Abstract
DNA-damage checkpoints sense and respond to genomic damage. Human Rad9 (hRad9), an evolutionarily conserved gene with multiple functions for preserving genomic integrity, plays multiple roles in fundamental biological processes, including the regulation of the DNA damage response, cell cycle checkpoint control, DNA repair, apoptosis, transcriptional regulation, exonuclease activity, ribonucleotide synthesis and embryogenesis. This review examines work that provides significant insight into the molecular mechanisms of several individual cellular processes which might be beneficial for developing novel therapeutic approaches to cancerous diseases with genomic instability.
Key Words: DNA damage, replication, checkpoint, cancer
INTRODUCTION
The human genome is exposed to variety of genotoxins which can elicit unscheduled replication. After DNA is damaged or replication is perturbed, cells respond by activation of evolutionarily conserved signal transduction pathways that delay cell cycle progression and induce repair of the damaged DNA. These signal transduction pathways include protein sensors that recognize aberrant DNA structures and activate kinases, thereby inducing phosphorylation cascades that ultimately lead to cell cycle arrest and DNA repair [1–4]. Failure of this cell cycle surveillance mechanism can cause genomic instability that eventually leads to cancer formation in mammals [5, 6].
hRad9 protein is the human homologue of Schizosaccharomyces pombe Rad9. Rad9 is a member of the checkpoint rad genes (rad1+, rad3+, rad9+, rad17+, rad26+ and hus1+) that are required for the S phase (DNA replication) and G2 (DNA mitosis) checkpoints [7–9]. Multifunctional participation of hRad9 has been demonstrated in the DNA damage sensor as the 9-1-1 complex not only in checkpoint signaling, but also in DNA repair via DNA polymerase β [10] or flap endonuclease 1 (FEN1) [11], and in apoptosis via potential binding to Bcl-2 or Bcl-xl [12, 13].
In this article, we note that hRad9 takes many roles including the regulation of the DNA damage response, cell cycle checkpoint control, DNA repair, apoptosis, transcriptional regulation, exonuclease activity, ribonucleotide synthesis and embryogenesis. Thus hRad9 preserves genomic integrity in many ways (Fig. (1)).
Fig. (1).
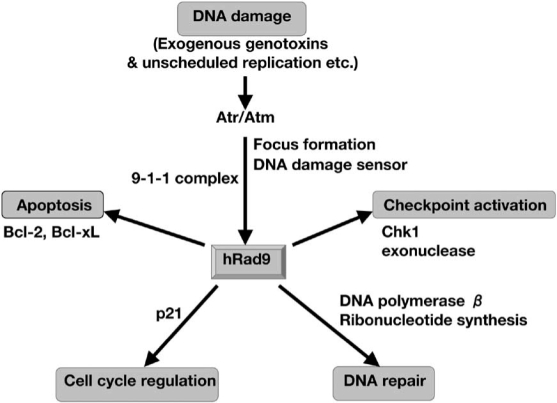
Multiple functions of hRad9 in DNA damage response. After DNA is damaged by exogenous genotoxins, unscheduled replication etc, the damage is sensed with Atr/Atm or foci of γ-H2AX and 9-1-1 complex. Rad9 regulates the P21 expression, supposed to be involved in G1/S checkpoint. The phosphorylation of C-terminus of Rad9 plays a role in the activation of Chk1, and the activation of Chk1 induces the stimulation of the G2/M checkpoint. Under the checkpoint activation, Rad9 is involved in DNA repair through DNA polymerase β activity and ribonucleotide synthesis. A termination of the Chk1 arrest may elicit the induction of apoptosis [49], or Rad9 participates in induction of apoptosis interacting with Bcl-2 and Bcl-xL.
DNA DAMAGE SENSOR
Cell cycle checkpoints are signal transduction pathways that maintain the proper order of cell cycle events. As was γ-H2AX is an important marker for detection of double-strand breaks [14] and has been used for evaluating DNA repair dynamics [15]. In mammalian cells, a signal initiated by two phosphatidylinositol 3-kinase-related kinases (PIKK), ataxia-telangiectasia mutated (Atm) and ataxia-telangiectasia mutated and Rad3-related (Atr), plays a central role in the checkpoint signaling pathways [16]. Atm and Atr are activated by genotoxins and phosphorylate downstream signaling proteins, including Chk1 and Chk2, two protein kinases that regulate checkpoint responses [17–19].
Like its yeast counterpart, hRad9 forms a ring-shaped, heterotrimeric complex with the hRad1 and hHus1 proteins [20, 21]. Each member of the hRad9-hRad1-hHus1 complex (known as the 9-1-1 complex) shares sequence homology with proliferating cell nuclear antigen (PCNA), a homo-trimer that encircles DNA and tethers DNA polymerase δ during DNA synthesis [21–25]. Hus1 and Rad1 are involved in checkpoint activation [21–27]. PCNA is loaded onto DNA by pentameric protein complex replication factor C (Rfc) [28], which is composed of one large subunit and four smaller subunits. Previous study indicates that the 9-1-1 complex is loaded onto DNA by a complex between hRad17 and the four small subunits of Rfc [26] and forms a DNA-damage focus, which acts to repair damage [26, 27, 29]. Since DNA damage induces hRad17-dependent association of 9-1-1 complex with chromatin, the 9-1-1 complex is believed to be involved in the direct recognition of DNA lesions during the initial stages of the checkpoint response. The 9-1-1 complex may associate with chromatin after DNA damage to transduce signals for DNA damage-activated checkpoint signaling pathways [27]. The C-terminal region of the hRad9 protein acts to transport the 9-1-1 complex into the nucleus [29]. hRad9 and Atm rapidly colocalize to regions containing DNA double-strand breaks after DNA damage [30, 31], and Atm can phosphorylate Rad9 [32]. hRad9 is phosphorylated directly by Atm at Ser-272 during ionizing radiation (IR)-induced G1/S checkpoint activation [32]. The other phosphorylation sites of the C-terminal region of hRad9 are also essential for Chk1 activation following hydroxyurea (HU), IR, and UV treatment [33], although the constitutive phosphorylation of hRad9 does not influence the stability of the 9-1-1 complex [33, 34].
EXONUCLEASE ACTIVITY
Recombinant hRad9 has recently been shown to possess 3’-5’ exonuclease activity, suggesting that exonucleolytic processing of primary DNA lesions by hRad9 may contribute to DNA damage checkpoint response in humans [35], although the exact mechanism remains to be investigated further.
CELL CYCLE REGULATION
Genotoxic stress induces stabilization and transient accumulation of wild-type p53 protein in mammalian cells, leading to increased expression of p53 down-stream genes such as P21/WAF1 [36, 37]. The G1/S checkpoint is activated immediately after DNA damage, and inhibits DNA-damaged cells from entering the S phase [30, 38]. The phosphorylation of hRad9 is essential for Chk1 activation at S to G2 checkpoints following HU, IR, and UV treatment [33], and Chk1 is phosphorylated at two residues (Ser-317 and Ser-345) located in a Ser/Thr-Gln-rich domain [39]. Thus the phosphorylation of hRad9 is required for S-phase and G2/M checkpoint activation [33], although a potential transactivating property of Rad9 to P21 promoter has been shown, which suggests that p21 is involved also in the G1/S checkpoint [40].
DNA SYNTHESIS (WITH DNA POLYMERASE β)
The 9-1-1 complex can physically interact with DNA polymerase β in vitro, and the 9-1-1 complex has a stimulatory effect on DNA polymerase β activity, suggesting the possibility that the 9-1-1 complex might attract DNA polymerase β to DNA damage sites, thus directly connecting checkpoints and DNA repair [10]. Also, 9-1-1 is a damage-specific activator of FEN1 [41]. Although each member of the 9-1-1 complex shares sequence homology with PCNA, PCNA and the 9-1-1 complex can independently bind to and activate FEN1 [11].
RIBONUCLEOTIDE SYNTHESIS
Immunoprecipitated Rad9 complex contains a 240 kDa protein that has been identified as carbamoyl phosphate synthetase/aspartate transcarbamoylase/dihydroorotase (CAD), a multienzymatic protein required for the de novo synthesis of pyrimidine nucleotides and cell growth. Further investigation revealed that only free Rad9, but not Rad9 within the 9-1-1 complex, binds to CAD. These findings suggest that Rad9 may play a role in ribonucleotide biosynthesis [42].
APOPTOSIS (Bcl-2, Bcl-xL)
The Schizosaccharomyces pombe Rad9 (SpRad9) protein contains a group of amino acids with similarities to the Bcl-2 homology 3 death domain, which is required for SpRad9 interaction with human Bcl-2 and apoptosis induction in human cells [12]. hRad9 interacts with the anti-apoptotic Bcl2-family proteins Bcl-2 and Bcl-xL, and induces apoptosis [13].
TRANSCRIPTIONAL REGULATION
Rad9 can control expression of a number of genes that might be involved in developmental processes. Rad9 may control embryonic development through regulation at the transcriptional level specifically of target genes critical for this process [40].
EMBRYOGENESIS
Mouse Rad9 (-/-) embryo fibroblasts were not viable. mRad9 is a key mammalian genetic element of pathways that regulate the cellular response to DNA damage, maintenance of genomic integrity, and proper embryonic development [43].
PHOSPHORYLATION OF hRad9
hRad9 is highly modified by phosphorylation in a constitutive manner in response to both DNA damage and cell cycle position, and this phosphorylation plays a critical role in checkpoint signaling [44]. hRad9 has multiple functions in DNA damage response through the involvement of its phosphorylations, including checkpoint activation and damage repair [32, 33, 44]. IR induces Atm-dependent phosphorylation of hRad9 at least at Ser-272, which was detected in an in vitro study [33]. Atm-mediated phosphorylation of hRad9 is required for IR-induced G1/S checkpoint activation [32]. Thr-292 of hRad9 is subject to Cdc2-dependent phosphorylation in mitosis. Furthermore, four other hRad9 phosphorylation sites (Ser-277, Ser-328, Ser-336, and Thr-355) are regulated in part by Cdc2. Phosphorylation at Ser-387 is a constitutive, prerequisite form of DNA damage-induced hyperphosphorylation of hRad9. Overexpression of these mutants blocks the interaction between hRad9 and the DNA damage-responsive protein TopBP1 and impairs the cellular response to DNA damage during the S phase [44]. hRad9 mutants lacking a Ser-272 phosphorylation site, which is phosphorylated in response to genotoxins, had no effect on survival or checkpoint activation in mRad9 (-/-) mouse ES cells [33], whereas the maintenance of basal phosphorylation of the C-terminus appears to be essential for hRad9-mediated Chk1 activation following HU, IR, and UV treatment [33]. Consistent with a role for Chk1 in S-phase arrest, HU- and UV-induced S-phase arrest was abrogated in hRad9 phosphorylation mutants [33]. The exact roles of hRad9 phosphorylation in the DNA damage network remain to be investigated.
CONCLUSIONS
The disruption of these functions contributes to the accumulation of genomic instability and characterizes cancerous cells [2, 45, 46]. Recent studies of tumors indicate that the RAD9 gene is located in the chromosomal region of 11q13, which is amplified and frequently overexpresses both mRNA and protein in breast and lung cancer [47, 48]; the up-regulations correlate with tumor size and local recurrence [47]; and silencing RAD9 expression by RNA interference inhibits its proliferation in vitro [47], leading to unscheduled replications after DNA damage. Such conditions contribute to pathology in carcinogenesis or tumor progression.
REFERENCES
- 1.Dasika GK, Lin SC, Zhao S, Sung P, Tomkinson A, Lee EY. DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene. 1999;18:7883–7899. doi: 10.1038/sj.onc.1203283. [DOI] [PubMed] [Google Scholar]
- 2.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 3.Wang JY. Cellular responses to DNA damage. Curr Opin Cell Biol. 1998;10:240–247. doi: 10.1016/s0955-0674(98)80146-4. [DOI] [PubMed] [Google Scholar]
- 4.Weinert T. DNA damage and checkpoint pathways: molecular anatomy and interactions with repair. Cell. 1998;94:555–558. doi: 10.1016/s0092-8674(00)81597-4. [DOI] [PubMed] [Google Scholar]
- 5.Hartwell L, Weinert T, Kadyk L, Garvik B. Cell cycle check-points, genomic integrity, and cancer. Cold Spring Harbor Symp Quant Biol. 1994;59:259–263. doi: 10.1101/sqb.1994.059.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 7.Caspari T, Carr AM. DNA structure checkpoint pathways in Schizosaccharomyces pombe. Biochimie (Paris) 1999;81:173–181. doi: 10.1016/s0300-9084(99)80050-9. [DOI] [PubMed] [Google Scholar]
- 8.Rhind N, Russell P. Mitotic DNA damage and replication check-points in yeast. Curr Opin Cell Biol. 1998;10:749–758. doi: 10.1016/s0955-0674(98)80118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart E, Enoch T. S-phase and DNA-damage checkpoints: a tale of two yeasts. Curr Opin Cell Biol. 1996;8:781–787. doi: 10.1016/s0955-0674(96)80078-0. [DOI] [PubMed] [Google Scholar]
- 10.Toueille M, El-Andaloussi N, Frouin I, Freire R, Funk D, Shevelev I, Friedrich-Heineken E, Villani G, Hottiger MO, Hubscher U. The human Rad9/Rad1/Hus1 damage sensor clamp interacts with DNA polymerase beta and increases its DNA substrate utilisation efficiency: implications for DNA repair. Nucleic Acids Res. 2004;32:3316–3324. doi: 10.1093/nar/gkh652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich-Heineken E, Toueille M, Tannler B, Burki C, Ferrari E, Hottiger MO, Hubscher U. The two DNA clamps Rad9/Rad1/Hus1 complex and proliferating cell nuclear antigen differentially regulate flap endonuclease 1 activity. J Mol Biol. 2005;353:980–989. doi: 10.1016/j.jmb.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Komatsu K, Hopkins K, Lieberman H, Wang H. Schizosaccharomyces pombe Rad9 contains a BH3-like region and interacts with the anti-apoptotic protein Bcl-2. FEBS Lett. 2000;481:122–126. doi: 10.1016/s0014-5793(00)01975-x. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu K, Miyashita T, Hang H, Hopkins KM, Zheng W, Cuddeback S, Yamada M, Lieberman HB, Wang HG. Human homologue of S. pombe Rad9 interacts with BCL-2/BCL-xL and promotes apoptosis. Nat Cell Biol. 2000;2:1–6. doi: 10.1038/71316. [DOI] [PubMed] [Google Scholar]
- 14.Sedelnikova OA, Pilch DR, Redon C, Bonner WM. Histone H2AX in DNA damage and repair. Cancer Biol Ther. 2003;2:233–235. doi: 10.4161/cbt.2.3.373. [DOI] [PubMed] [Google Scholar]
- 15.Kuhne M, Riballo E, Rief N, Rothkamm K, Jeggo PA, Lobrich M. A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 2004;64:500–508. doi: 10.1158/0008-5472.can-03-2384. [DOI] [PubMed] [Google Scholar]
- 16.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 17.Bartek J, Falck J, Lukas J. CHK2 kinase--a busy messenger. Nat Rev Mol Cell Biol. 2001;2:877–886. doi: 10.1038/35103059. [DOI] [PubMed] [Google Scholar]
- 18.McGowan CH. Checking in on Cds1 (Chk2): A checkpoint kinase and tumor suppressor. Bioessays. 2002;24:502–511. doi: 10.1002/bies.10101. [DOI] [PubMed] [Google Scholar]
- 19.Rhind N, Russell P. Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J Cell Sci. 2000;113:3889–3896. doi: 10.1242/jcs.113.22.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Onge RP, Udell CM, Casselman R, Davey S. The human G2 checkpoint control protein hRAD9 is a nuclear phosphoprotein that forms complexes with hRAD1 and hHUS1. Mol Biol Cell. 1999;10:1985–1995. doi: 10.1091/mbc.10.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkmer E, Karnitz LM. Human homologs of Schizosaccharomyces pombe rad1, hus1, and rad9 form a DNA damage-responsive protein complex. J Biol Chem. 1999;274:567–570. doi: 10.1074/jbc.274.2.567. [DOI] [PubMed] [Google Scholar]
- 22.Burtelow MA, Roos-Mattjus PM, Rauen M, Babendure JR, Karnitz LM. Reconstitution and molecular analysis of the hRad9-hHus1-hRad1 (9-1-1) DNA damage responsive checkpoint complex. J Biol Chem. 2001;276:25903–25909. doi: 10.1074/jbc.M102946200. [DOI] [PubMed] [Google Scholar]
- 23.Caspari T, Dahlen M, Kanter-Smoler G, Lindsay HD, Hofmann K, Papadimitriou K, Sunnerhagen P, Carr AM. Characterization of Schizosaccharomyces pombe Hus1: a PCNA-related protein that associates with Rad1 and Rad9. Mol Cell Biol. 2000;20:1254–1262. doi: 10.1128/mcb.20.4.1254-1262.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thelen MP, Venclovas C, Fidelis K. A sliding clamp model for the Rad1 family of cell cycle checkpoint proteins. Cell. 1999;96:769–770. doi: 10.1016/s0092-8674(00)80587-5. [DOI] [PubMed] [Google Scholar]
- 25.Venclovas C, Thelen MP. Structure-based predictions of Rad1, Rad9, Hus1 and Rad17 participation in sliding clamp and clamp-loading complexes. Nucleic Acids Res. 2000;28:2481–2493. doi: 10.1093/nar/28.13.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bermudez VP, Lindsey-Boltz LA, Cesare AJ, Maniwa Y, Griffith JD, Hurwitz J, Sancar A. Loading of the human 9-1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro. Proc Natl Acad Sci USA. 2003;18:1633–1638. doi: 10.1073/pnas.0437927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burtelow MA, Kaufmann SH, Karnitz LM. Retention of the human Rad9 checkpoint complex in extraction-resistant nuclear complexes after DNA damage. J Biol Chem. 2000;275:26343–26348. doi: 10.1074/jbc.M001244200. [DOI] [PubMed] [Google Scholar]
- 28.Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 29.Hirai I, Wang HG. A role of the C-terminal region of human Rad9 (hRad9) in nuclear transport of the hRad9 checkpoint complex. J Biol Chem. 2002;277:25722–2577. doi: 10.1074/jbc.M203079200. [DOI] [PubMed] [Google Scholar]
- 30.Greer DA, Besley BDA, Kennedy KB, Davey S. hRad9 rapidly binds DNA containing double-strand breaks and is required for damage-dependent topoisomerase II beta binding protein 1 focus formation. Cancer Res. 2003;63:4829–4835. [PubMed] [Google Scholar]
- 31.Xu ZX, Timanova-Atanasova A, Zhao RX, Chang KS. PML colocalizes with and stabilizes the DNA damage response protein TopBP1. Mol Cell Biol. 2003;23:4247–4256. doi: 10.1128/MCB.23.12.4247-4256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen MJ, Lin YT, Lieberman HB, Chen G, Lee EYHP. ATM-dependent phosphorylation of human Rad9 is required for ionizing radiation-induced checkpoint activation. J Biol Chem. 2001;276:16580–16586. doi: 10.1074/jbc.M008871200. [DOI] [PubMed] [Google Scholar]
- 33.Roos-Mattjus P, Hopkins KM, Oestreich AJ, Vroman BT, Johnson KL, Naylor S, Lieberman HB, Karnitz LM. Phosphorylation of human Rad9 is required for genotoxin-activated checkpoint signaling. J Biol Chem. 2003;278:24428–24437. doi: 10.1074/jbc.M301544200. [DOI] [PubMed] [Google Scholar]
- 34.Lindsey-Boltz LA, Bermudez VP, Hurwitz J, Sancar A. Purification and characterization of human DNA damage checkpoint Rad complexes. Proc Natl Acad Sci USA. 2001;98:11236–11241. doi: 10.1073/pnas.201373498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bessho T, Sancar A. Human DNA damage checkpoint protein hRAD9 is a 3’ to 5’ exonuclease. J Biol Chem. 2000;275:7451–7454. doi: 10.1074/jbc.275.11.7451. [DOI] [PubMed] [Google Scholar]
- 36.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 37.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–33. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 38.Friesner JD, Liu B, Culligan K, Britt AB. Ionizing radiation—dependent γ-H2AX focus formation requires Ataxia Telangiectasia Mutated and Ataxia Telangiectasia Mutated and Rad3-related. Mol Biol Cell. 2005;5:2566–2576. doi: 10.1091/mbc.E04-10-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 40.Yin Y, Zhu A, Jin YJ, Liu YX, Zhang X, Hopkins KM, Lieberman HB. Human RAD9 checkpoint control/proapoptotic protein can activate transcription of p21. Proc Natl Acad Sci USA. 2004;15:8864–8869. doi: 10.1073/pnas.0403130101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Brandt P, Rossi ML, Lindsey-Boltz L, Podust V, Fanning E, Sancar A, Bambara RA. The human Rad9-Rad1-Hus1 checkpoint complex stimulates flap endonuclease 1. Proc Natl Acad Sci USA. 2004;101:16762–7. doi: 10.1073/pnas.0407686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsey-Boltz LA, Wauson EM, Graves LM, Sancar A. The human Rad9 checkpoint protein stimulates the carbamoyl phosphate synthetase activity of the multifunctional protein CAD. Nucleic Acids Res. 2004;32:4524–30. doi: 10.1093/nar/gkh789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopkins KM, Auerbach W, Wang XY, Hande MP, Hang H, Wolgemuth DJ, Joyner AL, Lieberman HB. Deletion of mouse rad9 causes abnormal cellular responses to DNA damage, genomic instability, and embryonic lethality. Mol Cell Biol. 2004;24:7235–48. doi: 10.1128/MCB.24.16.7235-7248.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St Onge RP, Besley BD, Pelley JL, Davey S. A role for the phosphorylation of hRad9 in checkpoint signaling. J Biol Chem. 2003;278:26620–26628. doi: 10.1074/jbc.M303134200. [DOI] [PubMed] [Google Scholar]
- 45.Dash BC, El-Deiry WS. Cell cycle checkpoint control mechanisms that can be disrupted in cancer. Methods Mol Biol. 2004;280:99–161. doi: 10.1385/1-59259-788-2:099. [DOI] [PubMed] [Google Scholar]
- 46.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 47.Cheng CK, Chow LW, Loo WT, Chan TK, Chan V. The cell cycle checkpoint gene Rad9 is a novel oncogene activated by 11q13 amplification and DNA methylation in breast cancer. Cancer Res. 2005;65:8646–8654. doi: 10.1158/0008-5472.CAN-04-4243. [DOI] [PubMed] [Google Scholar]
- 48.Maniwa Y, Yoshimura M, Bermudez VP, Yuki T, Okada K, Kanomata N, Ohbayashi C, Hayashi Y, Hurwitz J, Okita Y. Accumulation of hRad9 protein in the nuclei of nonsmall cell lung carcinoma cells. Cancer. 2005;103:126–132. doi: 10.1002/cncr.20740. [DOI] [PubMed] [Google Scholar]
- 49.Zhang YW, Otterness DM, Chiang GG, Xie W, Liu YC, Mercurio F, Abraham RT. Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Mol Cell. 2005;19:607–618. doi: 10.1016/j.molcel.2005.07.019. [DOI] [PubMed] [Google Scholar]


