones chronically pre-treated with ethanol. Neurochem Int. 2002;40:585–591. doi: 10.1016/s0197-0186(01)00131-0. [DOI] [PubMed] [Google Scholar]
- 150.Nagy J, Kolok S, Dezs o P, Boros A, Szombathelyi Z. Differential alterations in the expression of NMDA receptor subunits following chronic ethanol treatment in primary cultures of rat cortical and hippocampal neurones. Neurochem Int. 2003;42:35–43. doi: 10.1016/s0197-0186(02)00062-1. [DOI] [PubMed] [Google Scholar]
- 151.Nagy J. Renaissance of NMDA receptor antagonists: Do they have a role in the pharmacotherapy for alcoholism? IDrugs: Invest Drugs J. 2004;7:339–350. [PubMed] [Google Scholar]
- 152.Nagy J. NR2B type of NMDA receptor subunits: a possible target in treatment for alcohol dependence. Curr Drug Targets CNS Neurol Disord. 2004;3:169–179. doi: 10.2174/1568007043337409. [DOI] [PubMed] [Google Scholar]
- 153.Nagy J, Horváth C, Farkas S, Kolok S, Szombathelyi Z. NR2B subunit selective NMDA antagonists inhibit neurotoxic effect of alcohol-withdrawal in primary cultures of rat cortical neurones. Neurochem Int. 2004;44:17–23. doi: 10.1016/s0197-0186(03)00100-1. [DOI] [PubMed] [Google Scholar]
- 154.Narita M, Soma M, Narita M, Mizoguchi H, Tseng L F, Suzuki T. Implications of the NR2B subunit-containing NMDA receptor localized in mouse limbic forebrain in ethanol dependence. Eur J Pharmacol. 2000;401:191–195. doi: 10.1016/s0014-2999(00)00428-3. [DOI] [PubMed] [Google Scholar]
- 155.Nash JE, Hill MP, Brotchie JM. Antiparkinsonian actions of blockade of NR2B-containing NMDA receptors in the reserpine-treated rat. Exp Neurol. 1999;155:42–48. doi: 10.1006/exnr.1998.6963. [DOI] [PubMed] [Google Scholar]
- 156.Nash JE, Fox SH, Henry B, Hill MP, Peggs D, McGuire S, Maneuf Y, Hille C, Brotchie JM, Crossman AR. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp Neurol. 2000;165:136–142. doi: 10.1006/exnr.2000.7444. [DOI] [PubMed] [Google Scholar]
- 157.Nestler EJ, Hope BT, Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- 158.Nestler EJ. Cellular responses to chronic treatment with drugs of abuse. Crit Rev Neurobiol. 1993;7:23–39. [PubMed] [Google Scholar]
- 159.Nestler EJ, Aghajanian GK. Molecular and Cellular Basisof Addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 160.Nestler EJ. Molecular basis of long-lived neural plasticity to drugs of abuse. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 161.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 162.Nikam SS, Meltzer LT. NR2B selective NMDA receptor antagonists. Curr Pharmaceut Des. 2002;8:845–855. doi: 10.2174/1381612024607072. [DOI] [PubMed] [Google Scholar]
- 163.Okabe S, Collin C, Auerbach JM, Meiri N, Bengzon J, Kennedy MB, Segal M, McKay RD. Hippocampal synaptic plasticity in mice overexpressing an embryonic subunit of the NMDA receptor. J Neurosci. 1998;18:4177–4188. doi: 10.1523/JNEUROSCI.18-11-04177.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Parsons CG, Danysz W, Hesselink M, Hartmann S, Lorenz B, Wollenburg C, Quack G. Modulation of NMDA receptors by glycine--introduction to some basic aspects and recent developments. Amino-Acids. 1998;14:207–216. doi: 10.1007/BF01345264. [DOI] [PubMed] [Google Scholar]
- 165.Parsons CG, Danysz W, Bartmann A, Spielmanns P, Frankiewicz T, Hesselink M, Eilbacher B, Quack G. Amino-alkyl-cyclohexanes are novel uncompetitive NMDA receptor antagonists with strong voltage-dependency and fast blocking kinetics: in vitro and in vivo characterization. Neuropharmacology. 1999;38:85–108. doi: 10.1016/s0028-3908(98)00161-0. [DOI] [PubMed] [Google Scholar]
- 166.Peoples RW, Weight FF. Cutoff in potency implicates alcohol inhibition of N-methyl-D-aspartate receptors in alcohol intoxication. Proc Natl Acad Sci USA. 1995;92:2825–2829. doi: 10.1073/pnas.92.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peoples RW, White G, Lovinger DM, Weight FF. Ethanol inhibition of N-methyl-D-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br J Pharmacol. 1997;122:1035–1042. doi: 10.1038/sj.bjp.0701483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer J S, Sucher NJ, Heinemann SF. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci. 2001; 21:1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Piasecki J, Koros E, Dyr W, Kostowski W, Danysz W, Bienkowski P. Ethanol-reinforced behaviour in the rat: effects of uncompetitive NMDA receptor antagonist, memantine. Eur J Pharmacol. 1998;354:135–143. doi: 10.1016/s0014-2999(98)00442-7. [DOI] [PubMed] [Google Scholar]
- 170.Popp RL, Lickteig R, Browning MD, Lovinger DM. Ethanol sensitivity and subunit composition of NMDA receptors in cultured striatal neurons. Neuropharmacology. 1998;37:45–56. doi: 10.1016/s0028-3908(97)00186-x. [DOI] [PubMed] [Google Scholar]
- 171.Popp RL, Lovinger DM. Interaction of acamprosate with ethanol and spermine on NMDA receptors in primary cultured neurons. Eur J Pharmacol. 2000;394:221–231. doi: 10.1016/s0014-2999(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 172.Premkumar LS, Auerbach AJ. Subconductance states of a mutant NMDA receptor channel kinetics, calcium, and voltage dependence. Gen Physiol. 1997;110:485–502. doi: 10.1085/jgp.109.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Prendergast MA, Harris BR, Mullholland PJ, Blanchard Ii JA, Gibson DA, Holley RC, Littleton JM. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of n-methyl-d-aspartate receptors. Neuroscience. 2004;124:869–877. doi: 10.1016/j.neuroscience.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 174.Quartaroli M, Fasdelli N, Bettelini L, Maraia G, Corsi M. GV196771A, an NMDA receptor/glycine site antagonist, attenuates mechanical allodynia in neuropathic rats and reduces tolerance induced by morphine in mice. Eur J Pharmacol. 2001;430:219–227. doi: 10.1016/s0014-2999(01)01278-x. [DOI] [PubMed] [Google Scholar]
- 175.Rameau AG, Akaneya Y, Chiu LY, Ziff EB. Role of NMDA receptor functional domains in excitatory cell death. Neuropharmacology. 2000;39:2255–2266. doi: 10.1016/s0028-3908(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 176.Rammes G, Mahal B, Putzke J, Parsons C, Spielmanns P, Pestel E, Spanagel R, Zieglgansberger W, Schadrack J. The anti-craving compound acamprosate acts as a weak NMDA-receptor antagonist, but modulates NMDA-receptor subunit expression similar to memantine and MK-801. Neuropharma-cology. 2001;40:749–760. doi: 10.1016/s0028-3908(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 177.Ravindran CRM, Ticku MK. Role of CpG islands in the up-regulation of NMDA receptor NR2B gene expression following chronic ethanol treatment of cultured cortical neurons of mice. Neurochem Int. 2005;46:313–327. doi: 10.1016/j.neuint.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 178.Ripley TL, Little HJ. Effects on ethanol withdrawal hyperexcitability of chronic treatment with a competitive N-methyl-D-aspartate receptor antagonist. J Pharmacol Exp Ther. 1995;272:112–118. [PubMed] [Google Scholar]
- 179.Risinger FO, Freeman PA, Greengard P, Fienberg AA. Motivational effects of ethanol in DARPP-32 knock-out mice. J Neurosci. 2001;21(1):340–348. doi: 10.1523/JNEUROSCI.21-01-00340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rock DM, Macdonald RL. The polyamine spermine has multiple actions on N-methyl-D-aspartate receptor single-channel currents in cultured cortical neurons. Mol Pharmacol. 1992;41:83–88. [PubMed] [Google Scholar]
- 181.Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem. 2001;276:44729–44735. doi: 10.1074/jbc.M102800200. [DOI] [PubMed] [Google Scholar]
- 182.Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- 183.Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283(1-3):177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- 184.Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93:1135–1140. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- 185.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 186.Rudolph JG, Walker DW, Iimuro Y, Thurman RG, Crews FT. NMDA receptor binding in adult rat brain after several chronic ethanol treatment protocols. Alcohol Clin Exp Res. 1997;21:1508–1519. [PubMed] [Google Scholar]
- 187.Rumbaugh G, Prybylowski K, Wang JF, Vicini S. Exon 5 and spermine regulate deactivation of NMDA receptor subtypes. J Neurophysiol. 2000;83(3):1300–1306. doi: 10.1152/jn.2000.83.3.1300. [DOI] [PubMed] [Google Scholar]
- 188.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 189.Schorge S, Colquhoun D. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci. 2003;23:1151–1158. doi: 10.1523/JNEUROSCI.23-04-01151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shelton KL, Balster RL. Effects of gamma-aminobutyric acid agonists and N-methyl-D-aspartate antagonists on a multiple schedule of ethanol and saccharin self-administration in rats. J Pharmacol Exp Ther. 1997;280:1250–1260. [PubMed] [Google Scholar]
- 191.Smothers CT, Mrotek JJ, Lovinger DM. Chronic ethanol exposure leads to a selective enhancement of N-methyl-D-aspartate receptor function in cultured hippocampal neurons. J Pharmacol Exp Ther. 1997;283:1214–1222. [PubMed] [Google Scholar]
- 192.Smothers CT, Clayton R, Blevins T, Woodward JJ. Ethanol sensitivity of recombinant human N-methyl-D-aspartate receptors. Neurochem Int. 2001;38(4):333–340. doi: 10.1016/s0197-0186(00)00094-2. [DOI] [PubMed] [Google Scholar]
- 193.Smothers CT, Woodward JJ. Effect of the NR3 subunit on ethanol inhibition of recombinant NMDA receptors. Brain Res. 2003;987:117–121. doi: 10.1016/s0006-8993(03)03315-8. [DOI] [PubMed] [Google Scholar]
- 194.Snell LD, Bhave SV, Tabakoff B, Hoffman PL. Chronic ethanol exposure delays the ‘developmental switch’ of the NMDA receptor 2A and 2B subunits in cultured cerebellar granule neurons. J Neurochem. 2001;78:396–405. doi: 10.1046/j.1471-4159.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 195.Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Spanagel R, Holter SM, Allingham K, Landgraf R, Zieglgansberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- 197.Spanagel R, Putzke J, Stefferl A, Schobitz B, Zieglgansberger W. Acamprosate and alcohol: II. Effects on alcohol withdrawal in the rat. Eur J Pharmacol. 1996;305:45–50. doi: 10.1016/0014-2999(96)00175-6. [DOI] [PubMed] [Google Scholar]
- 198.Spanagel R, Zieglgansberger W, Hundt W. Acamprosate and alcohol: III. Effects on alcohol discrimination in the rat. Eur J Pharmacol. 1996;305(1-3):51–56. doi: 10.1016/0014-2999(96)00176-8. [DOI] [PubMed] [Google Scholar]
- 199.Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18:54–59. [PubMed] [Google Scholar]
- 200.Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT. Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. Exp Neurol. 2000;163:239–243. doi: 10.1006/exnr.2000.7374. [DOI] [PubMed] [Google Scholar]
- 201.Stork O, Kojima N, Stork S, Kume N, Obata K. Resistance to alcohol withdrawal-induced behaviour in Fyn transgenic mice and its reversal by ifenprodil. Brain Res Mol Brain Res. 2002;105:126–135. doi: 10.1016/s0169-328x(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 202.Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proc Natl Acad Sci USA. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Taniguchi K, Shinjo K, Mizutani M, Shimada K, Ishikawa T, Menniti FS, Nagahisa A. Antinociceptive activity of CP-101,606, an NMDA receptor NR2B subunit antagonist Br. J Pharmacol. 1997;122:809–812. doi: 10.1038/sj.bjp.0701445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Thomas MP, Morrisett RA. Dynamics of NMDAR-mediated neurotoxicity during chronic ethanol exposure and withdrawal. Neuropharmacology. 2000;39:218–226. doi: 10.1016/s0028-3908(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 205.Thomson AM, West DC, Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985;313:479–81. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- 206.Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- 207.Trevisan L, Fitzgerald LW, Brose N, Gasic GP, Heinemann SF, Duman RS, Nestler EJ. Chronic ingestion of ethanol up-regulates NR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62:1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- 208.Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. Am J Psychiatry. 1995;152:332–340. doi: 10.1176/ajp.152.3.332. [DOI] [PubMed] [Google Scholar]
- 209.Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- 210.Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 211.Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 212.Whittington MA, Little HJ. Changes in voltage-operated calcium channels modify ethanol withdrawal hyperexcitability in mouse hippocampal slices. Exp Physiol. 1993;78:347–370. doi: 10.1113/expphysiol.1993.sp003690. [DOI] [PubMed] [Google Scholar]
- 213.Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- 214.Winkler A, Mahal B, Kiianmaa K, Zieglgansberger W, Spanagel R. Effects of chronic alcohol consumption on the expression of different NR1 splice variants in the brain of AA and ANA lines of rats. Mol. Brain Res. 1999;72:166–175. doi: 10.1016/s0169-328x(99)00218-1. [DOI] [PubMed] [Google Scholar]
- 215.Wirkner K, Poelchen W, Koles L, Muhlberg K, Scheibler P, Allgaier C, Illes P. Ethanol-induced inhibition of NMDA receptor channels. Neurochem Int. 1999;35:153–162. doi: 10.1016/s0197-0186(99)00057-1. [DOI] [PubMed] [Google Scholar]
- 216.De Witte P, Pinto E, Ansseau M, Verbanck P. Alcohol and withdrawal: from animal research to clinical issues. Neurosci Biobehav Rev. 2003;27:189–197. doi: 10.1016/s0149-7634(03)00030-7. [DOI] [PubMed] [Google Scholar]
- 217.De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. 2005;19:517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- 218.Woodward JJ. Overview of the effects of alcohol on the cerebral nervous system. Neurochem Int. 1999;35(2):93–94. [PubMed] [Google Scholar]
- 219.Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- 220.Wright JL, Gregory TF, Kesten SR, Boxer PA, Serpa KA, Meltzer LT, Wise LD, Espitia SA, Konkoy CS, Whittemore ER, Woodward RM. Parallel synthesis of a series of subtype-selective NMDA receptor antagonists. J Med Chem. 2000;43:3408–3419. doi: 10.1021/jm000023o. [DOI] [PubMed] [Google Scholar]
- 221.Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci. 2003;23(9):3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute Received: 02 May, 2005 Accepted: 18 July, 2005 ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003;27:1736–1742. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yenari MA, Bell TE, Kotake AN, Powell M, Steinberg GK. Dose escalation safety and tolerance study of the competitive NMDA antagonist selfotel (CGS 19755) in neurosurgery patients. Clin Neuropharmacol. 1998;21:28–34. [PubMed] [Google Scholar]
- 224.Zhang J, Chiodo LA, Freeman AS. Electrophysiological effects of MK-801 on rat nigrostriatal and mesoaccumbal dopaminergic neurons. Brain Res. 1992;590:153–163. doi: 10.1016/0006-8993(92)91091-r. [DOI] [PubMed] [Google Scholar]
- 225.Zhou ZL, Cai SX, Whittemore ER. 4-Hydroxy-1-[2-(4-hydroxyphenoxy)ethyl]-4-(4-methylbenzyl)piperidine: a novel, potent, and selective NR1/2B NMDA receptor antagonist. J Med Chem. 1999;42:2993–3000. doi: 10.1021/jm990246i. [DOI] [PubMed] [Google Scholar]
- 226.Zieglgansberger W, Hauser C, Putzke J, Spanagel R, Wetzel C. The enhanced excitability of central neurons following chronic alcohol intake in reduced by acamprosate. Alcohol Alcohol. 1995;30:552. [Google Scholar]


