Abstract
Electrical stimulation of phrenic afferent fibres in the dog elicits a reflex inhibition of efferent activity to the inspiratory intercostal muscles. However, electrical stimulation has a poor selectivity, so the sensory receptors responsible for this inhibition were not identified.
In the present studies, cranial forces were applied during spontaneous inspiration to the abdominal surface of the central, tendinous portion of the canine diaphragm to activate tension mechanoreceptors in the muscle. Vagal afferent inputs were eliminated by vagotomy.
The application of force to the central tendon caused a graded, reflex reduction in inspiratory intercostal activity, especially in external intercostal activity. This reduction was commonly associated with a decrease in inspiratory duration and was invariably attenuated after section of the cervical dorsal roots.
In contrast, no change in inspiratory intercostal activity was seen when high frequency mechanical vibration was applied to the central tendon to stimulate diaphragmatic muscle spindles.
These observations provide strong evidence that tension receptors in the diaphragm, but not muscle spindles, induce reflex inhibition of inspiratory intercostal activity. The expression of this reflex probably involves supraspinal structures.
Paralysis of the diaphragm is well known to elicit an increased activity in the inspiratory intercostal muscles leading to an increased inspiratory expansion of the ribcage (De Troyer & Kelly, 1982; De Troyer, 1992; Katagiri et al. 1994), but the mechanism of this compensation is as yet incompletely understood. It has been suggested that this increased activity was primarily related to an increased chemical respiratory drive (Stradling et al. 1987; Ninane et al. 1989), and indeed paralysis of the diaphragm in anaesthetized animals induces a decrease in tidal volume and an increase in arterial PCO2. However, Brichant & De Troyer (1997) have recently demonstrated that the increased inspiratory intercostal activity after selective blockade of the phrenic nerves in dogs is out of proportion to the increased arterial PCO2 (Pa,CO2). They postulated, therefore, that this increased activity was due, in part, to the release of an inhibition originating from the contracting diaphragm. Furthermore, based on the fact that many low-threshold mechanoreceptors in the diaphragm discharge during spontaneous or electrically induced muscle contraction (Corda et al. 1965; Jammes et al. 1986; Balkowiec et al. 1995), these investigators also speculated that this intercostal inhibition might arise in diaphragmatic Golgi tendon organs (Brichant & De Troyer, 1997).
This hypothesis was initially tested by stimulating electrically the proximal ends of the canine phrenic nerves (De Troyer, 1998). Electrical stimulation of phrenic afferents did elicit a powerful reflex reduction in inspiratory intercostal activity, thus confirming the existence of an inhibitory phrenic-to-intercostal reflex. However, electrical stimulation has a poor selectivity in differentiating the different afferents, and so the nature of the sensory receptors responsible for this reflex inhibition could not be assessed. In the present studies, we therefore set out to define the response of the canine inspiratory intercostal muscles to activation of diaphragmatic tension mechanoreceptors and muscle spindles. Tension receptors were activated by impeding diaphragmatic descent during spontaneous inspiration, and muscle spindles were stimulated by applying high frequency mechanical vibration to the central, tendinous portion of the muscle.
METHODS
Thirteen adult mongrel dogs (15–29 kg) anaesthetized with pentobarbitone sodium (initial dose, 25 mg kg−1i.v.) were studied, as approved by the Animal Ethics and Welfare Committee of the Brussels School of Medicine. The animals were placed in the supine posture and intubated with a cuffed endotracheal tube, and a venous cannula was inserted in the forelimb to give maintenance doses of anaesthetic. A catheter was also inserted in the femoral artery to monitor blood pressure and sample arterial blood periodically for blood gas analysis. The ribcage and intercostal muscles were exposed on the right side of the chest from the first to the seventh rib by deflection of the skin and underlying muscle layers, after which the vagi were isolated bilaterally in the neck, infiltrated with 2 % lidocaine (lignocaine) and sectioned.
The abdomen was then opened by a mid-line incision from the xiphisternum to the umbilicus, and a circular aluminium cap, 30 mm in diameter, was positioned between the cranial aspect of the left hepatic lobe and the central tendon. As shown in Fig. 1, this cap was fixed to the tip of a 30-cm-long bar (10 mm external diameter), the other end of which was attached to a strain gauge (model TC-2000-50, Kulite Semiconductor Inc., Ridgefield, NJ, USA) and a handle. Using this device, we could therefore apply different cranially oriented forces to the central tendon and impede in a graded manner the inspiratory descent of the diaphragmatic dome; the strain gauge measured force linearly in the range from 0 to 5 kg.
Figure 1. Diagram of experimental preparation.
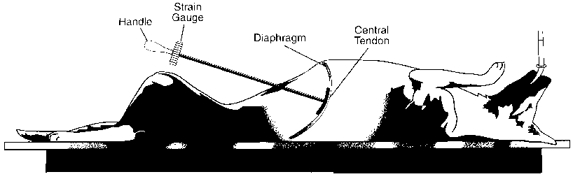
An aluminium cap fixed to the tip of a 30-cm-long bar was placed against the central, tendinous portion of the diaphragm so as to impede diaphragmatic descent during inspiration. The force applied was measured with the strain gauge.
Measurements
All measurements were obtained while the animal was breathing spontaneously. Airflow was measured at the endotracheal tube with a heated Fleisch pneumotachograph connected to a differential pressure transducer (Validyne, Northridge, CA, USA), and the changes in lung volume were obtained by integration of the flow signal. The force applied to the diaphragm was measured with the strain gauge connected to the cap, and the electromyograms of the parasternal intercostal, external intercostal and diaphragmatic muscles were recorded with pairs of silver hook electrodes spaced 3–4 mm apart. Each pair of electrodes was placed in parallel fibres. The parasternal intercostal electrodes were implanted in the bundles situated near the sternum in the third interspace, the external intercostal electrodes were implanted in the same interspace midway between the costochondral junction ventrally and the angle of the rib dorsally, and the diaphragmatic electrodes were placed in the ventral region of the left hemidiaphragm. The three electromyographic (EMG) signals were processed with amplifiers (model 830/1, CWE Inc., Ardmore, PA, USA), bandpass filtered below 100 and above 2000 Hz, and rectified before their passage through leaky integrators with a time constant of 0.2 s.
Protocol
Following instrumentation, the animal was allowed to recover for 30 min, after which measurements of tidal volume, force and EMG activity were made. The aluminium cap-strain gauge system was held manually throughout, so that the site and the force of application could be well controlled. Additionally, diaphragmatic EMG activity was continuously monitored on a loudspeaker, which provided the investigator with a precise phase reference. With each inspiration, therefore, the cap was slowly displaced caudally as the diaphragm was descending (no force), and during expiration the cap was displaced rostrally as the diaphragm was returning to its initial, resting position. Every 15–20 breaths, however, the normal inspiratory caudal displacement of the diaphragm was reduced for a single breath; the force thus applied on the central tendon was initiated at the onset of inspiration, increased gradually in magnitude until inspiration ceased, and was then released rather abruptly. Thirty to forty manipulated breaths covering a wide range of force were obtained in each animal. Instant increases in force during the course of inspiration were also applied.
Two additional procedures were performed.
Experiment 1
In six animals, we assessed the effect of cervical dorsal root section on the response of the parasternal and external intercostal muscles to the application of force on the diaphragm. After completion of the control procedure, the animal was repositioned to the prone posture, and the spinal cord was exposed through a dorsal laminectomy from C4 to T1. The dura was opened, and the dorsal roots corresponding to the fifth, sixth and seventh cervical segments were sectioned bilaterally. The incision was loosely closed and covered with warm mineral oil before the animal was returned to the initial supine posture.
The measurements and experimental procedure after cervical dorsal rhizotomy were essentially similar to those used in the control condition. However, we expected that diaphragmatic manipulation after rhizotomy would not elicit any change in parasternal or external intercostal inspiratory EMG activity, whereas, in fact, some response persisted (see Results). Therefore, in addition to tidal volume, force and EMG activity, we also measured the respiratory displacements of the fourth rib along the craniocaudal axis of the ribcage (axial rib motion); this measurement was obtained with a linear displacement transducer (Schaevitz Eng., Pennsauken, NJ, USA) connected to a hook screwed into the rib in the mid-axillary line, as previously described (De Troyer & Kelly, 1982).
Experiment 2
In five animals, we also assessed the response of the parasternal intercostal and external intercostal muscles to stimulation of diaphragmatic muscle spindles by high frequency mechanical vibration. When the control procedure was completed, vibrations were thus applied to the central tendon using a vibrator (model V. 101; LDS, Royston, UK) which was adjusted so that the amplitude of vibration was 1.5–2.5 mm. As for the strain gauge (Fig. 1), the vibrator was held manually outside the abdominal cavity and maintained continuously in contact with the central tendon via a 30-cm-long Plexiglass tubing; the area of contact between the tendon and the vibrator was approximately 4 cm2.
The site and the force of application of the vibrator on the central tendon was kept as constant as possible. As the animal was breathing, the vibrator was therefore alternatively displaced caudally and rostrally so as to follow the natural motion of the diaphragm, and every 15–20 breaths vibration was delivered for a single inspiration. The frequencies of vibration were set at 10, 40 and 70 Hz. In each animal, four trials were obtained with each frequency.
Although the animals were breathing spontaneously throughout the studies, they were maintained under light surgical anaesthesia. Supplementary doses of pentobarbitone sodium (1–2 mg kg−1i.v.) were given at intervals to ensure that there was no spontaneous movement of the fore- or hindlimbs, including during the application of force or vibration on the diaphragm, no pupillary light reflex and no flexor withdrawal of the forelimbs. Manipulation of the diaphragm did not alter blood pressure either. In addition, rectal temperature was maintained constant between 36 and 38°C with infrared lamps. At the end of the experiment, the animal was killed with an overdose of pentobarbitone sodium (30–40 mg kg−1i.v.).
Data analysis
Diaphragmatic EMG activity was used to indicate the time course of diaphragmatic contraction throughout the study and to assess inspiratory time (TI). The duration of inspiration was measured from the onset of the diaphragmatic electromyogram until peak activity; to allow comparison between the different animals, TI during each manipulated breath was expressed as a percentage of TI during the immediately preceding non-manipulated (control) breath. However, previous studies on isolated strips of canine diaphragm have shown that changes in muscle length introduce substantial alterations in EMG activity which are independent of neural drive. Specifically, for a given supramaximal or submaximal stimulation of the phrenic nerve, diaphragmatic EMG activity decreased as muscle length was increased (Kim et al. 1985). A similar effect of muscle length has been demonstrated during supramaximal stimulation of the phrenic nerves with single twitches in humans (Gandevia & McKenzie, 1986). Since the application of force on the central tendon during inspiration causes the diaphragmatic muscle fibres to shorten less than normally or to lengthen, measurements of peak diaphragmatic EMG activity might therefore have yielded a systematic underestimate of neural drive. Hence, alterations in this variable were not considered in the data analysis.
The application of force on the diaphragm should not produce such recording artefacts in the parasternal intercostal and external intercostal muscles. Phasic inspiratory EMG activity in these muscles during the manipulated and control breaths was therefore quantified by measuring the peak height of the integrated EMG signal in arbitrary units. As for the inspiratory time, the inspiratory EMG activity recorded during each manipulated breath was then expressed as a percentage of the activity recorded during the immediately preceding control breath; the changes thus seen during the application of force were therefore independent of the changes in chemical respiratory drive that might have occurred. In each individual animal in each condition, the changes in intercostal EMG activity and TI produced by the application of force on the central tendon were finally plotted against the force delivered at peak inspiration, and the slope and correlation coefficient (r) of the linear regressions was calculated.
Data were averaged for the animal group, and they are presented as means ±s.e.m. To assess the effect of dorsal rhizotomy, Student's paired t test was used to test the change in slope across the animal group; statistical assessment of the effect of vibration was also made by paired t test. The criterion for statistical significance was taken as a two-sided P < 0.05.
RESULTS
Effects of force on the diaphragm
The 13 animals of the study had a mean arterial PCO2 of 43.5 ± 1.7 mmHg and a mean arterial PO2 of 86.4 ± 2.6 mmHg, and the effects of applying a force on the central tendon for a single breath on the inspiratory EMG activity recorded from the external intercostal and parasternal intercostal muscles (integrated signals) are illustrated by the records of a representative animal in Fig. 2. When force was applied, so that the normal inspiratory descent of the diaphragm and tidal volume was reduced, there was a decrease in external intercostal EMG activity. In all animals, this reduction in inspiratory EMG activity increased gradually in magnitude as the force applied on the diaphragm was greater, and in six animals it was so prominent that EMG activity could be suppressed. In each case, the relationship between force and external intercostal activity could therefore be fitted by a linear function with a negative slope, as shown in Fig. 3A. The correlation coefficient (r) of this function ranged between 0.76 and 0.93 (mean ±s.e.m.= 0.84 ± 0.02; P < 0.001), and its slope (i.e. the change in inspiratory EMG activity produced by a force of 1.0 kg) varied between -8.3 and -69.3 % kg−1.
Figure 2. Example of the effects of force application on inspiratory intercostal activity.
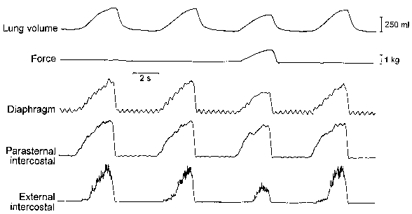
Traces obtained in a representative animal. The parasternal intercostal and external intercostal muscles (third intercostal space) were electrically active with the diaphragm during the inspiratory phase of the breathing cycle. When force was applied on the central tendon, the external intercostal EMG activity was reduced markedly and the parasternal intercostal activity was reduced moderately. Tidal volume was also reduced.
Figure 3. Response of the parasternal intercostal and external intercostal muscles to the application of force on the central tendon.
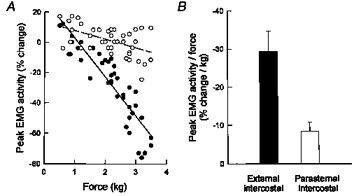
A, relationships between force and peak EMG activity of the external intercostal (•-•) and parasternal intercostal (^ - - ^) muscles in a representative animal. Each circle corresponds to a single manipulated breath, and EMG activity is expressed as a percentage change relative to the activity recorded during the immediately preceding unmanipulated breath. B, slopes of the relationships between force and change in EMG activity in 13 animals (mean values ±s.e.m.). Force caused a larger reduction in external intercostal activity than in parasternal intercostal activity (P < 0.001).
The application of force had less effect on the inspiratory EMG activity recorded from the parasternal intercostals (Figs 2 and 3). Thus parasternal intercostal activity remained unchanged throughout in three of 13 animals, and although it showed a gradual reduction (P < 0.05) in 10 animals, the slope of the relationship between force and parasternal intercostal activity was consistently smaller than that seen between force and external intercostal activity. For the animal group, therefore, whereas the slope of the latter relationship averaged -29.4 ± 5.3 % kg−1, the slope of the former was only -8.4 ± 2.4 % kg−1 (P < 0.001) (Fig. 3B).
Eight of the 13 animals did not show any consistent alteration in TI during the procedure, thus indicating that the reductions in external intercostal and parasternal intercostal activity essentially resulted from a decrease in the rate of rise (Fig. 2). In contrast, five animals had a moderate, gradual reduction in TI (Fig. 4A). In these animals, therefore, there was a direct relationship between the reduction in peak inspiratory intercostal EMG activity and the reduction in TI. As shown in Fig. 4B, however, the reduction in TI, when present, was much smaller in magnitude than the reduction in intercostal activity, in particular in external intercostal activity. Consequently, the reductions in intercostal activity in these animals were also primarily related to a decrease in the rate of rise, rather than a reduction in TI.
Figure 4. Example of changes in inspiratory duration during force application.
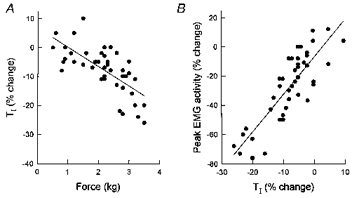
Same animal as in Fig. 3A. A, relationship between force and inspiratory duration (TI). Each circle corresponds to a single manipulated breath, and TI is expressed as a percentage change relative to TI during the preceding unmanipulated breath. Force in this animal induced a gradual reduction in TI (P < 0.001). There was therefore a relationship between the change in peak external intercostal EMG activity and the change in TI (P < 0.001), as shown in B. The slope of this relationship, however, was 2.53, thus indicating that the reduction in EMG activity was greater than the reduction in TI.
The instant application of force in the middle of inspiration further confirmed the real inhibition of inspiratory intercostal activity. As shown in Fig. 5, these applications elicited clear-cut, immediate reductions in external intercostal EMG activity and moderate reductions in parasternal intercostal activity. These reductions disappeared shortly after the force was released.
Figure 5. Changes in inspiratory intercostal EMG activity induced by instant application of force.
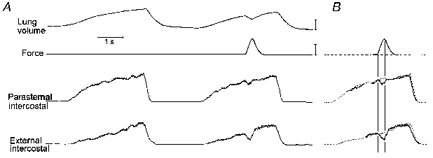
A, traces obtained during a control breath and a manipulated breath in a representative animal. B, the traces of the integrated EMG signals recorded during the manipulated breath (continuous traces) have been superimposed on the traces recorded during the control breath (dotted traces). The onset of force application (first vertical line) caused an abrupt reduction in external intercostal inspiratory EMG activity; activity increased as soon as force started to decline (second vertical line). Volume calibration: 250 ml; force calibration: 2 kg.
Effects of dorsal root section
The effects of sectioning the C5-C7 dorsal roots on the parasternal intercostal and external intercostal muscle response to the application of force on the diaphragm are illustrated in Fig. 6. Four of the six animals studied had a reduction in parasternal intercostal activity in the control condition, and this response was consistently abolished after dorsal root section (Fig. 6A). The reduction in TI that was present in two animals during control was also suppressed. On the other hand, external intercostal activity continued to decrease progressively in each animal (Fig. 6B). The persistent relationship between force and external intercostal activity, however, was invariably flatter than in the control condition. As a result, the slope for the six animals was reduced from -27.6 ± 8.7 to -7.7 ± 1.8 % kg−1 (P < 0.05).
Figure 6. Effects of dorsal root section.
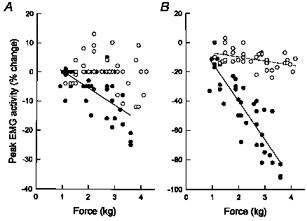
Relationships between force and peak parasternal intercostal (A) and external intercostal (B) EMG activity before (•-•) and after (^ - - ^) section of the C5-C7 dorsal roots in a representative animal. EMG activity is expressed as percentage change relative to the activity recorded during resting unmanipulated breathing. Sectioning the dorsal roots abolished the reduction in parasternal intercostal activity (no dashed line is shown) and attenuated the reduction in external intercostal activity.
To understand the residual reduction in external intercostal EMG activity, we compared in these animals the axial displacement of the fourth rib during the application of force with the axial rib displacement during unimpeded breathing. When force was applied on the diaphragm, there was a clear-cut, consistent increase in the inspiratory cranial motion of the rib (Fig. 7A), such that in each animal, the relationship between force and cranial rib motion could be fitted by a linear function with a positive slope (Fig. 7B). The correlation coefficient (r) of this function ranged between 0.82 and 0.96 (mean ±s.e.m.= 0.91 ± 0.02; P < 0.001), and its slope varied between 1.8 and 4.2 mm kg−1 (mean ±s.e.m.= 3.0 ± 0.4).
Figure 7. Changes in inspiratory rib motion during the application of force on the central tendon.
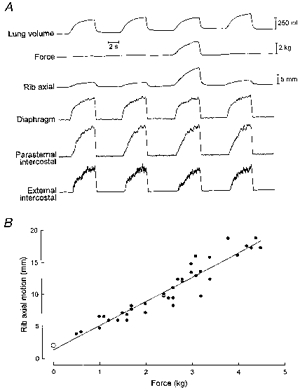
Data obtained in a representative animal after section of the C5-C7 dorsal roots. A, traces of axial rib motion (cranial motion upward) and parasternal intercostal and external intercostal EMG activity during two control unmanipulated breaths and a breath during which force was applied. The application of force induced an increased inspiratory cranial motion of the rib and a small reduction in external intercostal inspiratory EMG activity. B, relationship between force and inspiratory rib motion. ^, control unmanipulated breathing; •, single manipulated breaths.
Effects of diaphragmatic vibration
The records shown in Fig. 8 illustrate the response of the inspiratory intercostal muscles and the diaphragm to the application of vibration on the central tendon. Vibrating the diaphragm for a single breath did not induce any reduction in tidal volume in any animal, thus indicating that the force of application was moderate and did not significantly impede the inspiratory descent of the muscle, nor did it elicit any change in TI or in inspiratory intercostal activity. With the frequency of vibration set at 70 Hz, parasternal intercostal and external intercostal EMG activities in the five animals studied were still 101.9 ± 1.9 and 103.1 ± 2.4% of the control value, respectively. Yet, with vibration, there was an abrupt small increase in diaphragmatic EMG activity, which ceased as abruptly on removal of the vibrator.
Figure 8. Response of the parasternal intercostal and external intercostal muscles to high frequency vibration of the central tendon.
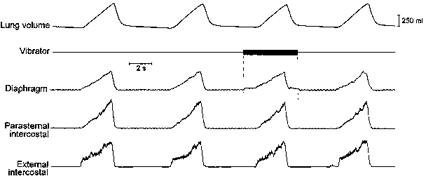
Traces obtained in a representative animal. The application of vibration on the central tendon (vertical dashed lines) induced an abrupt increase in diaphragmatic EMG activity but did not cause any alteration in parasternal intercostal or external intercostal inspiratory EMG activity. The frequency of vibration in this example was 70 Hz.
DISCUSSION
Although it is virtually impossible to block selectively the afferent inputs from tendon organs or to stimulate them selectively (Jami, 1992), earlier studies on limb muscles in cats by Lundberg & Winsbury (1960) have shown that afferent volleys from tendon organs in an active muscle can be substantially increased by muscle stretch. Extracellular recordings from cervical dorsal root ganglia in cats by Revelette et al. (1992) have also shown increased discharge frequencies from diaphragmatic tendon organs during stretching of the diaphragm by abdominal compression. This procedure, however, would affect a number of additional structures, such as the abdominal muscles, the abdominal viscera, and the large vessels in the abdominal cavity, and the confounding influence of these structures on inspiratory intercostal activity might be difficult to assess. In addition, most tension receptors in the diaphragm are situated in the immediate vicinity of or within the central tendon (Balkowiec et al. 1995). To test the hypothesis that diaphragmatic tension receptors may cause reflex inhibition of efferent activity to the inspiratory intercostal muscles (Brichant & De Troyer, 1997; De Troyer, 1998), it appeared therefore more appropriate to pull directly on the central tendon during inspiration than to compress the entire abdomen.
In agreement with this hypothesis, the application of force on the central tendon consistently induced a graded inhibition of the inspiratory intercostal muscles, in particular the external intercostals. Although there was a decrease in tidal volume, this inhibition was seen while force was applied for a single inspiration or in the middle of inspiration after cervical vagotomy, thus indicating that it results from a neural reflex mechanism independent of the chemical respiratory drive and of vagal afferent inputs. The attenuation of the external intercostal inhibition and the disappearance of the parasternal intercostal inhibition after section of the cervical dorsal roots provided definite evidence that this inhibitory reflex primarily originates from diaphragmatic receptors.
The intercostal inhibition induced by the application of force on the diaphragm is thus closely similar to that previously seen during electrical stimulation of phrenic afferents (De Troyer, 1998), and this suggests that both inhibitions are related to the same reflex mechanism. However, whereas electrical stimulation caused no consistent alterations in respiratory timing (De Troyer, 1998), the application of force was observed to induce a gradual reduction in inspiratory duration in five animals. This reduction suggests that the reflex inhibition elicited by this procedure has a supraspinal component, and indeed phrenic afferents are well known to project to various brainstem structures, in particular the respiratory neurones in the dorsal and ventral respiratory groups (Macron & Marlot, 1986; Speck & Revelette, 1987). The reason that no reduction in TI was seen during electrical stimulation is uncertain, but two explanations come to mind. First, it is notable that when present, the reductions in TI induced by the application of force became significant (>10 %) only with high forces (Fig. 4). This suggests that the expression of this supraspinal component requires a sufficient number of afferent volleys from diaphragmatic receptors, and it is possible that this number was not reached during electrical stimulation. Indeed, the electrical stimuli were applied exclusively to the C5 phrenic nerve roots and involved therefore only one-third of all phrenic afferents. The second possible explanation relates to the poor selectivity of electrical stimuli. In other words, the reduction in TI due to electrical stimulation of the afferents responsible for the reflex inhibition might have been obscured or at least reduced by the supraspinal effects of other afferents that were recruited as well.
Although the primary effect of force application may be to elicit increased afferent volleys from tension receptors in the diaphragm (Lundberg & Winsbury, 1960; Revelette et al. 1992), the procedure must also reduce or abolish the normal inspiratory shortening of the muscle fibres. Presumably, therefore, muscle spindles were triggered as well. Previous electrical recordings from phrenic nerve afferents in cats have shown that the ratio of muscle spindle afferents to tendon organ afferents was lower in these nerves than in most skeletal muscles (Corda et al. 1965). Histological studies in cats have also indicated that the diaphragm contains few muscle spindles (Duron et al. 1978). It would be tempting to conclude, therefore, that diaphragmatic muscle spindles would be unable to provide enough afferent input to cause marked inhibition of inspiratory intercostal activity. However, the spinal and supraspinal projections of diaphragmatic muscle spindles are essentially unknown, and regardless of their small number, their input could still be sufficient to inhibit inspiratory intercostal motoneurones. In addition, Balkowiec et al. (1995) have recently suggested that the proportion of spindle afferents in the cat phrenic nerves may be substantially higher than previously thought. The possibility existed, therefore, that the inhibitory response of the inspiratory intercostals to the application of force on the central tendon originated from muscle spindles, rather than tension mechanoreceptors.
There is abundant evidence that high frequency vibration, when applied to the belly of a limb muscle or to its tendon, is a potent, relatively selective stimulus of the primary spindle endings (Brown et al. 1967; Burke et al. 1976); the reflex muscle contraction due to this stimulation is conventionally referred to as the ‘tonic vibration reflex’. Applying vibration to the central tendon elicited a small increase in diaphragmatic EMG activity, thus suggesting, in agreement with the observations of Balkowiec et al. (1995), that a tonic vibration reflex may indeed be induced in the diaphragm. This issue, however, was beyond the scope of the present investigation, and the possibility of a recording artefact (that is, a contamination of the diaphragmatic EMG signal by the vibrator) was not totally excluded. More importantly, no reduction in parasternal intercostal or external intercostal inspiratory activity was seen in any animal during this procedure. This result does not necessarily exclude projections from diaphragmatic muscle spindles to the inspiratory intercostal motoneurones but indicates that the reflex inhibition of inspiratory intercostal activity produced by the application of force on the central tendon does primarily result from activation of tension receptors.
The question finally remains as to why the application of force on the central tendon continued to elicit a small reduction in external intercostal EMG activity after section of the cervical dorsal roots. Previous studies by Langford & Schmidt (1983) in rats have shown that some small-diameter phrenic afferents enter the spinal cord through the ventral roots, rather than the dorsal roots. Revelette et al. (1988) have reached a similar conclusion in dogs, and this therefore raises the possibility that ventral roots were one route of spinal cord entry for the response of the inspiratory intercostal muscles to the application of force on the central tendon. The afferent fibres entering the spinal cord through the ventral roots, however, are essentially unmyelinated (group IV) fibres, and in limb muscles, the receptors connected to such fibres are primarily activated by noxious stimuli and chemical agents, rather than by muscle contraction (Kaufman, 1995). Electrical recordings from group IV phrenic afferents in cats have similarly shown that no regular discharges can be recorded from such fibres during spontaneous (Jammes et al. 1986) or electrically induced diaphragmatic contraction (Jammes & Balzamo, 1992).
On the other hand, it is well established in the cat that the external intercostal muscles contain many muscle spindles whereas the parasternal intercostals are poorly supplied (Duron et al. 1978). Strong evidence has also been obtained suggesting that the external intercostal α-motoneurones, but not the parasternal intercostal α-motoneurones, receive abundant projections from mechanoreceptors in the costo-vertebral joints (De Troyer, 1996, 1997). These differences in spinal connections are such that, when the normal inspiratory cranial motion of the ribs is reduced by external rib manipulation in dogs, the decreased shortening of the inspiratory intercostal muscles causes an increased external intercostal EMG activity but does not alter parasternal intercostal activity. Conversely, increasing the inspiratory cranial motion of the ribs induces a decreased external intercostal activity without any change in parasternal intercostal activity (De Troyer, 1996, 1997). A small reduction in external intercostal activity, combined with a constant parasternal intercostal activity, was exactly what the application of force produced after dorsal root section, and current understanding of the mechanical action of the diaphragm on the ribcage suggests that a force applied on the central tendon during inspiration could indeed accentuate the inspiratory cranial displacement of the ribs.
The muscle fibres of the costal portion of the diaphragm insert on the upper margins of the lower ribs and are oriented cranially. When these fibres contract, they therefore generate a force that causes the lower ribs to move cranially. This force is usually referred to as the ‘insertional’ force of the diaphragm (De Troyer et al. 1982; Loring & Mead, 1982), and measurements of thoracoabdominal motion in quadriplegic subjects, in whom pacing of the phrenic nerves in the neck allows diaphragmatic activation to remain constant, have shown that the magnitude of this force is affected by the resistance provided by the abdominal contents to diaphragmatic descent (Danon et al. 1979; Strohl et al. 1984). Specifically, when abdominal resistance in such subjects is increased by a pneumatic cuff or an elastic binder, the dome of the diaphragm descends less and the muscle fibres remain oriented cranially throughout inspiration such that the inspiratory cranial displacement of the lower ribs is augmented. As shown in Fig. 7, the application of force on the central tendon similarly resulted in a graded increase in the inspiratory cranial displacement of the ribs. Based on the associated increase in the inspiratory shortening of the inspiratory intercostal muscles and the altered afferent inputs from rib joint mechanoreceptors (De Troyer, 1996, 1997), one may therefore account for the persistent reduction of external intercostal activity after dorsal root section.
The conclusion can thus be drawn that impeding diaphragmatic descent during inspiration induces a graded reduction in inspiratory intercostal activity partly through an increased elevation of the ribs but mostly through a reflex inhibition arising from low-threshold tension receptors in the diaphragm. This result, therefore, provides strong evidence for the contention that the increased inspiratory intercostal activity observed after phrenic nerve blockade is due, in part, to the removal of inhibitory inputs from diaphragmatic tension-sensitive receptors (Brichant & De Troyer, 1997). The morphology of the receptors, however, remains to be clarified. Many features of this reflex are consistent with the known response properties and reflex actions of Golgi tendon organs in limb muscles (Jami, 1992). Indeed, when activated, tendon organs in a particular extensor muscle inhibit extensor muscles acting at other joints, and so diaphragmatic tendon organs might well cause widespread inhibition of muscles inflating the lung. On the other hand, our previous studies involving electrical stimulation of phrenic afferents have failed to show any intercostal inhibition until the stimulus strength was increased to three times the motor threshold (i.e. was well above the threshold of activation of Ib fibres in limb muscles) (De Troyer, 1998). The speculation must be offered, therefore, that the reflex primarily arises either from tension-sensitive free nerve endings or from tendon organ-like receptors connected to afferents with group III or group II conduction velocities, as described in the cat (Balkowiec et al. 1995).
Acknowledgments
The authors gratefully acknowledge the National Heart, Lung, and Blood Institute (USA - grant HL 45 545) and the Research Committee of the Brussels School of Medicine for their financial support.
References
- Balkowiec A, Kukula K, Szulczyk P. Functional classification of afferent phrenic nerve fibres and diaphragmatic receptors in cats. The Journal of Physiology. 1995;483:759–768. doi: 10.1113/jphysiol.1995.sp020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichant JF, De Troyer A. On the intercostal muscle compensation for diaphragmatic paralysis in the dog. The Journal of Physiology. 1997;500:245–253. doi: 10.1113/jphysiol.1997.sp022014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Engberg I, Matthews PBC. The relative sensitivity to vibration of muscle receptors of the cat. The Journal of Physiology. 1967;192:773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contractile muscles. The Journal of Physiology. 1976;261:673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda M, von Euler C, Lennerstrand G. Proprioceptive innervation of the diaphragm. The Journal of Physiology. 1965;178:161–177. doi: 10.1113/jphysiol.1965.sp007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon J, Druz WS, Goldberg NB, Sharp JT. Function of the isolated paced diaphragm and the cervical accessory muscles in C1 quadriplegics. American Review of Respiratory Disease. 1979;119:909–919. doi: 10.1164/arrd.1979.119.6.909. [DOI] [PubMed] [Google Scholar]
- De Troyer A. The electro-mechanical response of canine inspiratory intercostal muscles to increased resistance: the cranial rib-cage. The Journal of Physiology. 1992;451:445–461. doi: 10.1113/jphysiol.1992.sp019172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A. Rib motion modulates inspiratory intercostal activity in dogs. The Journal of Physiology. 1996;492:265–275. doi: 10.1113/jphysiol.1996.sp021307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A. Role of joint receptors in modulation of inspiratory intercostal activity by rib motion in dogs. The Journal of Physiology. 1997;503:445–453. doi: 10.1111/j.1469-7793.1997.445bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A. The canine phrenic-to-intercostal reflex. The Journal of Physiology. 1998;508:919–927. doi: 10.1111/j.1469-7793.1998.919bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Kelly S. Chest wall mechanics in dogs with acute diaphragm paralysis. Journal of Applied Physiology. 1982;53:373–379. doi: 10.1152/jappl.1982.53.2.373. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Sampson M, Sigrist S, Macklem PT. Action of costal and crural parts of the diaphragm on the rib cage in dog. Journal of Applied Physiology. 1982;53:30–39. doi: 10.1152/jappl.1982.53.1.30. [DOI] [PubMed] [Google Scholar]
- Duron B, Jung-Caillol MC, Marlot D. Myelinated nerve fiber supply and muscle spindles in the respiratory muscles of cat: quantitative study. Anatomy and Embryology. 1978;152:171–192. doi: 10.1007/BF00315923. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McKenzie DK. Human diaphragmatic EMG: changes with lung volume and posture during supramaximal phrenic stimulation. Journal of Applied Physiology. 1986;60:1420–1428. doi: 10.1152/jappl.1986.60.4.1420. [DOI] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiological Reviews. 1992;72:623–666. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- Jammes Y, Balzamo E. Changes in afferent and efferent phrenic activities with electrically induced diaphragmatic fatigue. Journal of Applied Physiology. 1992;73:894–902. doi: 10.1152/jappl.1992.73.3.894. [DOI] [PubMed] [Google Scholar]
- Jammes Y, Buchler B, Delpierre S, Rasidakis A, Grimaud C, Roussos C. Phrenic afferents and their role in inspiratory control. Journal of Applied Physiology. 1986;60:854–860. doi: 10.1152/jappl.1986.60.3.854. [DOI] [PubMed] [Google Scholar]
- Katagiri M, Young RN, Platt RS, Kieser TM, Easton PA. Respiratory muscle compensation for unilateral or bilateral hemidiaphragm paralysis in awake canines. Journal of Applied Physiology. 1994;77:1972–1982. doi: 10.1152/jappl.1994.77.4.1972. [DOI] [PubMed] [Google Scholar]
- Kaufman M. Afferents from limb skeletal muscles. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. 2. New York, USA: Marcel Dekker; 1995. pp. 583–616. [Google Scholar]
- Kim MJ, Druz WS, Sharp JT. Effect of muscle length on electromyogram in a canine diaphragm strip preparation. Journal of Applied Physiology. 1985;58:1602–1607. doi: 10.1152/jappl.1985.58.5.1602. [DOI] [PubMed] [Google Scholar]
- Langford LA, Schmidt RF. An electron microscopic analysis of the left phrenic nerve in the rat. Anatomical Records. 1983;205:207–213. doi: 10.1002/ar.1092050211. [DOI] [PubMed] [Google Scholar]
- Loring SH, Mead J. Action of the diaphragm on the rib cage inferred from a force-balance analysis. Journal of Applied Physiology. 1982;53:756–760. doi: 10.1152/jappl.1982.53.3.756. 10.1063/1.329944. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Winsbury G. Selective adequate activation from muscle spindle and Golgi tendon organs. Acta Physiologica Scandinavica. 1960;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Macron JM, Marlot D. Effects of stimulation of phrenic afferent fibers on medullary respiratory neurons in cat. Neuroscience Letters. 1986;63:231–236. doi: 10.1016/0304-3940(86)90361-7. 10.1016/0304-3940(86)90361-7. [DOI] [PubMed] [Google Scholar]
- Ninane V, Farkas GA, Baer R, De Troyer A. Mechanism of rib cage inspiratory muscle recruitment in diaphragmatic paralysis. American Review of Respiratory Disease. 1989;139:146–149. doi: 10.1164/ajrccm/139.1.146. [DOI] [PubMed] [Google Scholar]
- Revelette R, Reynolds S, Brown D, Taylor R. Effect of abdominal compression on diaphragmatic tendon organ activity. Journal of Applied Physiology. 1992;72:288–292. doi: 10.1152/jappl.1992.72.1.288. [DOI] [PubMed] [Google Scholar]
- Revelette WR, Jewell LA, Frazier DT. Effect of diaphragm small-fiber afferent stimulation on ventilation in dogs. Journal of Applied Physiology. 1988;65:2097–2106. doi: 10.1152/jappl.1988.65.5.2097. [DOI] [PubMed] [Google Scholar]
- Speck DF, Revelette WR. Excitation of dorsal and ventral respiratory group neurons by phrenic nerve afferents. Journal of Applied Physiology. 1987;62:946–951. doi: 10.1152/jappl.1987.62.3.946. [DOI] [PubMed] [Google Scholar]
- Stradling JR, Kozar LF, Dark J, Kirby T, Andrey SM, Phillipson EA. Effect of acute diaphragm paralysis on ventilation in awake and sleeping dogs. American Review of Respiratory Disease. 1987;136:633–637. doi: 10.1164/ajrccm/136.3.633. [DOI] [PubMed] [Google Scholar]
- Strohl KP, Mead J, Banzett RB, Lehr J, Loring SH, O'Cain CF. Effect of posture on upper and lower rib cage motion and tidal volume during diaphragm pacing. American Review of Respiratory Disease. 1984;130:320–321. doi: 10.1164/arrd.1984.130.2.320. [DOI] [PubMed] [Google Scholar]


