Abstract
ATP-sensitive potassium (KATP) channels are composed of pore-forming Kir6.2 and regulatory SUR subunits. A truncated isoform of Kir6.2, Kir6.2ΔC26, expresses ATP-sensitive channels in the absence of SUR1, suggesting the ATP-inhibitory site lies on the Kir6.2 subunit.
We examined the effect on the channel ATP sensitivity of mutating the arginine residue at position 50 (R50) in the N-terminus of Kir6.2, by recording macroscopic currents in membrane patches excised from Xenopus oocytes expressing wild-type or mutant Kir6.2ΔC26.
Substitution of R50 by serine, alanine or glycine reduced the Ki for ATP inhibition from 117 μm to 800 μm, 1.1 mm and 3.8 mm, respectively. The single-channel conductance and kinetics were unaffected by any of these mutations. Mutation to glutamate, lysine, asparagine, glutamine or leucine had a smaller effect (Ki, ∼300–400 μm). The results indicate that the side chain of the arginine residue at position 50 is unlikely to contribute directly to the binding site for ATP, and suggest it may affect ATP inhibition by allosteric interactions.
Mutation of the isoleucine residue at position 49 to glycine (I49G) reduced the channel ATP sensitivity, while the mutation of the glutamate residue at position 51 to glycine (E51G) did not.
When a mutation in the N-terminus of Kir6.2ΔC26 that alters ATP sensitivity (R50S; Ki, 800 μm) was combined with one in the C-terminus (E179Q; Ki, 300 μm), the Ki for the apparent ATP sensitivity was increased to 2.8 mm. The Hill coefficient was also increased. This suggests that the N- and C-termini of Kir6.2 may co-operate to influence channel closure by ATP.
ATP-sensitive potassium (KATP) channels are found in pancreatic β-cells, heart, smooth and skeletal muscle and certain neurones (Ashcroft & Ashcroft, 1990). They are formed by the physical association of four inwardly rectifying K+ channel subunits (Kir6.2) with four sulphonylurea receptor subunits (either SUR1, SUR2A or SUR2B) (Inagaki et al. 1995, 1996; Sakura et al. 1995; Isomoto et al. 1996; Clement et al. 1997). Kir6.2 serves as an ATP-sensitive pore while SUR is a regulatory subunit that modulates the channel gating properties, enhances the apparent ATP sensitivity and acts as the target for sulphonylurea drugs, K+ channel openers and intracellular Mg2+ nucleotides, which modulate KATP channel activity (Tucker et al. 1997; Proks & Ashcroft, 1997). The balance between the stimulatory effects (mediated via SUR) and inhibitory effects (mediated via Kir6.2) of intracellular adenine nucleotides serves to couple the electrical activity of the cell to its metabolic status.
Although wild-type Kir6.2 does not express functional channels in the absence of SUR1, deletion of the last 26 amino acids (Kir6.2ΔC26) enables its independent functional expression (Tucker et al. 1997). The ability of ATP to inhibit Kir6.2ΔC26 currents demonstrates that the ATP inhibitory site does not lie on SUR1 and suggests that it may be located on Kir6.2. Additional support for this view is provided by the fact that mutations within this subunit may severely reduce the inhibitory effects of ATP (Tucker et al. 1997, 1998; Shyng et al. 1997). In this paper we explore the effect of mutating an N-terminal amino acid, the arginine residue at position 50 (Fig. 1), on the ATP sensitivity of Kir6.2ΔC26. We also examine the effects of mutating the amino acids on either side of this residue, and of combining mutation of R50 with a second mutation in the C-terminus of the channel (E179; Fig. 1) that also confers reduced ATP sensitivity.
Figure 1. Putative membrane topology of Kir6.2.
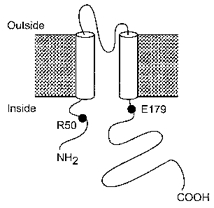
The positions of residue R50, which lies in the N-terminus, and of E179, which lies in the C-terminus, of the protein are indicated.
METHODS
A 26 amino acid C-terminal deletion of mouse Kir6.2 (Kir6.2ΔC26) was made by introduction of a stop codon at the appropriate residue (Tucker et al. 1997). Site-directed mutagenesis of Kir6.2ΔC26 was carried out by subcloning the appropriate fragments into the pALTER vector (Promega). Synthesis of capped mRNA was carried out using the mMessage mMachine in vitro transcription kit (Ambion, Austin, TX, USA). Amino acids are indicated by the single-letter code.
Female Xenopus laevis were anaesthetized with MS-222 (2 g l−1 added to the water). One ovary was removed via a mini-laparotomy, the incision sutured and the animal allowed to recover. Once the wound had completely healed, the second ovary was removed in a similar operation and the animal was then killed by decapitation whilst under anaesthesia. Immature stage V-VI Xenopus oocytes were manually defolliculated, injected with ∼2 ng of mRNA encoding wild-type or mutant forms of Kir6.2ΔC26 and studied 1–4 days after injection (Gribble et al. 1997).
Macroscopic currents were recorded from giant inside-out patches using an EPC-7 patch-clamp amplifier (List Electronik, Darmstadt, Germany) at 20–24°C (Gribble et al. 1997). The holding potential was 0 mV and currents were evoked by repetitive 3 s voltage ramps from -110 to +100 mV. Currents were filtered at 0.2 kHz, digitized at 0.5 kHz using a Digidata 1200 Interface and analysed using pCLAMP software (Axon Instruments). Single-channel currents were recorded from small inside-out patches, filtered at 1 kHz and sampled at 3 kHz.
The pipette solution contained (mM): 140 KCl, 1.2 MgCl2, 2.6 CaCl2, 10 Hepes (pH 7.4 with KOH) and the internal (bath) solution contained (mM): 110 KCl, 1.4 MgCl2, 30 KOH, 10 EGTA, 10 Hepes (pH 7.2 with KOH) and nucleotides as indicated. Solutions containing ATP were made up fresh each day and the pH was re-adjusted after addition of ATP. Rapid exchange of solutions was achieved by positioning the patch in the mouth of one of a series of adjacent inflow pipes placed in the bath.
Data analysis
The slope conductance was measured by fitting a straight line to the current-voltage relationship between -20 and -100 mV: the average of five consecutive ramps was calculated in each solution. ATP dose-response relationships were measured by alternating test and control solutions. Currents were corrected by subtraction of the background current measured in water-injected oocytes (∼5 pA at -100 mV). Conductance was expressed as a fraction of the mean of that obtained in control solution before and after ATP application. ATP dose-response curves were fitted to the Hill equation:
where [ATP] is the ATP concentration, Ki is the ATP concentration at which inhibition is half-maximal and h is the slope factor (Hill coefficient).
Single-channel currents were analysed using a combination of pCLAMP and in-house software written by Dr P. A. Smith (Oxford University). Single-channel current amplitudes were calculated from an all-points amplitude histogram. Channel activity (NPo) was measured as the mean patch current divided by the single channel current amplitude, for segments of the current records of ∼1 min duration. Open probability (Po) was calculated from NPo/N: where N is the number of channels in the patch, and was estimated from the maximum number of superimposed events. For analysis of single-channel kinetics, events were detected using a 50 % threshold level method. Burst analysis was carried out as described by Jackson et al. (1983).
All data are given as the mean ±s.e.m. The symbols in the figures indicate the mean and the vertical bars indicate one s.e.m. (where this is larger than the symbol). Statistical significance was tested by Student's t test or ANOVA, as appropriate.
RESULTS
Figure 2 shows that replacement of the arginine residue at position 50 of Kir6.2 with glycine (R50G) greatly decreases the inhibitory effect of ATP, as previously reported (Tucker et al. 1998). This residue lies within the N-terminal region of the protein and is predicted to be located intracellularly (Sakura et al. 1995). If the arginine side chain contributes directly to ATP binding, then its substitution by amino acids with altered charge, hydrophobicity and/or ability to form hydrogen bonds should have markedly different effects on the measured ATP sensitivity. Figure 3 shows that eight different mutations at position 50 produced a significant reduction in the sensitivity of the channel to 1 mM ATP. Figure 2B compares the mean dose-response curves for ATP inhibition of wild-type (wt) Kir6.2ΔC26 with Kir6.2ΔC26 containing the R50G, R50A and R50Q mutations. Mean data for all the mutant channels are given in Table 1. Half-maximal inhibition (Ki) of wtKir6.2ΔC26 was produced by 117 ± 6 μm ATP (n= 7). Of the eight different mutations introduced at position 50, substitution of serine, alanine or glycine caused the greatest shifts in ATP sensitivity, to a Ki of ∼800 μm or ∼1 mM or ∼4 mM, respectively. The other mutations produced much smaller shifts in ATP sensitivity. Replacement of the positively charged arginine with a negatively charged glutamate had only a small effect on ATP sensitivity (Ki, ∼300 μm), similar to that obtained when arginine was replaced by a positively charged (lysine) or a neutral (asparagine, glutamine or leucine) residue. This suggests that a positive charge at position 50 is not required for the high ATP sensitivity of the wild-type KATP channel. The Hill coefficients were not significantly different (by ANOVA), suggesting that for both wild-type and mutant channels the binding of a single ATP molecule is sufficient to cause inhibition.
Figure 2. Effect of ATP on wild-type and mutant Kir6.2ΔC26 currents.
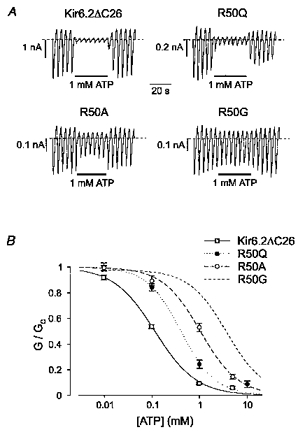
A, macroscopic currents recorded from inside-out patches excised from oocytes injected with mRNA encoding wild-type (wt) Kir6.2ΔC26, Kir6.2ΔC26-R50Q, Kir6.2ΔC26-R50A or Kir6.2ΔC26-R50G. Currents were elicited in response to a series of voltage ramps from -110 to +100 mV. ATP (1 mM) was added as indicated by the bars. B, mean ATP dose-response relationships for wtKir6.2ΔC26 (n= 7), Kir6.2ΔC26-R50Q (n= 5), Kir6.2ΔC26-R50A (n= 5) and Kir6.2ΔC26-R50G (data from Tucker et al. 1998) currents. The slope conductance (G) is expressed as a fraction of the mean (Gc) of that obtained in control solution before and after exposure to ATP. The lines are the best fit of the data to the Hill equation using the mean values for Ki and h given in Table 1.
Figure 3. Effect of ATP on wild-type and mutant Kir6.2ΔC26 currents.
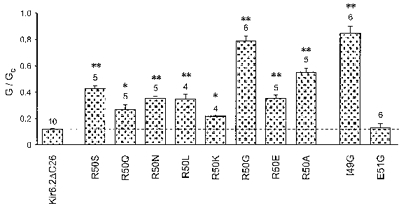
Mean conductance recorded in the presence of 1 mM ATP, expressed relative to the mean of that recorded before and after exposure to ATP from wtKir6.2ΔC26 and from Kir6.2ΔC26 containing the indicated mutations. The dashed line indicates the level of inhibition observed for wtKir6.2ΔC26. The number of patches is given above each bar. *P < 0.0005, **P < 0.00001vs. wtKir6.2ΔC26.
Table 1.
Effect of mutations on the ATP sensitivity of Kir6.2ΔC26
| Clone | K1 ATP | P | h | n | Side chain | Hydropathy |
|---|---|---|---|---|---|---|
| R | 117 ± 6 μM | — | 1.0 ± 0.04 | 7 | −(CH2)3NHC(NH2)NH3+ | −4.5 |
| R50E | 322 ± 19 μM | ** | 0.8 ± 0.06 | 4 | −CH2CH2COO− | −3.5 |
| R50K | 318 ± 47 μM | * | 1.1 ± 0.16 | 4 | −(CH2)4NH3+ | −3.9 |
| R50N | 406 ± 30 μM | ** | 0.9 ± 0.1 | 5 | −CH2CONH2 | −3.5 |
| R50Q | 407 ± 46 μM | ** | 1.2 ± 0.1 | 5 | −CH2CH2CONH2 | −3.5 |
| R50L | 425 ± 45 μM | ** | 0.8 ± 0.08 | 4 | −CH2CH(CH3)2 | 3.8 |
| R50S | 796 ± 76 μM | ** | 1.1 ± 0.09 | 5 | −CHOH | −0.8 |
| R50A | 1.05 ± 0.12 mM | ** | 1.1 ± 0.12 | 5 | −CH3 | 1.8 |
| R50G† | 3.77 ± 0.71 mM | ** | 1.1 ± 0.13 | 6 | −H | −0.4 |
| E179Q† | 296 ± 29 μM | ** | 1.3 ± 0.1 | 7 | — | — |
| R50S/E179Q | 2.8 ± 0.2 mM | ** | 1.6 ± 0.2 | 5 | — | — |
K1 ATP, ATP concentration at which inhibition is half-maximal; h, Hill coefficient for ATP inhibition; n, number of patches. Hydropathy values are taken from Kyte & Doolittle (1982).
*P < 0.0005
P < 0.00001, against wtKir6.2ΔC26 (for K1 value).
Data from Tucker et al. (1998), corrected for background.
The channel ATP sensitivity was not dependent on the extent of hydrophobicity of the residue at position 50, or its ability to form hydrogen bonds. Thus, although arginine is strongly hydrophilic (hydrophobicity, -4.5; Kyte & Doolittle, 1982) its substitution by the strongly hydrophobic residue leucine (hydrophobicity, +3.8) only decreased the Ki for ATP inhibition from ∼100 to ∼400 μm, whereas substitution by the less hydrophobic glycine residue (hydrophobicity, -0.4) shifted the Ki to ∼3.5 mM. Likewise, neither glycine nor leucine is able to form hydrogen bonds, yet the Ki values for Kir6.2ΔC26 channels containing these residues at position 50 differed by ∼10-fold. Furthermore, mutation of R50 to leucine resulted in a Ki for ATP inhibition similar to that produced by substitution of residues capable of hydrogen bonding (such as glutamate, glutamine, lysine and asparagine). The property that correlated most closely with ATP sensitivity was the size of the side chain of the amino acid at position 50. Residues with small side chains (like glycine, alanine and serine) were associated with lower ATP sensitivity than those with large side chains (arginine, glutamine). Because ATP sensitivity is not correlated with the ability of the amino acid side chain to form chemical interactions it seems unlikely that the arginine side chain interacts directly with ATP. One possible explanation is that the presence of a small residue at position 50 induces a conformational change in the N-terminus that influences either binding of ATP to a site elsewhere or the mechanism by which ATP binding causes channel closure.
Single-channel currents
One mechanism by which a mutation may indirectly alter the channel ATP sensitivity is by impairing the ability of the channel to close (Shyng et al. 1997; Tucker et al. 1998). We therefore compared the kinetic properties of single-channel wtKir6.2ΔC26 currents with those of the two most ATP-insensitive mutations: R50G and R50A (Fig. 4). The single-channel current, measured at -60 mV, was 4.2 ± 0.3 pA (n= 3) for Kir6.2ΔC26, 4.1 ± 0.1 pA (n= 3) for Kir6.2ΔC26-R50A and 4.3 ± 0.4 pA (n= 3) for Kir6.2ΔC26-R50G. As mutation of R50 does not modify the single-channel current amplitude, nor the extent of rectification of the macroscopic currents (see Fig. 2A), this residue probably does not contribute functionally to the pore. The single-channel kinetics were also unaffected by mutation of R50 (Fig. 4 and Table 2). This argues that the reduced ATP sensitivity is not a consequence of an impaired ability of the channel to close, and thus that the mutation is likely to interfere either with ATP binding or with the mechanism by which binding is linked to channel closure.
Figure 4. Single-channel currents for wild-type and mutant Kir6.2ΔC26 channels.
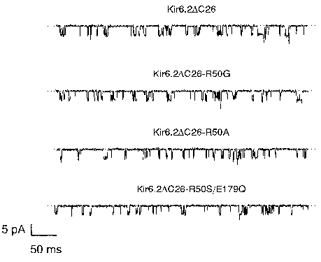
Single-channel currents recorded at -60 mV from an inside-out patch excised from an oocyte injected with mRNA encoding wtKir6.2ΔC26, Kir6.2ΔC26-R50G, Kir6.2ΔC26-R50A or Kir6.2ΔC26-R50S/E179Q. A kinetic analysis of these currents is given in Table 2.
Table 2.
Comparison of wild-type and mutant Kir6.2ΔC26 single-channel kinetics
| Clone (n= 3) | Po | τo(ms) | τC1(ms) | τC2(ms) | %C2 | Burst duration(ms) | Openings per burst |
|---|---|---|---|---|---|---|---|
| Kir6.2ΔC26 | 0.11 ± 0.03 | 0.79 ± 0.06 | 0.31 ± 0.03 | 12.6 ± 2.9 | 41 ± 8 | 2.4 ± 0.6 | 2.4 ± 0.3 |
| Kir6.2ΔC26-R50G | 0.14 ± 0.07 | 0.99 ± 0.09 | 0.32 ± 0.01 | 9.1 ± 3.1 | 36 ± 3 | 2.6 ± 0.5 | 2.1 ± 0.4 |
| Kir6.2ΔC26-R50A | 0.09 ± 0.02 | 0.71 ± 0.04 | 0.29 ± 0.01 | 17.1 ± 4.7 | 44 ± 7 | 2.8 ± 0.6 | 2.6 ± 0.4 |
| Kir6.2ΔC26-R50S/E179Q | 0.08 ± 0.02 | 0.75 ± 0.0 | 0.28 ± 0.01 | 9.4 ± 1.9 | 50 ± 2 | 2.7 ± 0.3 | 2.8 ± 0.3 |
| Kir6.2ΔC26-I49G | 0.13 ± 0.04 | 0.85 ± 0.07 | 0.29 ± 0.03 | 7.3 ± 1.8 | 32 ± 4 | 2.9 ± 0.2 | 2.7 ± 0.2 |
Kinetic parameters were measured at −60 mV, as described in Trapp et al. (1978). Channel openings are grouped into bursts of rapid openings and closings separated by long closings. Po is the channel open probability, τo the mean open time, τC1 the mean short closed time, τC2 the mean long closed time, %C2 the number of long closed times as a percentage of the total closed times. Burst duration, the duration of a burst of openings. The number of patches analysed was 3 in each case.
The open probability and single-channel kinetics of Kir6.2 are influenced by the presence of the sulphonylurea receptor (Proks & Ashcroft, 1997). This explains why the single-channel kinetics we observed are similar to those previously found for Kir6.2ΔC26, but differ from those reported for Kir6.2/SUR1 (Proks & Ashcroft, 1997), and for native β-cell KATP (Ashcroft & Rorsman, 1989) currents in having slightly shorter open times and long closed times of greater duration. The mean short closed times were similar.
Effects of mutations at positions adjacent to R50
We next examined the effect of mutating the residues adjacent to R50. There was no change in ATP sensitivity when the glutamate at position 51 was mutated to glycine (E51G; Fig. 3). Mutation of Q52 (to alanine) was also without effect (Tucker et al. 1998). However when the isoleucine at position 49 was changed to glycine, ATP produced significantly less inhibition (I49G; Figs 3 and 5). As this mutant did not produce large enough currents to measure the macroscopic current ATP dose-response curve, we measured the effect of ATP on the open probability of the single-channel currents at -60 mV (Fig. 5). These data were used to estimate the Ki for inhibition of the channel by ATP using the Hill equation, and gave a value of 5.4 ± 1.9 mM (n= 6). The reduced ATP sensitivity of the I49G mutant channel was not associated with a change in the single-channel kinetics (Fig. 5 and Table 2), suggesting that this mutation impairs either ATP binding or the mechanism by which binding is linked to channel closure. No marked effect on the channel ATP sensitivity was observed when either K47 or H46, or other N-terminal residues more distantly located from R50, were mutated (Tucker et al. 1998). Thus it appears that, within the N-terminus, R50 and I49 are of particular importance for the ATP sensitivity of Kir6.2ΔC26.
Figure 5. Single-channel currents for wild-type and mutant Kir6.2ΔC26 channels.
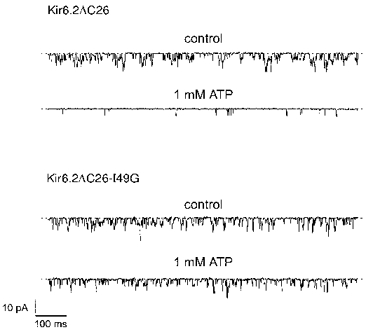
Single-channel currents recorded at -60 mV from an inside-out patch excised from an oocyte injected with mRNA encoding wtKir6.2ΔC26 or Kir6.2ΔC26-I49G, in the absence and presence of ATP as indicated. A kinetic analysis of these currents is given in Table 2.
Effects of combining mutations in the N- and C-termini
We have shown elsewhere that mutations in the C-terminus can markedly decrease the sensitivity of the channel to ATP (e.g. K185Q; Tucker et al. 1997, 1998). Thus mutations that affect the channel ATP sensitivity may occur in either the N- or C-terminus. One possible explanation for these results is that the N- and C-termini of Kir6.2 interact, and that this interaction is critical for the inhibitory effect of ATP. We therefore tested the effect of combining mutations in both the N- and C-termini of Kir6.2ΔC26. We selected two mutations that individually shift the ATP sensitivity by a small amount without affecting the channel kinetics: R50S in the C-terminus and E179Q in the N-terminus (Table 1; Tucker et al. 1998). When these two mutations were combined (R50S/E179Q), the single-channel conductance (3.9 ± 0.1 pA, n= 3) and kinetics (Fig. 4 and Table 2) were not significantly different from those of wtKir6.2ΔC26. However, the ATP sensitivity was further reduced (Fig. 6 and Table 1). The Ki for channel inhibition was ∼3 mM for the double mutant compared with ∼800 μm for R50S and ∼300 μm for E179Q (Table 1). The Hill coefficient was also increased, from 1.0 ± 0.04 (n= 7) for wtKir6.2ΔC26 to 1.6 ± 0.2 (n= 5) for the channel containing the double mutation. These effects are consistent with the idea that both the N- and the C-terminus are involved in channel inhibition by ATP.
Figure 6. Effects of mutations in both the N- and C-termini of Kir6.2ΔC26.
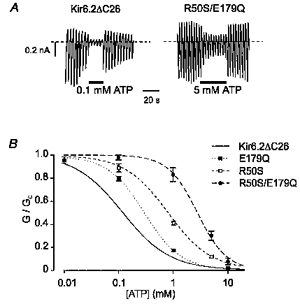
A, macroscopic wtKir6.2ΔC26 or Kir6.2ΔC26-R50S/E179Q currents recorded in response to a series of voltage ramps from -110 to +100 mV. ATP was added to the intracellular solution as indicated. B, mean ATP dose-response relationships for wtKir6.2ΔC26 currents (continuous line; same data as in Fig. 1), Kir6.2ΔC26-R50S currents (n= 5), Kir6.2ΔC26-E179Q currents (n= 7) and Kir6.2ΔC26-R50S/E179Q currents (n= 5). The slope conductance (G) is expressed as a fraction of the mean (Gc) of that obtained in control solution before and after exposure to ATP. The lines are the best fit of the data to the Hill equation using the mean values for Ki and h given in Table 1.
DISCUSSION
Our results demonstrate that mutation of the arginine residue at position 50 of Kir6.2ΔC26 to glycine, alanine or serine markedly reduces the channel ATP sensitivity. Other mutations produce lesser shifts in ATP sensitivity. None of the mutations altered the single-channel kinetics, suggesting they do not impair ATP sensitivity by interfering with channel gating, as suggested for other mutations in Kir6.2 (Shyng et al. 1997; Tucker et al. 1998). There was no correlation between the channel ATP sensitivity and either the charge, or the hydrophobicity or the hydrogen bonding capability of the side chain of the residue at position 50. However, there was some correlation between the size of the amino acid side chain and ATP sensitivity, larger side chains being associated with greater ATP sensitivity. These data strongly suggest that ATP does not interact directly with the positive charge of the arginine side chain. It is possible, however, that ATP binds to the peptide backbone and that mutation of the side chain influences the orientation of the backbone within the binding pocket. Alternatively, the effect of substitutions at position 50 may be mediated allosterically, by inducing a conformational change that interferes with ATP binding elsewhere in the molecule, or with the mechanism by which ATP binding is transduced into channel closure.
Mutation of the neighbouring residue, I49, to glycine also produced a large reduction in the ATP sensitivity of Kir6.2ΔC26, without affecting the channel kinetics. This suggests that both I49 and R50 may influence ATP sensitivity by a similar mechanism. However, mutation of E51 to glycine did not markedly alter the sensitivity of the channel to inhibition by ATP. We have shown elsewhere mutation of E51 to glutamine produces only a small reduction of ATP sensitivity (Tucker et al. 1998), shifting the Ki to an estimated value of ∼300 μm. Several other mutations in the N-terminus, including N41A, K47N, Q52A and P69R were without marked effect on the ATP sensitivity of Kir6.2ΔC26 (Tucker et al. 1998). These results support the view that, of the residues that lie within the N-terminus that have been tested, R50 and I49 may be uniquely important for the ATP sensitivity of Kir6.2ΔC26.
Mutations within the proximal C-terminus have been shown previously to affect the channel ATP sensitivity (Tucker et al. 1997, 1998). One possibility suggested by our data, therefore, is that R50 interacts with the C-terminus and thereby influences either the conformation of an ATP binding site, or the mechanism by which ATP binding is transduced into closure of the channel pore. This idea is not without precedent. Both N- and C-termini co-operate in the G-protein-mediated activation of the related inwardly rectifying Kir3.0 channel and have been shown to physically associate (Huang et al. 1995, 1997). Likewise, the N- and C-termini of the voltage-gated K+ channel Kv2.1 may interact to influence activation and inactivation (Pascual et al. 1997). Studies of cyclic nucleotide-gated (CNG) channels have also demonstrated that the N- and C-termini physically interact, and that N-terminal mutations may influence the ability of cyclic nucleotides to cause channel activation without affecting their ability to bind to their binding site in the C-terminus (Gordon & Zagotta, 1995; Varnum & Zagotta, 1997). We speculate, therefore, that interaction between the N- and C-termini of Kir6.2 may assist in channel closure in response to ATP, and that an allosteric change produced by the presence of small residues at position 50 may alter this interaction and thereby decrease the ATP sensitivity.
Acknowledgments
We thank The Wellcome Trust and the British Diabetic Association for support. F. M. G. is an Eli-Lilly Research Fellow and S. J. T. is a Wellcome Trust Research Fellow.
References
- Ashcroft FM, Ashcroft SJH. Properties and functions of ATP-sensitive K+ channels. Cellular Signalling. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. 10.1016/0898-6568(90)90048-F. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Progress in Biophysics and Molecular Biology. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Clement JP, IV, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Zagotta WN. Localization of regions affecting an allosteric transition in cyclic nucleotide-gated channels. Neuron. 1995;14:857–864. doi: 10.1016/0896-6273(95)90229-5. 10.1016/0896-6273(95)90229-5. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Ashfield R, Ämmälä C, Ashcroft FM. Properties of cloned ATP-sensitive K+ currents expressed in Xenopus oocytes. The Journal of Physiology. 1997;498:87–98. doi: 10.1113/jphysiol.1997.sp021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-L, Jan YN, Jan LY. Binding of the Gβγ subunit to multiple regions of G protein-gated inwardly rectifying K+ channels. FEBS Letters. 1997;405:291–298. doi: 10.1016/s0014-5793(97)00197-x. 10.1016/S0014-5793(97)00197-X. [DOI] [PubMed] [Google Scholar]
- Huang C-L, Slesinger PA, Casey PJ, Jan YN, Jan LY. Evidence that direct binding of Gβγ to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, IV, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulphonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, IV, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulphonylurea receptors determines the sensitivity of the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. 10.1016/S0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulphonylurea receptor forms with BIR (Kir6.2) a smooth muscle type of ATP-sensitive K+ channel. Journal of Biological Chemistry. 1996;271:24321–24325. doi: 10.1074/jbc.271.40.24321. 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Jackson MB, Wong BC, Morris CE, Lecar H, Christian CN. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophysical Journal. 1983;42:109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Pascual JM, Shieh CC, Kirsch GE, Brown AM. Contribution of the NH2 terminus of Kv2.1 to channel activation. American Journal of Physiology. 1997;273:C1849–1858. doi: 10.1152/ajpcell.1997.273.6.C1849. [DOI] [PubMed] [Google Scholar]
- Proks P, Ashcroft FM. Phentolamine block of KATP channels is mediated by Kir6.2. Proceedings of the National Academy of Sciences of the USA. 1997;94:11716–11720. doi: 10.1073/pnas.94.21.11716. 10.1073/pnas.94.21.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura H, Ämmälä C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel expressed in pancreatic β-cells, brain, heart and skeletal muscle. FEBS Letters. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Ferrigni T, Nichols CG. Control of rectification and gating of KATP channels by the Kir6.2 subunit. Journal of General Physiology. 1997;110:141–153. doi: 10.1085/jgp.110.2.141. 10.1085/jgp.110.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Proks S, Tucker SJ, Ashcroft FM. Molecular analysis of KATP channel gating and implications for channel inhibition by ATP. Journal of General Physiology. 1978;112:333–349. doi: 10.1085/jgp.112.3.333. 10.1085/jgp.112.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Proks P, Trapp S, Ryder TJ, Haug T, Reimann F, Ashcroft FM. Molecular determinants of KATP channel inhibition by ATP. EMBO Journal. 1998;17:3290–3296. doi: 10.1093/emboj/17.12.3290. 10.1093/emboj/17.12.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+-channels in the absence of the sulphonylurea receptor. Nature. 1997;378:179–183. doi: 10.1038/387179a0. 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Varnum MD, Zagotta WN. Interdomain interactions underlying activation of cyclic nucleotide-gated channels. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]


