Abstract
Resurfacing replacement represents the most conservative solution available for total arthroplasty of the hip. However, despite the excellent results reported by highly experienced surgeons, a small but not insignificant body of literature has been published on the more controversial aspects of this approach, mainly those related to the biological and mechanical vulnerability of the retained epiphysis. We report here our evaluation of most of the variables inherent to this procedure (surgical exposure, implant design, technical steps). Based on our results, we conclude that the short-term outcome is strongly related to the surgical approach and the relationship between implant design and cementing technique. Even if posterior approaches are currently widely accepted for resurfacing replacement, the ability to preserve the medial circumflex artery has been questioned, and an alternative exposure has been proposed with good results (antero-lateral, lateral and digastric trochanteric osteotomy). Moreover, a minimally invasive posterior approach could increase the risks of vascular damage. Alternatively, inner implant geometry could affect the distribution of cement over the epiphysis when other variables (direct or indirect cementing technique, viscosity) are not properly selected.
Résumé
Les techniques de resurfaçage sont aujourd’hui une solution conservatrice dans le cadre des prothèses totales de hanche. Cependant malgré l’excellent résultat publié par les auteurs une part non négligeable de la littérature fait état de controverses, notamment en ce qui concerne la fragilité biologique et mécanique de l’épiphyse fémorale. Les différents éléments d’appréciation de cette technique voie d’abord, dessin de l’implant, technique chirurgicale, permettent de conclure qu’à moyen terme, il existe une corrélation importante entre la voie d’abord, le dessin de l’implant et les techniques de cimentage. Même si les voies d’abord postérieures sont largement utilisées, il paraît essentiel de préserver l’artère circonflexe médiale, d’autres voies d’abord ont été proposées avec de bons résultats, voies antéro latérale, latérale, digastrique avec ostéotomie du grand trochanter. Les voies d’abord mini invasives peuvent aggraver les risques vasculaires. Le dessin de l’implant peut influencer la répartition du ciment au niveau de l’épiphyse, les autres variables notamment la technique de cimentage et la viscosité du ciment n’ont pas été évaluées.
Introduction
Currently accepted concepts in the area of total hip replacement are becoming increasingly focused on conservative procedures, both in surgical approaches and implants.
In terms of the design of a prosthesis, resurfacing hip replacement represents the less invasive femoral solution available for primary procedures. The maximum preservation of bone using this implant strongly recommends its use in young and active people, thereby saving most of bone stock for future revisions and conceding a functional joint restoration that falls within physiological range. However, fixation at the bone-implant interface and preservation of biological integrity of retained bone have been questioned as a result of early failures due to neck fractures and avascular necrosis.
The aim of this prospective study was to evaluate early implant behaviour by analysing the mechanisms of failure and related risk factors as these represent critical items in terms of the increased impact of an early revision in a young patient population.
Materials and methods
A total of 60 consecutive resurfacing hip replacements performed between 2001 and 2005 in 58 patients were prospectively evaluated during a mean follow-up period of 32 months (range: 2–44 months). Relative youth (range: 30–60 years), a high functional activity level, good bone stock and absence of morphological changes in the femoral head and neck were indications for this implant. The mean age of our patient cohort was 46.82 years (minimum: 30 years; maximum: 59 years; SD: 6.44 years). The pre-operative diagnosis was primary osteoarthritis of the hip in 57 hips (95%,) and post-traumatic arthritis in three hips (5%). Avascular necrosis of the femoral head and femoral dysplasia such as an extremely short femoral neck (<2 cm) were considered to be exclusion criteria.
A postero-lateral approach was used for all implants and all operations were carried out by the same surgeon. Four different prostheses were implanted: BHR (Bimar/Smith & Nephew), ASR (Johnson & Johnson/Depuy, Warsaw, Ind.), RECAP (Biomet, Parsippany, N.J.), MRS (Lima, Italy). Each implant consisted of an uncemented cup and a cemented femoral component.
Clinical evaluation, based upon Harris Hip Score (HHS) [11], was carried out pre-operatively and post-operatively (1, 3, 6 months and each year thereafter).
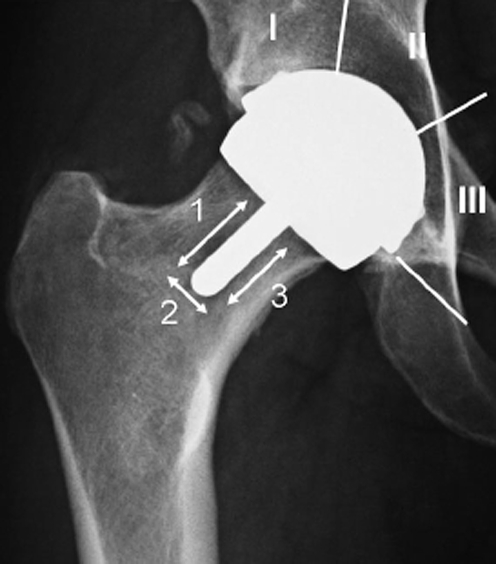
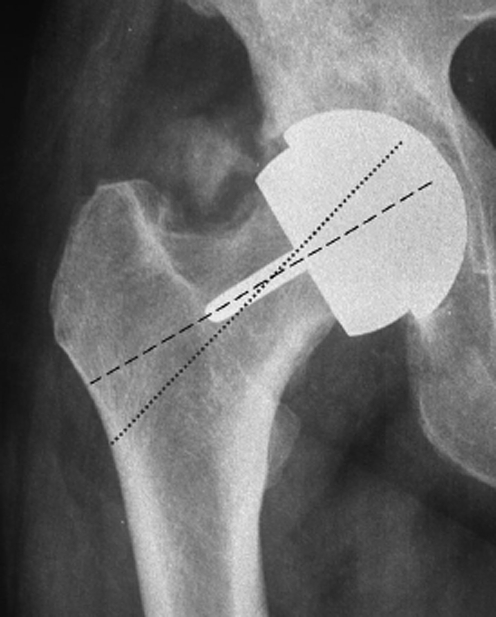
The results of radiological examinations (radiolucency, osteolysis, bone thickening, femoral notching) were analysed and registered on the basis of the Gruen scheme [3], (Fig. 1) while the implant orientation of the femoral components was related to the neck inclination (varus/valgus) (Fig. 2). Orientation in the lateral plane was evaluated but not systematically reported in this study because of technical difficulties in obtaining a reproducible projection for all patients. It is likely that a computed tomography approach would enable a more reliable evaluation and should be adopted in future studies.
Fig. 1.
Gruen scheme, used for topographic location of radiographic findings
Fig. 2.
References for Varus/valgus evaluation on AP view X-ray
We also evaluated the distance between the stem and medial and lateral neck cortex on antero-posterior (AP) radiograms (calculated by establishing a ratio between the distance observed and the known dimension of the femoral component) and related this parameter and valgus orientation to the presence of supero-lateral notching.
Heterotopic ossification was detected by standard methodology and classified using the Brooker method [6].
Complications, such as painful implants, fractures and loosening, were closely monitored and treated when assessed to be necessary. A subsequent revision procedure was considered to be an exclusion criterion, and the respective patient was excluded from the study cohort after revision.
Each implant and the complications eventually detected – if any – were related to the surgical technique, implant inner geometry (cylindrical or conical cross section), cementing technique and cement viscosity. Two types of cement were used:
low viscosity (LV);
high viscosity (HV).
Two different cementing techniques were adopted:
direct, with apposition of the cement directly on the femoral epiphysis;
indirect, in which the cement was applied inside the femoral component.
Two inner geometries of implant were classified:
cylindrical;
conical.
Table 1 presents a summary of the implant type, inner geometry, cementing technique and type of cement used in this study.
Table 1.
Summary of implant type, geometry, cementing technique and type of cement used in our case series
| Type | Number of patients | Geometry | Cementing techniquea | Type of cementb | ||
|---|---|---|---|---|---|---|
| IC | DC | HVC | LVC | |||
| A | 12 | Cylindrical | 12 | 0 | 0 | 12 |
| B | 5 | Cylindrical | 5 | 0 | 5 | 0 |
| C | 23 | Conical | 19 | 4 | 22 | 1 |
| D | 20 | Cylindrical | 20 | 0 | 20 | 0 |
aIC, Indirect; DC, direct
bHVC, high viscosity; LVC, low viscosity
A pulsed lavage was used in all cases to reduce local blood perfusion and improve cement bone penetration.
Retrieved implants were subjected to anatomo-pathological and histological examination to detect bone remodelling, avascular necrosis, morphology of the cement mantle and quality of bone cement interface.
Results
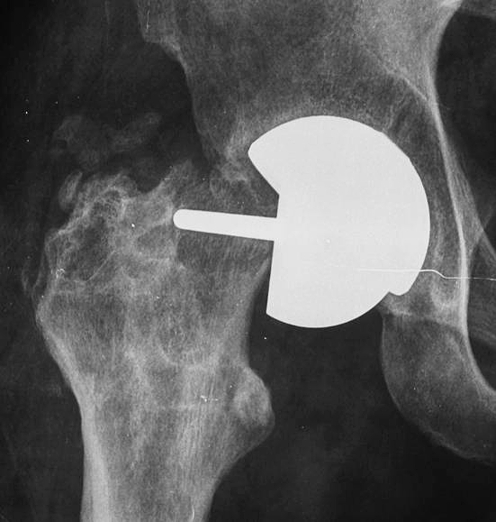
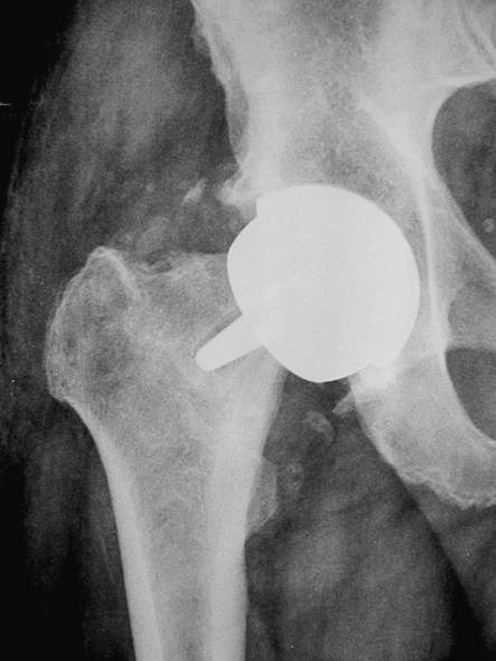
No patient was lost during the follow-up. Only one patient was operated on in another institution, but the indication for a revision procedure was clearly established. The survival rate at the end point was 91.66%, with five cases revised at 1.5, 8, 10, 11, and 12 months post-surgery, respectively. Indications for the revision procedure was femoral neck fracture in two cases Fig. 3, loosening of the femoral component in two cases (Fig. 4), painful femoral implant with incomplete radiolucency around the stem but resistance to conservative treatment (in the absence of clear radiographic signs of loosening) in one case.
Fig. 3.
Hip resurfacing: periprosthetic fracture
Fig. 4.
Hip resurfacing: loosening of the femoral component of the implant
It was quite remarkable that there was an absence of distinctive diagnostic signs, both clinical and radiographic, that anticipated the fractures (1.5 and 11 months post-surgery). One case was actually studied for joint stiffness associated with a slight but progressive opacity within the soft tissue around the hip visible on X-ray (which was not definable as Brooker ossification) 6 months following the initial surgery. The second patient was being monitored for mild pain that appeared 3 months after the initial surgery. This patient was not scheduled for revision due to a clinical remission at 5 months post-surgery with conservative treatment (prolonged abstention from weight-bearing and analgesic therapy) and at the time of the fracture was actually asymptomatic (12 months of follow-up).
The overall rate of complications (including X-ray changes and transient clinical impairment) was 11.66%, with 8.34% of these patients subsequently undergoing femoral implant revision, as stated above (See Table 2).
Table 2.
Failure of the diagnosis and subsequent treatment
| Failure diagnosis | Number of patients | Rate (%) | Treatment |
|---|---|---|---|
| Neck fracture | 2 | 3.33 | Both revised |
| Loosening | 2 | 3.33 | One revised, one pending |
| Painful implant, no clear loosening | 2 | 3.33 | One revised, one clinical remission |
| Stiff joint | 1 | 1.66 | Closely watched over |
| Total | 7 | 11.66 |
The appearance of symptoms in five of the seven more complicated cases (mean interval of appearance after surgery: 4 months) affected the clinical score, determining a bias on the mean value detected in our cohort. Two of these patients were subsequently excluded from the protocol following revision, one patient achieved a clinical remission, while the remaining two are unrevised and being closely monitored. The two fracture cases, as already stated, did not show clear signs of fracture before the complication and, therefore, they were only excluded from the protocol after the revision procedure was carried out.
The mean HHS in the pre-operative clinical evaluation was 55 (range: 44–62). The post-operative HHS for the whole cohort was 77.8 in the first month, 94 in the third month, 98 in the sixth month and 98.7 at 1 year. The mean HHS for uncomplicated cases was 78 in the first month, 95 in the third month, 100 in the sixth month and 100 at 1 year.
An evaluation of the implant orientation revealed a proper positioning, as defined by a variation from the physiological axis on the AP radiogram between ±5°. In the AP plane, 34 implants (56.66%) showed a neutral positioning, as defined by a variation from the physiological cervical axis of ±5°, three implants (5%) were found to have a varus positioning (mean: 7°; range: 5–10°) and 23 of the implants (38.33%) showed a valgus orientation (mean: 6.4°, range: 6–8°).
Two patients presented with supero-lateral notching (less than 3 mm) due to a stem located in the infero-medial quadrant (with the distal edge of the stem at a distance of 3 and 4.4 mm from the inner medial aspect of the cortex, respectively).
Two patients presented a similar radiographic finding associated with a valgus orientation of the femoral component of 8 and 7°, respectively.
Radiographic evaluation according to the Gruen method revealed an absence of radiolucency in 55 patients, while two patients were classified with a score 2 (radiolucency in zone 1), one received a score of 7 (incomplete radiolucency in zones 1– to 3) and two patients, considered to be loose, were given a score of 9 (complete radiolucency in zones 1–3: loosening). The patient with a score of 7 and one of the patients with a score of 9 underwent revision, while the second patient with a score of 9 is currently awaiting revision.
The association of HV cement applied inside a femoral component with a conical inner cross section was detected in five of the seven (85.7%) complicated cases (including the patient with pain relieved after 5 months). In the sixth case, a LV cement was used in association with the same component, while the seventh patient was treated with HV cement applied inside a cylindrical inner cross section.
A statistical analysis of the significance of these parameters (inner cross section, cement viscosity, cementing technique) as related to implant failure was conducted. The four implants used in this study were classified as types A, B, C and D, respectively, where only the Type C femoral component has a conical cross section; the remaining three types have a cylindrical cross section (Table 1). Viscosity of the cement used during the procedure (high/low) and the cementing technique (direct/indirect) were analysed for each type, and a statistical analysis on the association between these variables and clinical/radiographic complications was carried out.
The type of implant and, consecutively, inner geometry were determined to be significantly associated with complications for the Type C implant [chi-square test: χ2 = 9.2 (critical value for 3 df: 7.815), p ≤ 0.05] and conical inner cross section [Chi-square test: χ2 = 5.42 (critical value for 1 df: 3.84), p ≤ 0.05].
Cement viscosity was not shown to have a statistically significant correlation with complications, even if failure was detected in six of the seven cases with HV components. The prevalence of this kind of cement compared to the LV component (47 vs. 13) result in the frequency of complications being proportional to the number of cases [chi-square test: χ2 = 0.98 (critical value for 1 df: 3.84), p ≤ 0.05].
Cementing technique was found to have a casual correlation with complications, both for the whole series and for the Type C group, with the χ2 being very much lower than expected at p ≤ 0.05 (but even for p ≤ 0.75), respectively: for the chi-square test, χ2 = 0.0028 (critical value for 1 df: 3,84), p ≤ 0.05 for the whole series; for the chi-square test with Yates’ correction, χ2 = 0.5014 (critical value for 1 df: 3.84), p = 0.05 for Type C group.
Anato-pathological and histological examination of three retrieved specimens revealed extended signs of necrosis throughout the epiphysis (one fractured, two loosened, one with pseud-arthrosis hidden by the femoral component) that extended to the femoral neck. Macroscopic observation of the fourth specimen (aseptic loosening, actually under anato-pathological examination) revealed a wide resorption of the femoral epiphysis similar to those classified as necrotic (the data for the fifth case of anato-pathological evaluation, third in order of revision procedure; are not available because revision occurred in another institution).
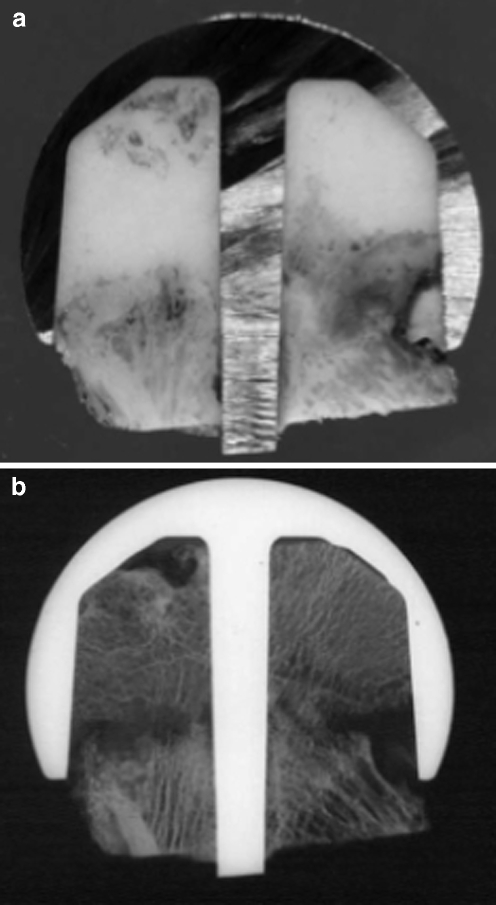
A polar concentration of HV cement was detected in three of these implants; this was associated to focal necrosis beneath the mantle, with a lack of equatorial cement interdigitation (Fig. 5) and with a massive LV cement penetration into the epiphyseal bone with extended aseptic necrosis (manifesting as pseud-arthrosis; Fig. 6a,b).
Fig. 5.
Retrieved specimen. Massive cement penetration into the proximal femoral epiphysis
Fig. 6.
a Polar concentration of cement beneath femoral component. Retrieved specimen. b Same case as in a, with evidence of femoral neck fracture manifesting as pseud-arthrosis hidden by the prosthetic component. Microradiography
Discussion
Resurfacing arthroplasty is currently considered to be the most conservative option for total hip joint reconstruction in young, active adults [7, 10]. However, even with international series based on a mid-term experiences of its application (in some cases with broader indications to arthritis, including avascular necrosis and dysplasia [2, 8]) showing excellent results, more than one concern has been expressed about the reproducibility of these results. In addition, although the definitive proposition of a hybrid implant (cementless acetabular cup, cemented femoral component) as the best solution for this procedure, which has developed since the earlier catastrophic attempts with the cemented cup [12] or cementless femoral component [9], has produced more homogeneous results in different series, controversy remains undiminished in some scientific communities. One reason for this is that, despite good to excellent mid-term survival rate and functional results, this procedure seems to be burdened by a considerable risk of early biological failure (within the first year).
Our series consisted of a population of patients selected on the basis of age (not over 60 years) and pathology (primary or secondary osteoarthritis). As much as possible, we excluded local conditions able to significantly influence the early behaviour of the components, such as low BMD, dysplasia, avascular necrosis and other major morphological or biological changes. Nevertheless, we report here an 11.66% rate of complicated cases within the first year of follow-up, with an 8.34% rate of revision [not including a patient who is currently being closely monitored; this could increase the revision (failure) rate to 10%]. These quite negative results, which are worse than most reported to date in international series [17–19], are quite dramatic considering the early presentation of the failures and the need to proceed with a revision within the first year after the initial surgery. A recent systematic review by Wyness [20] on the available evidence published before 2002 detected a revision rate between 0 and 14.3% [majority of revision surgery was due to fractures (56%), followed by loosening (19%), infection (11%), avascular necrosis (11%) and dislocation (3%)].
However, our excellent results in the uncomplicated cases have led us to be prudent before condemning resurfacing arthroplasty. As such, we have carefully analysed our failures in the search for possible reasons for such different behaviour for similar procedures.
The need of a wide postero-lateral surgical exposure to prepare the femoral epiphysis, with a circumferential capsular release, has been advocated as the possible origin of the critical interruption of blood supply that could lead to delayed avascular necrosis of the retained femoral head and lead to component loosening [14]. There are published reports of histological signs of avascular necrosis rate in about 92% in the retrieved cases revised for aseptic loosening or femoral neck fracture (12 of 13 cases revised) [15]. A relationship between the involution of the cervical or cephalic bone and loosening or fracture seems to be a fact, but it is not clear why this happens only in certain cases and not in others (in particular, in our series all the procedures were performed by the same surgeon using the same surgical exposure in the absence of technical variations in the access. Moreover, patients with avascular necrosis preceding the surgery were excluded from our protocol).
Although these discussions on the true impact of surgical exposure on the risk of developing a secondary necrosis have not resulted in an unambiguous conclusion, some authors have proposed the lateral approach as a possible alternative option, while other authors [14] recommend resurfacing procedures with a careful dissection of the capsule to preserve the retinacular vessels or suggest, even for resurfacing hip arthroplasty, other experimental approaches for intracapsular surgical procedures (digastric trochanteric osteotomy, with capsule exposure proximal to piriformis muscle) [16]. This last option should increase the surgeon’s awareness of experimental minimally invasive surgical exposure which could increase the risk of neurovascular damage [1].
There are, however, more conclusive proposals relating the mechanical failure of the femoral component to malpositioning or the cementing technique.
A proper alignment with the femoral neck represents a key point of the surgical technique, and is based upon slightly different instrumentation with each different implant. An improper alignment can impair mechanical resistance to neck fracture due to a proximal notching or a stress concentration at the edge of the neck [4]. An in vitro study on cadaveric specimens [13] reported a significant reduction of the load capable of fracturing the femoral neck in the presence of a proximal notching of 4 mm (4865 N compared to 7043 N); conversely, a 15 and 21% increase in the stress distribution at the anterior-superior and postero-superior aspect of neck, respectively was detected in the presence of 10° of varus positioning.
It is quite remarkable that our two fractured patients had neither a significant notching nor an evident varus positioning, while cases with proximal notching (<3 mm) or a case with severe varus alignment (10°) did not suffer from such a complication. Extended necrotic changes were observed in our fractured specimen, and the relationship between avascular necrosis and fracture risk (as suggested by several authors [15]) could surpass mechanical explanations for this complication.
An extremely interesting article recently published [5] has focused on a possible biological and mechanical phenomenon – super-imposition in the presence of notching. Using laser Doppler flowmetry, the authors measured the blood flow in 14 osteoarthritic femoral heads during routine total hip replacement surgery, before and after notching of the femoral neck. In ten hips they were able to detect a reduction in blood flow of more than 50% after simulated notching of the femoral neck due to damage to the extraosseous vessels. This result suggests that this intra-operative complication plays a role in pre-disposing the femoral head to avascular necrosis in addition to the well-known weakness of cortical neck.
An anato-pathological study of our revised cases and retrospective evaluation of the surgical steps suggests an alternative solution the interpretation of implant loosening: all available retrieved implants showed the association of a conical inner geometry with cement applied into the component itself (three HV, one LV).
The lower polar pressure developed during the positioning of a component with conical inner geometry filled with cement may cause a non-homogeneous distribution of cement, especially around the equatorial aspect of the epiphysis, while the HV of the cement may reduce its sliding at the bone-implant interface and bone-cement interdigitation. This improper technique could lead to a polar concentration of cement, leading to a lack of stability at the equator and excessive thermal stress of the bone beneath the polar cement mass.
However, the retrieved specimen in which LV cement was used inside the same inner geometry showed a massive penetration of cement into the epiphyseal bone. While this finding may contradict the assumption of a low polar pressure, the virtual absence of cement on the equatorial surfaces of the epiphysis itself was confirmed, even for this different cement component. On the other hand, an indirect cement application (inside the component) is suggested for the cylindrical inner surface because of the higher polar pressure that develops during positioning and the ability of this kind of cement to slide and achieve a better interdigitation with bone.
Statistical analysis of variables such as inner geometry (cylindrical/conical), cement viscosity (high/low) and cement application (direct/indirect) revealed that only the inner geometry has a significant correlation with implant early failure. The effects of the other variables were assessed as being non-significant.
The Type C implant produced the most complications (Type C, six complicated cases), and its peculiar inner geometry has been found to be associated with a significant increase in complication risk. On the other hand, the large number of procedures performed with this implant and the indirect application of cement (HV or LV) without complications make these last variables suggestive but not significantly related to failure risk.
With respect to cement distribution, we suggest the use of pulsed lavage and standing time before insertion of the definitive component in order to be able to determine an optimal cement penetration into the cancellous bone. The latter is significantly reduced by insufficient bone cleaning or an improper low density of the cement (unable to be correctly pressurised during component positioning).
The single failure of the Type B implant can be considered to be random.
Extensive osteonecrosis was observed in all of our retrieved implants: one was revised for a clear neck fracture, two showed aseptic loosening and one presented an undetected fracture at the head-neck junction (hidden by the femoral component on the X-ray pre-operative images) and evolving into peud-arthrosis. Even if the necrosis found in the last case could have developed as a consequence of the fracture, the importance of avascular involution of the epiphysis seems to be, as mentioned in the literature, a constant finding in failed resurfacing implants.
The role of surgical devascularisation during the postero-lateral approach or thermal necrosis caused by the cement is not clear, although our retrieved cases presented an abnormal concentration at the top of the femoral head or a massive penetration in the same structure.
Although several factors have been proposed to explain the non-homogeneous behaviour of resurfacing implants, the prerogative design of this study (same surgeon, same approach, exclusion of patients with local risk factors) and the weak relationship found between femoral component alignment and implant stability or neck mechanical failure have provided us with the opportunity to move our attention onto technical variables intrinsic to the performance of these procedures. We conclude that only a 360° proper surgical technique, based on a careful dissection of the peri-articular soft tissues, an optimal positioning of the femoral component and an adequate choice of femoral design, cement viscosity and cementing technique for a given implant can lay the foundations for the biological and mechanical success of femoral resurfacing components. The wrong choice/management of even one of these variables can explain failures not clearly referable to crude surgical errors.
Osteonecrosis seems to be involved in most implant failures, even if its role as the primary cause of loosening or fracture has not been clearly established. Future studies will likely focus on this aspect in retrieved specimens, but the key problem remains as to how to avoid an aseptic necrosis after this procedure. Our opinion is that changing the surgical approach from postero-lateral to another less damaging to the medial circumflex artery (such as the lateral approach or the anterior approach) could dramatically reduce this complication, while minimally invasive exposure could increase the risk of vessels being damaged as a result of the restricted visualisation of these structures.
In conclusion, a potential early failure of resurfacing arthroplasty can not be excluded, and the patient should be informed about risk prior to the surgery in accordance with informed consent principles and as a protection to surgeons from legal controversies.
Acknowledgements
We like express our gratitude to Dr. Chiara Pedone, Statistics, for her invaluable help with the statistical analysis of technical and clinical data.
Contributor Information
F. Falez, Phone: +39-06-68352309, FAX: +39-06-68352307, Email: f.falez@flashnet.it
F. Casella, Phone: +39-338-6205673, Email: filippo.casella@alice.it
References
- 1.Allison C (2005) Minimally invasive hip resurfacing. Issues Emerg Health Technol 65:1–4 [PubMed]
- 2.Amstutz HC, Su EP, Le Duff MJ (2005) Surface arthroplasty in young patients with hip arthritis secondary to childhood disorders. Orthop Clin North Am 36:223–230 [DOI] [PubMed]
- 3.Amstuz AC, Beaulè PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA (2004) Metal on metal hybrid surface arthroplasty: two to six years follow-up study. J Bone Joint Surg 86A:28–39 [PubMed]
- 4.Amstuz HC, Campbell PA, Le Duff MJ (2004) Fracture of the neck of the femur after surface arthroplasty of the hip. J Bone Joint Surg 86A:1874–1877 [DOI] [PubMed]
- 5.Beaule PE, Campbell PA, Hoke R, Dorey F (2006) Notching of the femoral neck during resurfacing arthroplasty of the hip: a vascular study. J Bone Joint Surg Br 88:35–39 [DOI] [PubMed]
- 6.Brooker AF, Bowermann JW, Robinson RA (1973) Ectopic ossification following total hip replacement: incidence and method of classification. J Bone Joint Surg 55A:1629 [PubMed]
- 7.Daniel J, Pynsent PB, McMinn DJ (2004) Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br 86:177–184 [DOI] [PubMed]
- 8.Dong T, Sun J, Qu Y, Wang Y (2005) Resurfacing arthroplasty for treatment of avascular necrosis of femoral head in young and middle-aged patient. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 19:707–709 [PubMed]
- 9.Duijsens AW, Keizer S, Vliet-Vlieland T, Nelissen RG (2005) Resurfacing hip prostheses resited failure analysis during a 16-year follow-up. Int Orthop 29:224–228 [DOI] [PMC free article] [PubMed]
- 10.Goldberg VM (2005) Surface replacement solutions for the arthritic hip. Orthopedics 28:943–944 [DOI] [PubMed]
- 11.Harris H (1969) Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg 51A:737–755 [PubMed]
- 12.Howie DW, McGee MA, Costi K, Graves SE (2005) Metal on metal resurfacing versus total hip replacement – the value of a randomized clinical trial. Orthop Clin North Am 36(2):195–201 [DOI] [PubMed]
- 13.Indelli PF, Vail TP, Dominguez D, Pickering T (2005) Resurfacing hip replacement: surgical technique and clinical results with minimum 1-year follow-up. In: 90th Natl Congr Ital Orthop Traumatol Soc, Florence, Italy
- 14.Lavigne M, Kalhor M, Beck M, Ganz R, Leunig M (2005) Distribution of vascular foramina around the femoral head and neck junction: relevance for conservative intracapsular procedures of the hip. Orthop Clin North Am 36:171–176 [DOI] [PubMed]
- 15.Little CP, Ruiz AL, Harding IJ, McLardy-Smith P, Gundle R, Murray DW, Athanasou NA (2005) Osteonecrosis in retrieved femoral heads after failed resurfacing arthroplasty of the hip. J Bone Joint Surg Br 87:320–323 [DOI] [PubMed]
- 16.Nork SE, Scar M, Pfander G, Beck M, Djonov V, Ganz R, Leunig M (2005) Anatomic considerations for choice of surgical approach for hip resurfacing arthroplasty. Orthop Clin North Am 36:163–170 [DOI] [PubMed]
- 17.Sharma H, Rana B, Watson C, Campbell AC, Singh BJ (2005) Femoral neck fractures complicating metal-on-metal resurfaced hips: a report of 2 cases. J Orthop Surg (Hong Kong) 13:69–72 [DOI] [PubMed]
- 18.Shimmin AJ, Back D (2005) Femoral neck fractures following Birmingham hip resurfacing. J Bone Joint Surg Br 87:463–464 [DOI] [PubMed]
- 19.Treacy RB, McBryde CW, Pynsent PB (2005) Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br 87:167–170 [DOI] [PubMed]
- 20.Wyness L, Vale L, McCormack K, Grant A, Brazzelli M (2004) The effectiveness of metal on metal hip resurfacing: a systematic review of the available evidence published before 2002 MC. Health Serv Res 4:39 [DOI] [PMC free article] [PubMed]