Abstract
The effects of prostaglandins E2 (PGE) and F2α (PGF) on membrane potential and isometric tension and cytoplasmic free calcium concentration ([Ca2+]i) and tension were studied in strips of uterine smooth muscle obtained from women undergoing Caesarean delivery at term and during established labour.
Prostaglandins (PGs) evoked a biphasic response. The excitatory component consisted of depolarization of the membrane, which initiated spike action potentials, an increase in [Ca2+]i and tension development. The membrane remained depolarized at −19 ± 1 mV for about 2 min, then repolarized abruptly, [Ca2+]i promptly returned to basal levels, and tension development ceased.
This component of the response to PGE or PGF was followed by a slow hyperpolarization which reached −85 ± 2 mV (n= 22) at term and −70 ± 2 mV (n= 9) during labour, and during which spontaneous action potentials and tension development did not occur.
Nifedipine (10−6 M) abolished spontaneous activity, abolished PG-induced action potentials and reduced the increase in [Ca2+]i (9 ± 3%, n= 6), the depolarization (10 ± 1 mV, n= 14), the tension (2 ± 1%, n= 14) and the hyperpolarization (9 ± 1 mV, n= 14, at term).
A variety of K+ channel blockers were without effect on the peak amplitude of the PG-induced hyperpolarization but the latter did not occur in the presence of ouabain (10−6 M) or in K+-free or low-Na+ solutions, suggesting an involvement of the Na+-K+-ATPase pump.
In conclusion, a substantial dependence on Ca2+ influx through voltage-operated Ca2+ channels accounts for the importance of membrane potential in regulating contractions in human uterine smooth muscle. The classical excitatory effect of PGE and PGF is followed by hyperpolarization involving the Na+-K+-ATPase pump. The hyperpolarization restricts the response to a single contraction and decreases the frequency of subsequent contractions. The amplitude of the hyperpolarization decreases during labour, allowing contraction frequency to increase. Its persistence at this time ensures complete relaxation between each single robust contraction, preventing spasm of the uterus that would restrict blood flow to the fetus during delivery.
Prostaglandins (PGs) are likely to play a role in human parturition. Oral or intramuscular administration of prostaglandins E2 (PGE) or F2α (PGF) precipitates uterine contraction and labour (Embrey, 1975; Keirse, 1992) and blockade of PG synthesis prolongs labour (Keirse, 1992; Olson et al. 1995). PGs are produced by tissues that are in close proximity to the uterus. Changes in the enzymes involved in the synthesis and metabolism of PGs in amnion, chorion and decidua can account for the increase in the concentrations of PGE and PGF in amniotic fluid prior to and progressively during labour (López-Bernal et al. 1987; Aitken et al. 1990; Romero et al. 1994; Hirst et al. 1995; Challis et al. 1997).
The mechanisms involved in the contractile response of human myometrium to PGs remain unresolved. In cultures of uterine smooth muscle cells obtained from pregnant women, PGF stimulated the production of inositol trisphosphate (InsP3) (Carrasco et al. 1996) and PGE similarly increased [Ca2+]i solely by releasing Ca2+ from stores and not via Ca2+ influx (Asboth et al. 1996). Yet in strips of pregnant human myometrium, neither PGF nor PGE were found to stimulate InsP3 production, despite the ability of oxytocin to do so in the same tissues (Schrey et al. 1988). In smooth muscle cells isolated from non-pregnant human myometrium, PGF evoked a transient increase in [Ca2+]i which was reduced to 50 % by verapamil, was entirely dependent on the presence of Ca2+∘ and did not involve mobilization of InsP3 (Molnar & Hertelendy, 1990). Moreover, patch clamp studies of isolated human myometrial cells have revealed a large inward current underpinned by both T- and L-type voltage-operated calcium channels (Inoue et al. 1990; Young et al. 1993). Although these reports provide elegant details on the properties of the ion channels, the relationship between membrane electrical activity, Ca2+ influx and contraction remains unresolved. The experiments of Kawarabayashi & Sugimori (1985) addressed this problem more directly by recording electrical activity via the sucrose gap technique simultaneously with tension in strips of human myometrium. They found that PGF changed the form of the action potential from simple spikes to a plateau type, or increased the duration of the depolarization in tissues in which the spontaneous action potential was already of the plateau type, and increased the amplitude of the associated contraction.
The aim of the present study was to re-examine the mechanisms by which PGE and PGF modulate contraction in human myometrium by simultaneous recording of membrane potential and tension and, in other experiments, [Ca2+]i and tension, in whole strip preparations immediately following excision of the tissue. Some of these results have been presented in abstract form (Parkington et al. 1998).
METHODS
Tissue preparation
Uterine smooth muscle was obtained from women undergoing Caesarean delivery. Tissue was collected only following written, informed consent, obtained by the research midwife prior to surgery. The research project and associated consent forms were approved by the Research and Ethics Committees of the Royal Women's Hospital. Tissue was obtained from women at term not in labour, that is, from 37–40 weeks of gestation, who were undergoing Caesarean delivery for fetal growth restriction, a previous Caesarean delivery or suspected cephalo-pelvic disproportion. Tissue was also obtained at Caesarean delivery during established labour in cases of fetal distress, breech presentation or cephalo-pelvic disproportion. In most cases spinal anaesthesia was used. The women at term and in labour were of similar age and had had one to three previous successful pregnancies. The tissues were from the upper edge of a lower segment, transverse incision. The tissue was collected from theatre by the research midwife and immediately taken to the laboratory in a capped container of cold, oxygenated physiological saline solution (PSS) (see below).
Strips of muscle (0.5 mm × 3 mm) were prepared using a dissecting microscope and transferred to a recording chamber. One end of each strip was attached to a force transducer (SensoNor 801) and, for electrophysiology, the other end was pinned to the rubber base of the recording chamber. This enabled the simultaneous recording of membrane potential and isometric tension. The chamber was transferred to the stage of an inverted microscope and the tissue was continuously perfused at 3 ml min−1 with PSS maintained at 35°C.
Membrane potentials were recorded using conventional intracellular microelectrodes filled with 1 M KCl and having resistances of 70–100 MΩ. The microelectrodes and reference electrode were connected to an Axoclamp-2B amplifier (Axon Instruments). Membrane potential and the occurrence of spontaneous increases in tension became regular within about 1 h of mounting the tissue in the bath.
For simultaneous measurement of [Ca2+]i and tension, the free end of the strip of tissue was fixed to the glass base of the recording chamber with a tiny drop of SupaGlue. The tissue was incubated in the acetoxymethyl ester (AM) form of the fluorescence probe fura-2 (5 μm) for 1 h at room temperature. The tissues were not perfused during this incubation, and hence 10 mM of bicarbonate in the PSS was replaced with 10 mM Hepes and the solution was buffered to pH 7.4. The solution also contained pluronic F-127, 0.01 %, to aid in the dispersal of fura-2 AM. At the end of the loading period the tissue was perfused with normal PSS for 30 min at 35°C to provide time for endogenous esterases to cleave the methyl ester from the fura-2 AM and to remove excess probe from the extracellular space. To record changes in [Ca2+]i, the tissues were irradiated with UV light alternately at 340 and 380 nm. The emission signal, at 505 nm, was collected by a photomultiplier tube and, following correction for background and autofluorescence obtained prior to loading with fura-2, the ratio of the signals at 340 and 380 nm, R340/380, was calculated. A chopper in the pathway of the excitation illumination selected the incident light at 100 Hz for each wavelength. The signal from the photomultiplier tube was sampled and demultiplexed, and three samples were averaged for each window to give one point every 10 ms for each wavelength. The data were further averaged to give an effective frequency response of 1 Hz. In all experiments, attempts were made to calibrate the signal, converting R340/380 to [Ca2+]i. Rmax was determined by permeabilizing the membrane with 2 × 10−5 M ionomycin in the presence of 2.5 mM Ca2+∘ and Rmin was determined in Ca2+-free solution containing 3 mM EGTA. The value of KD used was 224 nM. In some experiments R340/380 was used to indicate changes in [Ca2+]i due to the difficulties surrounding a determination of the absolute value of [Ca2+]i (Austin & Wray, 1995). For small changes in R340/380, the relationship with [Ca2+]i was approximately linear, but peak values of R340/380 would have underestimated the changes in [Ca2+]i.
In both experimental situations the tissues were stretched to optimal length, that is, until the increase in tension evoked by 0.5–1 min exposure to 100 mM KCl was maximal.
Solutions and drugs
Normal PSS contained (mM): NaCl, 120; KCl, 5; KH2PO4, 1; MgSO4, 1.2; NaHCO3, 25; CaCl2, 2.5; glucose, 11; bubbled with 95 % O2-5 % CO2. Zero Na+ solution contained (mM): N-methyl-D-glucamine (NMDG) or Tris, 135; KHCO3, 6; MgSO4, 1.2; CaCl2, 2.5; Hepes, 10; glucose, 11; buffered to pH 7.4 with HCl. In some experiments Na+∘ was reduced to 25 mM, in which case normal PSS was used but with 120 mM NMDG replacing NaCl. K+-free solution was made using NaCl as substitute in PSS. For most experiments, high-K+ solution was made by substituting NaCl with the isosmotic equivalent of KCl (see text for concentrations).
Dinoprost, a stable form of PGF, and PGE (gifts from Upjohn, Kalamazoo, MI, USA) were used in most experiments. The injectable forms of dinoprost (Upjohn) and salbutamol (10−2 M) (Glaxo, Melbourne, Australia) were also used. Stock solutions (in distilled water except where indicated) of nifedipine (10−2 M in DMSO), verapamil (10−2 M), indomethacin (10−2 M in Na2CO3), 4-aminopyridine (1 M), ouabain (10−2 M), tetraethylammonium (1 M), apamin (5 × 10−4 M), catechol (1 M), tetrodotoxin (10−4 M), Tris, NMDG (all from Sigma, St Louis, MO, USA), charybdotoxin (10−4 M; Auspep, Melbourne, Australia), glibenclamide (10−2 M in DMSO; a gift from Hoechst, Melbourne, Australia), iloprost (10−3 M in DMSO; a gift from Schering, Berlin, Germany) were used. Fura-2 AM (10−2 M in DMSO) and pluronic F-127 were obtained from Molecular Probes, Eugene, OR, USA. When dimethyl sulphoxide (DMSO) was used as diluent, the concentration of the stock solution was such that the final concentration of DMSO in the perfusate, 10−4 M, had no effect on membrane potential or tension in myometrium.
Data analysis
The electrophysiological and tension responses were stored on video cassette using a digital data recorder (VR-100, Instrutech Corp., Great Neck, NY, USA) for later analysis. The Ca2+ and tension data were captured on computer using the software and hardware of an AmLab (AmLab International, Sydney, Australia) which also enabled the tension and R340/380 to be recorded on a chart recorder on-line during the experiment. Data were analysed using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA, USA) which allowed analysis of variance and curve fitting. Student's t tests were performed using GraphPad Instat to compare means. The mean ± standard error of the mean is quoted and a statistical significance level of P < 0.05 was accepted throughout. Only one tissue was used from each woman (two tissues when electrophysiology and [Ca2+]i were both recorded) and the number of tissues studied, n, was used in analyses.
RESULTS
Effect of PGE and PGF on [Ca2+]i, tension and membrane potential at term
Exposure of tissues to PGE or PGF (both at 10−5 M, to achieve maximal responses) evoked an increase in [Ca2+]i, and an example of the effect of PGE is shown in Fig. 1. [Ca2+]i increased from 40 ± 11 nM (n= 6) at rest to a peak of 669 ± 58 nM (n= 6) within 10–20 s and then declined to a plateau of 248 ± 38 nM (n= 6) (37 % of the peak value). This Ca2+ response was associated with an increase in tension that also peaked in 10–20 s and subsequently declined to 41 ± 8 % (n= 10) of the peak. A return of [Ca2+]i to resting levels was followed by a prompt cessation of active tension development.
Figure 1. [Ca2+]i and tension during a spontaneous contraction and during exposure to 10−5 M PGE, in control and in the presence of nifedipine, in term human uterine smooth muscle.
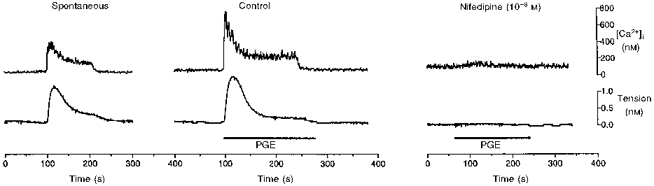
[Ca2+]i (upper trace) and tension (lower trace) were recorded simultaneously in tissues that had been loaded with fura-2. Each increase in tension, both spontaneous and in response to 1 min exposure to PGE (10−5 M), was preceded by an increase in [Ca2+]i. Nifedipine (10−6 M) markedly reduced the increase in both [Ca2+]i and tension evoked by PGE.
In another strip from the same samples of muscle used in the fura-2 study, and in samples from additional women, PGE and PGF (10−5 M for 1 or 3 min) depolarized the membrane from a resting potential of -59 ± 1 mV (n= 22) and initiated an action potential that consisted of a spike followed by depolarization of the smooth muscle to -19 ± 1 mV, which lasted for 2.3 ± 0.2 min (Fig. 2). Tension increased rapidly to a peak (134 ± 7 % of spontaneous tension development in n= 13 spontaneously active tissues) and then declined slowly to 69 ± 4 % (n= 22) of the peak value (particularly clearly illustrated in Figs 4 and 6), despite the persistence of the depolarization. The depolarization was terminated by a brisk repolarization to around -65 mV followed by abrupt cessation of tension development. The repolarization was followed by a further slow hyperpolarization to -85 ± 2 mV (n= 22). Spontaneous action potentials and increases in tension were suspended for 23 ± 3 min (n= 13), that is, for the duration of the hyperpolarization following 1 min exposure to 10−5 M PGF (Fig. 2). The normal interval between spontaneous increases in tension in the same tissues was 6 ± 1 min (n= 13). Responses evoked by PGE and PGF were indistinguishable.
Figure 2. Effect of PGE and PGF on membrane potential and tension in term human uterine smooth muscle.
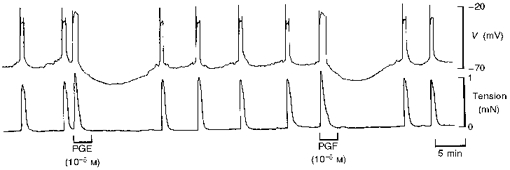
Exposure to PGE and PGF (3 min) evoked an action potential (upper trace) and an increase in tension (lower trace) in a strip of uterine smooth muscle. The action potential was followed by hyperpolarization that increased the interval to the next spontaneous action potential and rise in tension.
Figure 4. Prolonged exposure to PGF on membrane potential and tension in term human uterine smooth muscle.
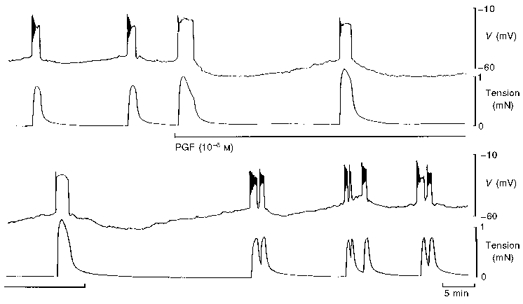
Application of PGF (10−6 M) for 30 min evoked large action potentials and increases in tension but caused an increase in membrane negativity between the action potentials. This resulted in a reduction in the frequency of spontaneous activity. The effect was rapidly reversible.
Figure 6. Effect of tetraethylammonium on the response to PGF in term human uterine smooth muscle.
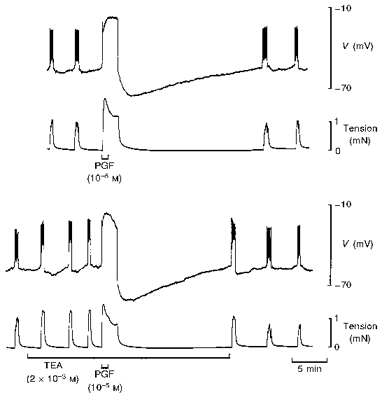
In control solution, PGF evoked an action potential followed by hyperpolarization that increased the interval before the next spontaneous action potential and increase in tension (upper traces). Tetraethylammonium (TEA) caused a 5–10 mV depolarization and increased the frequency of activity but had little effect on the response to PGF except to increase the rate of recovery from the hyperpolarization (lower traces).
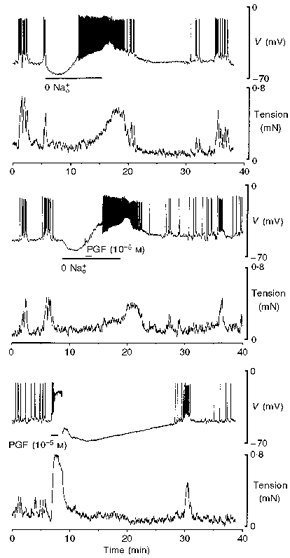
Nifedipine (10−6 M) promptly abolished all spontaneous increases in [Ca2+]i, action potentials and tension development. Both PGs evoked only a very small increase in [Ca2+]i (to 96 ± 28 nM, n= 6) in the presence of nifedipine (Fig. 1). Nifedipine reduced the depolarization in response to PGF (10−5 M) to 10 ± 1 mV (n= 14) in amplitude and the accompanying tension was reduced to 2 ± 1 % of control (Fig. 10). The PG-dependent hyperpolarization was also reduced to 9 ± 1 mV (n= 14) in amplitude in the presence of nifedipine (Fig. 10). Another class of Ca2+ channel blocker, verapamil (10−5 M), also abolished the action potential and markedly reduced the increase in tension evoked by PGF in three tissues (data not shown).
Figure 10. Effect of nifedipine on membrane potential and tension responses to PGF and K+-free solution in term human uterine smooth muscle.
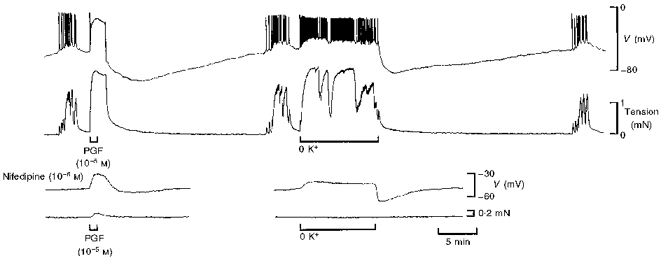
The PGF-dependent hyperpolarization and that which followed restoration of 6 mM K+ following perfusion in K+-free solution were similar in amplitude and time course (upper traces). Nifedipine (lower traces) abolished all action potential activity, revealed the depolarization evoked by PGF and by perfusion with K+-free solution, all but abolished PG-induced increases in tension and markedly attenuated the hyperpolarizations. All responses in the same cell.
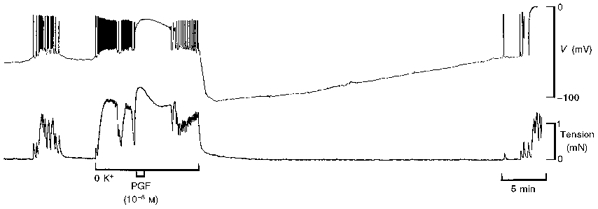
Low concentrations of PGF (10−8 to 10−7 M) evoked depolarization that was subthreshold for the initiation of action potentials (n= 4). In the absence of an action potential, the depolarization evoked by PGF was not followed by hyperpolarization (data not shown). Increasing [K+]o to 50 mM evoked an action potential and an increase in tension similar to those in response to PGF but the K+-induced action potential was not followed by hyperpolarization (Fig. 3). The response to PGF in the presence of 50 mM K+∘ was indistinguishable from the response to PGF in normal PSS (Fig. 3).
Figure 3. Comparison between responses to 50 mM K+∘ and PGF in term human uterine smooth muscle.
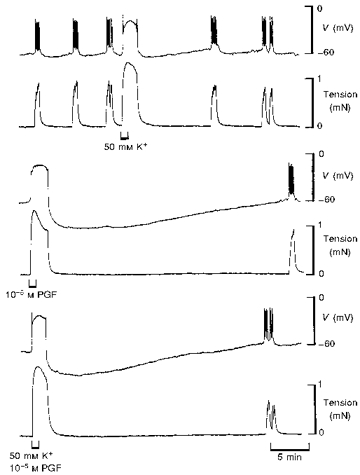
Increasing [K+]o to 50 mM evoked a membrane potential response which resembled a PG-induced action potential but hyperpolarization did not follow (upper traces). PGF alone (middle traces) or in solution containing 50 mM K+ (lower traces) evoked the usual action potential that was followed by hyperpolarization. All recordings were made in the same cell.
Prolonged application of PGs
The effect of prolonged (30–180 min) exposure to PGF was tested in tissues displaying regularly occurring spontaneous action potentials and increases in tension. An example illustrating the effects of 60 min exposure to 10−6 M PGF is shown in Fig. 4. The presence of PGF caused the membrane potential between action potentials to reach a more hyperpolarized level compared with control, and hence a longer time was required to reach threshold for the initiation of action potentials. This led to a decrease in the frequency of tension development. The amplitudes of the increases in tension were larger during exposure to PG compared with controls (Fig. 4). Figure 5 summarizes the results from 10 tissues; there was a concentration-dependent increase in the peak amplitude of the tension, an increase in the level of membrane potential negativity attained between successive action potentials and an increase in the interval between spontaneous increases in tension over the range 10−7 to 10−5 M PGF.
Figure 5. Prolonged exposure to PGF on tension, membrane potential and interval between spontaneous activity in term human uterine smooth muscle.
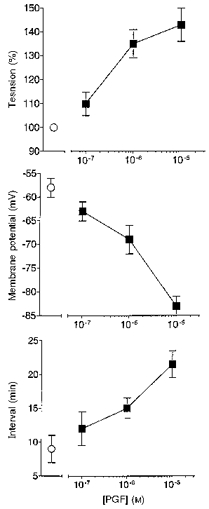
Ten spontaneously active tissues at weeks 37–39 of pregnancy were exposed to 10−7, 10−6 or 10−5 M PGF (2–3 concentrations per tissue), each for 30–180 min. The peak amplitude of the rise in tension, the most negative level of membrane potential attained and interval between action potentials and rises in tension during PG exposure were recorded (▪) (data from 6–7 tissues at each point). The values of these parameters in the same tissues prior to application of PGF are also shown (^).
Blockade of the synthesis of endogenous PGs by including 10−6 or 10−5 M indomethacin in the perfusing solution was without effect on the responses to 1 min or longer applications of PGE or PGF. In 7 of 39 tissues, the depolarization elicited by 100 mM K+ was followed by hyperpolarization of around 10 mV and when this occurred it was always abolished by 30 min exposure to 10−6 M indomethacin.
Response to PGs during labour
The responses to PGE and PGF during established labour were qualitatively similar to those observed at term, 37- 40 weeks of pregnancy, but quantitative differences were apparent. A comparison of the responses to 1 min application of 10−5 M PGF at term and during labour is summarized in Table 1. The duration of the action potential was significantly reduced during labour, while the level of the plateau of depolarization remained unchanged. The peak increase in tension accompanying the PG-induced action potential was significantly larger in tissues obtained during established labour while the decline in tension with time was smaller in labour. The level of membrane potential attained during the hyperpolarization which followed the action potential was significantly reduced during labour. Since there was a range of resting membrane potentials amongst the tissues, the amplitude of the hyperpolarization was measured by subtracting the value of the membrane potential attained during the peak of the hyperpolarization from the resting membrane potential immediately prior to the application of PGF. This amplitude was halved during labour. Despite this, the interval to the next increase in tension was 17 ± 2 min in the three in-labour tissues that displayed spontaneous increases in tension at an interval of 5 ± 1 min. Again, the responses to PGE and PGF were indistinguishable during labour.
Table 1.
Comparison of responses at term and during established labour
| PGF (10−5 M) | |||||||
|---|---|---|---|---|---|---|---|
| Plateau | Tension | Hyperpolarization | |||||
| Vrest(mV) | Level(mV) | Duration(min) | Amplitude(%) | Decline(to %) | Level(mV) | Amplitude(mV) | |
| Term (n= 22) | −59 ± 1 | −19 ± 1 | 2.3 ± 0.2 | 134 ± 7 | 69 ± 4 | −85 ± 2 | 22 ± 1 |
| Labour (n= 10) | −61 ± 2 | −22 ± 1 | 1.4 ± 0.2* | 190 ± 13* | 85 ± 5* | −70 ± 2* | 9 ± 1* |
Vrest: resting membrane potential. Plateau: the level of membrane potential attained during the plateau component of the action potential and the duration from spike upstroke to the initial repolarization. Tension: the peak amplitude of the increase in tension (as a percentage of spontaneous tension), and the level attained during the decline in tension (immediately prior to commencement of relaxation, as a percentage of the initial peak). Hyperpolarization: the level of membrane potential attained during the hyperpolarization and its amplitude (level minus Vrest). Observations at term (weeks 37–40 of pregnancy) and in established labour.
Significantly different from observations at term.
K+ channel blockers
The effects of a variety of K+ channel blockers on the PGE and PGF-dependent hyperpolarization were investigated in tissues obtained at term. The blockers tested were: tetraethylammonium (TEA; (1–5) × 10−3 M, n= 5), charybdotoxin (ChTx; 10−8 M, n= 2), glibenclamide (10−6 M, n= 4), apamin (10−7 M, n= 2), barium (3 × 10−5 and 10−4 M, n= 5), catechol (5 × 10−3 M, n= 2) and 4-aminopyridine (4-AP; (1–5) × 10−3 M, n= 4). Pretreatment with these blockers had no detectable effect on the peak amplitude of the PG-dependent hyperpolarization; an example using TEA is shown in Fig. 6.
The blockers reduced the duration of the hyperpolarization, that is, the recovery of the membrane potential to resting values was more rapid in the presence of some of the blockers. 4-AP and catechol reduced the recovery interval to 52 ± 3 % (n= 6) of control and TEA reduced its duration to 63 ± 4 % (n= 5) of control (Fig. 6). The duration of the hyperpolarization remained in excess of 87 ± 4 % (n= 10) of control in the presence of the remaining K+ channel blockers.
The K+ channel blockers were also capable of influencing the characteristics of the plateau phase of the PG-induced action potential. The effect of barium (10−4 M) was most dramatic, in that it almost doubled the duration of the plateau (1.8 ± 0.1-fold increase, n= 4). The duration of the plateau was increased in a more modest fashion by 4-AP and ChTx (1.2 ± 0.1-fold, n= 6). As a consequence, the duration of the accompanying increase in tension was prolonged, since relaxation did not occur until after the membrane potential had repolarized.
Blockade of the Na+-K+-ATPase pump
Blockade of the Na+ pump with ouabain pretreatment (3 × 10−7 to 10−5 M, n= 11) caused prompt (within 1 min) depolarization of the membrane and the initiation or an increase in frequency of action potentials (Fig. 7). PGF evoked the usual depolarization in the presence of ouabain but the hyperpolarization did not occur (Fig. 7). In three tissues, application of 10−6 M ouabain during the peak of the PG-dependent hyperpolarization caused prompt reversal of the hyperpolarization, further depolarization that initiated the firing of action potentials, and an increase in tension that was 81 ± 3 % (n= 5) of the PG-induced increase in tension (Fig. 8). Application of TEA (5 × 10−3 M) at a similar position in the hyperpolarization during the previous application of PG (n= 3) had little effect on membrane potential or tension (Fig. 8).
Figure 7. PG-dependent hyperpolarization did not occur in the presence of ouabain in term human uterine smooth muscle.
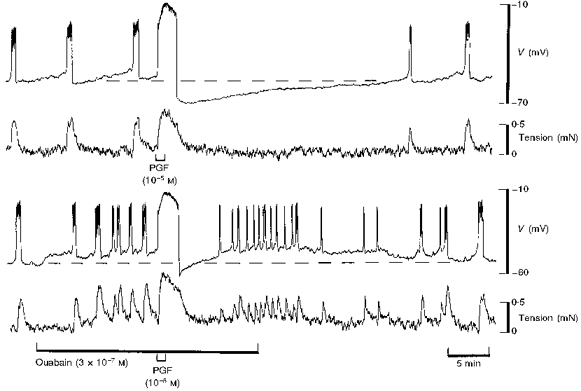
The action potential initiated by PGF was followed by prolonged hyperpolarization in control solution (upper panel). A modest concentration of ouabain (3 × 10−7 M) evoked depolarization and an increase in the frequency of firing of action potentials similar to application of TEA (see Fig. 6). PGF initiated a similar action potential as in control but no hyperpolarization occurred (lower panel). The resting membrane potential is indicated by the dashed line. For both panels, the upper trace is membrane potential and the lower trace is tension. Continuous impalement throughout.
Figure 8. Application of TEA or ouabain during the peak of the PG-dependent hyperpolarization in term human uterine smooth muscle.
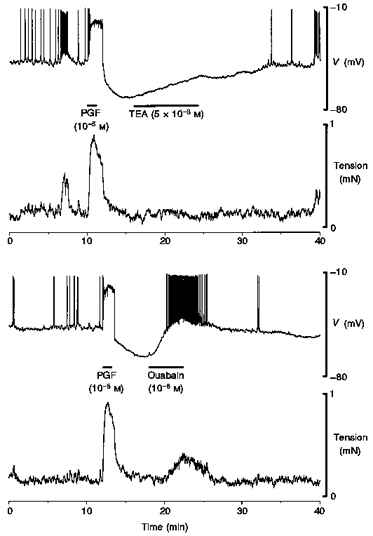
Application of TEA during the nadir of the PG-dependent hyperpolarization was without measurable effect on membrane potential or tension. In contrast, inclusion of ouabain during the nadir caused prompt repolarization, the firing of action potentials and an increase in tension.
Omission of K+ from the perfusing solution also suppresses activity of the Na+ pump and in the present study resulted in the firing of action potentials, similar to those observed in the presence of 10−6 M ouabain. K+-free solution inhibited the PGF-dependent hyperpolarization (n= 13) (Fig. 9). Restoration of normal 6 mM K+∘ following 4–12 min exposure to K+-free solution resulted in prompt hyperpolarization that peaked at 23 ± 2 mV (n= 6) more negative that the resting membrane potential, a level that was similar to the 20 ± 1 mV hyperpolarization evoked by PGF in the same tissues. Furthermore, the time courses of recovery from these two hyperpolarizations were remarkably similar (Fig. 10). Nifedipine abolished the action potentials and the increase in tension that occurred in K+-free solution, leaving a depolarization. The amplitude of the hyperpolarization that occurred upon the restoration of 6 mM K+ was reduced to 11 ± 1 mV (n= 5) by nifedipine, similar to the effect of the dihydropyridine on the PGF-induced hyperpolarization (Fig. 10).
Figure 9. PG-dependent hyperpolarization did not occur in K+-free solution in term human uterine smooth muscle.
Hyperpolarization followed the action potential in response to PGF in control solution. In K+-free solution no such hyperpolarization was observed.
Effect of PGF in low extracellular Na+
Involvement of the Na+-K+-ATPase would be expected to depend on [Na+]o through Na+ influx either at rest due to Na+ leakage into the cells, or in response to PGs. [Na+]o was lowered from 145 mM to 25 and 0 mM (NMDG substitution for 10 min in each test) in tissues obtained at term. Exposure to 25 mM Na+∘ caused prompt hyperpolarization of the membrane which peaked with an amplitude of 12 ± 1 mV (n= 6) in 1–2 min and thereafter returned to the normal resting membrane potential in about 7 min. This membrane potential response was not associated with any detectable change in tension. In 0 mM Na+∘ the membrane hyperpolarized transiently by 16 ± 1 mV (n= 9). The membrane potential returned to the resting level in four of the tissues, in which case there was no increase in tension. In the remaining five tissues the membrane continued to depolarize beyond the resting level, and spike action potentials occurred which were associated with a 17 ± 3 % increase in tension (relative to a K+-induced contraction) (Fig. 11). Nifedipine (10−6 M) abolished the action potentials and tension leaving a biphasic membrane potential response to 0 mM Na+∘. Tris was used as a Na+ substitute (0 mM Na+∘ for 10 min) in three of the experiments with identical results (data not shown). Upon restoration of normal PSS from low-Na+∘ solution, the membrane always depolarized to threshold for the initiation of action potentials and an increase in tension (see Fig. 11).
Figure 11. Response to PGF in solution containing 0 mM Na+∘ in term human uterine smooth muscle.
Lowering Na+∘ to 0 mM caused significant, but transient hyperpolarization. In 5 of 9 tissues the ensuing depolarization exceeded threshold for the firing of spike action potentials accompanied by a modest increase in tension (upper panel). Inclusion of PGF in 0 mM Na+∘ solution evoked only modest additional depolarization, no or a small additional increase in tension, and no after-hyperpolarization (middle panel). The control response to PGF illustrates a robust after-hyperpolarization in this cell (lower panel). For all three panels membrane potential is shown in the upper trace and tension in the lower trace. Impalement of the same cell throughout.
Application of PGF for 1 min at the peak of the hyperpolarization evoked by 25 mM Na+∘ resulted in approximately 2 mV depolarization and an increase in tension that was only 2 ± 1 % of the control PG response (data not shown). When applied after the membrane potential had returned to control levels, that is, 7 min in 25 or 0 mM Na+∘, PGF evoked depolarization of variable but reduced amplitude (ranging from 2 to 6 mV, n= 9) and an increase in tension that was 9 ± 2 % of control (Fig. 11). The after-hyperpolarization in response to PG did not occur in solution containing 25 or 0 mM Na+∘ (Fig. 11).
Perfusion with 25 mM Na+∘ was without effect on [Ca2+]i. In three of seven tissues, 0 mM Na+∘ solution for 10 min evoked a small, slow rise in [Ca2+]i that achieved 104 ± 6 % of basal and an accompanying increase in tension that reached 15 ± 1 % of that evoked by 100 mM K+ PSS (Fig. 12). In the remaining four tissues, exclusion of Na+ from the perfusate was without effect on [Ca2+]i or tension (data not shown). Following restoration of normal 135 mM Na+∘ in all tissues, there was a rebound increase in [Ca2+]i and tension (Fig. 12), similar to observations in five of the nine electrophysiological studies.
Figure 12. [Ca2+]i and tension in response to PGF in solution containing 0 mM Na+∘ in term human uterine smooth muscle.
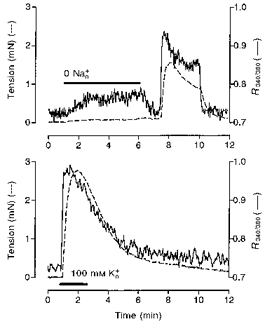
Lowering Na+∘ to 0 mM caused an increase in [Ca2+]i (upper panel) that was small compared with the increase evoked by 100 mM K+ (lower panel) in 2 of 6 tissues.
Tetrodotoxin (2 × 10−7 M) had no effect on spontaneous activity or on the amplitude of the spike, the sustained depolarization or the hyperpolarization that followed exposure to PGF (n= 3) (data not shown).
Effect of blockers in labour
Although an emphasis was placed on observations in tissues at term because of the larger amplitude of the PG-dependent hyperpolarization and the routine availability of tissue, the effects of K+ channel blockers (n= 3), ouabain (n= 2), K+-free solution (n= 5), and 0 mM Na+∘ (n= 2) were also examined in tissues obtained during established labour, the smaller numbers reflecting limited tissue availability. The effects of the blockers and of changing the ionic composition of the bathing solution were indistinguishable in tissues at term and during established labour.
Agents that activate the cyclic AMP system
Tissues were unresponsive to the stable prostacyclin analogue iloprost until 10−6-10−5 M, at which concentrations 1 min exposure evoked an action potential and large increase in tension that were followed by a modest hyperpolarization of 9 ± 2 mV (n= 3) (Fig. 13). Following recovery from the hyperpolarization action potentials occurred, but at first were not accompanied by rises in tension. The β2-adrenoceptor agonist salbutamol, 10−7 M for 1 min, hyperpolarized the smooth muscle by 5 ± 1 mV (n= 3) (data not shown). At 10−6 M, salbutamol evoked hyperpolarization that was 9 ± 1 mV in amplitude; spontaneous action potentials still occurred but the associated increase in tension was reduced by about half (Fig. 13).
Figure 13. Effect of the prostacyclin analogue iloprost and the β2-adrenoceptor agonist salbutamol on membrane potential and tension in term human uterine smooth muscle.
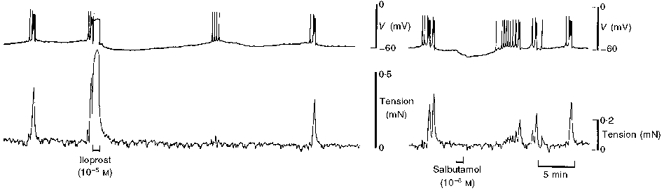
The stable prostacyclin analogue iloprost evoked an action potential similar to that observed in response to PGF, but this was followed by weak hyperpolarization. The tension associated with subsequent spontaneous action potentials was markedly suppressed. Salbutamol evoked modest hyperpolarization, without an action potential, and the tension associated with subsequent spontaneous action potentials was much reduced.
DISCUSSION
The present study revealed that the excitatory response evoked in human pregnant uterine smooth muscle by PGE and PGF, an action potential and large contraction, is followed by a profound hyperpolarization which limits excitation to a single contraction of 2–5 min duration and, in spontaneously active tissue, decreases the frequency of contractions.
The ionic mechanisms underlying the hyperpolarization include a significant contribution by the Na+-K+-ATPase. The hyperpolarization was insensitive to a variety of K+ channel blockers but was very sensitive to procedures that inhibit the Na+-K+-ATPase. Thus, it did not occur in tissues pretreated either with ouabain or in K+-free solution, while introduction of ouabain during the peak of the hyperpolarization resulted in its prompt reversal. Stimulation of the Na+ pump by restoration of normal 6 mM K+∘ following a period in K+-free solution resulted in hyperpolarization whose amplitude and time course were remarkably similar to the PG-dependent hyperpolarization. Furthermore, PGs did not evoke hyperpolarization in Na+-free solution. These results are consistent with the activation of the Na+-K+-ATPase following the action potential in the presence of PG.
The present study also demonstrates the pivotal role of voltage-operated Ca2+ channels (VOCCs) during contraction in pregnant human uterine smooth muscle at term and during established labour. Spontaneous and PG-induced contractions are underpinned by action potentials and a considerable increase in [Ca2+]i, all of which are abolished by drugs that block VOCCs, supporting previous observations in muscle strips (Forman et al. 1979; Schrey et al. 1988) and in freshly isolated smooth muscle cells (Molnar & Hertelendy, 1990) and is concordant with the effectiveness of dihydropyridines in suppressing uterine contractions in vivo (Kaul et al. 1985; Keirse, 1992). The pivotal role of VOCCs in contraction emphasizes the importance of membrane potential in human uterine smooth muscle and provides considerable significance to agents that modulate membrane potential, such as the Na+ pump.
PGE and PGF depolarize uterine smooth muscle in a variety of species. However, the role of VOCCs in the contractile response to PGs does not appear to assume the same significance in the uterus of other species since substantial contraction persists in the presence of VOCC blockers or Ca2+-free solution in tissue from rats (Reiner & Marshall, 1976), mice (Suzuki & Kuriyama, 1975) and guinea-pigs (Clegg et al. 1966; Coleman et al. 1988). In contrast, the major role of the PG-induced depolarization in human uterine muscle appears to be to increase the probability of opening of VOCCs.
In the present study, PGs evoked a single action potential and contraction, in contrast to the bursts of action potentials and multi-peaked contractions that have been observed previously in uterine muscle of mice (Osa et al. 1974), rats (Reiner & Marshall, 1976), guinea-pigs (Coleman & Parkington, 1988) and sheep (Parkington, 1985). A biphasic mechanical response to PGE, contraction followed by inhibition, has been described previously for human uterine muscle (Word et al. 1992; Senior et al. 1993). The present study demonstrates that an action potential underpins the initial contraction while a profound hyperpolarization accounts for the following inhibitory component. PG-dependent hyperpolarization has not been reported in other species.
Excitatory as well as inhibitory PG receptors occur in human uterine smooth muscle (Senior et al. 1993) and may mediate the depolarization and hyperpolarization, respectively. The dominant excitatory PG receptors on human uterine smooth muscle are the PGE type 3 and the PGF receptors, while PGE type 2, PGD and PGI receptors transduce relaxation (Senior et al. 1993). PGE activates the adenylyl cyclase- cyclic AMP system via its type 2 receptors. An effect of PGF on cyclic AMP has not been described. However, the receptor specificity of the native PGs is poor (Negishi et al. 1995). The relatively high concentrations of PGs required to effect a response in this study compound the problem of receptor specificity but the concentrations required are within the range previously needed by others to elicit a response in pregnant human myometrium in vitro (Kawarabayashi & Sugimori, 1985; Word et al. 1992). Agents that activate the adenylyl cyclase system have been shown to stimulate the Na+-K+-ATPase pump in toad stomach smooth muscle (Scheid & Fay, 1984). In the present study, the β-adrenoceptor agonist salbutamol and the inhibitory PGI, prostacyclin, which stimulate the cyclic AMP system, markedly suppressed the contraction associated with action potentials but evoked only modest hyperpolarization, effects similar to those reported for rat myometrium (Diamond & Marshall, 1969). Further work is required to clarify the types of receptors and any messenger systems involved in both the depolarization and the hyperpolarization evoked by these PGs.
The mechanisms by which PGE and PGF activate the Na+ pump may not be direct, but may be secondary to an increase in [Na+]i brought about by other means. Thus, ouabain-sensitive K+ (or rubidium) influx in vascular smooth muscle is sensitive to agents that inhibit the Na+-H+ exchanger (Kahn et al. 1988; Gupta et al. 1991). It is noteworthy that a change in the activity of Na+-H+ exchange is likely to have significant repercussions in relation to contraction in uterine smooth muscle through changes in cytoplasmic pH (Phoenix & Wray, 1993). A primary action of protein kinase C (PKC) on Na+-H+ or on Na+-K+-Cl− transporters, with a resulting increase in [Na+]i, may underlie subsequent stimulation of the Na+ pump (Little et al. 1986; Gupta et al. 1991). The Ca2+ dependence of some isoforms of PKC might explain the sensitivity of the hyperpolarization to nifedipine. Some isoforms of PKC require the presence of diacylglycerol, stimulated by PGs in human uterine muscle (Carrasco et al. 1996; Asboth et al. 1996). The prolonged nature of the PG-induced hyperpolarization would be in keeping with the involvement of such a second messenger system, although the similarly prolonged hyperpolarization which followed restoration of normal [K+]o following a period in K+∘-free solution casts doubt on that interpretation.
Another mechanism by which [Na+]i could increase is via Na+-Ca2+ exchange. Many smooth muscles contract when [Na+]o is lowered and this has been explained in terms of operation of the Na+-Ca2+ exchanger in reverse mode, which promotes an increase in [Ca2+]i. However, the depolarization elicited in such tissues by low [Na+]o calls this interpretation into question (Tomita & Pang, 1996). Lowering [Na+]o in the present study did not evoke a rise in [Ca2+]i and the muscle did not contract, in marked contrast with observations in myometrium of other species (mice, Osa et al. 1974; rats, Masahashi & Tomita, 1983; Savineau et al. 1987; guinea-pigs, Hart et al. 1992). The hyperpolarization of human uterine muscle upon lowering [Na+]o observed here and described previously (Inoue et al. 1990) could be explained by operation of Na+-Ca2+ exchange in reverse mode but such a response is also consistent with the changes in membrane potential predicted from the Goldman-Hodgkin-Katz equation. Assuming a relative permeability of 0.05 for Na+, 0.1 for Cl−, and 0.1 for Ca2+ yielded a resting membrane potential of -59 mV. Decreasing [Na+]o from 145 mM to 25 and 0 mM under these conditions was calculated to cause hyperpolarization of 11 and 14 mV, respectively, close to the 12 and 16 mV recorded. One possible interpretation of these findings is that the Na+-Ca2+ exchanger operates in forward mode during the PG-evoked action potential, consistent with the depolarization of the plateau, the decline in [Ca2+]i and the decline in the contraction during the plateau, and a possible increase in [Na+]i. The latter could then stimulate the Na+-K+-ATPase pump.
The membrane did not remain hyperpolarized for the entire 10 min period in low-Na+∘ solution but had recovered to resting or more depolarized levels within about 7 min. This is likely to reflect the leakage of Na+ out of the cells, in keeping with previous observations in other smooth muscles (Aickin & Brading, 1985; Aickin, 1987).
The level of membrane potential attained during the hyperpolarization, -85 mV, is remarkably close to the equilibrium potential for K+ reported previously for this tissue (-87 mV, Inoue et al. 1990). Pretreatment with blockers of a range of K+ channels was entirely without effect on the amplitude of the hyperpolarization. However, the blockers reduced the time for recovery from the hyperpolarization, implicating a K+ conductance late in the process.
The decrease in the amplitude of the hyperpolarization during labour could result from a decrease in the activity or density of Na+-K+-ATPase pumps at this time. Growth factors, glucocorticoids and thyroid hormone have been shown to stimulate or inhibit expression of the pump in a variety of cells, including smooth muscle (see O'Donnell & Owen, 1994). In the event of an indirect involvement of the Na+-H+ exchanger in the hyperpolarization, several growth factors and glucocorticoids have been found to modulate the expression and activity of this exchanger in smooth muscle (O'Donnell & Owen, 1994).
In conclusion, the pronounced hyperpolarization which follows the PG-induced action potential has a profound effect in limiting the response in human tissue to a single individual contraction and in increasing the interval between contractions in human uterine smooth muscle at term and during established labour. The overall effect is a decrease in the incidence of tetanic contraction that would lead to impairment of the blood supply to the feto-placental unit and thus jeopardize fetal wellbeing during labour (Wray, 1993). The effectiveness of the hyperpolarization arises from the reliance of the tissue on voltage-dependent Ca2+ influx for contraction. The Na+ pump is implicated in the hyperpolarization. The decline, and not disappearance, of the latter during labour allows the necessary increase in contraction frequency.
Acknowledgments
The authors thank Professors M. E. Holman and J. N. Pennefather and Dr M. Tare for constructive criticism of the manuscript and the obstetric and midwifery staff (especially Jenny Robinson and Jane Atkinson) of the Royal Women's Hospital involved in retrieval of the tissues. The Brockhoff Foundation very generously equipped the electrophysiological laboratory at the Royal Women's Hospital and the Ramaciotti Trustees donated the Axoclamp amplifier at Monash University. The project was funded by the National Health and Medical Research Council of Australia.
References
- Aickin CC. Investigation of factors affecting intracellular sodium activity in the smooth muscle of guinea-pig ureter. The Journal of Physiology. 1987;385:483–505. doi: 10.1113/jphysiol.1987.sp016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin CC, Brading AF. Advances in the understanding of transmembrane ionic gradients and permeabilities in smooth muscle obtained by using ion-selective micro-electrodes. Experientia. 1985;41:879–887. doi: 10.1007/BF01970005. [DOI] [PubMed] [Google Scholar]
- Aitken MA, Rice GE, Brennecke SP. Gestational tissue phospholipase A2 messenger RNA content and the onset of spontaneous labour in the human. Reproduction, Fertility and Development. 1990;2:575–580. doi: 10.1071/rd9900575. [DOI] [PubMed] [Google Scholar]
- Asboth G, Phaneuf S, Europe-Finner GN, Toth M, López Bernal AL. Prostaglandin E2 activates phospholipase C and elevates intracellular calcium in cultured myometrial cells: involvement of EP1 and EP3 receptor subtypes. Endocrinology. 1996;137:2572–2579. doi: 10.1210/endo.137.6.8641211. 10.1210/en.137.6.2572. [DOI] [PubMed] [Google Scholar]
- Austin C, Wray S. The effects of extracellular pH and calcium change on force and intracellular calcium in rat vascular smooth muscle. The Journal of Physiology. 1995;488:281–291. doi: 10.1113/jphysiol.1995.sp020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco MP, Phaneuf S, Asboth G, López Bernal A. Fluprostenol activates phospholipase C and Ca2+ mobilization in human myometrial cells. Journal of Clinical Endocrinology and Metabolism. 1996;81:2104–2110. doi: 10.1210/jcem.81.6.8964835. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Lye SJ, Gibb W. Prostaglandins and parturition. Annals of the New York Academy of Sciences. 1997;828:254–267. doi: 10.1111/j.1749-6632.1997.tb48546.x. [DOI] [PubMed] [Google Scholar]
- Clegg PC, Hall WJ, Pickles VR. The action of ketonic prostaglandins on the guinea-pig myometrium. The Journal of Physiology. 1966;183:123–144. doi: 10.1113/jphysiol.1966.sp007855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HA, McShane PG, Parkington HC. Gestational changes in the utilization of intracellularly stored calcium in the myometrium of guinea-pigs. The Journal of Physiology. 1988;399:13–32. doi: 10.1113/jphysiol.1988.sp017065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HA, Parkington HC. Induction of prolonged excitability in myometrium of pregnant guinea-pigs by prostaglandin F2α. The Journal of Physiology. 1988;399:33–47. doi: 10.1113/jphysiol.1988.sp017066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J, Marshall JM. A comparison of the effects of various smooth muscle relaxants on the electrical and mechanical activity of rat uterus. Journal of Pharmacology and Experimental Therapeutics. 1969;168:21–30. [PubMed] [Google Scholar]
- Embrey MP. The Prostaglandins in Human Reproduction. Edinburgh: Churchill Livingstone; 1975. [Google Scholar]
- Forman A, Andersson KE, Persson CG, Ulmsten U. Relaxant effects of nifedipine on isolated, human myometrium. Acta Pharmacologica et Toxicologica. 1979;45:81–86. doi: 10.1111/j.1600-0773.1979.tb02364.x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ruderman NB, Cragoe EJ, Jr, Sussman I. Endothelin stimulates Na+-K+-ATPase activity by a protein kinase C-dependent pathway in rabbit aorta. American Journal of Physiology. 1991;30:H38–45. doi: 10.1152/ajpheart.1991.261.1.H38. [DOI] [PubMed] [Google Scholar]
- Hart JD, Coleman HA, Parkington HC. Prostaglandin F2α and oxytocin differentially affect calcium and sodium permeabilities in uterine smooth muscle of guinea-pigs. Proceedings of the Australian Physiological and Pharmacological Society. 1992;23:108P. [Google Scholar]
- Hirst JJ, Teixeira FJ, Zakar T, Olson DM. Prostaglandin H synthase-2 expression increases in human gestational tissues with spontaneous labour onset. Reproduction, Fertility and Development. 1995;7:633–637. doi: 10.1071/rd9950633. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Nakao K, Okabe K, Izumi H, Kanda S, Kitamura K, Kuriyama H. Some electrical properties of human pregnant myometrium. American Journal of Obstetrics and Gynecology. 1990;162:1090–1098. doi: 10.1016/0002-9378(90)91322-4. [DOI] [PubMed] [Google Scholar]
- Kahn AM, Allen JC, Shelat H. Na+-Ca2+ exchange in sarcolemmal vesicles from bovine superior mesenteric artery. American Journal of Physiology. 1988;23:C441–449. doi: 10.1152/ajpcell.1988.254.3.C441. [DOI] [PubMed] [Google Scholar]
- Kaul AF, Osathanondh R, Safon LE, Frigoletto FDJ, Friedman PA. The management of preterm labor with the calcium channel-blocking agent nifedipine combined with the beta-mimetic terbutaline. Drug Intelligence and Clinical Pharmacy. 1985;19:369–371. doi: 10.1177/106002808501900507. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Sugimori H. Effects of oxytocin and prostaglandin F2α on pregnant human myometrium recorded by the single sucrose-gap method - comparison of an in vitro experiment and an in vivo trial. Asia-Oceania Journal of Obstetrics and Gynaecology. 1985;11:247–253. doi: 10.1111/j.1447-0756.1985.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Keirse MJNC. Inhibitors of prostaglandin synthesis for treatment of preterm labour. In: Drife JO, Calder AA, editors. Prostaglandins and the Uterus. London: Springer-Verlag; 1992. pp. 277–296. [Google Scholar]
- Little PJ, Cragoe EJ, Bobik A. Na-H exchange is a major pathway for Na influx in rat vascular smooth muscle. American Journal of Physiology. 1986;251:C707–712. doi: 10.1152/ajpcell.1986.251.5.C707. [DOI] [PubMed] [Google Scholar]
- López Bernal A, Hansell DJ, Alexander S, Turnbull AC. Steroid conversion and prostaglandin production by chorionic and decidual cells in relation to term and preterm labour. British Journal of Obstetrics and Gynaecology. 1987;94:1052–1058. doi: 10.1111/j.1471-0528.1987.tb02289.x. [DOI] [PubMed] [Google Scholar]
- Masahashi T, Tomita T. The contracture produced by sodium removal in the non-pregnant rat myometrium. The Journal of Physiology. 1983;334:351–363. doi: 10.1113/jphysiol.1983.sp014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar M, Hertelendy F. Regulation of intracellular free calcium in human myometrial cells by prostaglandin F2α: Comparison with oxytocin. Journal of Clinical Endocrinology and Metabolism. 1990;71:1243–1250. doi: 10.1210/jcem-71-5-1243. [DOI] [PubMed] [Google Scholar]
- Negishi M, Sugimoto Y, Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochimica et Biophysica Acta. 1995;1259:109–119. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- O'Donnell ME, Owen NE. Regulation of ion pumps and carriers in vascular smooth muscle. Physiological Reviews. 1994;74:683–721. doi: 10.1152/physrev.1994.74.3.683. [DOI] [PubMed] [Google Scholar]
- Olson DM, Mijovic JE, Sadowsky DW. Control of human parturition. Seminars in Perinatology. 1995;19:52–63. doi: 10.1016/s0146-0005(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Osa T, Suzuki H, Katase T, Kuriyama H. Excitatory action of synthetic prostaglandin E2 on the electrical activity of pregnant mouse myometrium in relation to temperature changes and external sodium and calcium concentrations. Japanese The Journal of Physiology. 1974;24:233–248. doi: 10.2170/jjphysiol.24.233. [DOI] [PubMed] [Google Scholar]
- Parkington HC. Some properties of the circular myometrium of the sheep throughout pregnancy and during labour. The Journal of Physiology. 1985;359:1–15. doi: 10.1113/jphysiol.1985.sp015571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkington HC, Tonta MA, Brennecke SP, Holman ME, Coleman HA. Prostaglandins hyperpolarize and suppress spontaneous contractions in human uterine smooth muscle. The Journal of Physiology. 1998;507.P:68P. [Google Scholar]
- Phoenix J, Wray S. Changes in frequency and force production of the human myometrium with alteration of pH and metabolism. Journal of Reproduction and Fertility. 1993;97:507–512. doi: 10.1530/jrf.0.0970507. [DOI] [PubMed] [Google Scholar]
- Reiner O, Marshall JM. Action of prostaglandin, PGF2α on the uterus of the pregnant rat. Naunyn-Schmiedeberg's Archives of Pharmacology. 1976;292:243–250. doi: 10.1007/BF00517384. [DOI] [PubMed] [Google Scholar]
- Romero R, Baumann P, Gonzalez R, Gomez R, Rittenhouse L, Behnke E, Mitchell MD. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. American Journal of Obstetrics and Gynecology. 1994;171:1613–1620. doi: 10.1016/0002-9378(94)90412-x. [DOI] [PubMed] [Google Scholar]
- Savineau JP, Mironneau J, Mironneau C. Influence of the sodium gradient on contractile activity in pregnant rat myometrium. General Physiology and Biophysics. 1987;6:535–560. [PubMed] [Google Scholar]
- Scheid CR, Fay FS. β-Adrenergic effects on transmembrane 45Ca fluxes in isolated smooth muscle cells. American Journal of Physiology. 1984;246:C431–438. doi: 10.1152/ajpcell.1984.246.5.C431. [DOI] [PubMed] [Google Scholar]
- Schrey MP, Cornford PA, Read AM, Steer PJ. A role for phosphoinositide hydrolysis in human uterine smooth muscle during parturition. American Journal of Obstetrics and Gynecology. 1988;159:964–970. doi: 10.1016/s0002-9378(88)80182-0. [DOI] [PubMed] [Google Scholar]
- Senior J, Marshall K, Sangha R, Clayton JK. In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. British Journal of Pharmacology. 1993;108:501–506. doi: 10.1111/j.1476-5381.1993.tb12832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kuriyama H. Effects of prostaglandin E2 on the electrical property of the pregnant mouse myometrium. Japanese The Journal of Physiology. 1975;25:201–215. doi: 10.2170/jjphysiol.25.201. [DOI] [PubMed] [Google Scholar]
- Tomita T, Pang Y. Membrane depolarization caused by sodium removal in the circular muscle of guinea-pig gastric antrum. In: Bolton TB, Tomita T, editors. Smooth Muscle Excitation. London: Academic Press; 1996. pp. 437–447. [Google Scholar]
- Word RA, Kamm KE, Casey ML. Contactile effects of prostaglandins, oxytocin, and endothelin-1 in human myometrium in vitro: Refractoriness of myometrial tissue of pregnant women to prostaglandins E2 and F2α. Journal of Clinical Endocrinology and Metabolism. 1992;75:1027–1032. doi: 10.1210/jcem.75.4.1400867. 10.1210/jc.75.4.1027. [DOI] [PubMed] [Google Scholar]
- Wray S. Uterine contraction and physiological mechanisms of modulation. American Journal of Physiology. 1993;264:C1–18. doi: 10.1152/ajpcell.1993.264.1.C1. [DOI] [PubMed] [Google Scholar]
- Young RC, Smith LH, McLaren MD. T-type and L-type calcium currents in freshly dispersed human uterine smooth muscle. American Journal of Obstetrics and Gynecology. 1993;169:785–792. doi: 10.1016/0002-9378(93)90006-5. [DOI] [PubMed] [Google Scholar]


