Abstract
The voltage- and time-dependent characteristics of the hyperpolarization-activated current (IH) and its contribution to the resting membrane potential of neonatal rat nodose sensory neurons were investigated using the whole-cell tight seal method of voltage and current clamp recording.
IH was found in all neonatal nodose neurons in vitro, contrary to previous reports where its presence was particular for A-type neurons. We used the presence of both tetrodotoxin-sensitive (TTX-S) and tetrodotoxin-resistant (TTX-R) sodium currents to distinguish C- from A-type neurons (TTX-S only). We obtained further support for the presence of IH in C-type neurons with experiments in which IH was demonstrated in a subset of neurons sensitive to capsaicin.
In both groups IH activated at potentials negative to −50 mV, developed slowly with time and was inhibited by 1–5 mm extracellular caesium. At −120 mV, IH activated with a fast time constant of 73 ± 3 ms in A-type neurons and 163 ± 37 ms in C-type neurons (P < 0.05). A second, slower time constant of 682 ± 83 ms was observed in A-type neurons and 957 ± 122 ms in C-type neurons.
A- and C-type neurons differed in the amplitude of IH. The mean magnitude of IH at −110 mV was −2338 ± 258 pA in A-type neurons but only -241 ± 40 pA (P < 0.001) in C-type neurons. This disparity persisted when currents were normalized for capacitance. The reversal potentials for IH were −39 ± 4 mV for A-type neurons and −37 ± 5 mV for C-type neurons (P > 0.05).
During current clamp recording IH caused time-dependent rectification in response to hyperpolarizing current injections from the resting membrane potential. CsCl abolished the rectification and hyperpolarized the resting potential of A-type neurons from −55 ± 3 mV to −61 ± 4 mV and C-type neurons from −62 ± 2 mV to −71 ± 3 mV. Taken together, the results in these studies indicate that IH contributes to the resting membrane potential in all nodose neurons.
A population of neurons whose cell bodies are contained within the nodose sensory ganglia serves as a primary afferent limb of the baroreceptor reflex. These neurons monitor arterial pressure by increasing their discharge when arterial pressure rises. Approximately 15 % of the baroreceptor neurons have myelinated axons, A-type neurons, while the remainder have unmyelinated axons, C-type neurons (Andresen et al. 1978). The two groups have quite different discharge properties in response to changes in arterial pressure (Brown et al. 1978). These discharge properties are the result of activation, inactivation, and/or deactivation of a diverse population of ion channels. Thus far, the kinetic properties of voltage-dependent calcium (Mendelowitz & Kunze, 1992; Bacal & Kunze, 1994), sodium (Baccaglini & Cooper, 1982; Ikeda et al. 1986; Ikeda & Schofield, 1987; Schild & Kunze, 1997) and outward potassium currents (Cooper & Shrier, 1989; McFarlane & Cooper, 1991) have been described and implemented in a model system that characterizes the contributions of individual ionic currents to firing patterns in A- and C-type neurons (Schild et al. 1994). In the absence of available experimental data, the model presented by Schild et al. (1994) rests on the assumption that the underlying current setting the resting membrane potential for nodose neurons is primarily due to a background leak current, permeable to both sodium and calcium cations.
In this study we sought the identity of the current(s) that set the resting membrane potential for nodose neurons. One of the current(s) that may contribute to the resting membrane potential is the hyperpolarization-activated current. The reason for this hypothesis is the following. When the neurons are given a hyperpolarizing current injection, there is an instantaneous hyperpolarization followed by a slow depolarization, described as time-dependent rectification (Stansfeld & Wallis, 1985; Puizillout & Gambarelli, 1989; Undem & Weinreich, 1993). This time-dependent rectification may be an indication of the hyperpolarization-activated current (If, Ih, Iq), similar to that established in sinoatrial node cells (DiFrancesco, 1985) and other central neurons (Spain et al. 1987; Bobker & Williams, 1989; McCormick & Pape, 1990a, b; Banks et al. 1993; Travagli & Gillis, 1994) and peripheral neurons (Ingram & Williams, 1994; Pearce & Duchen, 1994; Scroggs et al. 1994). This current is known to be active near -60 mV (Pape, 1996), which is also within the region of the resting membrane potential of nodose neurons. However, previous investigations have suggested that time-dependent rectification is prevalent only in A-type nodose neurons while C-type neurons show little to no time-dependent rectification (Stansfeld & Wallis, 1985; Puizillout & Gambarelli, 1989; Undem & Weinreich, 1993). This suggests that the resting membrane potential for C-type nodose neurons may be set by other ionic currents, such as a cation leak current used in a model of Schild et al. (1994).
Even though IH has been extensively characterized in cardiac cells and in a variety of neurons, there are differences in the kinetics, activation threshold and reversal potential of IH among various cell types (Pape, 1996). Therefore in the present study, we provide a detailed characterization of IH for both A- and C-type nodose neurons. In addition, we demonstrate that the hyperpolarization-activated cation current contributes an inward current at the resting membrane potential of neonatal rat nodose sensory neurons in both A- and C-type neurons, as identified by their selective pharmacological sensitivity to tetrodotoxin or capsaicin during voltage clamp measurements. The difference in properties of IH between A- and C-type neurons may explain the lack of time-dependent rectification in some nodose neurons, as previously suggested.
METHODS
Isolation of nodose neurons
Nodose ganglia were excised from neonatal (1–3 days old) Sprague- Dawley rats and the neurons were isolated and cultured, as previously described (Bacal & Kunze, 1994). Briefly, neonatal rats were asphyxiated by CO2 inhalation and the nodose ganglia were surgically removed. The dissected ganglia were placed in nodose complete medium (NCM) consisting of Dulbecco's modified Eagle's medium/F-12 (Gibco BRL), 5 % fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA), 0.1 % serum extender (Collaborative Research, Bedford, MA, USA) and 1 % penicillin/ streptomycin (Gibco BRL). The medium was replaced with Earle's balanced saline solution (EBSS; Gibco BRL) containing 5 mg ml−1 trypsin, 12 mM cysteine, 0.5 mM EDTA and 1.5 mM CaCl2, incubated for 30 min at 37°C. Following incubation, the medium was replaced with 3 ml NCM supplemented with 1.5 mg ml−1 albumin. The tissue was triturated with a fire-polished Pasteur pipette to disperse the cells and subsequently placed into 35 mm Petri dishes containing poly-D-lysine-treated glass coverslips. The cells were kept in a humidified incubator (5 % CO2-95 % air, at 37°C) and fed every other day with NCM. Some cultures were treated with NCM supplemented with 8 ng ml−1 nerve growth factor (NGF; Collaborative Research). No statistical difference was observed for the expression of IH among the cells with or without NGF. Therefore the data from the two groups of nodose neurons were pooled.
Electrophysiology
Electrophysiological experiments were performed on isolated neurons 1–2 days post-culture. By day 3, nodose neurons have developed processes; therefore space clamp was not possible. Isolated nodose sensory neurons were voltage and/or current clamped using the whole-cell tight seal patch technique. Polished electrodes were made from Corning no. 8161 or 7052 glass electrodes using a Narishige electrode puller. The electrodes, when filled with potassium aspartate solution, generally had a resistance of 1–3 MΩ for 8161 glass and 3–5 MΩ for 7052 glass. The reference electrode was a silver-silver chloride plug linked to the bath by a 150 mM KCl bridge. An Axopatch 1C patch clamp amplifier (Axon Instruments) was used with the 80 dB decade−1 low-pass filter set at a -3 dB frequency of 10 kHz. pCLAMP software was used to apply voltage and current clamp protocols in order to measure the whole-cell currents and whole-cell membrane potentials, respectively. The series resistance was generally between 3 and 12 MΩ. The capacitance and series resistance were approximately 40–60 % compensated where stated and no leak subtraction was performed. For data presented, the voltage error did not exceed ±3 mV. Junction potentials were corrected as described by Nehr (1992). A multibarrel gravity-fed perfusion system was used to exchange extracellular solutions at a rate of approximately 1 ml min−1. All recordings were done at room temperature.
Data analysis
Activation current traces were fitted with a biexponential function of the form:
using the Chebyshev technique within pCLAMP v. 6 software, where A0 is the offset constant, A1 and A2 are the amplitudes, and τ1 and τ2 are the respective time constants. The instantaneous tail currents at -80 mV were used to construct the steady-state activation curve for IH (see Figs 1C and 7C). The instantaneous tail currents were first normalized for each cell by using the formula:
where I-50 is the minimal current for IH and I-140 is the maximal current for IH. After normalizing the tail currents for each cell, the data points for each cell were fitted with a Boltzmann function:
where V½ is the half-activation potential of IH and kS is the slope factor.
Figure 1. Whole-cell voltage clamped recordings of the hyperpolarization-activated current (IH) in isolated neonatal rat nodose sensory neurons.

IH was measured in solution 1 (refer to Methods) in the absence (A) or presence (B) of 1 mM CsCl. The intracellular pipette contained high potassium aspartate solution (see Methods). The neuron was held at -40 mV and pulsed to different test potentials between -40 and -140 mV for 1 s duration in 10 mV step increments (inset in A). After each test potential, the neuron was stepped to -80 mV for 750 ms before returning to the holding potential. This voltage clamp protocol was used for subsequent studies regarding resting membrane potential and ionic contributions to IH, unless otherwise stated. C, representative current traces illustrating the caesium-sensitive current, attained by subtracting the current traces in the presence of CsCl (B) from the current traces in the absence of CsCl (A) at each test potential. D, mean ±s.e.m. (n= 16) current-voltage relationship measured at 1 s after start of test pulse in control (□) and in CsCl (▪) and resultant current (control - CsCl traces) at the beginning (^) and end of the pulse (•).
Figure 7. The Boltzmann fit of IH.
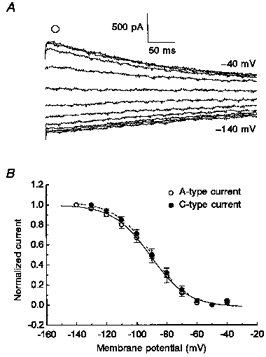
A, voltage dependence of activation determined at -80 mV step following return from different prepulse potentials (see Fig. 1). B, the mean normalized current from the instantaneous current traces at -80 mV (A, ^) was fitted with a Boltzmann function for A- (^) and C-type (•) neurons.
The reversal potential for each extracellular ionic condition during voltage clamp measurements was determined by calculating the membrane potential at the zero current. This was accomplished by a linear fit between two current measurements on each side of the zero current. Data are presented as means ±s.e.m. Statistical significance was determined at P < 0.05 using Student's t test.
Solutions
For voltage clamp studies, the following bath solutions were used. Solution 1 (mM): 137 NaCl, 5.4 KCl, 1 MgCl2, 0.02 CaCl2, 10 glucose and 10 Hepes, pH 7.4 with NaOH. Solution 2 was the same as solution 1 with equimolar substitution of NaCl by NMDG-Cl; and solution 3 was the same as solution 2 but NMDG-Cl was replaced by NMDG-MeSO4, CaCl2 by calcium gluconate and MgCl2 by magnesium gluconate. The pipette contained (mM): 145 potassium aspartate, 5 NaCl, 1.95 CaCl2, 2.2 EGTA, 2 MgCl2, 10 glucose and 5 Hepes, pH 7.3 with KOH.
For current clamp studies, the bath solution was the same as solution 1 except for the addition of 2 mM CaCl2; the pipette solution was the same as above. All chemicals, including tetrodotoxin and capsaicin, were purchased from Sigma.
RESULTS
Current-voltage characteristics of IH under voltage clamp
When a nodose neuron was voltage clamped at -40 mV, there was a small outward potassium current due to the activation of both the delayed rectifier potassium channels and the transient outward potassium channels, as previously reported in these neurons (Cooper & Shrier, 1989). As the neuron was hyperpolarized in 10 mV step increments, this outward potassium current deactivated within milliseconds and, subsequently, a slow non-inactivating inward current appeared (Fig. 1A). This hyperpolarization-activated inward current (IH) increased as the membrane potential was further hyperpolarized. The inward current approached a near steady level within 1 s. The voltage was then stepped to -80 mV from each test potential, resulting in the IH tail currents. The inward current at the hyperpolarized potentials was similar to the hyperpolarization-activated current found in other cells (DiFrancesco, 1985; Pape, 1996). In those reports, IH was sensitive to extracellular CsCl. Superfusion of nodose neurons with 1 or 5 mM CsCl added to the extracellular bath solution did not affect the outward potassium current at -40 mV (Fig. 1B). On the other hand, IH was completely inhibited in the presence of caesium. The inward IH tail currents, produced by the step to -80 mV, were also inhibited. To isolate the hyperpolarization-activated inward current from the outward potassium currents, the current traces in the presence of caesium were subtracted from the current traces in the absence of caesium (Fig. 1C). The caesium-sensitive current, representing IH, had an onset component that gradually increased towards a near steady value within 1 s. The I-V relation of the caesium-sensitive current at the near steady level showed that IH was activated at membrane potentials more negative than -50 mV. In a group of 16 neurons, IH reached a mean current amplitude of -542 ± 124 pA at -140 mV (Fig. 1D, n= 16).
In our study all nodose sensory neurons recorded under voltage clamp conditions expressed functional IH. This is in contrast with previous voltage recordings in nodose neurons where investigators found time-dependent rectification, an index of IH, only in A-type neurons and a few or no C-type neurons. In previous studies, the A- and C-type nodose neurons were distinguished according to the conduction velocity of their axons. We used the presence of both tetrodotoxin-resistant (TTX-R) and tetrodotoxin-sensitive (TTX-S) voltage-dependent sodium current to distinguish C-type neurons from A-type neurons (Stansfeld & Wallis, 1985; Schild & Kunze, 1997). The same voltage clamp protocol as described earlier was used to demonstrate IH (Fig. 2A). Cells were then held at -80 mV and stepped to depolarized potentials in 10 mV increments. There was a rapid activation and inactivation of the fast transient sodium currents at -40 mV and at more depolarized membrane potentials (Fig. 2B). Following the inactivation of the sodium currents, outward potassium currents appeared. It was not possible to obtain quantitative values for the sodium currents under these solution conditions due to poor voltage control. We were, however, able to demonstrate the elimination of the inward sodium current in 13 of 61 nodose neurons when 1 μm tetrodotoxin was perfused in the extracellular bath. The fast transient sodium current was completely inhibited while the outward potassium current was unaffected (Fig. 2C). These 13 TTX-S neurons were considered A-type neurons and had a large IH. On the other hand, the remainder of the population of nodose neurons (48/61) also displayed IH (Fig. 2D) but the sodium currents were only partially inhibited by 1 μm tetrodotoxin (Fig. 2F). The remaining inward current that activated near -20 mV was the TTX-R sodium current (Ikeda & Schofield, 1987; Schild & Kunze, 1997). The inward current was not a calcium or sodium current conducting through voltage-gated calcium channels, because the bath calcium concentration of 20 μm was sufficient to block sodium conductance through voltage-dependent calcium channels. Therefore, these neurons were classified as C-type neurons, since they expressed both the TTX-S and TTX-R sodium channels. No neurons were found to have only the TTX-R sodium current without the TTX-S sodium current.
Figure 2. IH is present in both A- and C-type neurons.
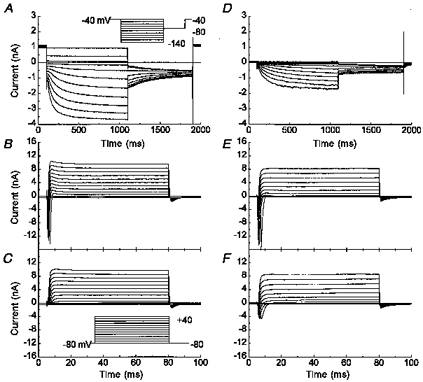
Representative whole-cell recording of two neurons, one with a prominent IH (A, 13 out of 61) and a second with a smaller IH (D, 48 out of 61). Voltage protocol shown in inset in A. B and C, the same neuron as illustrated in A, or E and F, the same neuron as in D, but with test potentials from -80 to +40 mV for 75 ms in 10 mV step interval from a holding potential of -80 mV (inset in C). The recordings were performed in solution 1 in the absence (A, B, D and E) or presence (C and F) of 1 μm tetrodotoxin. Since the sodium current was too fast and large, adequate voltage clamp was compromised.
Another pharmacological marker used to identify C-type neurons was capsaicin, a substance known to depolarize nociceptive neurons (Wood et al. 1988). Again using the IH voltage clamp protocol, we examined five nodose neurons that functionally expressed a very small amount of IH at the hyperpolarized potentials (Fig. 3A). When these neurons were held at -80 mV and step depolarized, they displayed the fast transient sodium currents and the kinetically slower outward potassium currents (Fig. 3B). When 10 μm capsaicin was perfused with the bath solution, a large inward current was induced at the holding potential (-80 mV). The capsaicin-induced current reversed near 0 mV, as expected for activation of a non-selective cation channel (Fig. 3C). The effect of capsaicin was reversible upon washout (Fig. 3D). All five capsaicin-sensitive neurons had both the TTX-S and TTX-R sodium currents.
Figure 3. IH was found in capsaicin-sensitive nodose neurons.
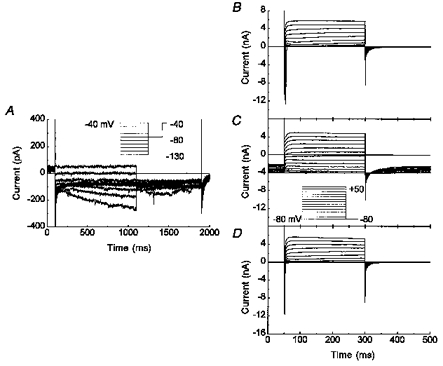
A, representative whole-cell recording of a neuron with a small magnitude IH at voltages between -40 and -130 mV (inset in A). B, C and D, the same neuron as in A but with test potentials from -80 to +50 mV for 250 ms in 10 mV step interval from a holding potential of -80 mV (inset in C). The recordings were performed in solution 1 in the absence (A, B and D) or presence (C) of 10 μm capsaicin. Notice the change in current and time scale with IH (A) or control and capsaicin treatment (B, C and D).
In comparing the current-voltage relationships, IH in A-type nodose neurons was significantly larger than that in C-type neurons. At -110 mV, IH in A-type nodose neurons had a mean amplitude of -2338 ± 258 pA while IH in C-type neurons had a mean amplitude of only -241 ± 40 pA (Fig. 4A, P < 0.001). The difference was not explained by cell size. Using capacitance measurement as an index for cell size, there was no correlation between the amplitude of IH and cell size (Fig. 4B). However, there was a tendency for A-type neurons to be larger than C-type neurons, where the mean capacitance was 53 ± 4 pF (n= 13) and 40 ± 2 pF (n= 48; P < 0.05), respectively. This was consistent with reports for dorsal root ganglia (Pearce & Duchen, 1994; Scroggs et al. 1994) but deviated from studies in nodose neurons (Carobi, 1996).
Figure 4. Magnitude of IH in tetrodotoxin-sensitive and -resistant neurons.
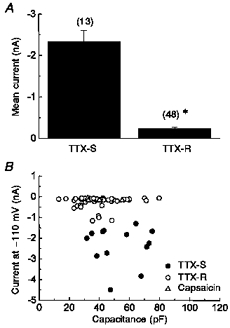
A, mean magnitude of IH at -110 mV for neurons with only tetrodotoxin-sensitive sodium currents (TTX-S) or for neurons with both tetrodotoxin-sensitive and -resistant sodium currents (TTX-R). *P < 0.001; n is given in parentheses. B, amplitude of IH at -110 mV was plotted with respect to the capacitance of the neuron. Neurons with only tetrodotoxin-sensitive sodium currents (•, n= 13), with tetrodotoxin-sensitive and -resistant sodium currents (^, n= 43; ▵, n= 5) or with capsaicin-sensitive channels (▵, n= 5) are illustrated.
The activation kinetics of IH were calculated by fitting the activation current traces at each hyperpolarized membrane potential to a biexponential function (Fig. 5A). In A-type neurons at -70 mV, the fast time constant for IH was 337 ± 38 ms with an amplitude of 151 ± 41 pA and the second time constant was 2184 ± 777 ms with an amplitude of 136 ± 47 pA (Fig. 5B and C, n= 7; notice the time held at each test potential was extended to 3.5 s). The amplitude of both components increased as the membrane potential was further hyperpolarized, with the fast τ component contributing to a larger extent than the slow τ component at -120 mV. In addition, both the slow and fast time constants decreased by more than 67 % as the membrane potential was hyperpolarized to -120 mV. Since IH was still increasing slightly even at 3.5 s, the slow time constant may be an underestimation, but protocols for longer durations compromised the whole-cell patch. Also, further hyperpolarization to -130 or -140 mV for greater than 2.5 s resulted in a loss of the tight seal.
Figure 5. IH is voltage and time dependent.
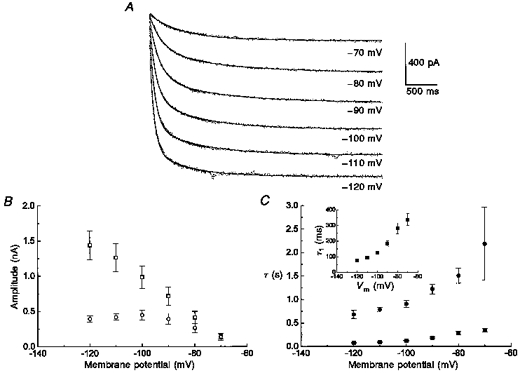
A, biexponential fits (continuous lines) of IH current traces of an A-type neuron at various test potentials for 3.5 s in a bath containing 2 mM CaCl2 to block the outward potassium tail-currents (see Fig. 10). B, amplitude 1 (□) and amplitude 2 (^) were plotted at each test potential (n= 7); C, the rate constants, τ1 (▪) and τ2 (•), were plotted at each test potential (n= 7) demonstrating the voltage- and time-dependent activation of IH; inset, the fast τ1 (▪) was plotted with respect to membrane potential (Vm) on an expanded time scale.
The small amplitude of IH in C-type neurons made it difficult to obtain fits to the data over the voltage range near threshold. Therefore, the activation kinetics for a group of C-type neurons were compared with A-type at -120 mV where the amplitude of the current was greatest. The activation kinetics of C-type neurons were slower than those for A-type neurons (Fig. 6). IH in A-type neurons had a fast τ component of 73 ± 3 ms with an amplitude of 1482 ± 237 pA (n= 6), while IH in C-type neurons had a fast time constant of 163 ± 37 ms and an amplitude of 38 ± 12 at -120 mV (n= 4; P < 0.05). The slow τ component for A-type neurons was 682 ± 83 ms with an amplitude of 393 ± 47 pA and that for C-type neurons, 957 ± 122 ms with an amplitude of 68 ± 14 pA (P < 0.05).
Figure 6. Activation kinetic of IH was different in tetrodotoxin-sensitive and -resistant neurons.
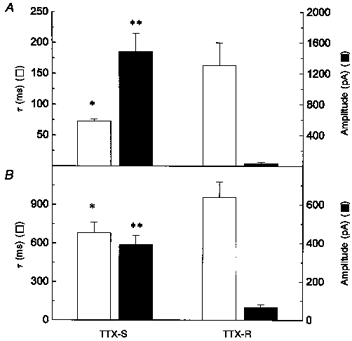
A, fast time constant of activation of IH (□) and its amplitude (▪) at -120 mV for neurons with only tetrodotoxin-sensitive sodium currents (TTX-S; n= 10) or neurons with both tetrodotoxin-sensitive and -resistant sodium currents (TTX-R; n= 10). B, second or slow time constant (□) and its amplitude (▪) of IH at -120 mV for neurons with only TTX-S sodium currents (n= 10) or neurons with both TTX-S and TTX-R sodium currents (n= 10). *P < 0.05; **P < 0.001 for comparison of TTX-S and TTX-R.
The voltage dependence of activation was not different between A- and C-type neurons. This was determined by fitting the amplitude of normalized tail currents at -80 mV (Fig. 7A) to a Boltzmann function. The Boltzmann fit illustrated that IH was active at membrane potentials more negative than -50 mV and approached saturation by -140 mV. For A-type neurons the half-maximal voltage of IH (V½) was -88 ± 3 mV with a slope factor (kS) of 10.7 ± 0.5 mV (n= 8); and for C-type neurons the values were V½= -87.4 ± 2.4 mV and kS= 11.8 ± 0.9 mV (n= 8).
Since IH was an inward current at -60 mV, this suggested that the reversal potential for IH was more depolarized than the reversal potential for potassium alone (EK= -83 mV). The reversal potential of IH was determined by activating IH at -120 mV and stepping thereafter in depolarizing increments of 10 mV towards -70 mV (Fig. 8A). At membrane potentials positive to -70 mV, the tetrodotoxin-sensitive and tetrodotoxin-resistant sodium currents were activated. This resulted in a loss of adequate voltage control and therefore made the calculation of the reversal potential for IH difficult at those potentials. Hence, only data points between -120 and -70 mV were used for calculating the reversal potentials for IH expressed in C-type neurons. The reversal potential for IH in A-type neurons were calculated from -120 to -50 mV in the presence of 1 μm tetrodotoxin to inhibit the sodium current. Furthermore, the same protocol was repeated for each neuron in the presence of extracellular caesium in order to eliminate the contributions of other currents (Fig. 8B and C). The instantaneous currents of the caesium-sensitive traces (Fig. 8D) were best fitted with a linear regression. The extrapolation of the linear regression to 0 pA gave a projected mean reversal potential of IH of -39 ± 3 mV in A-type neurons (n= 3) and -37 ± 5 mV in C-type neurons (n= 4; P > 0.05).
Figure 8. The reversal potential of IH.

The reversal potential was determined by clamping the neuron to -120 mV for 1 s and depolarizing in 10 mV increments to -70 mV (inset in B). The voltage protocol was performed on neurons perfused with solution 1 in the absence (A) or presence (B) of 5 mM CsCl. C, the isolation of IH was obtained from the difference between current traces in B and A.D, mean (n= 13) instantaneous currents of IH (C, •) for A- (^; n= 3) or C-type (•; n= 4) neurons was plotted with respect to membrane potential and a linear regression was performed.
Extracellular ions contributing to IH and the resting membrane potential
IH has well documented permeability characteristics. The following sets of experiments were performed in a mixed group of neurons, both A- and C-type, using these characteristics to obtain support for a role for IH in determining the resting membrane potential. Additionally, we explored possible contributions of other channels to the resting membrane potential.
When the nodose neurons were perfused with a bath containing the impermeant cation N-methyl-D-glucamine (Fig. 9B) instead of Na+ (Fig. 9A), no significant effect was seen on the peak tail currents of the outward potassium currents. IH, however, was markedly reduced in the absence of Na+. In a bath with K+ and Na+, IH was activated at -60 mV and more hyperpolarized, but in a bath with K+ and NMDG+, IH was not evident until -80 mV. In this group of cells, IH had a mean current amplitude of -1130 ± 299 pA at -140 mV in the presence of K+ and Na+, but decreased to -568 ± 152 pA when Na+ was replaced with NMDG+ (n= 15). The zero current potential of the nodose neurons in a bath containing both K+ and Na+ was -52 ± 1 mV (Fig. 9A) and shifted to a more hyperpolarized value, -57 ± 1 mV, when the sodium in the bath solution was replaced by the impermeant cation NMDG+ (Fig. 9C; n= 15; P < 0.005). This suggested that sodium ions permeated the IH channel, and also, that IH was active at the resting membrane potential of nodose sensory neurons. In addition, the application of TTX did not affect the conductance and amplitude of IH or alter the zero current potential (data not shown). When the extracellular saline contained sodium ions but no potassium ions (solution 1 without potassium chloride), IH was negligible (data not shown). This result was in agreement with a previous study in cardiac cells where the investigators reported that IH required the presence of extracellular potassium (Maruoka et al. 1994).
Figure 9. Decreasing Na+ affected both the zero current potential and IH.
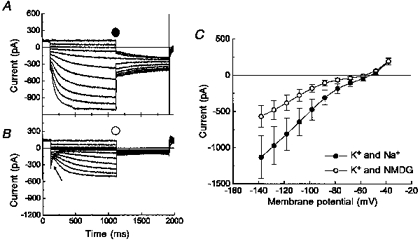
Whole-cell voltage clamped measurements of IH in the presence (A; solution 1) and absence (B; solution 2) of Na+ from -40 to -140 mV. C, mean (n= 15) currents measured at the steady-state component in the presence (•) and absence (^) of Na+. The arrow in B indicates the inward tail currents of the outward potassium currents.
Nodose neurons have a resting membrane potential close to the activation range of low threshold calcium channels. Therefore, we wanted to know if extracellular calcium ions contributed to the resting membrane potential and/or affected the conductance of IH. When the nodose neuron was perfused with an extracellular potassium solution (solution 2, Methods) containing only 20 μm CaCl2, a deactivation of the inward potassium tail currents (arrow in Fig. 10A) was followed by the activation of IH in response to hyperpolarizing voltage steps. When the same neuron was perfused with an extracellular potassium solution containing 2 mM CaCl2 (Fig. 10B), the inward potassium tail currents were inhibited, but the kinetics and amplitude of IH were not affected. Therefore, unlike sodium and potassium cations, calcium ions were not conducted through IH channels. However, the increase in Ca2+ caused a depolarizing shift in the zero current potential from -55 ± 2 to -48 ± 3 mV, when calculated in the presence of 20 μm and 2 mM CaCl2, respectively (Fig. 10C; n= 6; P < 0.005). This suggested that another inward current, such as the previously identified low threshold calcium current (Mendelowitz & Kunze, 1992), also contributes to the resting membrane potential of nodose neurons. Similar results were obtained when calcium was added to a bath containing both potassium and sodium ions, such that the zero current potential changed from -54 ± 3 mV to -48 ± 3 mV in the presence of 20 μm and 2 mM CaCl2, respectively (n= 6; P < 0.05).
Figure 10. Increasing Ca2+ affected the zero current potential but not IH.

Whole-cell voltage clamped recordings of nodose sensory neurons in the presence of 20 μm (A, solution 2) and 2 mM (B, solution 2) Ca2+ in the extracellular potassium solution. The arrow (A) showed the inward potassium tail currents. C, mean (n= 6) currents measured at the steady-state component in the presence of 20 μm (^) and 2 mM (•) Ca2+.
In other central and peripheral neurons, the combination of IH with other inward currents, such as the inward rectifier potassium current (IK(IR)) and/or a leak current, contributed to setting the resting membrane potential. The perfusion of BaCl2, a divalent salt that blocks IK(IR), inhibited the inward tail currents but had no effect on the steady-state current in nodose neurons. For example, the mean steady-state current was -831 ± 141 and -795 ± 149 pA in the absence and presence, respectively, of 100 μm BaCl2 at -140 mV (n= 8). This suggested that nodose neurons did not express IK(IR); however, the presence of IH may have masked the IK(IR), if the IK(IR) was substantially smaller than IH. To diminish IH without possibly affecting other potassium currents, we replaced extracellular chloride with gluconate in a bath without Na+ (Fig. 11). Previous investigators have shown that the replacement of extracellular chloride ions (Cl−) with large anions abolished IH in cardiac cells (Frace et al. 1992) and thalamic relay neurons (McCormick & Pape, 1990a). For nodose neurons, the replacement of Cl− with gluconate attenuated IH at all membrane potentials (Fig. 11B). The tail currents of outward potassium currents (arrow in Fig. 11B) were not affected by the removal of Cl− and furthermore, no IK(IR) was evident. The decrease in the amplitude of IH at the hyperpolarized membrane potentials also suggested that chloride ions were not permeable through IH channels, similar to a previous report in cardiac cells (Frace et al. 1992). The exchange of extracellular chloride with gluconate shifted the zero current potential from -59 ± 2 to -64 ± 3 mV (Fig. 11C; n= 6; P < 0.005), and interestingly, produced an increase in the outward potassium current. The latter may account for the shift in membrane potential toward EK. Even in the presence of both K+ and Na+, IH was attenuated when Cl− was substituted with gluconate and similar results were obtained when Cl− was replaced with the large anion aspartate (data not shown).
Figure 11. The replacement of Cl− with gluconate affected both the zero current potential and IH.

A, representative current traces of whole-cell recordings of a nodose neuron in a potassium solution containing either Cl− (solution 2; A) or gluconate (solution 3; B). The arrow (B) showed the inward potassium tail currents. C, mean (n= 6) currents measured at the steady-state component in the presence (^) and absence (•) of Cl−.
IH under current clamp measurements
From the voltage clamp experiments, IH appeared to be active at the resting membrane potential (Vrest). If this is the case, the inhibition of IH with caesium should cause a hyperpolarization of the nodose neurons while at rest. This was demonstrated using the whole-cell current clamp technique to measure the membrane potential in the absence or presence of caesium chloride. In physiological saline, the Vrest was -55 ± 3 mV for A-type neurons (n= 4) and -62 ± 2 mV for C-type neurons (n= 12). When relatively small hyperpolarizing current injections were given, the membrane potential was hyperpolarized to a near steady-state level (Fig. 12A). However, when greater hyperpolarizing currents were applied to the neurons, there was an initial hyperpolarization followed by a slow depolarization, described as time-dependent rectification. The slow kinetics of the depolarizing component were in agreement with the slow activation of IH. Upon perfusion with 5 mM CsCl (Fig. 12B), there was a significant hyperpolarization of the resting membrane potential to a mean of -61 ± 4 mV for A-type neurons (n= 4; with and without CsCl, P < 0.001) and -71 ± 3 for C-type neurons (n= 12; with and without CsCl, P < 0.001), suggesting the inhibition of an inward current at the Vrest. In addition, the time-dependent rectification during hyperpolarizing current injections was abolished in the presence of extracellular caesium. The input resistance, calculated from the linear regression of the I-V relation between 0 and -100 pA at the quasi-steady-state (Fig. 12C), was increased from 1.0 ± 0.3 GΩ (absence of CsCl) to 2.4 ± 0.2 GΩ (presence of CsCl) (n= 7; P < 0.001). The inhibition of an inward current was consistent with the inhibition of IH by CsCl in the voltage clamp studies.
Figure 12. Time-dependent rectification was caused by the activation of IH and was blocked by CsCl during current clamp recordings.

Representative whole-cell current clamped recordings of nodose sensory neurons in the absence (A, solution 1 with 2 mM CaCl2) or presence (B) of 5 mM CsCl during hyperpolarizing current injection from 0 to -40 pA in 10 pA step interval (inset in A). C, current-voltage relation of the same neuron at steady state in the absence (^) and presence (•) of 5 mM CsCl. Linear regression was performed to calculate the input resistance for the neuron. Similar results were obtained for 7 neurons.
DISCUSSION
IH contributes to the resting membrane potential of nodose sensory neurons
The resting membrane potential is determined by a steady-state relationship between the inward current(s) and outward current(s). In this study, we show that the hyperpolarization-activated inward current participates in determining the resting membrane potential of nodose sensory neurons. The evidence to support the hypothesis that IH is active at the resting membrane potential is as follows. First, IH has an activation threshold near -60 mV, a potential where nodose neurons are at rest, as shown in this study and in those of other investigators (Stansfeld & Wallis, 1985; Marsh et al. 1987; Puizillout & Gambarelli, 1989; Undem & Weinreich, 1993). Since IH does not inactivate, this implies that IH is tonically active at the resting membrane potential. Second, the replacement of extracellular Na+ or Cl−, which both reduce the contribution of IH to the total current, results in a shift of the zero current potential to a more hyperpolarized value. Finally, the strongest evidence showing the contribution of IH at the resting membrane potential is the hyperpolarization of the membrane potential and the decrease in membrane conductance upon perfusion with caesium, an inhibitor of IH.
At the concentrations used in this study CsCl (1–5 mM) blocks only IH at the resting membrane potential and consequently enables the outward current(s), presumably potassium current(s), to hyperpolarize the neurons. The neurons hyperpolarize to about -70 mV instead of EK (-83 mV), suggesting an additional inward current is present. This caesium-insensitive inward current was considered a small leak current introduced by an incomplete seal between the glass electrode and the cell membrane.
Outward current balancing inward current at the resting membrane potential
The Boltzmann fit of IH for both A- and C-type neurons indicates only about 2 % of this inward current is active at -60 mV. This suggests that the magnitude of the outward current opposing IH is probably very small at the resting membrane potential. Furthermore, this outward current must be a current that does not completely inactivate at prolonged steady-state conditions. An outward current that fits such criteria is the inward rectifier potassium current. Inwardly rectifying potassium currents have been found in central and other peripheral neurons, where they are important in setting the resting membrane potential (for example Scroggs et al. 1994). Inward tail currents were present upon hyperpolarization from -40 mV to more negative values. However, they were eliminated when a holding potential of -50 mV was used instead of -40 mV. This indicated that these inward currents result from potassium current that is active at -40 mV but not at -50 mV and that they are not the result of the inwardly rectifying potassium current. Furthermore, when we exposed nodose neurons to BaCl2, we observed no effect on the steady-state currents of nodose neurons, also suggesting the lack of an inward rectifier.
If the inward rectifier is smaller than IH though, the current may be masked by IH at the hyperpolarized potentials (-80 to -140 mV). However, when we substantially reduced IH by eliminating Na+ and replacing extracellular Cl− with gluconate, an inward rectifier potassium current was still not evident. These experiments suggest these neurons do not have an inward rectifier potassium current.
Other potassium channel inhibitors, such as α-dendrotoxin and charybdotoxin, were not effective at the resting membrane potential although they have been shown to block potassium channels in these neurons. Therefore, the outward current that balances IH at the resting membrane potential still needs to be determined for nodose neurons.
Expression of IH in nodose sensory neurons
There are several ways to explain the differences between our studies and those from other groups where IH was not observed in C-type neurons. Marsh et al. (1987) identified C-type sensory neurons using capsaicin. In those studies, no time-dependent rectification was present during hyperpolarizing current injection. Scroggs et al. (1994) and Pearce & Duchen (1994) suggested that an inward rectifier potassium current and not IH is present in capsaicin-sensitive neurons of the dorsal root ganglion. The most likely explanation for results of these studies resides in the small magnitude of IH in C-type neurons. The presence of seal leak in either current clamp or voltage clamp recordings can easily obscure the small IH. The activation of IH at near threshold potentials is slow and might not be apparent in that voltage range. At more negative potentials the appearance of inward rectification would be dependent on the relative contributions of IH to the total current at those potentials.
Ingram & Williams (1996) found the expression of IH only in medium-size to large nodose neurons isolated from adult guinea-pig and concluded that the small neurons did not have IH. However, they used caesium in the pipette to block outward potassium currents. Under these conditions the outward potassium currents become inward currents due to the change in the reversal potential for potassium ions, making it very difficult to isolate IH from potassium currents. In addition, IH is known to run down during whole-cell recordings (DiFrancesco, 1985; Ingram & Williams, 1996). The run-down of IH has the most impact on C-type neurons, since IH is substantially less in C-type neurons. In our experiments, the beginning of run-down was evident within 10–15 min after acquiring the whole-cell recording as monitored by changes in threshold for activation and in the amplitude of the current-voltage relationship. Therefore, no data were used when these changes were apparent. Finally, the age of the animal, culture conditions and/or the preparation of the neurons may be an explanation for the differences. There have been reports of developmental changes of IH in some species. Developmental changes are unlikely since in a preliminary subset of experiments in adult rat nodose neurons, all neurons, including A- and C-type neurons, expressed IH (data not shown). Additional support for the presence of IH in C-type neurons is provided in a recent study of unmyelinated vagal and sural nerve fibres. A caesium-sensitive and time-dependent inward rectification was demonstrated in these axons (Grafe et al. 1997).
Functional importance of IH
The resting membrane potential of nodose neurons lies between -50 and -65 mV, a range where a significant percentage of voltage-dependent sodium, calcium and potassium channels are inactivated. Hyperpolarization of the resting membrane potential may remove the inactivation of these voltage-dependent channels and consequently increase the number of channels available for activation during a depolarizing stimulus. On the other hand, a slight depolarization may increase the number of inactivated voltage-dependent channels, thereby reducing the ability of the neuron to discharge during an excitatory stimulus. In addition, IH is probably important during the after-hyperpolarization phase of the action potential, where IH opposes the ability of outward potassium currents to drive the membrane potential past the resting membrane potential. Weinreich & Wonderlin (1987) have shown that the inhibition of the slow after-hyperpolarization in rabbit nodose neurons consequently causes an increase in discharge during depolarizing current injections. Furthermore, the increase in excitability of the neuron is not due to a decrease in the conductance of the Ca2+-activated outward potassium channel, a current that participates in the slow after-hyperpolarization. Instead, the alteration in discharge is linked to a cAMP-dependent mechanism, identified by forskolin stimulation of adenylyl cyclase or by prostaglandin D2 or E2. Ingram & Williams (1996) have demonstrated that the amplitude of IH in adult nodose neurons is increased with prostaglandin E2 and cAMP analogues. This may explain the inhibition of the slow after-hyperpolarization in rabbit nodose neurons.
The major difference in the IH current between A- and C-type neurons is the magnitude of the current. The projected reversal potentials and the voltage dependence of activation are similar in A- and C-type cells and fall within the range previously reported in other neurons (Pape, 1996). An additional difference is that C-type neurons appear to activate more slowly than A-type. The activation process is voltage-dependent, increasing with hyperpolarization. We find that in both A- and C-type neurons IH activates with two time constants. Recently two groups have reported cloning from brain several members of what now appears to be a family of IH channels (Ludwig et al. 1998; Santoro et al. 1998). The heterologous expression of the cloned channels showed differences in voltage-dependent characteristics such as threshold and rate of activation. Such differences within the resting potential range can have substantial effects on neuronal function. Whether they exist in the native cell and whether A- and C-type neurons express distinct members of the IH family remain to be determined.
In conclusion, IH contributes towards setting the resting membrane potential of nodose sensory neurons at a critical range where a portion of voltage-dependent sodium, potassium, and calcium channels are inactivated. The modulation of the current may alter the excitability of the neuron by either increasing or decreasing the relative amount of inactivated channels. In addition, the input resistance at the resting membrane potential is very high (≥1 GΩ); therefore any abrupt increase of this cation inward current may induce enough current to elicit an action potential. The characterization of the time- and voltage-dependent properties of IH provided in the present study may now be used to refine the nodose neuronal model and to investigate the proposals for the functional role described above. The hyperpolarization-activated current has been integrated in a thalamocortical neuronal model (Bal & McCormick, 1996) where it is important for the oscillatory behaviour of the neuron. However, the current may have a different role when expressed in a non-oscillatory cell such as the nodose neuron.
Acknowledgments
The work was made possible by funding to D. L. K. from NIH HL36850.
References
- Andresen MC, Krauhs JM, Brown AM. Relationship of aortic wall baroreceptor properties during development in normotensive and spontaneously hypertensive rats. Circulation Research. 1978;43:728–738. doi: 10.1161/01.res.43.5.728. [DOI] [PubMed] [Google Scholar]
- Bacal K, Kunze DL. Dual effects of angiotensin II on calcium currents in neonatal rat nodose neurons. Journal of Neuroscience. 1994;14:7159–7167. doi: 10.1523/JNEUROSCI.14-11-07159.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini PI, Cooper E. Electrophysiological studies of new-born rat nodose neurones in cell culture. The Journal of Physiology. 1982;324:429–439. doi: 10.1113/jphysiol.1982.sp014122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron. 1996;17:297–308. doi: 10.1016/s0896-6273(00)80161-0. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA, Smith PH. Hyperpolarization-activated cation current (Ih) in neurons of the medial nucleus of the trapezoid body: voltage-clamp analysis and enhancement by norepinephrine and cAMP suggest a modulatory mechanism in the auditory brain stem. Journal of Neurophysiology. 1993;70:1420–1432. doi: 10.1152/jn.1993.70.4.1420. [DOI] [PubMed] [Google Scholar]
- Bobker DH, Williams JT. Serotonin augments the cationic current Ih in central neurons. Neuron. 1989;2:1535–1540. doi: 10.1016/0896-6273(89)90041-x. [DOI] [PubMed] [Google Scholar]
- Brown AM, Saum WR, Yasui S. Baroreceptor dynamics and their relationship to afferent fiber type and hypertension. Circulation Research. 1978;42:694–702. doi: 10.1161/01.res.42.5.694. [DOI] [PubMed] [Google Scholar]
- Carobi C. A quantitative investigation of the effects of neonatal capsaicin treatment on vagal afferent neurons in the rat. Cell and Tissue Research. 1996;283:305–311. doi: 10.1007/s004410050540. [DOI] [PubMed] [Google Scholar]
- Cooper E, Shrier A. Inactivation of A currents and A channels on rat nodose neurons in culture. Journal of General Physiology. 1989;94:881–910. doi: 10.1085/jgp.94.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarization-activated current, if. Origins and developments. Progress in Biophysical and Molecular Biology. 1985;46:163–183. doi: 10.1016/0079-6107(85)90008-2. 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Frace AM, Maruoka F, Noma A. Control of the hyperpolarization-activated cation current by external anions in rabbit sino-atrial node cells. The Journal of Physiology. 1992;453:307–318. doi: 10.1113/jphysiol.1992.sp019230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P, Quasthoff S, Grosskruetz J, Alzheimer C. Function of the hyperpolarization-activated inward rectification in non-myelinated peripheral rat and human axons. Journal of Neurophysiology. 1997;77:412–426. doi: 10.1152/jn.1997.77.1.421. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Schofield GG. Tetrodotoxin-resistant sodium current of rat nodose neurones: monovalent cation selectivity and divalent cation block. The Journal of Physiology. 1987;389:255–270. doi: 10.1113/jphysiol.1987.sp016656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR, Schofield GG, Weight FF. Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. Journal of Neurophysiology. 1986;55:527–539. doi: 10.1152/jn.1986.55.3.527. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Williams JT. Opioid inhibition of Ih via adenylyl cyclase. Neuron. 1994;13:179–186. doi: 10.1016/0896-6273(94)90468-5. 10.1016/0896-6273(94)90468-5. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Williams JT. Modulation of the hyperpolarization-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. The Journal of Physiology. 1996;492:97–106. doi: 10.1113/jphysiol.1996.sp021292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofman F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. The Journal of Physiology. 1990a;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. The Journal of Physiology. 1990b;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane S, Cooper E. Kinetics and voltage dependence of A-type currents on neonatal rat sensory neurons. Journal of Neurophysiology. 1991;66:1380–1391. doi: 10.1152/jn.1991.66.4.1380. [DOI] [PubMed] [Google Scholar]
- Marsh SJ, Stansfeld CE, Brown DA, Davey R, McCarthy D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience. 1987;23:275–289. doi: 10.1016/0306-4522(87)90289-2. 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Maruoka F, Nakashima Y, Takano M, Ono K, Noma A. Cation-dependent gating of the hyperpolarization-activated cation current in the rabbit sino-atrial node cells. The Journal of Physiology. 1994;477:423–435. doi: 10.1113/jphysiol.1994.sp020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz D, Kunze DL. Characterization of calcium currents in aortic baroreceptor neurons. Journal of Neurophysiology. 1992;68:509–517. doi: 10.1152/jn.1992.68.2.509. [DOI] [PubMed] [Google Scholar]
- Nehr E. Correction for liquid junction potentials in patch clamp experiments. Methods in Enzymology. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annual Review of Physiology. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Pearce RJ, Duchen MR. Differential expression of membrane currents in dissociated mouse primary sensory neurons. Neuroscience. 1994;63:1041–1056. doi: 10.1016/0306-4522(94)90571-1. 10.1016/0306-4522(94)90571-1. [DOI] [PubMed] [Google Scholar]
- Puizillout JJ, Gambarelli F. Electrophysiological and morphological properties of type C vagal neurons in the nodose ganglion of the cat. Journal of the Autonomic Nervous System. 1989;29:49–58. doi: 10.1016/0165-1838(89)90019-2. 10.1016/0165-1838(89)90019-2. [DOI] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- Schild JH, Clark JW, Hay M, Mendelowitz D, Andresen MC, Kunze DL. A- and C-type rat nodose sensory neurons: model interpretations of dynamic discharge characteristics. Journal of Neurophysiology. 1994;71:2338–2358. doi: 10.1152/jn.1994.71.6.2338. [DOI] [PubMed] [Google Scholar]
- Schild JH, Kunze DL. Experimental and modeling study of Na current heterogeneity in rat nodose neurons and its impact on neuronal discharge. Journal of Neurophysiology. 1997;78:3198–3209. doi: 10.1152/jn.1997.78.6.3198. [DOI] [PubMed] [Google Scholar]
- Scroggs RS, Todorovic SM, Anderson EG, Fox AP. Variation of IH, IIR, and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. Journal of Neurophysiology. 1994;71:271–279. doi: 10.1152/jn.1994.71.1.271. [DOI] [PubMed] [Google Scholar]
- Spain WP, Schwindt PC, Crill WE. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. The Journal of Physiology. 1987;57:1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Stansfeld CE, Wallis DI. Properties of visceral primary afferent neurons in the nodose ganglion of the rabbit. Journal of Neurophysiology. 1985;54:245–260. doi: 10.1152/jn.1985.54.2.245. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA. Hyperpolarization-activated current, Ih and Ikir in rat dorsal motor nucleus of the vagus neurons in vitro. Journal of Neurophysiology. 1994;71:1308–1317. doi: 10.1152/jn.1994.71.4.1308. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Weinreich D. Electrophysiological properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. Journal of the Autonomic Nervous System. 1993;44:17–34. doi: 10.1016/0165-1838(93)90375-5. 10.1016/0165-1838(93)90375-5. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Wonderlin WF. Inhibition of calcium-dependent spike after-hyperpolarization increases excitability of rabbit visceral sensory neurones. The Journal of Physiology. 1987;394:415–427. doi: 10.1113/jphysiol.1987.sp016878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Winter J, James IF, Rang HP, Yeats J, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. Journal of Neuroscience. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


