Abstract
The regulation of protein expression and activity has been for long time considered only in terms of transcription/translation efficiency. In the last years, the discovery of post-transcriptional and post-translational regulation mechanisms pointed out that the key factor in determining transcript/protein amount is the synthesis/degradation ratio, together with post-translational modifications of proteins. Polyubiquitinaytion marks target proteins directed to degradation mediated by 26S-proteasome. Recent functional genomics studies pointed out that about 5% of Arabidopsis genome codes for proteins of ubiquitination pathway. The most of them (more than one thousand genes) correspond to E3 ubiquitin ligases that specifically recognise target proteins. The huge size of this gene family, whose members are involved in regulation of a number of biological processes including hormonal control of vegetative growth, plant reproduction, light response, biotic and abiotic stress tolerance and DNA repair, indicates a major role for protein degradation in control of plant life.
Key Words: E3 ubiquitin ligase, post-translational regulation, plant
INTRODUCTION
The ubiquitination cascade is one of the main pathways of post-translational regulation of gene expression in eucari-otic cells, in which ubiquitin is bond to a lysine residue of a target protein. Ubiquitin itself has different lysine residues: one of them, tipically Lys-48, can be further conjugated by another ubiquitin moiety in a processive manner to form a polyubiquitin chain. Being a reversible form of covalent modification, ubiquitination acts very rapidly on target protein in different ways. The best characterised function of ubiquitination is the degradation of target proteins through the 26S proteasome. Besides the elimination of aberrant or truncated proteins for cellular housekeeping, this pathway regulates the amount of active proteins, which depends on the synthesis/degradation ratio. An example is the human PARKIN protein, involved in autosomal recessive familial Parkinson’s disease, which functions as E3 ligase promoting the degradation of a synaptic vesicle-associated septin [1, 2]. Furthermore, other regulatory functions (i.e. protein activation) of ubiquitin have been described over the past few years [3]. The consequences of protein ubiquitination might depend on the number of ubiquitin units in the chain linked to the target protein and on the lysine residue of ubiquitin utilised for the formation of polyubiquitin chain. For example, Lys-63 (K63)-linked polyubiquitin chains have been shown to mediate human protein kinase activation, DNA repair and vesicle trafficking. Monoubiquitination has also shown to be involved in nuclear targeting of human proteins interacting with DNA repair enzymes [3]. How ubiquitination can regulate the activity of target proteins is still unclear: ubiquitination could change the conformation of target proteins, otherwise a monoubiquitin moiety or a distinct polyubiquitin chain could serve as specific protein-protein interaction domain to recruit proteins carrying various ubiq-uitin-binding domains.
The ubiquitin attachment to target proteins is mediated by a three-steps enzymatic cascade. In the first step a thioester bond between ubiquitin and the ubiquitin-activating enzyme E1, is formed in an ATP-dependent reaction. Subsequently, ubiquitin is transferred to the ubiquitin-conjugating enzyme E2. Then, the transfer of ubiquitin to the target protein occurs in presence of the ubiquitin ligase enzyme E3, which specifically recognises the target proteins. An isopeptide bond is formed between the carboxyl terminus of ubiquitin and the ε-amino group of a lysine residue on the target protein. The efficient polyubiquitination is facilitated by multiubiquitin chain assembly factors (E4) which transfer additional ubiquitin moieties [4].
Many genomic studies have been carried out for E3 ubiquitin ligases in plants, particularly in species whose genome has been completely sequenced as Arabidopsis and rice. More than one thousand E3 ubiquitin ligase members have been predicted in Arabidopsis (Table 1). The huge size of the E3 ubiquitin ligase gene family suggests its role giving specificity to the system, each E3 ubiquitin ligase acting for the ubiquitination of a particular target protein. This review will present the current status on functional genomic studies aimed to characterize the large family of E3 ubiquitin ligases in relation to the main biological processes where they are involved.
Table 1.
Genomic Organization of E3 Ubiquitin Ligase Gene Family in Arabidopsis
| E3 sub group | Abbreviation | Number of genes | References |
|---|---|---|---|
| Homology to E6-AP C-Terminus | HECT | 7 | [17] |
| Really Interesting New Gene | RING | 499 | [23–26] |
| Plant U-box | PUB | 49 | [39–41] |
| CULLIN | CUL | 11 | [42–44] |
| Arabidopsis Skp1-related | ASK | 21 | [47–49] |
| Cyclin F proteins | F-box | 724 | [43, 50, 51, 66] |
| Bric a brac, Tramtrack and Broad complex | BTB | 81 | [44, 54] |
| CULLIN4-Damaged DNA-Binding Protein | CUL4-DDB | 5 | [28, 57] |
| Anaphase Promoting Complex | APC | 18 | [35] |
| Total | 1415 |
E3 LIGASE ORGANIZATION
E3 ubiquitin ligases are classified into two groups depending on the presence of a HECT (Homology to E6-AP C-Terminus – Fig. 1a) or a RING (Really Interesting New Gene)/U-box domain. RING proteins can act as single components containing both the active site and the binding pocket for the E2-ubiquitin intermediate (Fig. 1b), or as components of multisubunit complexes which in plants include SCF (SKP1-CULLIN-F-box), CUL3 (CULLIN 3)-BTB/POZ (Bric a brac, Tramtrack and Broad complex/Pox virus and Zinc finger), CUL4-DDB1 (UV-Damaged DNA-Binding Protein 1) and APC (Anaphase Promoting Complex) complexes, as shown in Fig. (1c, d, e and f). While RING/U-box E3s generally act as molecular adapters between E2 and target proteins, the HECT E3s form a covalent bond with ubiquitin before transferring it to the protein substrate, using a conserved Cys of the HECT domain to form a ubiquitin-E3 thiole-ester intermediate [5].
Fig. (1).
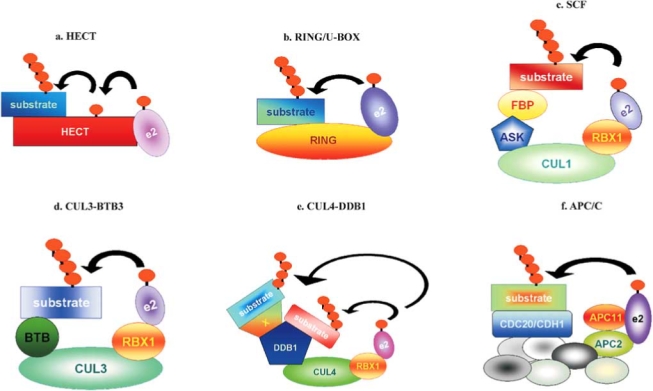
Organization of E3 ubiquitin ligases. CULLIN and CULLIN-like proteins are in green and RING proteins are in red/orange; small linked circles represent polyubiquitin chains. (a) and (b): E3 single components. (c)–(f): E3 multi subunit complexes.
The multi-subunit E3 ligases are CULLIN and RING finger-based protein complexes. These enzymes are constituted by two functional modules. The catalytic module is composed by a RING-finger subunit (RBX1- RING Box protein 1 - or APC11) interacting with the E2 enzyme. The second module (adapter) specifically recognises target substrates for ubiquitination. The same catalytic module can be associated to many different adapters, therefore many different E3 complexes are formed, which in turn catalyse the ubiquitination of different substrates. The two modules are brought together by a CULLIN (or CULLIN-like, APC2) protein which acts as a molecular scaffold and also defines the E3 class. The E3 SCF complexes contain four core components: SKP1 (S-phase Kinase associated Protein 1, named ASK1, for Arabidopsis Skp1-related in plants), CUL1, a F-box protein and RBX1. SCF structure has been resolved by Zheng et al. [6] in yeast and mammals. RBX1 catalyses the synthesys of polyubiquitin chains and, together with CUL1, forms a catalytic core complex recruiting a cognate E2. The CULLIN subunit acts as a large scaffold protein that ensures optimal presentation of the substrate to the E2 enzyme. It binds both RBX1 and the linker protein SKP1. The SKP1 protein serves as an adapter between CUL1 and the variable F-box protein, which binds the substrate [7–9]. The second family of the multisubunit E3 complexes is defined by the association among the highly conserved CULLIN family member, CUL3, a BTB/POZ domain protein, and the RBX1 protein [10]. In the current animal model for CUL3-BTB E3 ligases, BTB proteins function as substrate-specific adapters, which bind CUL3 through its BTB-domain and interact with the substrate through an associated protein-protein interaction domain [11]. RBX1 binds the E2 enzyme probably leading to its allosteric activation [12]. The BTB/POZ domain appears to assume a three-dimensional structure similar to the CUL1 interaction domain of the SKP1 adapter proteins [9], therefore it has been suggested that BTB proteins function as SKP1/F-box hybrid proteins that deliver the targets to CUL3.
CUL4 protein is a core component of a new class of E3 ubiquitin ligases that regulate replication and transcription in mammals. These E3 ubiquitin ligases also contain the DDB1 and RBX1 proteins. CUL4-DDB1 may recruit substrates directly, or through additional factors, such as the complex formed by De-Etiolated-1 (DET1) and Constitutively Pho-tomorphogenic-1 (COP1) proteins [13].
The APC is a multiprotein complex, constituted by eleven subunits conserved in all eukaryotes, involved in the regulation of the cell cycle progression by degrading cyclins. Two APC proteins, APC2, a distant member of the CULLIN family, and the RING-finger protein APC11 form the minimal ubiquitin ligase module of the APC. The two proteins interact with each other and with E2 ubiquitin conjugating enzymes, and together are able to catalyse ubiquitination of proteins in vitro, although without substrate specificity [14]. Stage-specific activation of APC as well as selection and binding of the substrates depend on a WD40 protein able to interact with the substrate. Two types of WD40 proteins have been identified, Cdc20 (Cell division cycle) and Cdh1 (Cadherin 1), known as Ccs52 (Cell cycle switch 52) in plants, and two different complexes can be formed depending on which WD40 protein interacts with the other APC subunits. In human APC, the interaction between the WD40 proteins and the APC core is mediated by two additional subunits (APC3 and APC7) [15].
E3 LIGASE COMPONENTS
HECT E3 Ubiquitin Ligases
The HECT E3 family contains proteins characterised by the presence of a conserved 350 amino-acid C terminal domain, called HECT from the first described protein, the human E6-Associated Protein (E6-AP), involved in the ubiquit-ination and degradation of p53 upon its association with the papillomavirus protein E6 [16].
Whereas mammals have greatly expanded the use of HECT E3 type, having a gene family of about 50 elements, no comparable expansion is evident in Arabidopsis, where according to a consensus HECT sequence-based search, only seven HECT genes, named UPL1-UPL7 are present [17] (Supplementary Table S1). UPL genes have been grouped in four subfamilies based on intron/exon positions, length of the corresponding protein and presence of additional protein motifs for subcellular localization (i.e. transmembrane domains) or interaction with other components of ubiquitination pathway or with glycosylated proteins (i.e. C-type lectin-binding domain) [17]. These domains, potentially important for target specificity are upstream to the C-terminal HECT domain and appear co-evoluted with the HECT domain.
Members belonging to different Arabidopsis UPL subfamilies often appear more similar to HECT E3 ubiquitin ligases from other species than to each other, suggesting that these subfamilies arose before the split of the animal, fungal and plant kingdoms and that they have catalytic activities that cannot be replaced by other E3 types. By means of an in vitro polyubiquitination assay it has been showed that the C-terminal HECT domain of UPL1 is necessary and sufficient to conjugate ubiquitin in a reaction that requires the positionally conserved cysteine within the HECT domain, an E1, and an E2 enzyme specifically belonging to the UBC8 family [18].
RING-Finger E3 Ubiquitin Ligases
RING containing proteins represent, together with F-box proteins, the most abundant E3 ubiquitin ligase gene families. The RING finger motif was first identified in the protein product of the human gene RING1 — Really Interesting New Gene 1 — [19].
The RING domain contains fours pairs of zinc ligands binding co-ordinately two zinc ions [20, 21]. The zinc ligands are formed by cysteine and hystidine residues placed at proper distance in a typical cross-brace structure. The two main RING domains, C3HC4 and C3H2C3, contain a conserved His at metal ligand position 4, but differ for the presence of either a Cys or His residue, respectively, at metal ligand position 5. The RING domains were shown to be essential for E3 ubiquitin ligase activity of human RING-containing proteins [22].
Only a limited number of RING proteins with a predicted or known biological function have been characterised in plants (Supplementary Table S2; Table 2). The complete sequence of the Arabidopsis and rice genomes gave new chances for large gene families characterisation in plants. Systematic searches for RING-finger domain containing proteins yielded about 500 distinct sequences in Arabidopsis, which should represent an exhaustive list of RING genes in this species [23–26].
Table 2.
E3 Ubiquitin Ligases Involved in Plant Growth and Development and Known Targets
| E3 ubiquitin ligase | E3 type | Biological processes | Interactions with other complex components | Demonstrated E3 ligase activity in vitro and E2 partners | Target proteins | References |
|---|---|---|---|---|---|---|
| FIP2 (Arabidopsis thaliana) | BTB | pollen tube extension, actin cytoskeleton | AFH1 | [95] | ||
| UFO (Arabidopsis thaliana) | F-box | floral development | APETALA3 | [81, 91, 104] | ||
| FKF1 (Arabidopsis thaliana) | F-box | flowering in response to photoperiod | ASK1,2,11,14 | CDF1/2 | [105–107] | |
| LKP2 (Arabidopsis thaliana) | F-box | flowering time, circadian clocks | ASK1,2,3,4,5,11,14,20 | CDF3, TOC | [87, 106] | |
| ETO1/EOL1/EOL2 (Arabidopsis thaliana) | BTB | ethylene biosynthesis | CUL3a | ACS5 | [67] | |
| BT4 (Arabidopsis thaliana) | BTB | SA and H2O2 response | AtBET10 | [94] | ||
| BT2 (Arabidopsis thaliana) | BTB | SA and H2O2 response | AtBET10 | [94] | ||
| BT1 (Arabidopsis thaliana) | BTB | SA and H2O2 response | CUL3a | AtBET10, AtBET9 | [94] | |
| ARIA (Arabidopsis thaliana) | BTB | ABA response | ABF2 | [108] | ||
| TIR1 (Arabidopsis thaliana) | F-box | auxin receptor | ASK1/2, AtCUL1 | IAA1/AXR5 | [46, 109–112] | |
| SLY1/SNE (Arabidopsis thaliana) | F-box | GA signaling | RGA, GAI, RGL2 (DELLA proteins) | [84, 113] | ||
| GID2 (Oryza sativa) | F-box | GA signaling | OsSkp15(ASK1) | OsSLR1 | [114, 115] | |
| EBF1/2 (Arabidopsis thaliana) | F-box | ethylene signaling | ASK1/2 | EIN3/EIL; ERF | [85, 116, 117] | |
| AIP2 (Arabidopsis thaliana) | RING-H2 | ABA signaling | UBC8, 10, 11, 28, 29, 30, UbcH5B | ABI3 | [118] | |
| SINAT5 (Arabidopsis thaliana) | RING-HCa | auxin signaling | AtUBC9a | NAC1 | [119] | |
| XBAT32 (Arabidopsis thaliana) | RING | auxin transport; root initiation | UbcH5B | XBAT32 | [120] | |
| COP1 (Arabidopsis thaliana) | RING-HCa | photomorphogenesis | UBC9 | LAF1, HY5, HYH, cry2, phyA, COL3; HFR1; AtMYB21 | [28, 98] | |
| CIP8 (Arabidopsis thaliana) | RING-H2 | photomorphogenesis | UBC8, 10, 11, 28, 29, 30 | HY5 HYH | [28] | |
| DET1 (Arabidopsis thaliana) | CUL4 complex | photomorphogenesis | LHY | [121] | ||
| NPH3 (Arabidopsis thaliana) | BTB | phototropism (hypocotil) | RPT2 | [122] | ||
| RPT2 (Arabidopsis thaliana) | BTB | phototropism (root), stomatal opening | PHOT1; NPH3 | [69, 123] | ||
| DET1 (Lycopersicum esculentum) | CUL4 complex | photomorphogenesis | H2B | [124] | ||
| HOS1 (Arabidopsis thaliana) | incomplete RING | cold stress | UBC8 | ICE1 | [31, 33] | |
| CHIP (Arabidopsis thaliana) | U-BOX | temperature stress | UbcH5a | denatured proteins; PP2A | [40] | |
| NPR1 (Arabidopsis thaliana) | BTB | salicilic and jasmonic acid response | TGA | [79, 125–129] | ||
| PRT1 (Arabidopsis thaliana) | RING | N-end rule pathway degradation | N-end rule substrates (aromatic termini) | [130] | ||
| APC2 (Arabidopsis thaliana) | Cullin-related | cell cycle regulation | APC11; CDC23 | D-box proteins | [36] | |
| SKP2 (Arabidopsis thaliana) | F-box | cell cycle regularion | E2Fc | [131] | ||
| AhSLF-S2 (Oryza sativa) | F-box | self-incompatibility | ASK1; CULLIN1-like | S-Rnases | [61] | |
| SFB (Prunus dulcis) | F-box | self-incompatibility | S-Rnases | [132] |
Kosarev et al. [23] identified 365 genes grouped into six clusters based on sequences similarity and RING domain features. A more extended search, performed by Stone et al. [25] led to the identification of 469 genes, characterised by 3 RING-type (RING-H2, RING-Hca and RING-HCb) and five modified RING-type domains (RING-C2, RING-v, RING-D, RING-S/T and RING-G) based on the nature and on the distance between the metal ligand residues. The majority of analysed proteins contained only the RING-finger domain (about 150 sequences) or a RING motif associated with one or more transmembrane domains (about 120 sequences). In the remaining sequences, the RING motif was found associated to a number of known domains which may interact with the target protein, or act as regulatory components. Many RING-associated domains, such as coiled-coil or zinc finger C×2C×5C×2C motifs and Ankyrin repeats, are potentially involved in the protein-protein interaction mechanisms. Furthermore, some domains are supposed to interact with specific classes of proteins, i.e. the WD40 repeats show specificity for ser/thr phosphoproteins [27].
A well characterised RING finger E3 ubiquitin ligase is COP1, which functions as central switch in light control of Arabidopsis seedling development. COP1 protein contains three structural domains: a RING finger, followed by a coiled-coil domain and seven WD40 repeats at the C-terminus. A large set of proteins interacting with COP1 have been identified. In particular, it has been proven that the RING domain mediates the interaction with COP10, a protein functioning as E2 ubiquitin conjugating enzyme, and CIP8 (COP1 Interacting Protein 8), another E3 ligase also involved, together with COP1, in the ubiquitination of the transcription factor HY5 (long hypocotyl phenotype 5). The coiled coil domain allows the dimerisation of COP1 and its interaction with a number of polypeptides, such as SPA (Su-pressor of phyA) proteins, involved in the modulation of COP1 activity. Finally, the C-terminal WD40 repeats are involved in the recognition of the ubiquitination targets: the transcription factors HY5, HYH (HY5-like protein H) and HFR1 (long Hypocotyl in Far Red 1) and the photoreceptors cry2 and phyA [28].
In some cases the RING domain was found associated to putative nucleic acid binding motifs such as C2H2 and C3H1 (DNA-binding zinc finger domains), or KH (K Homology) and RRM (RNA Recognition Motif). Notably, one RING finger protein (At3g54460) is predicted to contain an F-box domain [25].
ARIADNE proteins are characterized by the IBR (In Between Ring) domain, a motif with the pattern C6HC, usually located between two RING domains. The Arabidopsis ARI-ADNE family is formed by 16 genes [24]; twelve of them clustered together by Stone et al. [25].
ATL gene family members were originally isolated as genes rapidly induced in response to elicitors [29]. They contain a RING-H2 type domain with a particular signature: a highly conserved proline spaced out a residue upstream from the third zinc ligand, and a highly conserved tryptophan spaced out three residues downstream from the sixth zinc ligand. The analysis of Serrano et al. [26] led to the identification of 80 ATL proteins in Arabidopsis, most of them clustered together according to Kosarev et al. [23] and to Stone et al. [25].
For this review, we have listed 499 RING finger proteins (Supplementary Table S1), by considering, besides those collected by Stone et al. [25], 27 sequences annotated as RING proteins by Kosarev et al. [23] and Mladek et al. [24], and three individually characterised RING proteins: CER3 [30], HOS1 (High Expression of Osmotically Responsive Gene 1) [31], and PEX12 (Peroxisomal biogenesis protein 12) [32]. These latter sequences, although not perfectly satisfying the criteria imposed by Stone et al. [25], could represent potentially active E3 ubiquitin ligases. HOS1 has been recently shown to be active in the ubiquitination of the cold-related transcription factor ICE1 (Inducer of CBF Expression) [33].
Despite extensive studies on the RING protein family have been performed only in Arabidopsis, some evidences exist that the organization of this family can vary in different plant species. The modified RING domain RING-D appears to be characteristic of Arabidopsis (or dicots) species, since a similarity search carried out in rice genome failed to find proteins with the same domain [25].
The Arabidopsis RING gene collection also comprises three genes coding for proteins acting as subunits of large complexes: AtRbx1;1 and AtRbx1;2 whose protein products participate in assembling of SCF, CUL3-BTB and CUL4-DDB complexes [34] (Supplementary Table S1), and the APC11 gene, involved in the formation of the APC complex [35, 36].
U-Box E3 Ubiquitin Ligases
The U-box motif, originally identified in the yeast UFD2 (Ubiquitin Fusion Degradation 2) protein [37], is a RING finger-related domain lacking the zinc-chelating residues. One of best known plant U-box containing proteins is the Brassica ARC1 (ARM Repeat Containing protein 1) protein, an E3 ubiquitin ligase, acting downstream of S receptor kinase to promote ubiquitination and protein degradation during the rejection of self-incompatible Brassica sp. pollen [38]. Besides the U-box domain, ARC1 also contains several ARM (Armadillo) repeats. The association between U-box domain and ARM repeats is frequent in Arabidopsis, where 49 U-box genes have been identified, with 41 of them also showing ARM repeats (AtPUB-ARM genes - Supplementary Table S1) [39–41]. Although the U-box lacks the zinc-chelating residues, molecular modelling predicts that a system of salt bridges and hydrogen bonds may stabilize the U-box scaffold into a structure similar to the RING finger without the association of ions [39]. In fact, when six At-PUB-ARM proteins were tested in an in vitro polyubiquitination assay, all proteins proved to be active as E3 ubiquitin ligases [41].
The CULLIN Family
The CULLIN family encompasses at least six genes in humans and in Caenorhabditis elegans. A sequence similarity search in the Arabidopsis genome revealed the presence of at least 11 predicted CULLIN-related genes [42–44] (Supplementary Table S1). However, intact canonical C- and N-terminal domains were retrieved only for six CULLIN proteins, whereas four conserve only the N-terminal domain. EST-based evidences for gene expression have been reported only for five of the complete CULLIN genes, while the sixth CULLIN exhibited an apparent frame-shift in the coding region when compared to the most related CUL2, and may therefore not be functional. The putative functional CULLIN genes have been named CUL1, CUL2, CUL4, and CUL3A/CUL3B. The two closely related Arabidopsis CUL3 are subunits of CUL3/BTB complexes [45], whereas the Arabidopsis CUL1 and the related CUL2 protein showed association with the SCF class of E3 ubiquitin ligases [46]. In Arabidopsis, no ortholog for animal CUL2 has been identified. Five remaining truncated Arabidopsis CULLIN proteins await assignment, furthermore the unusual organization of these shorter forms raises the intriguing possibility that plants contain other CUL-based E3 ubiquitin ligases that are kingdom-specific [44].
SKP1-Like Family
The Arabidopsis genome contains 21 Arabidopsis SKP1 (ASK) genes [47–49] (Supplementary Table S1). Based on sequence similarity and phylogenetic analysis, they have been grouped in eight classes, and, with the exception of ASK20 and ASK21, the ASK genes appear to descend from a common ancestor [43, 48, 49].
F-Box Family
F-box proteins are characterized by a N-terminal 60-residue F-box conserved domain, originally described in the Cyclin F protein [7]. The F-box domain interacts with the SKP subunit of the SCF complex. The first 40 residues represent the core of the SKP-binding site, while the other 20 residues represent a variable domain conferring binding preferences toward SKP proteins. The C-terminal portion of the F-box proteins typically contains a variable protein–protein interaction module that presumibly participates in substrate recognition and binding, thus conferring specificity to the SCF complex.
Blast searches on the complete Arabidopsis genome for genes predicted to encode the F-box consensus sequence led to the identification of about seven hundred genes (703 according to Risseeuw et al. [43], 694 according to Gagne et al. [50] and 568 according to Kuroda et al. [51]), a number that could allow for the specific ubiquitination of many different substrates. A complete list of all F-box genes of Arabidopsis is presented in Supplementary Table S1. A phylogenetic analysis based on the F-box domain sequences, grouped the F-box protein genes in five families (A-E), whose A-C families are further divided into 18 subfamilies, giving 20 distinct classes. Residues conserved among the 20 families correspond with those implicated in the SKP association, while the additional positions conserved within the subfamilies could be important for preferential binding of different ASK proteins [50].
The analysis of the C-terminal region revealed an array of potential protein-protein interacting domains, including Leucine-Rich Repeat (LRR), Kelch, WD-40, ARM repeats, ThetracoPeptide Repeats (TPR), TUBBY, actin-related motif, DEXDc (DEAD-like helicase), and the metal-binding site Jmj-C (jumonji) domains [50]. The LRR and Kelch repeats are the most widely represented. Nevertheless, a large number of the Arabidpsis F-box proteins had C-terminal regions with no obvious similarity to known motifs. The output of the F-box phylogenetic tree suggests a general co-evolution of the F-box motif with the target interaction domain [50]. A survey of genomic arrangements revealed that events of tandem duplications of chromosomal regions should have played a major role in creating this sheer size family. However, sequence comparisons and genetic analyses suggest that most of the proteins do not have functional paralogs, that is F-box proteins, although with similar F-box motifs and C-terminal domains, have unique functions and so distinct targets [50].
The high number of F-box genes (significantly higher in plants than in other eukaryotes) and some associations between F-box and protein-protein interacting domains unique to plants may indicate that plants exploited the F-box family to broad their ability for specific target recognition.
BTB Family
The BTB/POZ domain was originally identified as a conserved motif present in Drosophila melanogaster ZID (Zinc finger protein with Interaction Domain) gene [52]. A variety of functional roles have been proved for this domain, including transcriptional regulation, cytoskeleton dynamics, ion channel assembly and gating and targeting proteins for ubiquitination. In most of these functional classes, the BTB domain acts as a protein-protein interacting module able both to self-associate and to interact with other proteins [53].
A search on Arabidopsis genome identified about 80 loci encoding proteins with one or more BTB motifs. We compared the two published lists [44, 54] to obtain an updated collection with 81 loci (Supplementary Table S1). Nearly all genes appear singletons, with just one example of apparent tandem duplication. Most of the BTB proteins harbour additional protein domains, either upstream or downstream of the BTB domain, acting as protein interactors (Ankyrin repeats, Meprin And TRAF Homologous domain – MATH -, ARM and TPR repeats), DNA binding motifs (Myb repeats, Transcriptional Adapter putative Zinc finger - TAZ), as well as the plant specific Nonphototropic Hypocotil domain (NPH3) and the potassium channel tetramerisation domain. The different plant BTB-domain proteins have been phylogenetically classified in various subgroups, and a general co-evolution between the BTB domain and the other associated protein domains have been evidenced [44, 54].
CUL4-DDB1 Subunits
Although the CUL4 protein has been conserved during evolution in fission yeast, plants, worms, flies and mammals, only recently evidences have shown a CUL4 dependent E3 ligases activity in Arabidopsis. Currently, knowledge from human allowed identifying plant orthologs for all the subunits of the human CUL4-DDB1 complexes (Supplementary Table S1). Two highly related DDB1 genes, named DDB1a and DDB1b were found in Arabidopsis [55]; other subunits are DET1 [55], COP10, COP1 [56] and DDB2 [57].
APC Complex Subunits
Hortologous genes have been individuated in Arabidopsis for all known vertebrates APC subunits, in some cases with gene duplication events [35]. The functional APC complex is constituted by eleven subunits. Among them, Arabidopsis APC2 and APC11 form the catalitic core [36]; the Cdc27 gene, homologous to the human APC3 gene, mediates, together with other Cdc subunits, the interaction between the APC core and the WD40 protein, Cdc20 or Ccs52. Many different APC complexes can be assembled, considering the existence in Arabidopsis of two Cdc27 genes (Cdc27A and Cdc27B/HOBBIT), three Ccs52 (Ccs52A1, Ccs52A2 and Ccs52B) and six Cdc20 genes, all shown to be part of APC complex in vivo in Arabidopsis [35, 58].
E3 UBIQUITIN LIGASES: EXPRESSION PATTERNS AND MOLECULAR INTERACTIONS
Genomic studies indicate that plant E3 ubiquitin ligase gene family comprises about 1500 members (Table 1). A further complexity derives from the possibility of different functional combinations among the different subunits within E3 complexes as well as among the E3 ubiquitin ligases and different E2 conjugation enzymes. Indeed, the E2 gene family, characterised by the UBC domain, comprises 37 genes in Arabidopsis [59].
Many studies have supplied evidences that the most of the genes coding for E3 ubiquitin ligases are indeed active in a number of plant tissues: nearly all RING, U-box, ASK and BTB proteins are expressed [26, 44, 48, 49, 60], while only for less than half F-box genes there is an evidence of expression [50, 61].
Some E3 ubiquitin ligases or E3 components are ubiquitously expressed in all analysed tissues, while others show more restricted expression patterns which could be related to a particular function in plant development.
A wide expression analysis carried out on all E3 ubiquitin ligases annotated in this work (Supplementary Table S1) in different physiological conditions may help to understand and quantify the key role of the ubiquitination pathway in plant life. The analysis was performed in silico by screening all Arabidopsis 22K microarray data available at the GENE-VESTIGATOR site (https://www.genevestigator.ethz.ch – Metanalyser - Supplementary Table S3; Fig. 2) [62]. A gene was considered up- or down-regulated when transcript amount increased or decreased of at least two times in treated samples respect to control samples. The physiological conditions tested included biotic, abiotic and light stresses, various chemical treatments (i.e. hormone inhibitors), hormone response, senescence, sugars supply and nutrient starvation. About the 70% of E3 genes were present as probeset in the GENEVESTIGATOR dataset, but probeset for only 43% of F-box genes were found, according to EST-based expression data reported by Gagne et al. [50]. The expression level of only 128 E3 genes was not found affected by any of the considered treatment conditions. Genes not yet reported as expressed may be characterised by a very low or spatially/temporally restricted expression profile; alternatively, some of them could be pseudogenes.
Fig. (2).
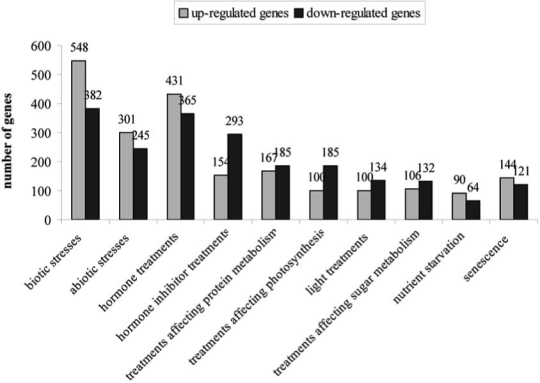
Changes in genes expression of E3 ubiquitin ligase genes following different conditions. A gene was considered up- or down-regulated when transcript amount increased or decreased of at least two times in treated samples respect to control samples. Up-regulated genes are reported in grey, down-regulated genes in black. Number of up- and down-regulated genes is indicated for each condition. The conditions considered are: biotic stresses (bacteria, fungi, insects, mycorrhiza, nematodes, syringolin, chitin); abiotic stresses (anoxia/ipoxia, cold, drought, heat, wounding, ozone, H2O2, osmotic, oxidative and salt stresses); hormone treatments (cytokinin, salicylic acid, jasmonate, auxin, gibberellin 3, ethylene, brassinosteroids, 1-aminocyclopropane-1-carboxylic acid (ACC) and ABA), hormone inhibitors (inhibitors of auxin, gibberellin, ethylene and brassinosteroids), treatments affecting protein metabolism (cycloheximide as inhibitor of protein synthesis, MG13 as inhibitor of proteasome), treatments affecting photosynthesis (high CO2, PNO8 as photosynthesis inhibitor, norflurazon as inhibitor of carotenoids synthesis), light treatments (dark, blue, far red, red, UV), treatments affecting sugar metabolism (glucose and sucrose supply), nutrient starvation (potassium, nitrogen, sulphur) and senescence. A gene was considered up- or down-regulated by a given condition when its expression changed following at least one of the treatments grouped in the considered condition. Expression data were obtained from GENEVESTIGATOR (2/14/2008 12:02PMhttps://www.genevestigator.ethz.ch – Metanalyser) [62].
Alternative splicing is one possibility to increase the number of proteins and their translation efficiency. An alternative splicing event was detected for ARI15 due to the existence of two different mRNA forms with or without the last intron within the 3’UTR, whose relative amount depended to some extent on the analysed tissue [24]. A similar intron retention event was described for other two Arabidopsis RING genes (At4g39140 and At2g21500) and for the homologous RING gene of durum wheat, 6G2, whose mRNA retained the last 3’UTR-located intron following exposure to abiotic stresses [63]. For several BTB Arabidopsis genes, available ESTs predicted the presence of multiple splice variants, although only for two genes, the protein coding region was predicted to be altered [44].
The expression analysis may supply important indications on the function of E3 ligases. AtRbx1 genes, participating to the assembly of CUL1-SCF and CUL3-BTB complexes, were found expressed in all plant organs, above all in tissues containing actively dividing cells, suggesting that ubiquitination may be involved in the turnover of key cell cycle regulatory plants proteins [34].
The three Ccs52 genes, which can form three different APC complexes, are expressed in different times during the progression cell cycle, furthermore Ccs52 proteins showed different specificity for different Arabidopsis cyclins, strongly indicating that APC complex composition and activity could be tightly regulated to control cell cycle progression by destroying different cyclins at the proper phase of the cell cycle [58].
Many ASK genes are expressed in the Arabidopsis gametophytes, mostly in the male one, suggesting some functional redundancy of the ASK genes and/or formation of different SCF complexes, based on the specificity of interaction between ASKs and F-box proteins during pollen grain development [49, 48].
Co-expression or interaction data on different E3 ubiquitin ligases and E2 conjugating enzymes, or different subunits of larger complexes, could implicate their functional association to control a specific biological process. Forty-five RING proteins representative of most RING and modified RING domains resulted to be active in an in vitro polyubiquitination assay carried out by using AtUBC8 as E2. The mutation of an amino acid at metal ligand positions C3, H4 and H/C5 of two RING-H2 proteins and the treatment of RING proteins with zinc chelators disrupted the ubiquitin ligase activity of the RING domain, demonstrating that also in plants RING proteins require an intact zinc-coordinating RING domain to mediate E2-dependent protein ubiquitination [25]. When the same set of RING proteins was assayed in combination with 15 different Arabidopsis UBC genes coding for E2 conjugating enzymes, a variable degree of specificity between E3 and E2 enzymes was observed. The UBC34 showed the highest specificity, being active in combination with only two E3 ubiquitin ligases. On the other hand, the most generic E2 conjugating enzymes were members of the UBC8 group, active with both canonical and modified RING types [59]. This experiment also showed that the same E3 ubiquitin ligase could act in association with different E2 conjugating enzymes suggesting that the activity of a single E3 component could depend not only on its own expression, but also on availability of a number of other proteins, E2, target and regulatory factors. The AIP2 (ABI3 interacting protein) E3 ligase mediates the ubiquitination of the transcription factor ABI3 involved in ABA (Abscisic Acid) signaling. However, whereas AIP2 transcripts were found ubiquitously in all plant tissues, ABI3 was exclusively expressed in developing and mature siliques and seeds. Therefore the same E3 ubiquitin ligase could act in different tissues and have different functions depending on the availability of the target protein [64].
The function of large complexes E3 ligases is a much more complicated task, because the E3 activity depends on the presence of a number of different subunits. Many E3 complexes have been characterised giving evidences supporting their critical role in plant life. Detailed studies have shown that the correct expression of some subunits can regulate the accumulation of the interacting proteins, or stabilise their interaction. For instance, RBX1 dsRNA lines contained a significant reduction of CUL1 protein level, suggesting that RBX1 protein is necessary to accumulate a stable SCF complex [34]. Furthermore, the presence of the F-box protein may stabilise interaction between CULLIN and the respective ASK adapter in SCF complexes. Indeed only weak interactions were observed by means of yeast two hybrid analysis for nearly all Arabidopsis SKP-1 related proteins (ASK1/2/3/4, ASK13/14/15/16 and ASK18/19), but a strong interaction between some ASK proteins and CUL1 was observed in presence of EID1 (Empfindlicher im Dunkelroten Licht 1 – i.e. more sensitive in far-red light) F-box [65]. Therefore, SCF assembly could be a two-step association process, in which the F-box protein first associates with the ASK adapter to form a unit that afterwards binds to the CULLIN core complex.
Despite the combinatorial possibilities for SCF complexes are very extensive, resulting from alternative association of 21 ASK proteins with 700 F-box proteins, a relatively limited number of functional SCF complexes with different ASK proteins and F-box combinations can be formed [43, 50]. For some F-box proteins no physical interactions were detected suggesting other functions rather than polyubiquitination [66], or requirement of the target or additional factors to associate with the rest of the SCF complex [50]. This possibility has been verified for some F-box proteins, which require another factor besides ASK/SKP1 for the interaction with CUL1 [43]. Additional evidences also suggest that higher order SCF complexes could be formed, involving more than one F-box protein. F-box dimerisation would allow more possibilities for regulation of the process, as well as higher level of potential combinatorial diversity.
Various interaction studies have been performed to support the presence of CUL3-BTB E3 complexes in Arabidopsis. Both CUL3A and CUL3B proteins interact with RBX1 and with a limited number of BTB protein [54, 67, 68]. However, the lack of interaction of CUL3 with some BTB-domain proteins indicates that BTB-proteins could function also in a CUL3-independent context. A more detailed analysis has been accomplished on the six members of the Arabi-dopsis BTB/POZ-MATH family (AtBPM1-6) [68], testing different interactions between AtBPM and CUL3 subunits. According to Dieterle et al. [54], only few AtBPM proteins were able to assemble with CUL3A and CUL3B. Interestingly, each AtBPM protein was able to form homodimers and heterodimers with all other members of the family, as reported also for some other BTB proteins, as hypothesised also for F-box proteins in SCF complexes [45, 69]. If verified in planta, this finding could have a potent consequence. Indeed, although limited interactions between CUL3 and BTB proteins reduce the combinatorial possibilities for BTB/POZ-dependent E3 ligases, the possibility of BTB/POZ homodimerization and heterodimerization supports a potentially very large number of distinct E3 ubiquitin ligases, rivalling the number of SCFs-type E3s.
Interaction studies allowed to verify the presence of the CUL4-DDB1 complexes in plants. Arabidopsis CUL4 forms complexes with DDB1 proteins, RBX1, DET1 and DDB2 in vitro and in planta [70]. DDB1a associates with DET1 and COP10, forming a stable protein complex, called CDD [56]. Furthermore, in dark conditions, the CDD complex directly interacts with COP1 and with the COP9 signalosome, a complex with regulatory function, able to post translationally modify the CULLIN subunit of E3 complexes [71]. In this case COP1, whose intrinsic ubiquitin ligase activity has been proved in vitro [72], could function as an adapter subunit of a larger E3 complex, maintaining the capability to bind both the E2 enzyme and substrates [56]. The COP1 interactors, including transcription factors and photoreceptors [28], suggest a strong involvement of the CUL4-DDB1 dependent ubiquitination mechanism in photomorphogenesis process.
UBIQUITIN-MEDIATED DEGRADATION: A RECURRENT THEME IN PLANT LIFE CYCLE
Most aspects of plant life cycle are somehow regulated by ubiquitin-mediated degradation of key proteins. This feature gets critical when target proteins are short-lived regulatory components, like enzymes directing rate-limiting steps, signaling receptors and transcriptional factors [73].
Most of the E3 ubiquitin ligases characterised up-to-date act in regulating plant development and hormone signaling [74, 75] (Table 2). Notably, an effect frequently observed in E3 ligase Arabidopsis insertional mutants is the arrest of embryo development. For four T-DNA insertional lines for ATL-RING genes only hemizygous lines were recovered, suggesting that the mutated genes could be essential for plant viability [26]. The embryo lethality associated to gene disruption was also described for other RING E3 ubiquitin ligases, as the Arabidopsis RIE1 [76] and PEX12. The analysis of RNA interference plants with partial reduction of the PEX12 transcript, exhibiting impaired peroxisome biogenesis and function, addressed the PEX12 function to the regulation of the peroxisome development [77].
Since RBX1, ASK and CUL proteins participate to form several E3 complexes, they could be involved in the control of many different processes, therefore, in absence of functional redundancy, many loss of function mutants may have lethal phenotype. Indeed, Arabidopsis mutants containing T-DNA insertions in the CUL1 gene displayed an arrest in early embryogenesis, before the first cell division of both embryo and endosperm cells [42]. Consistently, both the transcript and the product of the CUL1 gene were found to accumulate in embryos. The CUL1 protein was localized mainly in the nucleus, weakly present in the cytoplasm during interphase and co-localized with the mitotic spindle in metaphase. CUL3A and CUL3B appear functional redundant, since cul3a and cul3b mutants are viable and, consistently, they have largely overlapping expression patterns. However, the disruption of both the CUL3A and CUL3B genes affected both embryo pattern formation and endosperm development, causing embryo lethality [44, 45, 78]. Although arrest at the heart stage was predominant, block of embryogenesis occurring at multiple stages of embryo development indicated a general growth inhibition of the embryo. CUL4-DDB1 complex also appears essential, being the ddb1b mutation embryo lethal, although Arabidopsis contains two highly related DDB1 proteins [55]. According to RBX1 involvement in the formation of SCF, CUL3-BTB and CUL4-DDB1 complexes, severe phenotypes were observed for Arabidopsis RBX1 dsRNA lines, consistently with the suppression level [34], including the block of seedling development, death of young seedlings, or severe dwarf phenotypes. These findings strongly supported the essential role of E3 complexes in many plant biological processes activated during plant embryogenesis and post-embryonic development.
Deregulation of RBX1 protein level in dsRNA lines and mutations in ASK1 gene led also to phenotypes with reduced auxin response and decreased jasmonate response, similar to those observed in the axr1, tir1, and coi1 mutants, substantiating the role of RBX1 and ASK1 as components the SCFTir1 and SCFCoi1 complexes, known to be involved in auxin and jasmonate signaling respectively [46, 79, 80].
Ask1 mutants and RNAi lines showed also severe flower defects [49], in accordance to previous evidences of association between ASK1 and the F-box protein UFO, involved in flower development and male sterility [81–83]. Although a role of ASK1 in GA-signal transduction is not yet demonstrated, the SCF-complex SLY (SLEEPY) regulates GA response [84] and the RNAi line showed also features similar to those found in GA-deficient or insensitive mutants [49]. ASK1 was found to interact also with EID1, a F-box involved in phytocrome-A specific light signaling [65]. The F-box proteins EBF1/2 (EIN3-Binding F-box) [85] known to belong to the ethylene signaling pathway, and ORE9 (ORESARA9 - “long living”), are involved in the regulation of plant leaf senescence [86], whereas the F-box protein LKP2 (LOV KELCH Protein 2) and ZTL (ZEIT- LUPE) are involved in the regulation of circadian timings [87, 88]. Very recently the proteomes of the Arabidopsis wild type and ask1 mutant flower buds have been compared evidencing ten proteins involved in photomorphogenesis, circadian oscillation, post-translation process, stress-responses and cell expansion or elongation, all processes being misregulated in the mutant [89].
Although the implication of ASK1 in various cell processes, ask1 mutants are viable. In a similar manner, although the ASK2 protein was found to be associated with several F-box proteins, such as TIR1 (Transport-Inhibitor Response 1) [46], COI1 (Coronatine-insensitive) [90], UFO (Unusual Floral Organ) [91], and EID1 [92], ask2 mutant is morphologically similar to wild type. Therefore partially functional redundancy has been evocated to explain the viability of the two mutants. Indeed, the ask1-ask2 double mutation affects cell division and cell expansion/elongation, resulting in delayed embryogenesis and seedlings lethality, and demonstrating a vital role for ASK1 and ASK2 in embryogenesis and postembryonic development [93].
Functional analyses have been accomplished for some BTB proteins linking individual BTB proteins to several processes in plants. The best characterised BTB protein is ETO1 (Ethylene-Overproducer 1), a protein involved in the control of ethylene biosynthesis. As reported above, it is able to interact with CUL3A, likely forming a BTBETO1. Moreover it was found to target for degradation of the ACC synthase ACS5, the rate limiting enzyme of the ethylene biosynthesis and the first reported substrate for a plant CUL3-based E3 [67].
For other Arabidopsis BTB proteins, possible functions have been inferred biochemically, as the transcriptional regulation activity of some members of BTB family, characterised by a calmodulin-binding domain, in response to H2O2 and salicylic acid treatments [94]. FIP2 (Rab11-Family of Interacting Protein 2) protein was found to interact with Arabidopsis Formin Homology 1, AFH1 [95], a protein required for polar pollen tube extension [96]. The involvement of E3 ubiquitin ligases in signaling of hormones as ABA, jasmonic and salicilic acids indicates a strong functional role of ubiquitination pathway in plant response to environmental clues. The most studied system is represented by COP1/CIP8 and their role in regulation of photomorphogenesis [28, 97, 98]. The role of E3 ubiquitin ligases in response to plant pathogen has also been widely described [99–102]. Regarding response to abiotic stresses, some E3 ubiquitin ligases regulate stress response acting also in hormone independent pathways, as HOS1 ubiquitinating the key regulator of cold stress response ICE1 [33].
A recent work [70] has proven that Arabidopsis CUL4 participates in important processes such as the cell cycle, light-dependent growth control, modulation of chromosomal structure and DNA repair. Cul4 mutants are also severely affected in different aspects of the development: a reduced level of CUL4 expression leads to a reduced number of lateral roots, to abnormal vascular tissue and stomatal development, and to weakly altered response to photomorphogenic stimuli.
In silico expression analysis indicated that the E3 ubiquitin ligases gene family could be involved in much more biological processes than those known until now since changes in E3 gene expression were found in response to a wide range of growing conditions (Table 2, Fig. 2, Supplementary Table S3). A role of E3 ubiquitin ligases has been widely described in auxin, gibberellins, ethylene and ABA responses, nevertheless the expression data also indicate a strong involvement of E3 ubiquitin ligases in biological processes mediated by brassinosteroids (231 and 70 genes up- and down regulated respectively, Supplementary Table S3). Genes belonging to all E3 ubiquitin ligase sub families were reported as regulated by these hormones, including one HECT gene, suggesting a new role for this group whose function is very poorly understood in plants.
Particularly interesting is the case of APC complexes. They have been originally described as involved in the cell cycle progression [35]. Recently, loss of function mutants for the HOBBIT/Cdc27B gene, homologous of APC3 human subunit, were found unable to degrade the AXR3/IAA17 repressor of auxin response, indicating that the APC complex is also involved in hormone response [103]. Expression analyses suggest a role for these complexes in pathways controlled by many other hormones (some APC subunits are up-regulated by ABA, ACC, brassinosteroids, gibberellins and auxin and down-regulated by ethylene, salicylic acid and cytokinins). Furthermore, APC subunits were found up-regulated by light treatments, and down-regulated by all abiotic stresses except anoxia and cold (Supplementary Table S3).
A lower number of E3 ubiquitin ligase genes are also regulated by abiotic stresses, light and photosynthetic activity. Furthermore, the in silico expression analysis has provided evidences for the involvement of E3 ubiquitin ligases in other processes not previously described as potentially regulated by ubiquitination such as senescence, ipoxia/anoxia, sugar regulated pathways and nutrient starvation (Supplementary Table S3; Fig. 2).
Unfortunately, despite the huge knowledge obtained on large E3 ligase family in plants from a genomic point of view, wide information are still lacking about individuation of target proteins, that remains the most intriguing, and poorly understood aspect of the ubiquitination pathway, which, by specifically affecting many components of cellular regulation, can participate to the fine tuning of cellular response to the variable life conditions.
Supplementary Material
E3 ubiquitin ligases involved in plant growth and development
In silico expression analysis carried out on all E3 ubiquitin ligases annotated in this work (Supplementary Table S1) in different physiological conditions in Arabidopsis.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Agriculture (Projects AGRONANOTECH and FRUMISIS) of Italy. We are grateful to Mr. Astesano and Mr. Ambroggi for the valuable contribution to the collection and analysis of expression data.
REFERENCES
- 1.Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol. 1999;9(24):1458–67. doi: 10.1016/s0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]
- 2.Rankin CA, Joazeiro CA, Floor E, Hunter T. E3 ubiquitin-protein ligase activity of Parkin is dependent on cooperative interaction of RING finger (TRIAD) elements. J Biomed Sci. 2001;8(5):421–9. doi: 10.1007/BF02255952. [DOI] [PubMed] [Google Scholar]
- 3.Sun L, Chen ZJ. The novel functions of ubiquitination in signaling. Curr Opin Cell Biol. 2004;16:119–126. doi: 10.1016/j.ceb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 5.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–33. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 6.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SY, Pagano M, Conaway RC, Conaway JW, Harper JW, Pavletich NP. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 7.Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86(2):263–74. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 8.Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 9.Schulman BA, Carrano AC, Jeffrey PD, Bowen Z, Kinnucan ERE, Finnin MS, Elledge SJ, Harper JW, Pagano M, Pavletich NP. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature. 2000;408:381–386. doi: 10.1038/35042620. [DOI] [PubMed] [Google Scholar]
- 10.Pintard L, Willems A, Peter M. Cullin-based ubiquitin ligases: Cul3-BTB complexes join the family. EMBO J. 2004;23(8):1681–7. doi: 10.1038/sj.emboj.7600186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer R, Wee S, Anderson S, Yates JR, III, Wolf DA. BTB/POZ domain proteins are putative substrate adapters for cullin 3 ubiquitin ligases. Molecular Cell. 2003;12:783–790. doi: 10.1016/s1097-2765(03)00341-1. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa M, Ohta T, Xiong Y. Activation of UBC5 ubiquitinconjugating enzyme by the RING finger of ROC1 and assembly of active ubiquitin ligases by all cullins. J Biol Chem. 2002;277:15758–15765. doi: 10.1074/jbc.M108565200. [DOI] [PubMed] [Google Scholar]
- 13.McCall CM, Hu J, Xiong Y. Recruiting substrates to Cullin 4-dependent ubiquitin ligases by DDB1. Cell Cycle. 2005;4(1):27–9. doi: 10.4161/cc.4.1.1396. [DOI] [PubMed] [Google Scholar]
- 14.Gmachl M, Gieffers C, Podtelejnikov AV, Mann M, Peters JM. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc Natl Acad Sci USA. 2000;97:8973–8. doi: 10.1073/pnas.97.16.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vodermaier HC, Gieffers C, Maurer-Stroh S, Eisenhaber F, Peters JM. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr Biol. 2003;2(13):1459–68. doi: 10.1016/s0960-9822(03)00581-5. [DOI] [PubMed] [Google Scholar]
- 16.Huibregtse JM, Scheffner M, Beaudenon S, Howley PM. A family of proteins structurally and functionally related to the E6AP ubiquitin-protein ligase. Proc Natl Acad Sci USA. 1995;92:2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downes BP, Stupar RM, Gingerich DJ, Vierstra RD. The HECT ubiquitin-protein ligase (UPL) family in Arabidopsis: UPL3 has a specific role in trichome development. Plant J. 2003;3(6):729–742. doi: 10.1046/j.1365-313x.2003.01844.x. [DOI] [PubMed] [Google Scholar]
- 18.Bates PW, Vierstra RD. UPL1 and 2, two 405 kDa ubiquitin-protein ligases from Arabidopsis thaliana related to the HECT-domain protein family. Plant J. 1999;20(2):183–195. doi: 10.1046/j.1365-313x.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 19.Freemont PS, Hanson IM, Trowsdale J. A novel cysteinerich sequence motif. Cell. 1991;64:483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- 20.Barlow PN, Luisi B, Milner A, Elliott M, Everett R. Structure of the C3HC4 domain by 1H-nuclear magnetic resonance spectroscopy. A new structural class of zinc-finger. J Mol Biol. 1994;237(2):201–11. doi: 10.1006/jmbi.1994.1222. [DOI] [PubMed] [Google Scholar]
- 21.Borden KL. RING domains: master builders of molecular scaffolds? J Mol Biol. 2000;295(5):1103–12. doi: 10.1006/jmbi.1999.3429. [DOI] [PubMed] [Google Scholar]
- 22.Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad, Sci USA. 1999;96(20):11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosarev P, Mayer KFX, Hardtke CS. Evaluation and classification of RING-finger domains encoded by the Arabidopsis genome. Genome Biol. 2002;3(4):0016.1–0016.12. doi: 10.1186/gb-2002-3-4-research0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mladek C, Guger K, Hauser MT. Identification and characterization of the ARIADNE gene family in Arabidopsis. A group of putative E3 ligases. Plant Physiol. 2003;131:27–40. doi: 10.1104/pp.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone SL, Hauksdóttir H, Troy A, Herschleb J, Kraft E, Callis J. Functional analysis of the RING-Type ubiquitin ligase family of Arabidopsis . Plant Physiol. 2005;137:13–30. doi: 10.1104/pp.104.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano M, Parra S, Alcaraz LD, Guzmán P. The ATL gene family from Arabidopsis thaliana and Oryza sativa comprises a large number of putative ubiquitin ligases of the RING-H2 type. J Mol Evol. 2006;62:434–445. doi: 10.1007/s00239-005-0038-y. [DOI] [PubMed] [Google Scholar]
- 27.Orlicky S, Tang X, Willems A, Tyers M, Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112:243–256. doi: 10.1016/s0092-8674(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 28.Yi C, Deng XW. COP1 - from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15(11):618–25. doi: 10.1016/j.tcb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Salinas-Mondragon RE, Garciduenas-Pina C, Guzman P. Early elicitor induction in members of a novel multigene family coding for highly related RING-H2 proteins in Arabidopsis thaliana. Plant Mol Biol. 1999;40:579–590. doi: 10.1023/a:1006267201855. [DOI] [PubMed] [Google Scholar]
- 30.Hannoufa A, Negruk V, Eisner G, Lemieux B. The CER3 gene of Arabidopsis thaliana is expressed in leaves, stems, roots, flowers and apical meristems. Plant J. 1996;10:459–467. doi: 10.1046/j.1365-313x.1996.10030459.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee H, Xiong L, Gong Z, Ishitani M, Stevenson B, Zhu JK. The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo-cytoplasmic partitioning. Genes Dev. 2001;15:912–924. doi: 10.1101/gad.866801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zolman BK, Monroe-Augustus M, Silva ID, Bartel B. Identification and Functional Characterization of Arabidopsis PEROXIN4 and the Interacting Protein PEROXIN22. Plant Cell. 2005;17(12):3422–3435. doi: 10.1105/tpc.105.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong CH, Agarwal M, Zhang Y, Xie Q, Zhu JK. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc Natl Acad, Sci USA. 2006;103:8281–8286. doi: 10.1073/pnas.0602874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lechner E, Xie D, Grava S, Pigaglio E, Planchais S, Murray JAH, Parmentier Y, Mutterer J, Dubreucq B, Shen WH, Genschik P. The AtRbx1 protein is part of plant SCF complexes, and its down-regulation causes severe growth and developmental defects. J Biol Chem. 2002;277(51):50069–50080. doi: 10.1074/jbc.M204254200. [DOI] [PubMed] [Google Scholar]
- 35.Capron A, Okresz L, Genschik P. First glance at the plant APC/C, a highly conserved ubiquitin-protein ligase. Trends Plant Sci. 2003;8(2):83–9. doi: 10.1016/S1360-1385(02)00028-6. [DOI] [PubMed] [Google Scholar]
- 36.Capron A, Serralbo O, Fulop K, Frugier F, Parmentier Y, Dong A, Lecureuil A, Guerche P, Kondorosi E, Scheres B, Genschik P. The Arabidopsis anaphase-promoting complex or cyclosome: molecular and genetic characterization of the APC2 subunit. Plant Cell. 2003;15:2370–2382. doi: 10.1105/tpc.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohi MD, Vander Kooi CW, Rosenberg JA, Chazin WJ, Gould KL. Structural insights into the U-box, a domain associated with multiubiquitination. Nat Struct Biol. 2003;10:250–255. doi: 10.1038/nsb906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone SL, Anderson EM, Mullen RT, Goring DR. ARC1 is an E3 ubiquitin ligase and promotes the ubiquitination of proteins during the rejection of self-incompatible Brassica pollen. Plant Cell. 2003;15:885–898. doi: 10.1105/tpc.009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azevedo C, Santos-Rosa MJ, Shirasu K. The U-box protein family in plants. Trends Plant Sci. 2001;6:354–358. doi: 10.1016/s1360-1385(01)01960-4. [DOI] [PubMed] [Google Scholar]
- 40.Luo J, Shen G, Yan J, He C, Zhang H. AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment. Plant J. 2006;46:649–657. doi: 10.1111/j.1365-313X.2006.02730.x. [DOI] [PubMed] [Google Scholar]
- 41.Mudgil Y, Shiu SH, Stone SL, Salt JN, Goring DR. A large complement of the predicted Arabidopsis ARM repeat proteins are members of the U-Box E3 ubiquitin ligase family. Plant Physiol. 2004;134:59–66. doi: 10.1104/pp.103.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen W, Parmentier Y, Hellmann H, Lechner E, Masson ADJ, Granier F, Lepiniec L, Estelle M, Genschik P. Null mutation of AtCUL1 causes arrest in early embryogenesis in Arabidopsis. Mol Biol Cell. 2002;13:1916–1928. doi: 10.1091/mbc.E02-02-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, Estelle M, Somers DE, Crosby WL. Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J. 2003;34(6):753–767. doi: 10.1046/j.1365-313x.2003.01768.x. [DOI] [PubMed] [Google Scholar]
- 44.Gingerich DJ, Gagne JM, Salter DW, Hellmann H, Estelle M, Ma L, Vierstra RD. Cullins 3a and 3b assemble with members of the broad complex/tramtrack/bric-a-brac (BTB) protein family to form essential ubiquitin-protein ligases (E3s) in Arabidopsis. J Biol Chem. 2005;280(19):18810–18821. doi: 10.1074/jbc.M413247200. [DOI] [PubMed] [Google Scholar]
- 45.Figueroa P, Gusmaroli G, Serino G, Habashi J, Ma L, Shen Y, Feng S, Bostick M, Callis J, Hellmann H, Deng XW. Arabidopsis has two redundant Cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes in vivo. Plant Cell. 2005;17:1180–1195. doi: 10.1105/tpc.105.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M. Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev. 1999;13:1678–1691. doi: 10.1101/gad.13.13.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farras R, Ferrando A, Jasik J, Kleinow T, Okresz L, Tiburcio A, Salchert K, del Pozo C, Schell J, Koncz C. SKP1-SnRK protein kinase interactions mediate proteasomal binding of a plant SCF ubiquitin ligase. EMBO J. 2001;20:2742–2756. doi: 10.1093/emboj/20.11.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marrocco K, Lecureuil A, Nicolas P, Guerce P. The Arabidopsis SKP1-like genes present a spectrum of expression profiles. Plant Mol Biol. 2003;52:715–727. doi: 10.1023/a:1025056008926. [DOI] [PubMed] [Google Scholar]
- 49.Zhao D, Ni W, Feng B, Han T, Petrasek MG, Ma H. Members of the ASK gene family exhibit a variety of expression patterns and may play diverse roles in Arabidopsis. Plant Physiol. 2003;133:203–217. doi: 10.1104/pp.103.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci USA. 2002;99(17):11519–24. doi: 10.1073/pnas.162339999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuroda H, Takahashi N, Shimada H, Seki M, Shinozaki K, Matsui M. Classification and expression analysis of Arabidopsis F-Box-Containing protein genes. Plant Cell Physiol. 2002;43:1073–1085. doi: 10.1093/pcp/pcf151. [DOI] [PubMed] [Google Scholar]
- 52.Bardwell VJ, Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994;8:1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- 53.Stogios PJ, Downs GS, Jauhal JS, Nandra SK, Privé GG. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005;6(10):R82. doi: 10.1186/gb-2005-6-10-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dieterle M, Thomann A, Renou JP, Parmentier Y, Cognat V, Lemonnier G, Muller R, Shen WH, Kretsch T, Genschik P. Molecular and functional characterization of Arabidopsis Cullin 3A. Plant J. 2005;41(3):386–99. doi: 10.1111/j.1365-313X.2004.02302.x. [DOI] [PubMed] [Google Scholar]
- 55.Schroeder DF, Gahrtz M, Maxwell BB, Cook RK, Kan JM, Alonso JM, Ecker JR, Chory J. De-etiolated 1 and damaged DNA binding protein 1 interact to regulate Arabidopsis photomorphogenesis. Curr Biol. 2002;12:1462–1472. doi: 10.1016/s0960-9822(02)01106-5. [DOI] [PubMed] [Google Scholar]
- 56.Yanagawa Y, Sullivan JA, Komatsu S, Gusmaroli G, Suzuki G, Yin J, Ishibashi T, Saijo Y, Rubio V, Kimura S, Wang J, Deng XW. Arabidopsis COP10 forms a complex with DDB1 and DET1 in vivo and enhances the activity of ubiquitin conjugating enzymes. Genes Dev. 2004;18(17):2172–2181. doi: 10.1101/gad.1229504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koga A, Ishibashi T, Kimura S, Uchiyama Y, Sakaguchi K. Characterization of T-DNA Insertion Mutants and RNAi Silenced Plants of Arabidopsis thaliana UV-damaged DNA Binding Protein 2 (AtUV-DDB2) Plant Mol Biol. 2005;61(1-2):227–240. doi: 10.1007/s11103-006-6408-z. [DOI] [PubMed] [Google Scholar]
- 58.Fülöp K, Tarayre S, Kelemen Z, Horváth G, Kevei Z, Nikovics K, Bakó L, Brown S, Kondorosi A, Kondorosi E. A-rabidopsis anaphase-promoting complexes: multiple activators and wide range of substrates might keep APC perpetually busy. Cell Cycle. 2005;4(8):1084–1092. [PubMed] [Google Scholar]
- 59.Kraft E, Stone SL, Ma L, Su N, Gao Y, Lau OS, Deng XW, Callis J. Genome analysis and functional characterization of the E2 and RING-type E3 ligase ubiquitination enzymes of Arabidopsis. Plant Physiol. 2005;139(4):1597–611. doi: 10.1104/pp.105.067983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai CP, Lee CL, Chen PH, Wu SH, Yang CC, Shaw JF. Molecular analyses of the Arabidopsis TUBBY-Like protein gene family. Plant Physiol. 2004;134:1586–1597. doi: 10.1104/pp.103.037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiao H, Wang H, Zhao L, Zhou J, Huang J, Zhang Y, Xue Y. The F-box protein AhSLF-S2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum. Plant Cell. 2004;16:582–595. doi: 10.1105/tpc.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis Microarray Database and Analysis Toolbox. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mastrangelo AM, Belloni S, Barilli S, Reperti B, Di Fonzo N, Stanca AM, Cattivelli L. Low temperature promotes intron retention in two e-cor genes of durum wheat. Planta. 2005;221:705–715. doi: 10.1007/s00425-004-1475-3. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Garreton V, Chua NH. The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev. 2005;19:1532–1543. doi: 10.1101/gad.1318705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marrocco K, Zhou Y, Bury E, Dieterle M, Funk M, Genschik P, Krenz M, Stolpe T, Kretsch T. Functional analysis of EID1, an F-box protein involved in phytochrome A-dependent light signal transduction. Plant J. 2006;45(3):423–438. doi: 10.1111/j.1365-313X.2005.02635.x. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Dong L, Zhang Y, Zhang Y, Wu W, Deng X, Xue Y. Genome-wide analysis of S-Locus F-box-like genes in Arabidopsis thaliana. Plant Mol Biol. 2004;56:929–945. doi: 10.1007/s11103-004-6236-y. [DOI] [PubMed] [Google Scholar]
- 67.Wang KLC, Yoshida H, Lurin C, Ecker JR. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature. 2004;428:945–950. doi: 10.1038/nature02516. [DOI] [PubMed] [Google Scholar]
- 68.Weber H, Bernhardt A, Dieterle M, Hano P, Mutlu A, Estelle M, Genschik P, Hellmann H. Arabidopsis AtCUL3a and AtCUL3b form complexes with members of the BTB/POZ-MATH protein family. Plant Physiol. 2005;137:83–93. doi: 10.1104/pp.104.052654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inada S, Ohgishi M, Mayama T, Okada K, Tatsuya S. RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell. 2004;16:887–896. doi: 10.1105/tpc.019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernhardt A, Lechner E, Hano P, Schade V, Dieterle M, Anders M, Dubin MJ, Benvenuto G, Bowler C, Genschik P, Hellmann H. CUL4 associates with DDB1 and DET1 and its downregulation affects diverse aspects of development in Arabidopsis thaliana. Plant J. 2006 doi: 10.1111/j.1365-313X.2006.02810.x. doi: 10.1111/J.1365-313X.2006. 02810.x. [DOI] [PubMed] [Google Scholar]
- 71.von Arnim AG. On again - off again: Cop9 signalosome turns the key on protein degradation. Curr Opin Plant Biol. 2003;6:520–529. doi: 10.1016/j.pbi.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, Deng XW. The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 2003;17(21):2642–7. doi: 10.1101/gad.1122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smalle J, Vierstra RD. The ubiquitin 26S Proteasome proteolytic pathway. Ann Rev Plant Biol. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- 74.Moon J, Parry G, Estelle M. The Ubiquitin-Proteasome. Pathway and Plant Development. Plant Cell. 2003;16:3181–3195. doi: 10.1105/tpc.104.161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomann A, Dieterle M, Genschik P. Plant CULLIN-based E3s: Phytohormones come first. FEBS Lett. 2005;579:3239–3245. doi: 10.1016/j.febslet.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 76.Xu R, Li QQ. A RING-H2 zinc-finger protein gene RIE1 is essential for seed development in Arabidopsis. Plant Mol Biol. 2003;53(1-2):37–50. doi: 10.1023/b:plan.0000009256.01620.a6. [DOI] [PubMed] [Google Scholar]
- 77.Fan J, Quan S, Orth T, Awai C, Chory J, Hu J. The Arabidopsis PEX12 Gene Is Required for Peroxisome Biogenesis and Is Essential for Development. Plant Physiol. 2005;139:231–239. doi: 10.1104/pp.105.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomann A, Brukhin V, Dieterle M, Gheyeselinck J, Vantard M, Grossniklaus U, Genschik P. Arabidopsis CUL3A and CUL3B genes are essential for normal embryogenesis. Plant J. 2005;43(3):437–448. doi: 10.1111/j.1365-313X.2005.02467.x. [DOI] [PubMed] [Google Scholar]
- 79.Beckers GJ, Spoel SH. Fine-Tuning Plant Defence Signalling: Salicylate versus Jasmonate. Plant Biol (Stuttg) 2006;8(1):1–10. doi: 10.1055/s-2005-872705. [DOI] [PubMed] [Google Scholar]
- 80.Ren C, Pan J, Peng W, Genschik P, Hobbie L, Hellmann H, Estelle M, Gao B, Peng J, Sun C, Xie D. Point mutations in Arabidopsis Cullin1 reveal its essential role in jasmonate response. Plant J. 2005;(4):514–24. doi: 10.1111/j.1365-313X.2005.02394.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhao D, Yu Q, Chen M, Ma H. The ASK1 gene regulates B function gene expression in cooperation with UFO and LEAFY in Arabidopsis. Genes Dev. 2001;128:2735–2746. doi: 10.1242/dev.128.14.2735. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Wu H, Liang G, Yang M. Defects in nucleolar migration and synapsis in male prophase I in the ask1-1 mutant of Arabidopsis. Sex Plant Reprod. 2004;16:273–282. [Google Scholar]
- 83.Wang Y, Yang M. The ARABIDOPSIS SKP1-LIKE1 (ASK1) protein acts predominately from leptotene to pachytene and represses homologous recombination in male meiosis. Planta. 2006;223:613–617. doi: 10.1007/s00425-005-0154-3. [DOI] [PubMed] [Google Scholar]
- 84.McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, Steber CM. The Arabidopsis SLEEPY1 gene encodes a putative F-Box subunit of an SCF E3 ubiquitin ligase. Plant Cell. 2003;15(5):1120–1130. doi: 10.1105/tpc.010827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell. 2003;115:679–689. doi: 10.1016/s0092-8674(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 86.Woo HR, Chung KM, Park JH, Oh SA, Ahn T, Hong SH, Jang SK, Nam HG. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell. 2001;13:1779–1790. doi: 10.1105/TPC.010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yasuhara M, Mitsui S, Hirano H, Takanabe R, Tokioka Y, Ihara N, Komatsu A, Seki M, Shinozaki K, Kiyosue T. Identification of ASK and clock-associated proteins as molecular partners of LKP2 (LOV kelch protein 2) in Arabidopsis. J Exp Bot. 2004;55(405):2015–2027. doi: 10.1093/jxb/erh226. [DOI] [PubMed] [Google Scholar]
- 88.Han L, Mason M, Risseeuw EP, Crosby WL, Somers DE. Formation of an SCF(ZTL) complex is required for proper regulation of circadian timing. Plant J. 2004;40(2):291–301. doi: 10.1111/j.1365-313X.2004.02207.x. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Ni W, Ge X, Zhang J, Ma H, Cao K. Proteomic identification of potential target proteins regulated by an ASK1-mediated proteolysis pathway. Cell Res. 2006;16(5):489–98. doi: 10.1038/sj.cr.7310060. [DOI] [PubMed] [Google Scholar]
- 90.Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang D, Xie D. The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell. 2002;14:1919–1935. doi: 10.1105/tpc.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Samach A, Klenz JE, Kohalmi SE, Risseeuw E, Haughn GW, Crosby WL. The UNUSUAL FLORAL ORGANS gene of Arabidopsis thaliana is an F-box protein required for normal patterning and growth in the floral meristem. Plant J. 1999;20:433–445. doi: 10.1046/j.1365-313x.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 92.Dieterle M, Zhou YC, Schafer E, Funk M, Kretsch T. EID1, an F-box protein involved in phytochrome A-specific light signalling. Genes Dev. 2001;15:939–944. doi: 10.1101/gad.197201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu F, Ni W, Griffith ME, Huang Z, Chang C, Peng W, Ma H, Xie D. The ASK1 and ASK2 genes are essential for Arabidopsis early development. Plant Cell. 2004;16:5–20. doi: 10.1105/tpc.017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du L, Poovaiah BW. A novel family of Ca2+/calmodulin-binding proteins involved in transcriptional regulation: Interaction with fsh/Ring3 class transcription activators. Plant Mol Biol. 2004;54:549–569. doi: 10.1023/B:PLAN.0000038269.98972.bb. [DOI] [PubMed] [Google Scholar]
- 95.Banno H, Chua NH. Characterization of the Arabidopsis formin-like protein AFH1 and its interacting protein. Plant Cell Physiol. 2000;41:617–626. doi: 10.1093/pcp/41.5.617. [DOI] [PubMed] [Google Scholar]
- 96.Cheung AY, Wu Hm. Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell. 2004;16:257–269. doi: 10.1105/tpc.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoecher U. Regulated proteolysis in light signalling. Curr Op Plant Biol. 2005;8:469–476. doi: 10.1016/j.pbi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Roig-Villanova I, Bou J, Sorin C, Devlin PF, Martínez-García JF. Identification of primary target genes of phytochrome signaling. Early transcriptional control during shade avoidance responses in Arabidopsis. Plant Physiol. 2006;141:85–96. doi: 10.1104/pp.105.076331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Devoto A, Muskett PR, Shirasu K. Role of ubiquitination in the regulation of plant defence against pathogens. Curr Opin Plant Biol. 2003;6(4):307–311. doi: 10.1016/s1369-5266(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 100.Mahmut T, Yemm A, Holub E. The role of proteolysis in R gene mediated defence in plants. Mol Plant Pathol. 2003;4(4):287–296. doi: 10.1046/j.1364-3703.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 101.Tör M, Yemm A, Holub E. The role of proteolysis in R gene mediated defence in plants. Mol Plant Pathol. 2003;4:287–296. doi: 10.1046/j.1364-3703.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 102.Delaure SL, Broekaert WF, De Bolle MF, Cammue BP. The role of ethylene in host - pathogen interactions. Annu Rev Phytopathol. 2006;44 doi: 10.1146/annurev.phyto.44.070505.143440. doi: 10.1146/ annurev.phyto. 44.070505. 143440. [DOI] [PubMed] [Google Scholar]
- 103.Blilou I, Frugier F, Folmer S, Serralbo O, Willemsen V, Wolkenfelt H, Eloy NB, Ferreira PC, Weisbeek P, Scheres B. The Arabidopsis HOBBIT gene encodes a CDC27 homolog that links the plant cell cycle to progression of cell differentiation. Genes Dev. 2002;16:2566–2575. doi: 10.1101/gad.237302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X, Feng S, Nakayama N, Crosby WL, Irish V, Deng XW, Wei N. The COP9 signalosome interacts with SCF UFO and participates in Arabidopsis flower development. Plant Cell. 2003;15(5):1071–82. doi: 10.1105/tpc.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nelson DC, Lasswell J, Rogg LE, Cohen MA, Bartel B. FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell. 2000;101(3):331–40. doi: 10.1016/s0092-8674(00)80842-9. [DOI] [PubMed] [Google Scholar]
- 106.Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science. 2005;309(5732):293–7. doi: 10.1126/science.1110586. [DOI] [PubMed] [Google Scholar]
- 107.Takato I, Schultz TF, Harmon FG, Ho LA, Kay SA. FKF1 F-Box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science. 2005;309:293–297. doi: 10.1126/science.1110586. [DOI] [PubMed] [Google Scholar]
- 108.Kim S, Choi HI, Ryu HJ, Park JH, Kim MD, Kim SY. ARIA, an Arabidopsis arm repeat protein interacting with a transcriptional regulator of abscisic acid-responsive gene expression, is a novel abscisic acid signaling component. Plant Physiol. 2004;136(3):3639–48. doi: 10.1104/pp.104.049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes Dev. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature. 2001;414(6861):271–6. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- 111.Kepinsky S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 112.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 113.Strader LC, Ritchie S, Soule JD, McGinnis KM, Steber CM. Recessive-interfering mutations in the gibberellin signaling gene SLEEPY1 are rescued by overexpression of its homologue, SNEEZY. Proc Natl Acad Sci USA. 2004;101(34):12771–6. doi: 10.1073/pnas.0404287101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gomi K, Sasaki A, Itoh H, Ueguchi-Tanaka M, Ashikari M, Kitano H, Matsuoka M. GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice. Plant J. 2004;37(4):626–34. doi: 10.1111/j.1365-313x.2003.01990.x. [DOI] [PubMed] [Google Scholar]
- 115.Itoh H, Sasaki A, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, Matsuoka M. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science. 2003;299(5614):1896–8. doi: 10.1126/science.1081077. [DOI] [PubMed] [Google Scholar]
- 116.Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell. 2003;115(6):667–77. doi: 10.1016/s0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- 117.Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra RD. Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA. 2004;101(17):6803–8. doi: 10.1073/pnas.0401698101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chua NH, Garreton V, Zhang X. The AIP2 E3 ligase acts as a novel negative regulator of ABA signalling by promoting ABI3 degradation. Genes Dev. 2005;19(13):1532–43. doi: 10.1101/gad.1318705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie Q, Guo HS, Dallman G, Fang S, Weissman AM, Chua NH. SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature. 2002;419(6903):167–70. doi: 10.1038/nature00998. [DOI] [PubMed] [Google Scholar]
- 120.Nodzon LA, Xu WH, Wang Y, Pi LY, Chakrabarty PK, Song WY. The ubiquitin ligase XBAT32 regulates lateral root development in Arabidopsis. Plant J. 2004;40(6):996–1006. doi: 10.1111/j.1365-313X.2004.02266.x. [DOI] [PubMed] [Google Scholar]
- 121.Song HR, Carre IA. DET1 regulates the proteasomal degradation of LHY, a component of the Arabidopsis circadian clock. Plant Mol Biol. 2005;57:761–771. doi: 10.1007/s11103-005-3096-z. [DOI] [PubMed] [Google Scholar]
- 122.Motchoulski A, Liscum E. Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science. 1999;286(5441):961–4. doi: 10.1126/science.286.5441.961. [DOI] [PubMed] [Google Scholar]
- 123.Sakai T, Wada T, Ishiguro S, Okada K. RPT2. A signal transducer of the phototropic response in Arabidopsis. Plant Cell. 2000;2:225–36. doi: 10.1105/tpc.12.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benvenuto G, Formiggini F, Laflamme P, Malakhov M, Bowler C. The photomorphogenesis regulator DET1 binds the amino-terminal tail of histone H2B in a nucleosome context. Curr Biol. 2002;12(17):1529–34. doi: 10.1016/s0960-9822(02)01105-3. [DOI] [PubMed] [Google Scholar]
- 125.Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88(1):57–63. doi: 10.1016/s0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- 126.Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR. The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell. 2003;15:2181–2191. doi: 10.1105/tpc.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CMJ. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell. 2003;15:760–770. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fan W, Dong X. In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. Plant Cell. 2002;14(6):1377–89. doi: 10.1105/tpc.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou JM, Trifa Y, Silva H, Pontier D, Lam E, Shah J, Klessig DF. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol Plant Microbe Inter. 2000;13:191–202. doi: 10.1094/MPMI.2000.13.2.191. [DOI] [PubMed] [Google Scholar]
- 130.Stary S, Yin XJ, Potuschak T, Schlogelhofer P, Nizhynska V, Bachmair A. PRT1 of Arabidopsis is a ubiquitin protein ligase of the plant N-end rule pathway with specificity for aromatic amino-terminal residues. Plant Physiol. 2003;133(3):1360–6. doi: 10.1104/pp.103.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.del Pozo JC, Boniotti MB, Gutierrez C. Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(AtSKP2) pathway in response to light. Plant Cell. 2002;14(12):3057–71. doi: 10.1105/tpc.006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ushijima K, Sassa H, Dandekar AM, Gradziel TM, Tao R, Hirano H. Structural and transcriptional analysis of the self-incompatibility locus of almond: identification of a pollen-expressed F-box gene with haplotype-specific polymorphism. Plant Cell. 2003;15(3):771–81. doi: 10.1105/tpc.009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
E3 ubiquitin ligases involved in plant growth and development
In silico expression analysis carried out on all E3 ubiquitin ligases annotated in this work (Supplementary Table S1) in different physiological conditions in Arabidopsis.


