Abstract
Eight subjects performed two-legged exercise, one leg with low and the other with normal muscle glycogen content. The purpose was to study the effect of low initial muscle glycogen content on the metabolic response during 1 h of exercise and 2 h of recovery. This model allows direct comparison of net fluxes of substrates and metabolites over the exercising legs receiving the same arterial inflow.
Muscle glycogen breakdown during exercise was 60% lower in the leg with a reduced pre-exercise glycogen concentration and the rate of glucose uptake during exercise was 30% higher.
The amount of pyruvate that was oxidized during exercise was calculated to be approximately 450 mmol in the low-glycogen leg and 750 mmol in the normal-glycogen leg, which suggests more fat and amino acid oxidation in the low-glycogen leg.
During exercise, there was a significant release of amino acids not metabolized in the muscle, e.g. tyrosine and phenylalanine, only from the low-glycogen leg, suggesting an increased rate of net protein degradation in this leg.
The release of tyrosine and phenylalanine from the low-glycogen leg during the exercise period and the change in their muscle concentrations yield a net tyrosine and phenylalanine production rate of 1.4 and 1.5 mmol h−1, respectively. The net rate of protein degradation was then calculated to be 7–12 g h−1.
The results suggest that the observed differences in metabolism between the low-glycogen and the normal-glycogen leg are induced by the glycogen level per se, since the legs received the same arterial supply of hormones and substrates.
It is a well-known fact that fat and carbohydrates are the main fuels during sustained exercise. Protein and amino acid oxidation contributes less than 5–10 % to the energy yield (Lemon, 1987). However, several reports have indicated that the degradation of protein increases when the pre-exercise carbohydrate stores are reduced. For example, the excretion of nitrogen in sweat was considerably greater when the exercise was performed with reduced, as compared with elevated, initial muscle glycogen stores (Lemon & Mullin, 1980). Furthermore, the release of amino acids from the exercising leg was reported to be greater when the muscle glycogen level was low (Van Hall, 1996). However, in the design of the latter study with normal and low muscle glycogen legs performing repeatedly and after each other, the metabolic situation was different and assigning a specific role for low muscle glycogen is not obvious. The aromatic amino acids, tyrosine and phenylalanine, are neither degraded nor synthesized by skeletal muscle and could therefore be used as indicators of the balance between protein synthesis and protein degradation. Measurements of the release of aromatic amino acids from the muscle along with a change in the muscle level of these amino acids could give an indication of whether a net rate of protein degradation, i.e. a net protein loss, occurs during exercise with a low initial level of muscle glycogen.
Reduced levels of muscle glycogen have also been reported to result in lower rates of glycogenolysis during sustained submaximal exercise (Gollnick et al. 1972; Gollnick et al. 1981; Hargreaves et al. 1995; Van Hall et al. 1995; Weltan et al. 1998). However, the picture is less clear concerning the effect of the pre-exercise muscle glycogen concentration on glucose uptake. Gollnick et al. (1981) observed a greater glucose extraction in the leg that commenced the exercise with low glycogen levels as compared with the leg that started the exercise with normal muscle glycogen stores, whereas, in another study, no effect of muscle glycogen levels on glucose uptake was reported (Hargreaves et al. 1995).
The main purpose of the present work was to find out if a low initial level of muscle glycogen induces a reliance on protein and amino acid metabolism during exercise as compared with the situation when exercise starts with normal levels of muscle glycogen. The subjects exercised with both legs the morning after they had performed one-legged exercise in the evening before in order to lower the muscle glycogen content in one leg. This experimental model enabled us to study the effect of different muscle glycogen levels on the uptake and/or release of amino acids and the change in the muscle concentration of amino acids with the same arterial supply to both legs and the same hormonal response during exercise. In addition, measurements of the leg exchange of glucose, lactate, free fatty acids, glycerol and ammonia were made.
METHODS
Subjects
Eight healthy male subjects participated in this study after giving written, informed consent, having been fully informed of the risks involved. They were all recreational cyclists, and none of them were involved in regular exercise training. Their mean ±s.e.m. age was 25 ± 1 years, height was 182 ± 3 cm, weight was 81 ± 4 kg and maximal oxygen uptake (VO2,max) was 3.74 ± 0.14 l min−1. The study was approved by the Ethics Committee of the municipalities of Fredriksberg and Copenhagen.
Preliminary tests
The preliminary exercise tests were performed on a mechanically braked cycle ergometer (Monark 816E, Varberg, Sweden). One week before the experiment, the oxygen uptake of the subjects was determined at three submaximal work rates, together with their VO2,max, using an on-line system (MedGraphics, Spiropharma A/S, Klampenborg, Denmark). The subjects exercised at a pedalling rate of 60 r.p.m. A work rate amounting to approximately 70 % of VO2,max was calculated from these measurements.
Glycogen reduction exercise
During the 2 days preceding the experiment, the subjects were on a standardized meat free diet with 12.6 MJ day−1 (3000 kcal day−1; (carbohydrate 57 % of energy, fat 30 % and protein 13 %) and they were instructed to refrain from physical training. On the evening before the experiment, the subjects performed cycle exercise with one leg on a Krogh ergometer. They performed 45 min of exercise (pedalling at 60 r.p.m.) at a work rate of 120 ± 6 W, demanding an oxygen uptake of 2.14 ± 0.11 l min−1 (heart rate, 147 ± 4 beats min−1). After 5 min of rest they performed interval exercise, 5 × 2 min at a work rate of 147 ± 7 W, demanding an oxygen uptake of 2.41 ± 0.12 l min−1 (heart rate, 162 ± 4 beats min−1). Thereafter, the subjects performed interval exercise with both arms on an arm ergometer, 5 × 3 min of maximal exercise at a work rate of 204 ± 11 W (heart rate, 159 ± 2 beats min−1). The purpose of the last-mentioned exercise protocol was to reduce the glycogen content in muscles of the upper body in order to reduce the resynthesis of muscle glycogen in the exercised leg during the 12 h rest period between the evening exercise and the experiment the following morning. During the one-legged exercise, the oxygen uptake was measured after 15 and 35 min of the continuous exercise and during the second or third period of the interval exercise using an on-line system (see above). The heart rate was monitored continuously during the exercise period using a Sport Tester PE-3000 heart rate computer. After this exercise the subjects remained fasted until the experiment the next morning. A similar exercise protocol has been reported to give a reduced muscle glycogen level the following morning (Van Hall et al. 1995).
Experimental protocol
The subjects reported to the laboratory in the morning after fasting overnight (see above). Catheters were advanced proximally in one femoral artery and in both femoral veins (six subjects), 2 and 4 cm distal to the inguinal ligament, respectively. A thermistor was placed in the two venous catheters (approx. 8 cm proximal to the tip) which also had side holes for infusing saline or withdrawing blood. In two subjects, catheters were placed in one femoral vein only due to technical difficulties, and in the femoral artery. The arterial catheter was advanced 10 cm upstream and connected to a blood-pressure transducer and monitor (Patient Data Monitor 565A, Medicoline, Valby, Denmark). ECG chest electrodes were attached to the subject to monitor the heart rate. Force transducers were attached to the pedals to register the pedal force. The signals were recorded along with the heart rate, blood pressure and blood flow on a Gould TA 200 recorder.
The subjects then exercised for 60 min on a cycle ergometer in a semirecumbent position at a work rate of 164 ± 7 W. The pulmonary oxygen uptake was 2.58 ± 0.11 l min−1, which corresponds to 69 ± 2 % of the subjects’ upright VO2,max. It has been reported, however, that the maximal oxygen uptake during exercise in a recumbent position only reaches 90 % of the VO2,max obtained during upright exercise (Kjaer et al. 1987). The relative work rate in the present study is therefore more likely to correspond to approximately 75 % of VO2,max. The exercise period was followed by a recovery period of 2 h with the subjects in the supine position. During the recovery period, the subjects remained fasted; they were only given water to drink.
Measurements
Blood flow was measured in both femoral veins using the thermodilution technique, as described by Andersen & Saltin (1985) at rest, repeatedly during exercise and early recovery. Blood samples were drawn simultaneously from the femoral artery and from both femoral veins at rest, after 20, 40 and 60 min of exercise and repeatedly during the recovery period (after 5, 15, 30, 60, 90 and 120 min). The 60 min samples during exercise were drawn during the last minute of exercise. Muscle biopsy samples were taken from the lateral part of the quadriceps muscle of both legs at rest, immediately after exercise and 30, 60 and 120 min after the completion of exercise. The samples were immediately frozen in liquid nitrogen and stored at -80°C until analysed.
The pulmonary oxygen uptake was measured at rest and after approximately 10, 30 and 50 min of exercise using an on-line system (see above). Heart rate and blood pressure were monitored continuously during exercise.
Calculations
The oxygen content of arterial and venous blood was calculated from the measurements of haemoglobin concentration and oxygen saturation. The oxygen uptake over the legs was then calculated as blood flow multiplied by the arteriovenous difference in oxygen. The rate of exchange of glucose, lactate, ammonia and amino acids in the legs was calculated as the blood flow multiplied by the arteriovenous difference in blood (glucose and lactate) or plasma concentration (ammonia and amino acids) for these variables. The use of blood flow instead of plasma flow in the latter calculations was considered the most appropriate since the concentrations of ammonia and free amino acids are similar in plasma and whole blood, with the exception of glutamate, aspartate and taurine which are severalfold higher in red blood cells than in plasma (Hagenfeldt & Arvidsson, 1980). The plasma flow (blood flow × (1 - haematocrit)) was used to estimate the flux of free fatty acids and glycerol in the legs since these are only transported in plasma.
The total net exchange of substrates and amino acids during exercise and recovery was determined as the area under the curve of the exchange-time relationship. The rates of uptake or release at two consecutive time points were averaged, multiplied by the time span and summed for the whole exercise or recovery period.
Blood analyses
Blood samples were drawn in heparinized syringes. Analyses of oxygen saturation, haemoblogin, haematocrit, glucose and lactate were performed directly on whole blood. Arterial blood for insulin and catecholamine determinations was added to Eppendorf tubes containing 30 μl of 200 mM EGTA and 30 μl of a mixture of 200 mM glutathione (GSH) and 250 mM EGTA, respectively, centrifuged at 9000 g for 3 min and the plasma was stored at -80°C until analysed. Insulin was measured by radioimmunoassay (Insulin RIA 100, Amersham Pharmacia Biotech, Uppsala, Sweden) and catecholamines were analysed by high performance liquid chromatography (HPLC) with electrochemical detection according to Hjemdahl et al. (1979). The remaining blood was added to Eppendorf tubes and centrifuged at 9000 g, and the plasma was stored at -80°C. Growth hormone was measured in plasma using radioimmunoassay (ELSA-HGH, CIS bio international, France).
For amino acid measurements, the plasma samples were deproteinized with 0.6 M perchloric acid (PCA; 1 : 5) and centrifuged at 9000 g for 2 min, and the supernatant was stored at -80°C until analysed. The concentration of amino acids was measured by reversed-phase HPLC as described by Pfeifer et al. (1983), with orthophthalaldehyde (OPA) as the derivatizing agent.
Plasma free fatty acid, glycerol and ammonia concentrations were measured using a Cobas-Bio analyser (Hoffman LaRoche, Basel, Switzerland).
Muscle analyses
The biopsy specimens, mean weight 29 mg (7–69 mg), were freeze-dried and extracted in 0.6 M PCA (approx. 1: 50) and centrifuged at 9000 g for 2 min. Both the pellet and the supernatant were stored at -80°C. The amino acid concentration in the supernatant was analysed by the same method as the one used for plasma samples (see above). The muscle glycogen concentration was measured both in the supernatant and in the pellet from the PCA extract using the method described by Leighton et al. (1989). The total glycogen concentration is given as the sum of these measurements. Lactate concentration was measured in the PCA supernatant according to Lowry & Passonneau (1972).
Statistics
Conventional methods were employed to calculate mean values and s.e.m. Differences between the low-glycogen and the normal-glycogen leg concerning the net exchange of substrates and amino acids during exercise and recovery have been evaluated by comparing the areas under the time-uptake or time-release curves for the analysed variables. The comparison between the areas was then made by using either Student's t test for paired observations or the non-parametric Wilcoxon signed-ranked test when there was an obvious skewed distribution of the data. The same tests were also employed to compare flux rates at rest and to determine whether the exchange rates were different from zero. The muscle data were analysed using a two-way analysis of variance (ANOVA) with repeated measures to determine differences between the low-glycogen and the normal-glycogen leg and to evaluate changes over time. When a significant effect was indicated, Fisher's protected least significant difference test was used to determine where the significance occurred. A probability level of P < 0.05 was employed due to the relatively small number of subjects.
RESULTS
Cardiovascular parameters
Table 1 shows the pulmonary oxygen uptake, heart rate, leg blood flow and oxygen uptake at rest, during exercise and recovery after exercise. No differences were detected in blood flow between the normal- and the low-glycogen leg. Therefore, average values for the two legs were calculated at rest, during exercise and after exercise and used in the following calculations. At rest, the extraction of oxygen was 44 ± 9 % greater for the leg with normal muscle glycogen content, giving a higher oxygen uptake in this leg than in the leg with reduced muscle glycogen content. During the exercise period, there was no difference in oxygen extraction, blood flow or oxygen uptake between the two legs. During the recovery period, blood flow was measured repeatedly during the first 15 min and no difference between the two legs was found. Nor was there any difference in oxygen extraction between the legs during the whole recovery period. No difference in the pedal force between the two legs could be detected, i.e. the same amount of work was done throughout the exercise by the low-glycogen and the normal-glycogen leg. The pedal force averaged 29 ± 2 kg for the low-glycogen and 29 ± 3 kg for the normal-glycogen leg.
Table 1.
Pulmonary oxygen uptake (VO2), R value, heart rate, leg blood flow and leg oxygen uptake at rest, during exercise and during recovery after exercise
| Exercise | Recovery | |||||
|---|---|---|---|---|---|---|
| Rest | 10 min | 30 min | 50 min | 5 min | 15 min | |
| Pulmonary VO2 (l min−1) | 0.29 ± 0.02 | 2.51 ± 0.13 | 2.59 ± 0.12 | 2.56 ± 0.06 | — | — |
| R | 0.72 ± 0.02 | 0.86 ± 0.02 | 0.82 ± 0.02 | 0.82 ± 0.02 | — | — |
| Heart rate (beats min−1) | 60 ± 3 | 147 ± 3 | 160 ± 4 | 167 ± 5 | — | — |
| Leg blood flow (l min−1) | 0.29 ± 0.02 | — | 8.0 ± 0.3 | — | 0.8 ± 0.1 | 0.6 ± 0.1 |
| Leg VO2 (ml min−1) | ||||||
| Low | 12 ± 2 | — | 1240 ± 44 | — | 39 ± 5 | 24 ± 3 |
| Normal | 17 ± 3* | — | 1260 ± 41 | — | 39 ± 2 | 27 ± 3 |
Low and Normal indicate reduced and normal pre-exercise muscle glycogen concentrations. Values are means ± S.E.M. for eight subjects, except for values of leg blood flow and leg oxygen uptake where the number of subjects is six.
P <0.05 for low- vs. normal-glycogen leg.
Substrate exchange in the legs
In six of the subjects catheters were placed in both the right and left veins (see Methods). All comparisons between the two legs are therefore made in these subjects.
Glucose
Glucose was taken up by both legs at rest. The rate of uptake in the normal-glycogen leg was 48 ± 2 μmol min−1 and 76 ± 3 μmol min−1 (P > 0.05) in the low-glycogen leg. During exercise, the rate of glucose uptake increased in both legs; the average rate of uptake was 1.9 mmol min−1 in the low-glycogen leg and 1.5 mmol min−1 in the normal-glycogen leg, giving a 30 % larger uptake during exercise in the leg with a reduced initial muscle glycogen content. During the 2 h recovery period after exercise, the rate of uptake was higher than at rest in both legs. At 2 h of recovery the rate of uptake was 134 ± 4 μmol min−1 by the low-glycogen leg and 120 ± 2 μmol min−1 (P > 0.05) by the normal-glycogen leg. The total uptake of glucose during the 2 h recovery period was 30 ± 4 mmol by the low-glycogen leg and 26 ± 4 mmol by the normal-glycogen leg (Fig. 1A).
Figure 1. Exchange of glucose, lactate and ammonia at rest, during exercise and recovery.
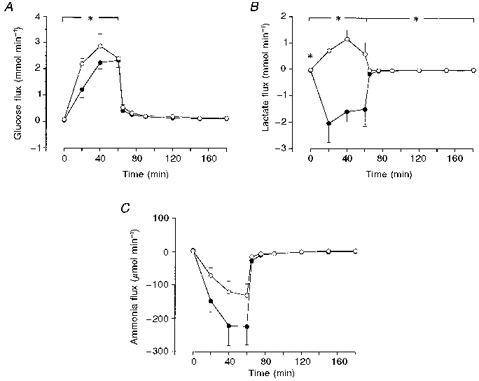
^, low-glycogen leg; •, normal-glycogen leg. Values are means ±s.e.m. for six subjects. *P < 0.05 for low- vs. normal-glycogen leg.
Lactate
There was a higher rate of release of lactate from the muscles of the normal-glycogen leg than from the low-glycogen leg at rest: 43 ± 6 and 21 ± 5 μmol min−1, respectively. During exercise, the average rate of release of lactate from the normal-glycogen leg increased to 1.3 ± 0.3 mmol min−1, whereas there was an uptake of lactate of 0.60 ± 0.3 mmol min−1 by the low-glycogen leg. Furthermore, during the recovery period, the rate of release of lactate from the normal-glycogen leg was higher than from the low-glycogen leg. At 5 min of recovery, the rate of release was 45 ± 1 μmol min−1 in the low-glycogen leg and 170 ± 4 μmol min−1 in the normal-glycogen leg. The total release from the normal-glycogen leg during the recovery period was 10 ± 3 as compared with 2.2 ± 1 mmol from the low-glycogen leg (Fig. 1B).
Free fatty acids and glycerol
Fatty acids and glycerol were released at similar rates by both legs at rest, free fatty acids at a rate of 10 μmol min−1 and glycerol at a rate of 6 μmol min−1. During exercise, there was an uptake of fatty acids and a release of glycerol from both legs. The average rate of uptake of fatty acids was 175 μmol min−1 during exercise, similar in the low- and the normal-glycogen leg. Glycerol was released from both legs during exercise and in the recovery period. The rate of release during exercise was 77 ± 29 and 49 ± 10 μmol min−1 (P > 0.05) from the low-glycogen and the normal-glycogen leg, respectively.
Ammonia
At rest, there was a small uptake of ammonia by the legs, 2–4 μmol min−1, which was similar for both legs. There was a release of ammonia from both legs during exercise, 80 μmol min−1 from the low-glycogen leg and 148 μmol min−1 (P > 0.05) from the normal-glycogen leg, giving a total release of 4.8 mmol from the low-glycogen leg and 8.9 mmol from the normal-glycogen leg. Ammonia was also released from both legs during the recovery period (Fig. 1C).
Amino acids
At rest, there was a release of several amino acids from the legs; only glutamate was taken up by the legs. Alanine was the only amino acid that showed a difference between the two legs: the release was greater from the normal-glycogen leg at rest. During exercise, there was an increased rate of release of alanine from both legs and of glutamine from the low-glycogen leg, whereas the release of glutamine from the normal-glycogen leg was not statistically significant, i.e. the release was not different from zero (Tables 2 and 3). There was also an increased rate of release from the low-glycogen leg of the amino acids that are not metabolized by the muscle (e.g. tyrosine and phenylalanine), whereas some subjects showed an uptake of these amino acids in the normal-glycogen leg, giving a non-significant rate of release from this leg during exercise. A direct comparison between the exchange of amino acids in the two legs during exercise did not show any statistically significant differences. In the recovery period, there was a release of most amino acids from both legs, except for glutamate, which was taken up by the muscle, and taurine, tryptophan and the branched-chain amino acids, which were neither released nor taken up by the muscle (Tables 2 and 3).
Table 2.
Exchange of amino acids (μmol min−1) in the low-glycogen leg at rest, during exercise and repeatedly during the recovery period
| Recovery | ||||||
|---|---|---|---|---|---|---|
| Amino acid | Rest | Exercise | 5 min | 15 min | 30–120 min | Recovery |
| Glutamate | 13 ± 2* | 38 ± 7* | 18 ± 4 | 13 ± 3 | 13 ± 3 | P < 0.05 |
| Serine | 2 ± 1 | −22 ± 11 | −2 ± 3 | −8 ± 4 | −2 ± 1 | n.s. |
| Glutamine | −18 ± 6* | −219 ± 69* | −35 ± 17 | −56 ± 21 | −22 ± 7 | P < 0.05 |
| Histidine | −2 ± 0.5* | −29 ± 10* | −1 ± 3 | −7 ± 3 | −1 ± 0.6 | P < 0.05 |
| Glycine | −4 ± 2 | −76 ± 29* | −13 ± 5 | −19 ± 6 | −11 ± 2 | P < 0.05 |
| Threonine | −2 ± 2 | −48 ± 16* | −7 ± 4 | −13 ± 4 | −5 ± 2 | P < 0.05 |
| Alanine | −18 ± 2* | −226 ± 54* | −33 ± 15 | −61 ± 21 | –31 ± 6 | P < 0.05 |
| Taurine | 0 ± 0.2 | −17 ± 13 | −2 ± 1 | −4 ± 2 | −1 ± 0.6 | n. s. |
| Arginine | −3 ± 0.5* | −28 ± 11* | −2 ± 2 | −7 ± 3 | −3 ± 1 | P < 0.05 |
| Tyrosine | –1 ± 0.5 | −21 ± 7* | −2 ± 2 | −5 ± 3 | −1 ± 0.5 | P < 0.05 |
| Methionine | −1 ± 0.2* | −10 ± 4* | −2 ± 1 | −3 ± 1 | −1 ± 0.4 | P < 0.05 |
| Valine | 0 ± 3 | −75 ± 43 | 6 ± 12 | −12 ± 10 | 4 ± 3 | n. s. |
| Tryptophan | 0 ± 0.6 | −16 ± 8* | 0 ± 1 | −2 ± 2 | 0 ± 0.3 | n. s. |
| Phenylalanine | −1 ± 0.4* | −22 ± 10* | −2 ± 2 | −5 ± 2 | −1 ± 0.5 | P < 0.05 |
| Isoleucine | −1 ± 0.5 | −24 ± 13* | 2 ± 3 | −4 ± 3 | 1 ± 1 | n. s. |
| Leucine | −1 ± 1 | −40 ± 25 | 2 ± 6 | −8 ± 6 | 1 ± 2 | n. s. |
| Lysine | −3 ± 2 | −59 ± 18* | −1 ± 4 | −13 ± 6 | −5 ± 2 | P < 0.05 |
The subjects performed cycle exercise for 1 h with a reduced muscle glycogen content in one leg. The values given are means ± S.E.M. for six subjects.
P < 0.05 indicates a difference from zero flux rates.
Table 3.
Exchange of amino acids (μmol min−1) in the normal-glycogen leg at rest, during exercise and repeatedly during the recovery period
| Recovery | ||||||
|---|---|---|---|---|---|---|
| Amino acid | Rest | Exercise | 5 min | 15 min | 30–120 min | Recovery |
| Glutamate | 13 ± 1* | 39 ± 8* | 15 ± 3 | 14 ± 2 | 14 ± 3 | P < 0.05 |
| Serine | 1 ± 1 | −21 ± 34 | −7 ± 4 | −10 ± 4 | −5 ± 1 | P < 0.05 |
| Glutamine | −27 ± 5* | −97 ± 126 | −58 ± 17 | −62 ± 19 | −36 ± 6 | P < 0.05 |
| Histidine | −3 ± 1* | −13 ± 18 | −6 ± 3 | −6 ± 3 | −3 ± 0.5 | P < 0.05 |
| Glycine | −6 ± 1* | −44 ± 41 | −15 ± 6 | −20 ± 6 | −12 ± 2 | P < 0.05 |
| Threonine | −4 ± 1* | −20 ± 26 | −14 ± 4 | −15 ± 4 | −10 ± 2 | P < 0.05 |
| Alanine | −30 ± 4* | −195 ± 71* | −55 ± 11 | −69 ± 22 | −41 ± 9 | P < 0.05 |
| Taurine | −1 ± 0.3 | −16 ± 12 | −5 ± 2 | −5 ± 2 | −1 ± 0.6 | n. s. |
| Arginine | −3 ± 1* | −24 ± 14 | −5 ± 2 | −9 ± 3 | −4 ± 1 | P < 0.05 |
| Tyrosine | −2 ± 0.4* | −8 ± 14 | −5 ± 2 | −5 ± 2 | −2 ± 0.5 | P < 0.05 |
| Methionine | −1 ± 0.2* | −8 ± 6 | −3 ± 1 | −3 ± 1 | −2 ± 0.3 | P < 0.05 |
| Valine | 0 ± 1 | −47 ± 68 | −6 ± 9 | −14 ± 10 | 0 ± 3 | n.s. |
| Tryptophan | 0 ± 0.2 | −12 ± 11 | −1 ± 1 | −3 ± 2 | −1 ± 0.3 | n. s. |
| Phenylalanine | −2 ± 0.3* | −15 ± 13 | −4 ± 1 | −6 ± 2 | −2 ± 0.4 | P < 0.05 |
| Isoleucine | −1 ± 0.5 | −12 ± 19 | −1 ± 3 | −4 ± 3 | 0 ± 1 | n. s. |
| Leucine | −2 ± 1 | −20 ± 35 | −2 ± 5 | −9 ± 6 | 0 ± 2 | n.s. |
| Lysine | −4 ± 1* | −45 ± 20 | −7 ± 6 | −15 ± 6 | −6 ± 1 | P < 0.05 |
The subjects performed cycle exercise for 1 h with a reducedmuscle glycogen content in one leg. The values given are means ± S.E.M. for six subjects.
P < 0.05 indicates a difference from zero flux rates.
Muscle concentrations
The water content of the biopsy samples was not significantly different at rest and after exercise. It averaged 75.6 % at rest and 76.8 % immediately after exercise. The average water content when all biopsy samples were included was 76.3 %.
Glycogen
The concentrations of glycogen before exercise were 167 ± 28 and 317 ± 40 μmol (kg dry wt)−1 (P < 0.05) in the low-glycogen and normal-glycogen leg, respectively. Thus, the evening exercise protocol led to an approximately 50 % lower level of muscle glycogen the following morning. The decrease in muscle glycogen during exercise was smaller in the low-glycogen leg; it averaged 99 ± 18 in the low-glycogen leg and 207 ± 22 μmol (kg dry wt)−1 (P < 0.05) in the normal-glycogen leg (Table 4).
Table 4.
Muscle concentrations (mmol (kg dry wt)−1) of glycogen and lactate in biopsy samples taken from the vastus lateralis at rest, immediately after exercise and repeatedly during the recovery period
| Glycogen concentration | Lactate concentration | |||
|---|---|---|---|---|
| Low | Normal | Low | Normal | |
| Rest | 167 ± 28 | 317 ± 40 | 7.1 ± 1.8 | 7.5 ± 1.2 |
| After exercise | 77 ± 17* | 101 ± 31* | 10 ± 1.6 | 14 ± 3.3 |
| 0.5 h after exercise | 98 ± 17* | 146 ± 35* | 5.3 ± 0.9 | 10 ± 2.3 |
| 1 h after exercise | 111 ± 19* | 131 ± 41* | 6.6 ± 1.4 | 5.8 ± 0.9 |
| 2 h after exercise | 132 ± 29 | 139 ± 38* | 7.8 ± 1.6 | 8.1 ± 1.9 |
Low and Normal indicate reduced and normal initial muscle glycogen concentrations. The values given are means ± S.E.M. for eight subjects.
*P < 0.05vs. resting value.
Lactate
The concentrations of lactate were slightly, but non-significantly, elevated in both legs after exercise, and after 30 min of recovery the concentrations had returned to resting levels (Table 4).
Amino acids
No differences in the resting concentrations of amino acids were found between the normal- and the low- glycogen leg. During exercise, there was a similar increase or no change in the level of most amino acids in the low-glycogen and the normal-glycogen leg. The only amino acid that showed a difference between the two legs was aspartate: a significantly greater increase in the concentration was found in the low-glycogen leg during exercise (Fig. 2A). For glutamine, histidine, glycine, taurine and the branched-chain amino acids (valine, isoleucine and leucine), there was no difference in the concentrations before and after exercise and the levels remained approximately constant during the 2 h recovery. One amino acid decreased during exercise: glutamate - a decrease of about 65 % was found in both legs. During recovery, the level increased in the first 30 min to approximately 60 and 75 % of the resting level in the low-glycogen leg and the normal-glycogen leg, respectively, and thereafter remained constant (Tables 5 and 6, Fig. 2B).
Figure 2. Change in muscle concentration of some selected amino acids during exercise and recovery.
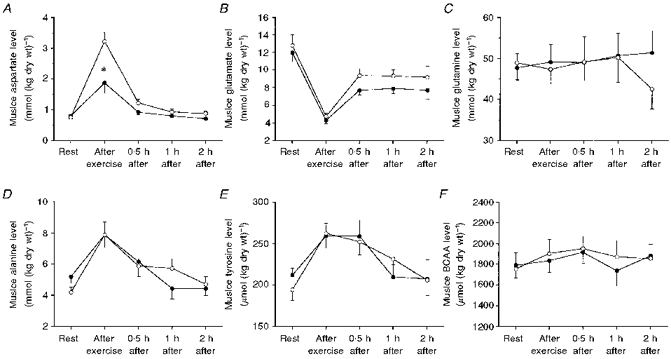
Samples were taken from the vastus lateralis muscle. ^, low-glycogen leg; •, normal-glycogen leg. Values are means ±s.e.m. for eight subjects. *P < 0.05 for low- vs. normal-glycogen leg.
Table 5.
Concentrations (μmol (kg dry wt)−1) of free amino acids in biopsy samples taken from the vastus lateralis muscle of the low-glycogen leg at rest, immediately after exercise and repeatedly during the recovery period
| Amino acid | Rest | After exercise | 0.5 h after | 1 h after | 2 h after |
|---|---|---|---|---|---|
| Aspartate | 757 ± 52 | 3222 ± 300* | 1221 ± 123* | 931 ± 87 | 883 ± 81 |
| Glutamate | 12810 ± 1230 | 4730 ± 360* | 9380 ± 800* | 9330 ± 720* | 9190 ± 1280* |
| Serine | 1457 ± 112 | 1882 ± 216* | 1807 ± 155* | 1727 ± 152 | 1381 ± 128 |
| Glutamine | 48920 ± 4150 | 47350 ± 3570 | 49170 ± 4570 | 50240 ± 6100 | 42500 ± 4840 |
| Histidine | 463 ± 104 | 1586 ± 108 | 1653 ± 127 | 1562 ± 100 | 1420 ± 176 |
| Glycine | 2715 ± 149 | 3115 ± 269 | 3130 ± 187 | 3060 ± 246 | 2778 ± 415 |
| Threonine | 1469 ± 114 | 1864 ± 176* | 1815 ± 135* | 1779 ± 172 | 1406 ± 150 |
| Alanine | 4190 ± 310 | 7870 ± 840* | 5870 ± 470* | 5730 ± 620* | 4700 ± 500 |
| Taurine | 52700 ± 3930 | 51650 ± 3860 | 57420 ± 4400 | 55260 ± 4460 | 58260 ± 8760 |
| Arginine | 1011 ± 130 | 1148 ± 136* | 1119 ± 141 | 1004 ± 107 | 950 ± 141 |
| Tyrosine | 194 ± 13 | 262 ± 18* | 252 ± 15* | 231 ± 23* | 206 ± 19 |
| Methionine | 104 ± 7 | 138 ± 11* | 133 ± 7* | 106 ± 8 | 91 ± 15 |
| Valine | 886 ± 77 | 957 ± 73 | 965 ± 67 | 929 ± 82 | 897 ± 61 |
| Phenylalanine | 187 ± 13 | 257 ± 15* | 241 ± 12* | 223 ± 19* | 205 ± 20 |
| Isoleucine | 283 ± 25 | 314 ± 17 | 324 ± 16 | 301 ± 20 | 315 ± 24 |
| Leucine | 587 ± 60 | 632 ± 47 | 662 ± 43 | 641 ± 65 | 643 ± 59 |
| Lysine | 1905 ± 128 | 2182 ± 126* | 2104 ± 151 | 1889 ± 102 | 1623 ± 175* |
The subjects performed cycle exercise for 1 h with a reduced muscle glycogen content in one leg. The values given are means ± S.E.M. for eight subjects.
P < 0.05 as compared with rest.
Table 6.
Concentrations (μmol (kg dry wt)−1) of free amino acids in biopsy samples taken from the vastus lateralis muscle of the normal-glycogen leg at rest, immediately after exercise and repeatedly during the recovery period
| Amino acid | Rest | After exercise | 0.5 h after | 1 h after | 2 h after |
|---|---|---|---|---|---|
| Aspartate | 797 ± 84 | 1873 ± 337* | 924 ± 87 | 801 ± 51 | 714 ± 41 |
| Glutamate | 11980 ± 1020 | 4290 ± 390* | 7700 ± 530* | 7910 ± 570* | 7720 ± 980* |
| Serine | 1700 ± 213 | 1856 ± 143 | 1918 ± 166 | 1661 ± 170 | 1547 ± 146 |
| Glutamine | 47700 ± 3530 | 49130 ± 4330 | 49030 ± 6320 | 50 700 ± 5520 | 51410 ± 5410 |
| Histidine | 1334 ± 126 | 1405 ± 88 | 1451 ± 151 | 1381 ± 118 | 1426 ± 117 |
| Glycine | 3415 ± 349 | 2989 ± 145 | 3473 ± 256 | 3120 ± 233 | 2960 ± 222 |
| Threonine | 1764 ± 175 | 1861 ± 136 | 1940 ± 142 | 1649 ± 134 | 1555 ± 121 |
| Alanine | 5190 ± 440 | 7870 ± 800* | 6150 ± 960 | 4430 ± 660 | 4420 ± 430 |
| Taurine | 50910 ± 6340 | 51950 ± 3690 | 57640 ± 5150 | 51790 ± 4570 | 47790 ± 3140 |
| Arginine | 905 ± 146 | 1051 ± 120 | 1013 ± 208 | 1005 ± 158 | 905 ± 123 |
| Tyrosine | 212 ± 8 | 259 ± 15* | 259 ± 19* | 210 ± 15 | 207 ± 23 |
| Methionine | 115 ± 12 | 132 ± 6 | 135 ± 10 | 100 ± 6 | 95 ± 7 |
| Valine | 896 ± 56 | 928 ± 59 | 937 ± 46 | 809 ± 59 | 895 ± 84 |
| Phenylalanine | 206 ± 12 | 297 ± 37* | 263 ± 22* | 204 ± 14 | 219 ± 25 |
| Isoleucine | 287 ± 21 | 303 ± 18 | 324 ± 19 | 278 ± 19 | 328 ± 35 |
| Leucine | 607 ± 52 | 603 ± 37 | 653 ± 44 | 542 ± 43 | 656 ± 85 |
| Lysine | 1643 ± 151 | 1944 ± 98 | 1855 ± 241 | 1799 ± 181 | 1648 ± 158 |
The subjects performed cycle exercise for 1 h with a reduced muscle glycogen content in one leg. The values given are means ± S.E.M. for eight subjects.
P < 0.05 as compared with rest.
Hormonal changes
Figure 3 shows the changes in the arterial concentrations of insulin, growth hormone and noradrenaline during exercise and recovery after exercise. The concentration of insulin decreased by approximately 50 % during exercise, increased immediately after exercise but decreased again during the recovery period to about the same level as after exercise. The concentration of growth hormone increased by about 170 % during exercise but decreased after the exercise to a level that was about 75 % below the resting level during the last hour of recovery. The arterial concentration of catecholamines increased severalfold during exercise but rapidly returned to the basal level after exercise.
Figure 3. Changes in arterial concentrations of insulin, growth hormone and noradrenaline during exercise and recovery.
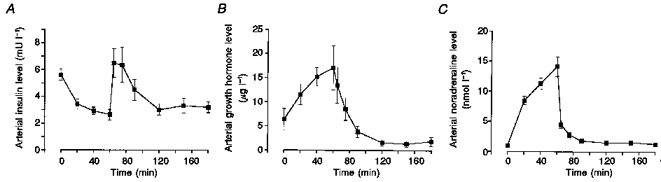
Values are means ±s.e.m. for eight subjects.
DISCUSSION
Exercise induced the same increase in the concentration of the aromatic amino acids, tyrosine and phenylalanine, in muscle biopsy samples from both legs. However, only from the low-glycogen leg was there a release of these amino acids by the muscle, as measured by arteriovenous differences and blood flow. This suggests that a reduced initial muscle glycogen level causes a net rate of protein degradation in muscle, which is in agreement with the results of other studies (Lemon & Mullin, 1980; Van Hall 1996). However, in these studies, experiments with reduced and normal glycogen levels were performed on separate occasions and it cannot be ruled out that the reduced initial muscle glycogen level induced a hormonal environment which was responsible for the increased rate of protein degradation (e.g. low levels of insulin). In the present study, both legs were exposed to the same hormonal environment and thus a hormonal effect can be ruled out.
The net rate of production of amino acids not metabolized in the muscle, e.g. tyrosine and phenylalanine, has been used to estimate the net rate of protein degradation in the low-glycogen leg during 60 min of exercise. The release of tyrosine and phenylalanine during the exercise period (arteriovenous difference in plasma concentration multiplied by the blood flow) and the change in their muscle concentration (dividing the dry weight concentration by 4 and assuming an active muscle mass of 7 kg) give a net production rate of 1.3 ± 0.4 mmol h−1 of tyrosine (240 ± 72 mg) and 1.5 ± 0.6 mmol h−1 (240 ± 100 mg) of phenylalanine. The net rate of protein degradation was then calculated from the observation that muscle protein contains 2.1 and 3.3 % of tyrosine and phenylalanine, respectively (Clowes et al. 1980). A net protein degradation of 12 ± 3.4 g h−1 was calculated from the tyrosine data and 7.3 ± 3.0 g h−1 from the phenylalanine data.
There was a release of alanine from both legs during exercise and also an increase in the alanine concentration in muscle, which were similar in the two legs. However, the exchange of glutamine was not as consistent as that of alanine; a release of glutamine was found only in the low-glycogen leg and no significant release was found in the other leg. This is slightly different from the results of studies employing sustained exercise with the knee extensors, in which a release of both alanine and glutamine was reported (Graham et al. 1991; MacLean et al. 1994; Graham et al. 1995). It is possible that in the present study, an increased rate of protein degradation in the low-glycogen leg will increase the level of glutamate. The ammonia produced could then be incorporated into glutamate to form glutamine and this may explain why there is a release of glutamine from the low-glycogen leg. As the three branched-chain amino acids are known to be metabolized by skeletal muscle, an uptake of these amino acids during exercise would be expected. However, a release in both legs, although not statistically significant, was found during exercise, which might be an effect of an increased rate of net protein degradation, at least in the low-glycogen leg.
The only amino acid in the muscle that was affected differently by exercise in the two legs was aspartate. The increase in asparate concentration was about twice as high in the leg with the reduced initial muscle glycogen level. The reason for this is unclear, but one possible explanation could be that more fatty acids and branched-chain amino acids are oxidized in the low-glycogen leg. This may cause a rise in the level of acetyl-CoA and oxaloacetate in the muscle, which will then produce aspartate.
There was a higher rate of oxygen uptake in the normal-glycogen leg at rest, but also a much higher rate of lactate and alanine release from this leg than from the low-glycogen leg. This indicates that the rate of glycolysis is increased in the normal-glycogen leg at rest. The breakdown of glycogen during exercise was greater in the leg that commenced the exercise with a normal glycogen content than in the leg that started the exercise with a reduced muscle glycogen content. This finding, i.e. that higher initial levels of muscle glycogen cause a higher rate of muscle glycogenolysis during exercise, is in agreement with earlier reports (Gollnick et al. 1981; Hargreaves et al. 1995). The finding is consistent with the view that the glycogen level in muscle can regulate the rate of glycogenolysis. Similar findings from studies in perfused rat muscle have been reported (Hespel & Richter, 1992).
There was a higher rate of glucose uptake in the low- glycogen leg during exercise than in the normal-glycogen leg. This is similar to the finding reported by Gollnick et al. (1981), while in other studies no such effect has been found (Hargreaves et al. 1995). It is possible that the exercise the previous evening might have increased the number of glucose transporters which will contribute to give a higher rate of glucose uptake the following morning. However, the time between the evening exercise and the following morning exercise was at least 14 h, which makes it less likely that the increased glucose uptake is a result of the evening exercise rather than the availability of muscle glycogen. Also during the recovery period, there was a tendency for a higher glucose uptake in the leg with a reduced initial glycogen level. Similar results have been reported in the perfused rat muscle: the rate of glucose uptake after exhausting exercise was 60–80 % higher in animals in which muscle glycogen was kept low than in those in which glycogen was raised by feeding carbohydrate (Fell et al. 1982).
There was the expected release of lactate from the normal-glycogen leg during exercise. In contrast, there was an uptake of lactate by the low-glycogen leg during exercise. Similar findings have been reported by Gollnick et al. (1981). In that study, measurements of RQ over the leg indicated that more fat and, therefore, less carbohydrate was oxidized in the low-glycogen leg. The amount of pyruvate oxidized in both legs in the present study can be calculated from the degradation of glycogen, the lactate produced or taken up, the glucose taken up and the alanine released: pyruvate oxidation amounted to approximately 450 mmol in the low-glycogen leg and 750 mmol in the normal-glycogen leg, which suggests more oxidation of fat in the low-glycogen leg. The total amount of carbohydrates oxidized during exercise, calculated from the pulmonary oxygen uptake and R value, agrees well with the value obtained from the sum of pyruvate oxidation in the two legs.
In summary, the present study shows that when exercise starts with a low level of muscle glycogen this leads to a higher rate of glucose uptake, an uptake of lactate and a net degradation of protein in the leg muscle. Since the normal- and low-glycogen legs received the same arterial supply, the results suggest that the glycogen level per se is responsible for these changes.
Acknowledgments
The present study was supported by grants from the Danish National Research Foundation and from Pripps Bryggerier, Sweden. The authors are grateful to Heidi Hansen, Gunilla Hedén, Karin Juel, Inge-Lise Kring, Carsten Nielsen and Merete Vannby for their excellent technical assistance.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. The Journal of Physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes GHA, Randall HT, Cha C-J. Amino acid and energy metabolism in septic and traumatized patients. Journal of Parenteral and Enteral Nutrition. 1980;4:195–205. doi: 10.1177/014860718000400225. [DOI] [PubMed] [Google Scholar]
- Fell RD, Terblanche SE, Ivy JL, Young JC, Holloszy JO. Effect of muscle glycogen content on glucose uptake following exercise. Journal of Applied Physiology. 1982;52:434–437. doi: 10.1152/jappl.1982.52.2.434. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Pernow B, Essén B, Jansson E, Saltin B. Availability of glycogen and plasma FFA for substrate utilization in leg muscle of man during exercise. Clinical Physiology. 1981;1:27–42. [Google Scholar]
- Gollnick PD, Piehl K, Saubert CW, Armstrong RB, Saltin B. Diet, exercise, and muscle glycogen. Journal of Applied Physiology. 1972;33:421–425. doi: 10.1152/jappl.1972.33.4.421. [DOI] [PubMed] [Google Scholar]
- Graham TE, Kiens B, Hargreaves M, Richter EA. Influence of fatty acids on ammonia and amino acid flux from active human muscle. American Journal of Physiology. 1991;261:E168–176. doi: 10.1152/ajpendo.1991.261.2.E168. [DOI] [PubMed] [Google Scholar]
- Graham TE, Turcotte LP, Kiens B, Richter EA. Training and muscle ammonia and amino acid metabolism in humans during prolonged exercise. Journal of Applied Physiology. 1995;78:725–735. doi: 10.1152/jappl.1995.78.2.725. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L, Arvidsson A. The distribution of amino acids between plasma and erythrocytes. Clinica Chimica Acta. 1980;100:133–141. doi: 10.1016/0009-8981(80)90074-1. 10.1016/0009-8981(80)90074-1. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, McConell G, Proietto J. Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise in humans. Journal of Applied Physiology. 1995;78:288–292. doi: 10.1152/jappl.1995.78.1.288. 10.1063/1.360672. [DOI] [PubMed] [Google Scholar]
- Hespel P, Richter EA. Mechanisms linking glycogen concentration and glycogenolytic rate in perfused contracting rat skeletal muscle. Biochemical Journal. 1992;284:777–780. doi: 10.1042/bj2840777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjemdahl P, Daleskog M, Kahan T. Determination of plasma catecholamines by high performance liquid chromatography with electrochemical detection: Comparison with a radioenzymatic method. Life Sciences. 1979;25:131–138. doi: 10.1016/0024-3205(79)90384-9. 10.1016/0024-3205(79)90384-9. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Secher NH, Bach FW, Galbo H. Role of motor center activity for hormonal changes and substrate mobilization in humans. American Journal of Physiology. 1987;253:R687–695. doi: 10.1152/ajpregu.1987.253.5.R687. [DOI] [PubMed] [Google Scholar]
- Leighton B, Blomstrand E, Challiss RAJ, Lozeman FJ, Parry-Billings M, Dimitriadis GD, Newsholme EA. Acute and chronic effects of strenuous exercise on glucose metabolism in isolated, incubated soleus muscle of exercised-trained rats. Acta Physiologica Scandinavica. 1989;136:177–184. doi: 10.1111/j.1748-1716.1989.tb08650.x. [DOI] [PubMed] [Google Scholar]
- Lemon PWR. Protein and exercise: update 1987. Medicine and Science in Sports and Exercise. 1987;19:S179–190. [PubMed] [Google Scholar]
- Lemon PWR, Mullin JP. Effect of initial muscle glycogen levels on protein catabolism during exercise. The Journal of Physiology. 1980;48:624–629. doi: 10.1152/jappl.1980.48.4.624. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- MacLean DA, Graham TE, Saltin B. Branched- chain amino acids augment ammonia metabolism while attenuating protein breakdown during exercise. American Journal of Physiology. 1994;267:E1010–1022. doi: 10.1152/ajpendo.1994.267.6.E1010. [DOI] [PubMed] [Google Scholar]
- Pfeifer R, Karol R, Korpi J, Burgoyne R, McCourt D. Practical application of HPLC to amino acid analysis. American Laboratory. 1983;15:77–84. [Google Scholar]
- Van Hall G. Maastricht, The Netherlands: University of Limburg; 1996. Amino acids, ammonia and exercise in man. PhD Thesis. [Google Scholar]
- Van Hall G, Saltin B, Van der Vusse GJ, Söderlund K, Wagenmakers AJM. Deamination of amino acids as a source of ammonia production during prolonged exercise. The Journal of Physiology. 1995;489:251–261. doi: 10.1113/jphysiol.1995.sp021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltan SM, Bosch AN, Dennis SC, Noakes TD. Influence of muscle glycogen content on metabolic regulation. American Journal of Physiology. 1998;274:E72–82. doi: 10.1152/ajpendo.1998.274.1.E72. [DOI] [PubMed] [Google Scholar]


