Abstract
4-Hydroxy-trans 2-nonenal (HNE) is one of the most abundant and toxic lipid aldehydes formed during lipid peroxidation by reactive oxygen species. We have investigated the genotoxic effects of HNE and its regulation by cellular glutathione (GSH) levels in human erythroleukemia (K562) cells. Incubation of K562 cells with HNE (5–10 μM) significantly elicited a 3- to 5-fold increased DNA damage in a time and dose dependent manner as measured by comet assay. Depletion of GSH in cells by L-Buthionine-[S,R]-sulfoximine (BSO) significantly increased HNE-induced DNA damage, whereas supplementation of GSH by incubating the cells with GSH-ethyl ester significantly decreased HNE-induced genotoxicity. Further, over-expression of mGSTA4-4, a HNE detoxifying GST isozyme, significantly prevented HNE-induced DNA damage in cells, and ablation of GSTA4-4 and aldose reductase with respective siRNAs further augmented HNE-induced DNA damage. These results suggest that the genotoxicity of HNE is highly dependent on cellular GSH/GST/AR levels and favorable modulation of the aldehyde detoxification system may help in controlling the oxidative stress- induced complications.
Keywords: HNE, DNA Damage, GSH, GST, aldose reductase, comet assay
1. Introduction
Increased reactive oxygen species (ROS) are known to cause DNA damage and related cytotoxicity (Slupphaug et al., 2003; Cooke et al., 2003). ROS -induced DNA damage has also been shown to be a major contributor to the patho-physiology of a number of diseases including cardio-vascular, cancer, neuro-degenerative, autoimmune and diabetic complications (Cooke et al., 2003; Evans et al., 2004; Bashir et al., 1993; Dandona et al., 1996). Normally ROS are readily detoxified by cellular anti-oxidative system but excessive ROS would cause increased lipid-peroxidation and lipid aldehyde formation. One of the most toxic and abundant lipid aldehydes in biological system is 4-hydroxy-trans2-nonenal (HNE), formed by the oxidation of ω-6-poly-unsaturated fatty acids in the plasma membrane (Esterbauer et al., 1991; Uchida, 2003; Pryor and Porter, 1990). HNE in the nucleus could form adducts with DNA as well as proteins, resulting in cytotoxicity and genotoxicity (Feng et al., 2004). All four bases of DNA are the targets for HNE adduct formation (Bont and Larebeke, 2004; Chung et al., 1996). At the low concentrations HNE is metabolized rapidly to less reactive compounds e.g. glutathione (GSH)-conjugates (Esterbauer et al., 1991) in the outer periphery of the cytoplasm so that the resultant gradient would not be enough to diffuse into the nucleus. However, under oxidative stress HNE generated endogenously in cells by oxidation of membrane lipids could reach an in-vivo concentration up to 10 μM or more (Esterbauer et al., 1991; Uchida, 2003). At such concentrations, in already stressed cells having decreased levels of GSH, HNE would have enough gradient to enter the nucleus and react with nuclear proteins and nucleotide bases. HNE can react and form exocyclic propano- adducts by reaction with purine and pyrimidine bases thereby modify their functions. For example HNE could react with DNA to form 4-HNE-dG (6-(1-hydroxyhexanyl)-8-hydroxy-1,N(2)-propano-2-deoxyguanisine), which could cause DNA damage and possibly inhibit DNA repair and cause carcinogenesis (Douki and Ames, 1994; Feng et al., 2003 and 2004).
Glutathione-S-transferase (GST) catalyzed conjugation of lipid aldehydes (such as HNE) with GSH and their reduction by aldose reductase (AR) are the major defense against oxidative stress –induced cytotoxicity (Sharma et al., 2004; Awasthi et al., 2005; Pladzyk et al, 2006). GSTs catalyze the conjugation of GSH via its sulfhydryl group to electrophilic centers of a wide variety of substrates including HNE. GST -catalyzed GS-lipid aldehyde conjugation is the main mechanism of detoxification of endogenous lipid aldehydes including HNE as well as the metabolism of xenobiotics (Ishikawa et al., 1986; Hartley et al., 1995). GS-lipid aldehydes such as GS-HNE are readily reduced by AR to corresponding GS-lipid alcohols such as GS-DHN (Srivastava et al., 2001; Ramana et al., 2006). Both GS-HNE and GS-DHN are actively transported out of the cells (Sharma et al., 2002). Thus adduction of toxic aldehydes with GSH and their reduction by AR and active transport out of the cells by transport system may be the major defense against the oxidative stress-induced cytotoxicity, genotoxicity and a number of related diseases. In the present study using comet assay, we have investigated HNE-induced DNA damage in human erythrolukemia cell line (K562) by regulating cellular GSH levels. We have shown that increasing the cellular GSH and/or GSTA4-4 (that efficiently conjugates lipid-aldehydes and GSH) significantly prevented HNE-induced DNA damage, whereas siRNA ablation of AR promoted HNE-induced DNA damage. Our results suggest that GSH/GST/AR system could regulate genotoxicity caused by HNE-induced oxidative stress.
Materials and methods
Materials
Phosphate-buffered saline (PBS), RPMI-1640 medium, Penicillin/Streptomycin (P/S), Fetal Bovine Serum (FBS) and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were purchased from GIBCO Inc. (Grand Island, NY). 4-HNE was purchased from Cayman Chemical Co. (Ann Arbor, MI). Glutathion-ethyl ester (GSH-ester), and Buthionine-[S,R]-sulfoximine (BSO) were purchased from Sigma Chemical Co. (St. Louis, MO). Sodium dodecyl sulfate (SDS), and acrylamide were purchased from Bio-Rad (Richmond, CA). The CometAssay™ kit was purchased from Trevigen, Inc. (Gaithersburg, MD). TransFast Transfection Reagent was purchased from Promega Co (Madison, WI). The antibodies against the recombinant mGSTA4-4 expressed in Escherichia coli were raised in rabbits (Zimniak et al., 1994). All other reagents were of analytical grade.
Cell culture and stable transfection of K562 cells
The K562 cells were grown in RPMI-1640 medium supplemented with 10% (v/v) FBS and 1% (v/v) P/S at 37°C in a humidified atmosphere of 5% CO2 (v/v). K562 cells were transfected with pRC/CMVmGSTA4-4 using liposome-based Trans-Fast transfection reagent. Stable transfectants were isolated by selection on 400 mg/ml G418 for ~2 weeks. Several G418-resistant stable clones were selected for further characterization and were maintained in medium containing 100 mg G418/ml. Single clone stable transfectant was established in two ways. The first was sequential dilution into 96-well plate, such that only a single cell was seeded in each well. The single clone lines were also established by plating in soft agarose with subsequent selection of viable colonies directly from Agarose. The transfected cells expressed >3 fold more GSTA4-4 than wild type K562 cells.
Ablation of GSTA4-4 and AR with siRNA
Immediately prior to transfection K562 cells were plated at an approximate density to attain >70% confluence in 0.25 ml RPMI-1640 medium supplemented with 10% FBS in a 24 -well plate. The cells were transiently transfected with ON-TARGETplus SMARTpool duplex GSTA4-siRNA (final concentration 30nM) containing a mixture of four siRNAs targeting a single gene (Dhanrmacon, Lafayette, CO) and with AR-siRNA (AATCGGTGTCTCCAACTTC AA) or scrambled-siRNA (AAAATCTCCCTAAATCATACA; control) (final concentration 50nM) using the TransIT-TKO transfection reagent (Mirus, Madison, WI) as suggested by the supplier. Briefly, for each well, respective amount of siRNA was diluted in 50 μl serum-free medium to attain the respective final concentrations of siRNAs and incubated with 2 μl TransIT-TKO transfection reagent for 20 min at room temperature. The transfection mixture was added drop-wise to the respective wells, and incubated at 37°C for 48 h. After incubation, the medium was replaced with fresh medium (serum-free) and stimulated with HNE as indicated. Changes in the expression of AR were estimated by Western blot analysis using anti-AR polyclonal antibodies.
Treatment of the cells
Stock solution of HNE in dichloromethane was dried under N2, dissolved in PBS and the concentration was determined at 221nm using a spectrophotometer. HNE was added to cell suspensions containing 2×106 cells/ml to achieve a final concentration of 5 or 10 μM. The cells treated with corresponding amount of PBS served as negative control. Cell suspensions with HNE were incubated for varied time points as indicated in a shaking water bath at 37°C. The cell suspensions were centrifuged and the viability of the cells was tested by trypan-blue exclusion method. The cell pellets were washed with PBS and resuspended in PBS, an aliquot was taken up in low melting Agarose, dispersed onto slides, and then processed according to the comet assay protocol.
Determination of DNA damage (Comet assay)
The comet assays were performed essentially as described by suppliers manual. Briefly, the cell pellets were resuspended in PBS and cells were mixed with 1% low melting point (LMP) Agarose in 1:10 ratio. Immediately, 75 μl of the cell suspension was pipetted and dispersed onto CometSlides specialy treated to enhance the adherence of low melting point Agarose. The slides were placed at 4°C in dark for 30 min. After the Agarose solidified, slides were immersed in a lysis solution (10 mM Tris–HCl, 100 mM EDTA (pH 10), 2.5 M NaCl, 1% sodium lauryl sarcosinate, 1% Triton X-100,) for at least 60 min. The excess lysis buffer from the slides was tapped off and the slides were washed twice with 1X Tris-buffered EDTA solution (TBE) for 5 min each. The slides were placed in a horizontal electrophoresis chamber and covered with TBE buffer. Electrophoresis was carried out at the rate of 1.0 V/cm for 20 min. The slides were removed from the electrophoresis chamber, washed in deionized water for 5 min and dipped in 70% alcohol for 5 min. Subsequently the slides were air dried, stained with SYBR-Green (1 μl/ml; 30 μl per slide) and mounted. The comet pictures were taken using epifluoroscence (epi800) microscope. At least 40 comets were observed per slide and each experiment was repeated independently at least three times. All steps of the Comet assay were conducted under red light.
Scoring
Microscopic evaluation of the comet images was quantified using the image analysis system of Comet Score™ (TriTek Corp.). At least 40 comets were evaluated per slide and the percentage of fluorescence in the tail (tail intensity (%)) was scored. In addition tail- moments and olive-moments were also scored in some cases.
Statistics
All data represent the mean ± SD. Statistical significance of difference between untreated control and treated groups was analyzed by student T-test using Microsoft excel software. Differences were considered statistically significant at P < 0.05.
Results
Dose and time dependent effect of HNE on DNA damage in K562 cells
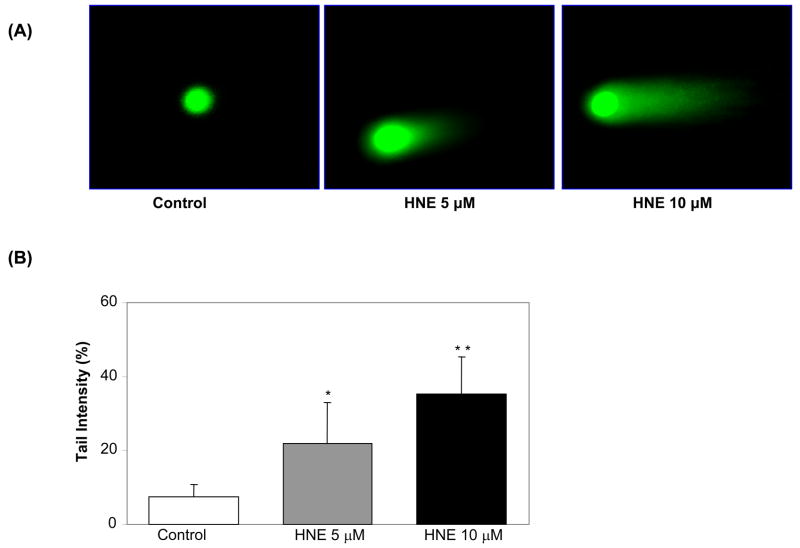
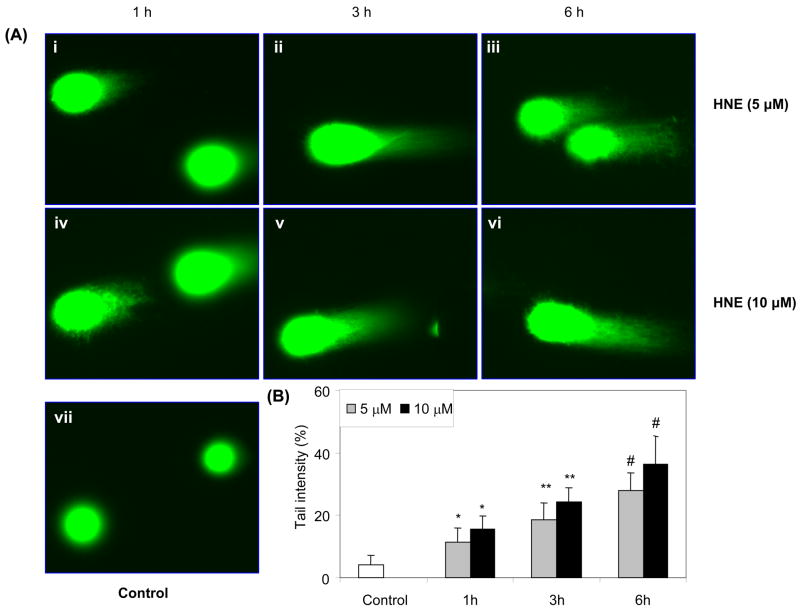
Using K562 cells and comet assay we have shown that 10 μM HNE elicited a significant DNA damage within 3 h (Fig. 1A). The control cells showed none or minimum basal DNA damage. The scoring of comets revealed the extent of HNE–induced DNA breaks in cells in terms of tail intensity (percent of total cell DNA found in the comet tail). As shown in fig. 1B, 5 μM HNE caused approximately 2.5-fold and 10 μM HNE caused 5-fold increase in tail intensity as compared to control cells treated with carrier (PBS). Next, we determined the time - dependent effect of HNE on DNA damage in K562 cells. As shown in fig. 2A both doses of HNE (5 and 10 μM) induced DNA damage in a time dependent manner. The comet tails were visible in both 5 and 10 μM HNE treated cells as early as 1 h after HNE treatment but at 10 μM HNE caused much more damage as compared to 5 μM as measured by tail length. The scoring of comets in terms of tail intensity as shown in fig. 2B also showed increase in DNA damage with time reaching approximately 6-fold after 6 h as compared to control cells treated with carrier (PBS) alone. These results demonstrate that HNE causes a concentration- and time- dependent increase in DNA damage in human erythroleukemia (K562) cells.
Fig. 1.
Dose-dependent genotoxic effects induced by HNE in K562 cells. (A) K562 cells were incubated with 5 and 10 μM HNE for 3 h and single cell gel-electrophoresis was performed to assess the DNA damage. The figure shows the representative comet tails indicative of DNA damage. (B) The bars indicate % DNA in tail (mean ± S.D.) from three independent experiments, each mean was calculated from three to four parallel slides, 40 comets were evaluated per slide. (*P < 0.01, **P < 0.001 vs control).
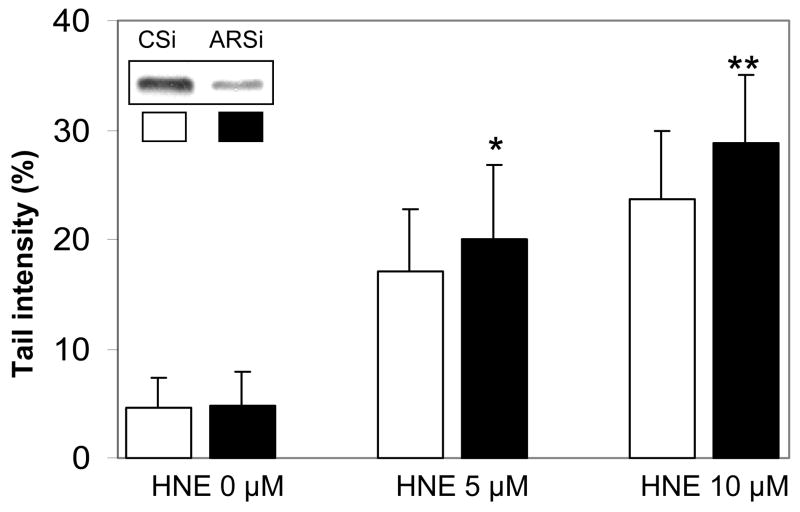
Fig. 5.
Effect of AR ablation by AR siRNA transfection on HNE-induced genotoxicity in K562 cells. K562 cells were transfected with control- and AR-siRNA and incubated for 48 h for silencing of AR mRNA. The cells were washed with PBS and treated with 5 and 10 μM HNE for 3 h followed by single cell gel-electrophoresis to assess the DNA damage. The bars show percent tail intensities (mean ± S.D.), and 50 comets were evaluated per slide from 4 experiments. *P < 0.02 vs respective C-siRNA groups. The inset represents Western blots for AR proteins in control- (Csi) and AR- siRNA (ARsi) transfected K562 cells.
Fig. 2.
Time-dependent genotoxic effects induced by HNE in K562 cells. (A) K562 cells were incubated with 5 and 10 μM HNE for 1, 3 and 6 h and single cell gel-electrophoresis was performed to assess the DNA damage. The figure shows the representative comet tails indicative of DNA damage. (B) The bar diagram shows tail intensity (% DNA in tail) (mean ± S.D.) from three independent experiments. The results are given as mean± S.D. The mean in each case was calculated from three to four parallel slides, 40 comets were evaluated per slide. (*P < 0.05; **P < 0.01; #P < 0.001 vs control).
Modulation of cellular GSH levels regulates HNE- induced DNA damage
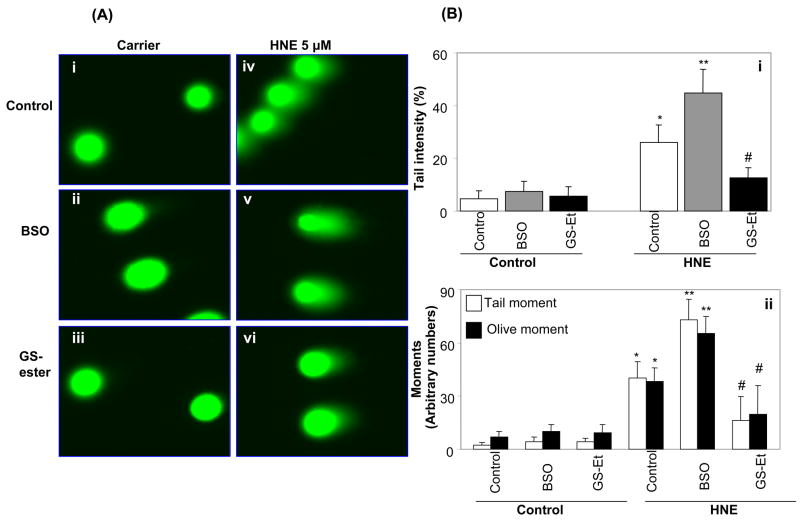
To examine the effect of cellular GSH on HNE-induced genotoxicity, the GSH in K562 cells was depleted by pre-treatment with 100 μM BSO for 16 h, which quenched >80% of the cellular GSH (data not shown). GSH depleted K562 cells were treated with 5 μM HNE for 3 h. As shown in fig. 3A, GSH depletion by BSO significantly enhanced HNE -induced DNA damage as compared to HNE alone (Fig. 3Av and iv). Supplementation of K562 cells with cell permeable GSH-ester significantly prevented HNE -induced DNA damage (Fig. 3Avi). The comet scores in terms of tail intensity showed a significant increase in BSO pre-treated cells over HNE alone-treated cells whereas in GSH-ester pre-treated cells, HNE-induced DNA damage was significantly prevented (Fig. 3Bi). We also scored other parameters that are commonly measured with the comet assay e.g. tail- moment and olive- moment which indicate the severity of the DNA damage. As shown in fig 3Bii HNE- induced tail- and olive -moments were significantly elevated in cells pretreated with BSO as compared to control cells, whereas GSH-ester-pretreated cells showed significantly low tail- and olive- moments. The control cells, not treated with HNE, had nominal value of tail- and olive-moments suggesting that pre-treatement with either BSO or GS-ester had no significant effect on either tail intensity or on tail- and olive-moments. These results demonstrate that adequate cellular GSH levels are crucial for prevention against HNE-induced DNA damage.
Fig. 3.
Effect of GSH depletion and supplementation on genotoxic effects induced by HNE in K562 cells. (A) K562 cells pretreated with 100 μM BSO for 16 h and with 1mM GSH-ethyl-ester (GS-ester) for 1 h and incubated with 5 μM HNE for 3 h and single cell gel electrophoresis was done to assess the DNA damage. The figure shows representative comet tails from each group indicative of DNA damage. B(i) shows tail intensity and (B)(ii) shows the tail- and olive-moments from same samples (mean ± S.D.). Three independent experiments were carried out and each mean was calculated from three to four parallel slides, 40 comets were evaluated per slide. (*P < 0.01 vs control, **P < 0.01 vs HNE alone; #P<0.001 vs HNE+BSO).
Over-expression of mGSTA4-4 in K562 cells protects against HNE- induced DNA damage
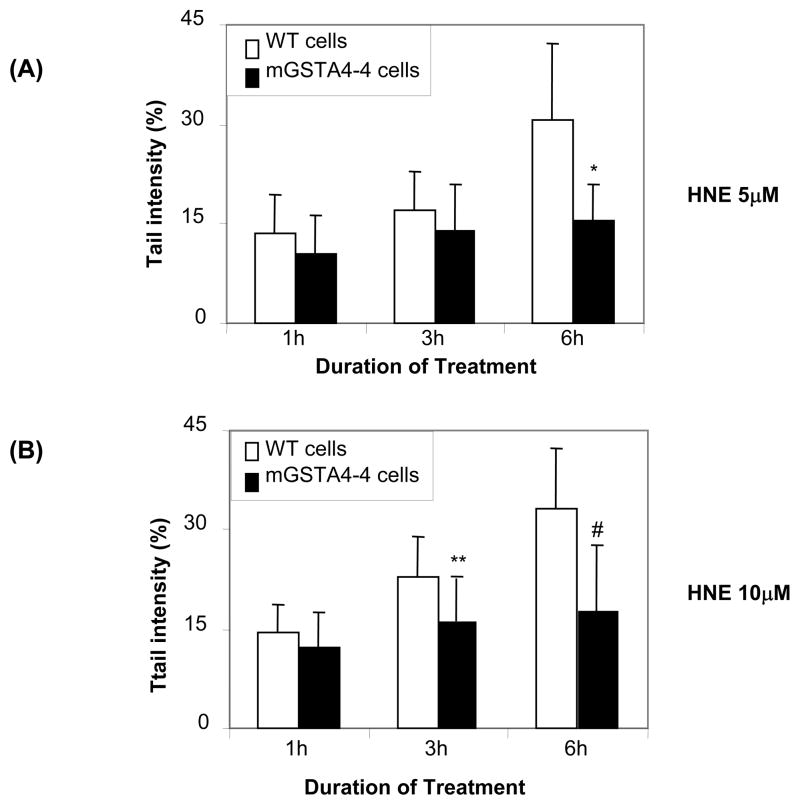
Transfection of K562 cells with mGSTA4-4, which is known to have high substrate specificity for the conjugation of lipid aldehydes with GSH (Hubatsch et al., 1998; Burns et al., 1999), significantly prevented HNE-induced DNA damage (Fig 4). While HNE caused a significant increase in tail intensity in wild type cells, mGSTA4-4 transfected cells showed significantly reduced tail intensities against both doses (5 and 10 μM) of HNE (Fig. 4A and B). To examine the effect of decreased GSTA4-4 activity on HNE-induced genotoxicity in K562 cells, we ablated GSTA4-4 protein in K562 cells by using GSTA4-4 siRNA containing a mixture of four siRNAs targeting GSTA4-4 gene. The results shown in fig 4C insert indicate approximately 90% ablation of GSTA4-4 protein by using siRNA. Further, depletion of GSTA4-4 caused significant (~2 fold) increase in HNE-induced DNA damage as compared to control siRNA transfected cells as determined by percentage of DNA in the comet tail as quantified by tail intensity (Fig. 4C). These results thus indicate that GSTA4-4 offers a significant protection against HNE -induced DNA damage in K562 cells.
Fig. 4.
Effect of overexpression ans depletion of GSTA4 on genotoxic effects induced by HNE in K562 cells. Stable mGSTA4-4 transfected K562 cells were treated with 5 μM (A) and 10 μM (B) of HNE for 1, 3 and 6 h followed by single cell gel-electrophoresis to assess the DNA damage. (C) The GSTA4-4 siRNA transfected cells and control siRNA transfected cells were treated with 5 and 10 μM of HNE for 3 h and comet assay was performed to assess the DNA damage. The bars show tail intensities (mean ± S.D.) from three independent experiments, each mean was calculated from three to four parallel slides, 50 comets were evaluated per slide. *P < 0.01 vs 5 μM HNE for 6 h; **P < 0.05 vs 10 μM HNE for 3 h; #P< 0.01 vs 10 μM HNE for 6 h; # #P < 0.01 vs respective Csi groups. The inset in figures represents Western blots for GSTA4-4 proteins in wild type (WT), and mGSTA4-4 (A and B) and control- (Csi) GSTA4-4 -siRNA (GSTA4si) (C) transfected K562 cells.
Ablation of AR with siRNA in K562 cells augments HNE- induced DNA damage
AR is a known aldehyde detoxifying enzyme as has been shown by us earlier (Srivastava et al., 1999; Pladzyk et al., 2006). In K562 cells, ablation of AR with AR-siRNA, which inhibited AR expression by more than 80% as compared to scrambled siRNA (control siRNA), augmented the HNE induced DNA damage (Fig. 5). A significant increase in percent tail intensity in AR siRNA transfected K562 cells was observed at 5 μM HNE (P < 0.05), however the augmentation was more at 10 μM HNE (P < 0.01). These results indicate that AR offers a significant protection against HNE -induced DNA damage in K562 cells and that siRNA ablation of AR augments the lipid aldehyde induced genotoxicity.
Discussion
Genetic alteration and DNA damage during oxidative stress are major risk factors in many disease conditions (Cooke et al., 2003; Evans et al., 2004). HNE, the main aldehyde generated under oxidative stress, has been shown to elicit cytotoxicity in cells leading to apoptosis, proliferation or differentiation in a concentration dependent manner (Cheng et al., 1999; Haynes et al., 2001). Although the genotoxic effects of HNE have been investigated earlier in many studies (Schaeferhenrich et al., 2003; Knoll et al., 2005; Glei et al., 2007), the concentrations of HNE (>100μM) used to study its genotoxic effects are barely attainable in in-vivo conditions during oxidative stress. For K562 cells the IC50 value of HNE is approximately 9 μM, which is very close to the concentration of HNE at which 50% inhibition of DNA, RNA, and protein synthesis occurs (Barrera et al., 1987). In the present study, we have used 5 and 10 μM of HNE that corresponds to in-vivo concentration during oxidative stress (Poli et al., 1985). We have used single cell gel electrophoresis, also known as comet assay, which is a highly sensitive method to quantify as well as assess the extent of DNA damage in cells. This assay also depicts the severity of DNA damage (Collins, 2004; Speit and Hartmann, 2005; Patel et al., 2006). The results of present study demonstrate that HNE causes DNA damage in a concentration- and time-dependent manner (Fig 1). The genotoxic effects of HNE have been studied in many cell types and it has been shown that HNE forms conjugates with all four bases of DNA and DNA repair proteins altering their integrity and functions leading to mutations and DNA strand breaks (Bont and Larebeke, 2004; Chung et al., 1996). This phenomenon can lead to gene alterations causing potential tumor formation or carcinogenesis, and other pathological conditions.
Increased cellular GSH is known to protect the cells from HNE-induced cytotoxicity and DNA damage (Nakajima et al., 2002; Cao et al., 2003; Ebert et al., 2001). Under oxidative stress GSH is oxidized to GSSG, which is transported out (Srivastava and Beutler, 1969; Meister et al., 1986) resulting in a significant decrease in cellular GSH levels. At the same time oxidative stress causes increased lipid peroxidation and lipid aldehyde formation, which coupled with decreased availability of GSH, further exacerbates the cytotoxicity of lipid aldehydes. Supplementing the cells with GSH protects cells against HNE-induced cytotoxicity (Cao et al., 2003). In the present study we have demonstrated that, depleting the cellular GSH by pre-treating the cells with BSO significantly increased the HNE-induced DNA damage. This indicates that in the absence or decreased levels of GSH, HNE could enter the nucleus and cause DNA damage. In contrast, GSH supplementation to the cells significantly prevented the HNE-induced DNA damage (fig. 3). Lander et al., (2006) have shown that HNE can diffuse to the organelle from an exogenous source besides interacting to a multitude of different cellular proteins including receptor tyrosine kinases, intracellular GSH pool in the cytosol and regulatory elements upstream of NF-κB in nucleus (Uchida, 2003). Though it is not clear how HNE migration to the nucleus is regulated by GSH, it could be stipulated that GS-HNE conjugates formed during oxidative stress are transported to the nucleus. Unlike other oxidative free radicals such as O2− and hydroxyl radicals which have very short half life such that they are active at the site of formation, HNE is a hydrophobic and relatively stable molecule. In order to cross the nuclear membrane HNE would require forming conjugate with hydrophilic molecules such as GSH. The conjugate of HNE with GSH i.e. GS-HNE could migrate to nucleus. Once in the nucleus, GS-HNE need to split, making HNE free that would react and form adducts with DNA bases. However, we have demonstrated that in GSH - depleted cells (by pre-treatment with BSO), HNE-induced DNA damage significantly increased suggesting that HNE transport to nucleus through GS-HNE formation may not be necessary. Also, in GSH supplemented cells HNE-induced DNA damage decreased significantly suggesting that GS-HNE is most likely transported out of the cells rather than transported to the nucleus. Another possibility could be that being a stable molecule HNE could accumulate excessively in a state of oxidative stress and defuse to the nucleus in a concentration gradient manner. Also in a stressed cell, HNE could be generated locally by the oxidation of unsaturated lipid molecules in the nuclear membrane further exacerbating its DNA damaging effect.
Although GSH readily conjugates with HNE, in the presence of GST this reaction is expedited several folds (Alin et al., 1985; Zimniak et al., 1994). Since GST also has glutathione peroxidase activity (Awasthi et al., 1980) that reduces lipid peroxides with the mediation of GSH, this enzyme plays a significant role in modulating the intracellular concentrations of HNE by catalyzing its conjugation with GSH. We have demonstrated that overexpression of GST efficiently protected (Fig. 4A and 4B), while depletion of GSTA4-4 by siRNA significantly increased DNA damage induced by HNE (Fig. 4C) suggesting that GST -mediated conjugation of HNE with GSH is a major detoxification pathway against lipid aldehyde-induced DNA damage. Thus, the increase in GST levels, especially GSTA4-4, under oxidative stress could be body’s defense since it would protect the cellular proteins from HNE adduction as well as from HNE –induced DNA damage by 1) reduction of lipid peroxides to corresponding alcohols, 2) decreasing the levels of lipid aldehydes by conjugation with GSH followed by active transport of GS-lipid aldehydes conjugates.
We have shown in our earlier studies that AR efficiently catalyzes the reduction of major toxic and abundant lipid peroxidation products such as HNE into the corresponding alcohols (Srivastava et al., 1998; 2000). In concert with this observation, we have also shown that over-expression of AR in HLEC offered significant protection against aldehyde-induced cytotoxicity (Pladzyk et al., 2006). In the present study genetic ablation of AR by siRNA aggravated HNE-induced damage which suggests the protective role of AR against HNE-induced DNA damage in K562 cells. Although, HNE-induced DNA damage in AR ablated cells was significant, there was only a marginal difference from control, possibly due to dual function of AR. On one hand AR reduces GS-lipid aldehyde conjugates and mediates oxidative stress signals initiated by cytokines, growth factors and hyperglycemia (Srivastava et al., 2005) on the other hand it protects cells by reducing the toxic lipid aldehydes (Srivastava et al., 1998; 2000). Further, depletion of AR could protect cells from oxidative damage by decreasing the levels of ROS as observed in vascular smooth muscle cells (VSMC) and macrophages (Srivastava et al., 2005; Ramana et al., 2006). We have also shown that inhibition of AR prevents phosphorylation of various kinases that are responsible for the activation of transcription factors required for cell survival and death (Ramana et al., 2004 and 2005). Ruef et al., (Ruef et al., 2000) have also shown that inhibition of AR protects VSMC form HNE-induced apoptosis. Thus, in summary, the results of this study suggest that 1) HNE caused dose- and time- dependent increase in DNA damage 2) the genotoxic effect of HNE could be ameliorated by modulating the cellular GSH levels and 3) over-expression of HNE-detoxifying enzymes such as GSTA4-4 and AR could be main cellular defense against oxidative stress-induced genotoxicity.
Acknowledgments
This study was supported by National Institutes of Health (NIH) Grants GM71036 (to K.V.R.) and DK36118 (to S.K.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alin P, Danielson UH, Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985;179:267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Ansari GA, Awasthi S. Regulation of 4-hydroxynonenal mediated signaling by glutathione S-transferases. Methods Enzymol. 2005;401:379–407. doi: 10.1016/S0076-6879(05)01024-4. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Dao DD, Saneto RP. Interrelationship between anionic and cationic forms of glutathione S-transferases of human liver. Biochem J. 1980;191:1–10. doi: 10.1042/bj1910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera G, Martinotti S, Fazio V, Manzari V, Paradisi L, Parola M, Frati L, Dianzani MU. Effect of 4-hydroxynonenal on c-myc expression. Toxicol Pathol. 1987;15:238–240. doi: 10.1177/019262338701500219. [DOI] [PubMed] [Google Scholar]
- Bashir S, Harris G, Denman MA, Blake DR, Winyard PG. Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann Rheum Dis. 1993;52:659–666. doi: 10.1136/ard.52.9.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bont RD, Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169 – 185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- Bruns CM, Hubatsch I, Ridderström M, Mannervik B, Tainer JA. Human glutathione transferase A4-4 crystal structures and mutagenesis reveal the basis of high catalytic efficiency for the toxic lipid peroxidation products. J Mol Biol. 1999;288:427–439. doi: 10.1006/jmbi.1999.2697. [DOI] [PubMed] [Google Scholar]
- Cao Z, Hardej D, Trombetta LD, Li Y. The role of chemically induced glutathione and glutathione S-transferase in protecting against 4-hydroxy-2-nonenal-mediated cytotoxicity in vascular smooth muscle cells. Cardiovasc Toxicol. 2003;3:165–177. doi: 10.1385/ct:3:2:165. [DOI] [PubMed] [Google Scholar]
- Cheng J, Singhal SS, Saini M, Singhal J, Piper JT, Van Kuijk FJGM, Zimniak P, Awasthi YC, Awasthi S. Effects of mGST A4 Transfection on 4-Hydroxynonenal-Mediated Apoptosis and Differentiation of K562 Human Erythroleukemia Cells. Arch Biochem Biophys. 1999;372:29–36. doi: 10.1006/abbi.1999.1479. [DOI] [PubMed] [Google Scholar]
- Chung FL, Chen HJ, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 2004;26:249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- Douki T, Ames BN. An HPLC-EC assay for 1,N2-propano adducts of 2-deoxyguanosine with 4-hydroxynonenal and other α,β-unsaturated aldehydes. Chem Res Toxicol. 1994;7:511–518. doi: 10.1021/tx00040a006. [DOI] [PubMed] [Google Scholar]
- Ebert MN, Beyer-Sehlmeyer G, Liegibel UM, Kautenburger T, Becker TW, Pool-Zobel BL. Butyrate-induces glutathione S-transferase in human colon cells and protects from genetic damage by 4-hydroxynonenal. Nutr Cancer. 2001;4:156–164. doi: 10.1080/01635581.2001.9680627. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: Induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Amin S, Tang MS. Mutational spectrum and genotoxicity of the major lipid peroxidation product, trans-4-hydroxy-2-nonenal, induced DNA adducts in nucleotide excision repair-proficient and -deficient human cells. Biochemistry. 2003;42:7848–7854. doi: 10.1021/bi034431g. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Tang M. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: A possible mechanism for lipid peroxidation-induced carcinogenesis. Proc Natl Acad Sci U S A. 2004;101:8598–8602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei M, Schaeferhenrich A, Claussen U, Kuechler A, Liehr T, Weise A, Marian B, Sendt W, Pool-Zobel BL. Comet Fluorescence in situ Hybridization Analysis for Oxidative Stress–Induced DNA Damage in Colon Cancer Relevant Genes. Toxicol Sci. 2007;96:279–284. doi: 10.1093/toxsci/kfl197. [DOI] [PubMed] [Google Scholar]
- Hartley DP, Ruth JA, Peterson DR. The hepatocellular metabolism of 4-hydroxynonenal by alcohol dehydrogenase, aldehyde dehydrogenase, and glutathione S-transferase. Arch Biochem Biophys. 1995;316:197–205. doi: 10.1006/abbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Brune B, Townsend AJ. Apoptosis in RAW 264.7 cells exposed to 4-hydroxy-2-nonenal: dependence on cytochrome C release but not p53 accumulation. Free Radic Biol Med. 2001;30:884–894. doi: 10.1016/s0891-5849(01)00476-2. [DOI] [PubMed] [Google Scholar]
- Hubatsch I, Ridderström M, Mannervik B. Human glutathione transferase A4-4: an α class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem J. 1998;330:175–179. doi: 10.1042/bj3300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Esterbauer H, Sies H. Role of cardiac glutathione transferase and of the glutathione S-conjugate export system in biotransformation of 4-hydroxynonenal in the heart. J Biol Chem. 1986;261:1576–1581. [PubMed] [Google Scholar]
- Knoll N, Ruhe C, Veeriah S, Sauer J, Glei M, Gallagher EP, Pool-Zobel BL. Genotoxicity of 4-hydroxy-2-nonenal in human colon tumor cells is associated with cellular levels of glutathione and the modulation of glutathione S-transferase A4 expression by butyrate. Toxicol Sci. 2005;86:27–35. doi: 10.1093/toxsci/kfi171. [DOI] [PubMed] [Google Scholar]
- Landar A, Zmijewski JW, Dickinson DA, Goffe CL, Johnson MS, Milne GL, Zanoni G, Vidari G, Morrow JD, Darley-Usmar VM. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2006;290:H1777–H1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME, Hwang O. Intracellular cysteine and glutathione delivery systems. J Am Coll Nutr. 1986;5:137–151. doi: 10.1080/07315724.1986.10720121. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Zou LB, Yan Y, Mizuno M, Nabeshima T. Interleukin-6 protects PC12 cells from 4-hydroxynonenal-induced cytotoxicity by increasing intracellular glutathione levels. Free Radic Biol Med. 2002;32:1324–1332. doi: 10.1016/s0891-5849(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Patel S, Pandey AK, Bajpayee M, Parmar D, Dhawan A. Cypermethrin-induced DNA damage in organs and tissues of the mouse: evidence from the comet assay. Mutat Res. 2006;607:176–183. doi: 10.1016/j.mrgentox.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Pladzyk A, Ramana KV, Ansari NH, Srivastava SK. Aldose reductase prevents aldehyde toxicity in cultured human lens epithelial cells. Exp Eye Res. 2006;83:408–416. doi: 10.1016/j.exer.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J. 1985;227:629–638. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA, Porter NA. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic Biol Med. 1990;8:541–543. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappaB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–829. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- Ruef J, Liu SQ, Bode C, Tocchi M, Srivastava S, Runge MS, Bhatnagar A. Involvement of aldose reductase in vascular smooth muscle cell growth and lesion formation after arterial injury. Arterioscler Thromb Vasc Biol. 2000;20:1745–1752. doi: 10.1161/01.atv.20.7.1745. [DOI] [PubMed] [Google Scholar]
- Schaeferhenrich A, Beyer-Sehlmeyer G, Festag G, Kuechler A, Haag N, Weise A, Liehr T, Claussen U, Marian B, Sendt W, Scheele J, Pool-Zobel BL. Human adenoma cells are highly susceptible to the genotoxic action of 4-hydroxy-2-nonenal. Mutat Res. 2003;526:19–32. doi: 10.1016/s0027-5107(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sharma A, Yang Y, Awasthi S, Singhal SS, Zimniak P, Awasthi YC. Functional reconstitution of Ral-binding GTPase activating protein, RLIP76, in proteoliposomes catalyzing ATP-dependent transport of glutathione conjugate of 4-hydroxynonenal. Acta Biochim Pol. 2002;49:693–701. [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. 2004;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- Slupphaug G, Kavli B, Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res. 2003;531:231–251. doi: 10.1016/j.mrfmmm.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Speit G, Hartmann A. The comet assay: a sensitive genotoxicity test for the detection of DNA damage. Methods Mol Biol. 2005;291:85–95. [PubMed] [Google Scholar]
- Srivastava S, Chandra A, Wang LF, Seifert WE, Jr, DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J Biol Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Conklin DJ, Liu SQ, Prakash N, Boor PJ, Srivastava SK, Bhatnagar A. Identification of biochemical pathways for the metabolism of oxidized low-density lipoprotein derived aldehyde-4-hydroxy trans-2-nonenal in vascular smooth muscle cells. Atherosclerosis. 2001;158:339–350. doi: 10.1016/s0021-9150(01)00454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Dixit BL, Cai J, Sharma S, Hurst HE, Bhatnagar A, Srivastava SK. Metabolism of lipid peroxidation product, 4-hydroxynonenal (HNE) in rat erythrocytes: role of aldose reductase. Free Radic Biol Med. 2000;29:642–651. doi: 10.1016/s0891-5849(00)00351-8. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Watowich SJ, Petrash JM, Srivastava SK, Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Beutler E. The transport of oxidized glutathione from the erythrocytes of various species in the presence of chromate. Biochem J. 1969;114:833–837. doi: 10.1042/bj1140833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Zimniak P, Singhal SS, Srivastava SK, Awasthi S, Sharma R, Hayden JB, Awasthi YC. Estimation of genomic complexity, heterologous expression, and enzymatic characterization of mouse glutathione S-transferase mGSTA4-4 (GST 5.7) J Biol Chem. 1994;269:992–1000. [PubMed] [Google Scholar]