Abstract
This investigation was to determine the extent to which retinal ‘on’ bipolar cells contribute to the adaptive changes that occur with light, which enable the rod visual system to operate over a wide range of ambient light intensities, and to elucidate the underlying adaptive mechanism.
Whole-cell voltage clamp recordings were obtained from bipolar cells in dark-adapted dogfish retinal slices. Current responses to brief flashes and steps of light were analysed. ‘On’ bipolar cell inward current light responses are mediated by a metabotropic glutamate receptor linked to the control of a cGMP cascade, with cGMP opening cation channels. Outward current responses to light of ‘off’ bipolar cells are mediated by the closure of ionotropic glutamate receptor channels.
When Ca2+ buffer was omitted from the patch pipette solution, ‘on’ bipolar cells rapidly desensitized to steps of light as dim as one rhodopsin molecule bleached per rod per second (1 Rh* s−1), whereas ‘off’ bipolar cells did not desensitize. Responses of ‘on’ bipolar cells to flashes in the presence of dim backgrounds recovered after a delay, but with diminished sensitivity, i.e. the cells adapted.
With the Ca2+ chelator BAPTA in the patch pipette solution, step responses of ‘on’ bipolar cells were sustained and flash responses following steps showed rapid recovery. Buffering Ca2+ in the patch pipette solution to 1 μm prevented desensitization, whereas 50 μm free Ca2+ reduced the ‘on’ bipolar cell flash responses, suppressed inward dark current and decreased input conductance.
We conclude that a major component of adaptation of the visual system is due to a reduction in gain at the rod-‘on’ bipolar cell synapse as a result of Ca2+ loading of the dendrites when their cGMP-gated cation channels open with light.
The rod visual system is extraordinary not only in its great sensitivity in the dark-adapted state, when it is capable of the detection of a few photons, but also in its ability to operate over a range of light intensities about a million times greater than absolute threshold (Aguilar & Stiles, 1954). High sensitivity in the dark-adapted state is achieved by high gain in phototransduction in rods (Pugh & Lamb, 1993) and in synaptic transduction in ‘on’ bipolar cells (Shiells & Falk, 1994), each involving a cGMP cascade linked to rhodopsin or to a metabotropic glutamate receptor (mGluR), respectively. This mGluR has been cloned and identified as mGluR6 (Nakajima et al. 1993). Psychophysical studies have indicated that the visual threshold rises at background light intensities too weak to induce significant adaptation in rod photoreceptors. Thresholds double at backgrounds when only one out of three rods absorb one photon per second, remarkable given that each rod in the human eye contains about 108 rhodopsin molecules (Rushton, 1965). To operate over such a wide range of light intensities there appears to be light- and time-dependent control of gain, or adaptation, in both photoreceptor and ‘on’ bipolar cell cGMP cascades.
Rod photoreceptors are relatively depolarized in the dark, releasing glutamate from their synaptic terminals at a high rate. Glutamate activates the ‘on’ bipolar cell mGluR6, which in turn activates a G-protein and phosphodiesterase (PDE) leading to the hydrolysis of cGMP and thus a reduction in cGMP-activated conductance. The closure of cGMP-activated cation channels results in a relatively hyperpolarized state in the dark. Light hyperpolarizes rods, shutting down glutamate release, so ‘on’ bipolar cells depolarize due to an increase in cGMP-activated conductance (Shiells & Falk, 1990; Nawy & Jahr, 1990; de la Villa et al. 1995). This system inverts the rod photoresponse giving rise to the ‘on’ pathway of the visual system. The hyperpolarizing rod response is conserved on synaptic transmission to ‘off’ bipolar cells since these cells possess non-desensitizing ionotropic glutamate receptors (Shiells & Falk, 1994).
Voltage gain in synaptic transmission from rods to ‘on’ bipolar cells is in the order of 100-fold when fully dark adapted (Ashmore & Falk, 1980). High gain in this system results from the coupling of single mGluRs to the control of a large number of cGMP-activated channels by the biochemical gain inherent in the cGMP cascade and the larger voltage change which results because most of these channels are closed in the dark (Shiells & Falk, 1994). In comparison, the voltage gain in synaptic transmission to ‘off’ bipolar cells is only in the order of 10-fold (Falk, 1989). The problem arises, however, as to how the ‘on’ system, and hence the rod visual system as a whole, can function over a wide range of light intensities if the voltage gain in synaptic transmission were maintained at a constant high level. ‘On’ bipolar cell responses would rapidly run into saturation with increasing light intensity. By whole-cell recording from bipolar cells in dark-adapted retinal slices in the absence and presence of Ca2+ chelator in the patch pipette solutions, we now show that the synaptic amplification is reduced with light adaptation by the influx of Ca2+ through their cGMP-activated channels. A preliminary report has been published (Shiells & Falk, 1998).
METHODS
Dogfish, Scyliorhinus canicula, were dark adapted overnight and stunned then decapitated and pithed before removing the eyes under dim red illumination. Whole-cell voltage clamp recordings were obtained from bipolar cells on, or just below the surface of dark-adapted slices prepared from the virtually all-rod retina as previously described (Shiells & Falk, 1990). The slices were continuously superfused with oxygenated Ringer solution at 16-18°C, and were viewed under infrared illumination. The Ringer solution contained (mM): NaCl, 260; KCl, 3; CaCl2, 4; NaHCO3, 20; MgSO4, 0.5; urea, 350; D-glucose, 10; Hepes, 5; buffered to pH 7.7 when bubbled with 95 % O2:5 % CO2. Patch pipettes were coated with a heated mixture of parafilm, mineral oil and wax to improve gigaseal formation, and when filled had resistances of 2-3 MΩ. K+-based patch pipette solutions contained (mM): KCl, 280; MgSO4, 5; Hepes, 10; urea, 350; buffered to pH 7.3, to which was added 1 mM ATP and 1 mM GTP just before the experiment. Cs+-based patch pipette solution replaced KCl with (mM): CsCl, 127; caesium methane sulphonate, 100; and TEA, 20. The Ca2+ chelator BAPTA was added (at 5 mM) to the patch pipette solutions when required. A computer program (MaxChelator) was used to calculate the amount of CaCl2 to add to yield a range of free Ca2+ concentrations, taking into consideration buffering by 1 mM ATP and GTP. At 1 μm free Ca2+ there is good buffering since the BAPTA concentration is very much greater than the free Ca2+ level. However, the buffering at 50 μm free Ca2+ is imperfect but the situation is somewhat improved since the patch pipette volume is very much greater than the cell volume. Following gigaseal formation and subsequent rupture of the membrane patch to establish the whole-cell mode, the dark membrane potential was measured in current clamp. Cells were then voltage-clamped to their dark potential (which was corrected for the tip potential) and responses to steps of light were obtained as soon as possible before there was any change in the intracellular medium, as well as after equilibration. In some experiments retinal slices were stimulated by green test flashes of 20 ms duration from a tungsten lamp positioned below the preparation and this was calibrated by mounting a photodiode in the same position as the preparation. The light absorbed by the rods (rhodopsin molecules bleached per rod (Rh*) per flash) was estimated from previous ‘on’ bipolar cell measurements in the eyecup (Ashmore & Falk, 1980), because of self-screening by the rods since they were illuminated transversely, and the variable thickness of the slice (150-250 μm). The slice preparation is not, however, entirely equivalent to the eyecup in that during slicing rods were sometimes dislodged. Furthermore, the retinal slices were not so completely dark-adapted as the eyecup, especially in the absence of pigment epithelium to regenerate rhodopsin. The light intensity required to give half-maximal ‘on’ bipolar cell flash responses was taken to correspond to one Rh*, the half-saturation intensity (I½) of the voltage responses and the b-wave (Ashmore & Falk, 1980). This estimate was reasonable if we assume that cells in the slice correspond to the lower end of the range of sensitivities obtained in the eyecup, where I½ was about 1 Rh*. Much briefer flashes (0.2 or 2 ms) were applied using green light-emitting diodes (LEDs; peak emission wavelength, 530 nm) in intensity-response measurements. The light emitted by the LED was calibrated with a photodiode to increase by factors of 2 over a range of 10 light intensities by adjusting 10 variable resistors in series with a battery. For step illumination, another green LED was mounted above the preparation.
RESULTS
Figure 1 illustrates whole-cell voltage clamp recordings obtained from bipolar cells in dark-adapted retinal slices exposed to moderately bright steps of light bleaching 200 Rh* s−1. Traces A and B are from ‘on’ and ‘off’ bipolar cells recorded within 20 min of each other from the same retinal slice under similar dark-adapted conditions. Trace A shows transient inward ‘on’ bipolar cell current responses to test flashes bleaching 2 Rh*, mediated by a rise in cGMP-activated conductance (Shiells & Falk, 1990). Application of the light step for 2 min induced a large transient inward current followed by a rapid decline towards zero current during the step, suggesting the presence of some mechanism acting to decrease cGMP-activated conductance in the presence of light. Following the step exposure, the responses to test flashes were much reduced, then showed some recovery and had faster time courses (upper inset) compared with control. The mean (±s.e.m.) reduction in ‘on’ bipolar cell flash response amplitudes, compared with control at the same time interval after similar adaptive steps, was 65 ± 9 % (n= 5), and showed slow but only partial recovery over the next 20-30 min. Test flashes were not superimposed on these bright steps; experiments of this type are shown later in Fig. 3 against dimmer background illumination.
Figure 1. Desensitization of ‘on’ bipolar cell light responses is blocked by BAPTA.
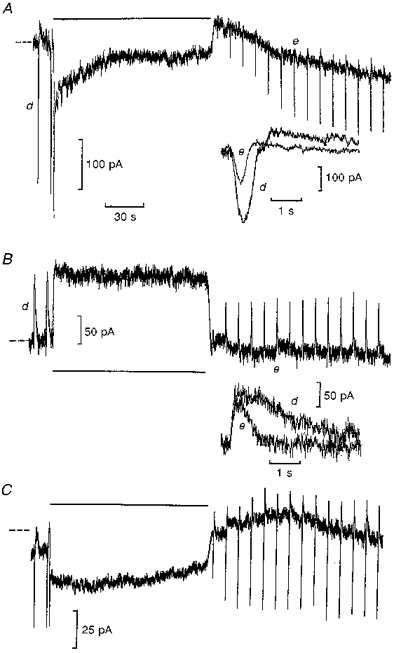
Traces A and B were recorded from ‘on’ and ‘off’ bipolar cells, respectively, from the same retinal slice without BAPTA, whilst C shows an ‘on’ bipolar cell recording with 5 mM BAPTA included in the patch pipette solution (all K+-based). Each cell was voltage clamped at zero current (dashed lines) to their dark potentials (-34, -29 and -28 mV, respectively). Each trace shows current responses (inward ‘on’ and outward ‘off’ bipolar cell responses) to test flashes bleaching 2 rhodopsin molecules per rod (2 Rh*), then light steps bleaching 200 Rh* s−1 were applied for 2 min (indicated by the horizontal bars). Traces A and B begin 30 s after going whole-cell, and the insets show flash responses before (d) and after (e) the steps as indicated on expanded time scales. Trace C, from an ‘on’ bipolar cell recorded in a different retinal slice, begins 11 min after going whole-cell, on full equilibration with the patch pipette solution containing 5 mM BAPTA with no added Ca2+.
Figure 3. Adaptation of ‘on’ bipolar cell flash responses by dim backgrounds.
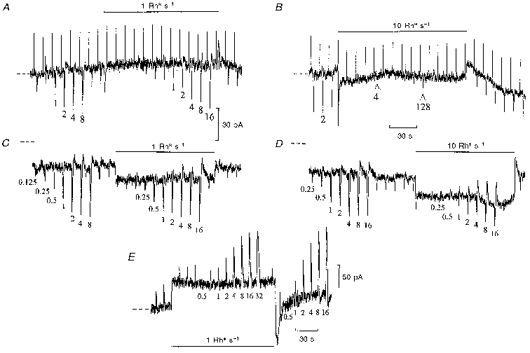
Whole-cell recordings from ‘on’ bipolar cells without (A and B) and with (C and D) 5 mM BAPTA with no added Ca2+ in the patch pipette solution (Cs+-based), voltage clamped to their dark potentials (-27 and -36 mV, respectively) at zero current (dashed lines). The test flash intensities (indicated below the responses) were increased by factors of 2 in darkness and in the presence of backgrounds bleaching 1 and 10 Rh* s−1 as indicated. Records B and D continue without break from A and C, respectively. The upward deflections in panels A and B are current responses to 1 mV voltage command pulses. At the arrow the test flash intensity was increased from 2 to 4 Rh* and then raised by factors of 2 to 128 Rh*. The input conductance remained relatively constant (30 nS) during the recording. Record C begins 10 min after membrane rupture on full equilibration with BAPTA. Input conductance increased from 31 nS in the dark to 36 nS with the step in C and from 28 to 33 nS in D. Inward current shifts from zero level (dashed lines) were induced by BAPTA.E, similar recording from an ‘off’ bipolar cell voltage clamped to its dark potential (-39 mV), with no Ca2+ chelator included in the patch pipette solution. This recording was obtained in the same retinal slice as the ‘on’ bipolar cell illustrated in A and B.
The ‘off’ bipolar cell (Fig. 1B) responded to light steps with only sustained outward currents, due to the closure of non-desensitizing ionotropic glutamate receptor-gated channels (Shiells & Falk, 1994). At light offset there was a small transient inward rebound and then flash responses recovered rapidly but were also briefer in duration (lower inset). The mean reduction in ‘off’ bipolar cell flash responses was by only 22 ± 7 % (n= 5) following similar bleaches. The speeding up of flash responses following the step would indicate some light adaptation occurring in rods (Baylor et al. 1979). However, the observation that ‘on’ bipolar cell responses were reduced by a much greater extent, and for a longer duration, than those of the ‘off’ bipolar cells suggests that the ‘on’ bipolar cell possesses an additional adaptive mechanism to that occurring in rods.
The rapid decline of ‘on’ bipolar cell step responses and adaptation of flash responses could be blocked by increasing the effective Ca2+ buffering of the cell by inclusion of the Ca2+ chelator BAPTA in the patch pipette solution. Figure 1C shows a whole-cell recording from an ‘on’ bipolar cell on full equilibration with the patch pipette solution containing BAPTA with no added Ca2+. Step responses showing rapid declines were recorded just after going whole-cell, then on full equilibration with BAPTA there was a small inward shift (25 ± 5 pA (n= 5)) in the dark current from zero level (dashed lines). The initial rapid transient of the response to the light step was followed by a sustained inward current. Following the light step, flash responses recovered to control amplitude within 30 s, then increased to 125 % of control amplitude over the next minute (an increase was observed in 3 out of 5 cells). Outward current rebounds were observed on the flash responses with BAPTA and were more pronounced following the step exposure. These results suggest that the entry of Ca2+, probably via the cGMP-activated channels which open with light, acts to desensitize ‘on’ bipolar cell light responses, since rapid buffering of the Ca2+ influx by BAPTA (τ= 17 ms) blocked the rapid decline of the sustained component of the step response and desensitization of subsequent flash responses.
To determine the concentration of free Ca2+ required to induce desensitization of ‘on’ bipolar cell light responses, whole-cell recordings were obtained with the free Ca2+ in the patch pipette solution buffered with BAPTA to concentrations of 1 and 50 μm (Fig. 2). With 1 μm free Ca2+ there was little change in the dark current, peak flash responses or membrane conductance over the duration of 15 min recording (B) (n= 3). In contrast, with free Ca2+ at 50 μm (A) a gradual decrease in inward dark current (29 ± 6 pA (n= 4)) and input conductance (32 ± 5 to 15 ± 3 nS (n= 4)) was observed, which was accompanied by a decrease in light responses. The slow time course of response suppression was probably due to the time taken for equilibration of the dendrites with the patch pipette solution. The inset shows scaled peak light responses before (c) and after (d) equilibration with 50 μm free Ca2+. There was no significant change in their time course, distinctly different from photoreceptor responses, which speed up with light adaptation (Baylor et al. 1979). The absence of any effect with 1 μm Ca2+ suggests that this concentration may be close to the dark level, as is the case in photoreceptors (Matthews et al. 1985). Higher concentrations of free Ca2+ (> 100 μm) induced a more rapid and complete block of light responses, whilst some reduction was observed with lower concentrations of 10-20 μm.
Figure 2. Raising intracellular free Ca2+ desensitizes ‘on’ bipolar cell light responses.
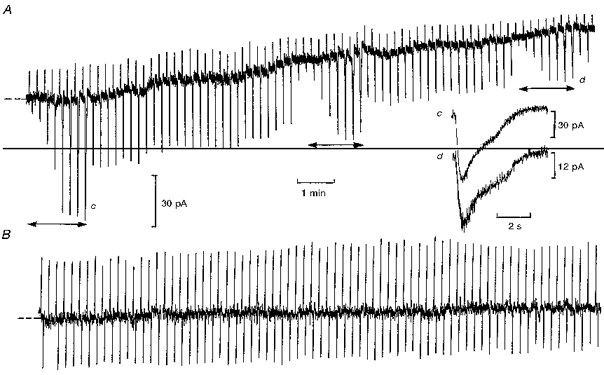
Whole-cell recordings from ‘on’ bipolar cells with 50 μm (A) and 1 μm (B) free Ca2+ in the patch pipette solution (Cs+-based) containing 5 mM BAPTA. The records begin 30 s after going whole-cell, with the cells voltage clamped to their dark potentials (-29 mV (A) and -26 mV (B)). Inward current responses were elicited by 1 Rh* test flashes (A) and 2 Rh* test flashes (B). In A, the intensity-response relation was determined at intervals (horizontal arrows) by applying light flashes which increased by factors of 2 from 0.125 to 16 Rh*. The upward deflections are current responses to 0.5 mV voltage command pulses. There was a decrease in input conductance from 43 to 14 nS as Ca2+ diffused into the cell, which was accompanied by an outward current of 35 pA from the initial dark level. The inset shows the time course of peak flash responses before (c) and after (d) equilibration with 50 μm Ca2+, as indicated.
A well-known characteristic of background light adaptation is the recovery of responses, but with a lower sensitivity, after a period of marked desensitization which may last tens of minutes, especially at low temperatures (Dowling & Ripps, 1970; Green et al. 1975). To demonstrate light adaptation occurring in ‘on’ bipolar cells independently from rods, flash responses were superimposed on very dim backgrounds (1 Rh* s−1), too dim to induce significant desensitization of rods, in the absence of Ca2+ chelator in the patch pipette solution (Fig. 3A). The intensity-response relation was measured in darkness and during the application of the dim background step. At step onset, there was a small inward current transient, after which the membrane current returned close to zero. Test flash responses were suppressed for 30 s by the dim background, then recovered to about half their initial amplitude, indicating a time-dependent recovery from desensitization. On increasing the test flash intensity, the same inward current response could be obtained at twice the light intensities as that elicited in darkness, which was suggestive of true adaptation. At step offset there was a small outward transient followed by recovery of flash response sensitivity to control. With the ten times brighter background (Fig. 3B), only very small responses could be obtained on increasing the test flash intensities to much higher levels. There was a longer lasting and more profound desensitization, especially at higher step intensities (Fig. 1A), with slow recovery which was difficult to follow over tens of minutes. The slow time course of recovery of ‘on’ bipolar cell flash responses was similar to the recovery of the b-wave, a reflection of ‘on’ bipolar cell activity (Falk & Shiells, 1995) recorded from skate retina following bright adapting steps (Dowling & Ripps, 1970).
When the same experiment was repeated on an ‘on’ bipolar cell equilibrated with BAPTA with no Ca2+ in the patch pipette solution (Fig. 3C), no desensitization was observed. This cell initially showed desensitization to steps just after membrane rupture, and the influx of BAPTA induced an increase in inward current shown as a displacement from zero current level. The dim light step induced a sustained inward current, and the reduction in maximum flash response amplitude could be accounted for by superposition of flash responses in the dark onto the background shift in current induced by the step. The 0.5 and 1 Rh* flash responses superimposed on the 1 Rh* s−1 step, however, showed no reduction and were thus significantly larger than in the dark when measured from the dark current level (increases were also observed following 200 Rh* s−1 steps in Fig. 1C).
With the brighter 10 Rh* s−1 background (Fig. 3D), BAPTA blocked the much more profound desensitization observed with the same background (B). The flash responses superimposed on the step showed a marked change in waveform with the appearance of large outward current rebounds, also observed in flash responses following bright steps (Fig. 1C) with BAPTA. ‘Off’ bipolar cells showed no initial suppression of the test flash response with dim backgrounds (Fig. 3E), and the reduction in peak amplitude could be accounted for simply by superposition onto the shift in current induced by the background illumination. The reduction in their dim light response amplitude was only by about 10 %, confirming little reduction in rod sensitivity with the 1 Rh* s−1 background.
Figure 4 summarizes averaged intensity-response relations determined in darkness and against backgrounds from ‘on’ bipolar cell responses obtained just after going whole-cell (Fig. 4A, control), on equilibration with 5 mM BAPTA with no added Ca2+ (Fig. 4B), and from ‘off’ bipolar cells (Fig. 4C). The control intensity-response series (Fig. 4A) shows that there is a small shift in the curve to the right induced by the 1 Rh* s−1 background, almost by a factor of two, with a reduction in peak amplitude. With BAPTA (Fig. 4B), the 1 Rh* s−1 background shifts the curve to the left, confirming that there is no reduction in sensitivity induced by the background when the rise in intracellular Ca2+ is suppressed. The dark intensity-response relation obtained from ‘off’ bipolar cells (Fig. 4C) shows that they are less sensitive to light than ‘on’ bipolar cells, and do not exhibit adaptation with the 1 Rh* s−1 backgrounds since the response reduction over the whole range of light intensities by 1 and 10 Rh s−1 backgrounds can be accounted for by superposition onto the background shifts in current.
Figure 4. Intensity-peak response relations from bipolar cells (modulus of current, pA) in the dark and against dim backgrounds shown on a logarithmic intensity axis to base 2.
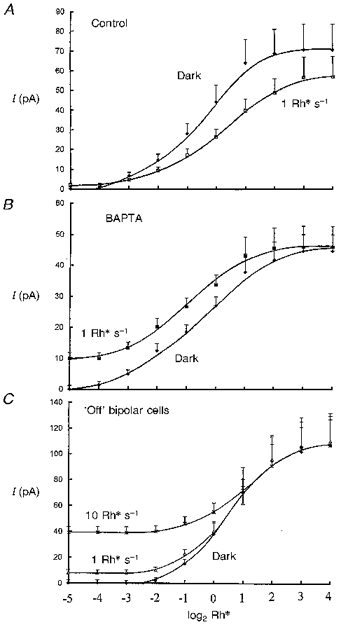
Flash responses were measured in the dark and against backgrounds from the dark current level to their peak amplitudes, so that superposition of responses could be tested. Each 1 Rh* s−1 or 10 Rh* s−1 intensity-response series was compared to its dark series (as in Fig. 3) determined immediately before or after the steps and these were averaged (+s.e.m. shown). Panel A shows control data, fitted by eye (12 intensity-response runs) from 12 ‘on’ bipolar cells, obtained just after going whole-cell in the dark and in the presence of 1 Rh* s−1 backgrounds. The 1 Rh* s−1 curve shows a small shift to the right with a reduction in peak response amplitude. Panel B shows similar data (7 runs) from 5 ‘on’ bipolar cells on full equilibration with 5 mM BAPTA with no added Ca2+, each obtained at least 10 min after going whole-cell. The 1 Rh* s−1 curve is shifted to the left, with no reduction in peak response amplitude. Panel C shows data (5 runs) from 5 ‘off’ bipolar cells, obtained just after going whole-cell in the presence of 1 Rh* s−1 and 10 Rh* s−1 backgrounds. These responses superimpose on the shifts in currents induced by the backgrounds.
DISCUSSION
The results show clear dependence of ‘on’ bipolar cell light responses on the degree of intracellular Ca2+ buffering. Desensitization of light responses was blocked by the inclusion of BAPTA in the patch pipette solutions. Consistent with these results, isolated ‘on’ bipolar cell current responses did not desensitize to concentration jumps of glutamate when EGTA was used as the intracellular Ca2+ buffer (Shiells & Falk, 1994). ‘Off’ bipolar cells gave sustained responses to steps of glutamate and to steps of light, which produced profound desensitization of ‘on’ bipolar cells. Consistent with desensitization occurring in ‘on’ bipolar cell but not ‘off’ bipolar cell inputs, adaptation with dim light was observed in ‘on’ ganglion cells of frog and cat retina but not in ‘off’ ganglion cells (Enroth-Cugell & Shapley, 1973; Donner et al. 1991). The results suggest that during light adaptation ‘on’ bipolar cell dendrites receive a Ca2+ load due to the opening of cGMP-activated channels, whose conductance is not reduced by external Ca2+ (Shiells & Falk, 1992). Relatively high concentrations of Ca2+ (10-50 μm) were required to reduce their light responses when introduced via the patch pipettes. Recent direct measurements of the free Ca2+ rise by fluorescence imaging of initially dark-adapted carp retinal slices have confirmed a large increase in free Ca2+ with light in the outer plexiform layer (Yamada et al. 1998). This rise was suppressed by 2-amino-4-phophonobutyrate (APB), a selective agonist for the ‘on’ bipolar cell mGluR6 (Shiells et al. 1981). A rise in Ca2+ of this order highly localized to ‘on’ bipolar cell dendrites is likely, given the locus of cGMP-activated channels and the components of the cGMP cascade.
The decrease in input conductance accompanying the suppression of inward dark current and light responses with elevated free Ca2+ suggests that there may be a direct action on the channels. We now have evidence for voltage-dependent block of ‘on’ bipolar cell cGMP-activated channels by intracellular Ca2+ (R. A. Shiells & G. Falk, in preparation). Furthermore, inhibitors of Ca2+-activated protein kinase C were found to block the desensitization induced by 50 μm Ca2+ and induced increases in flash response amplitudes against dim backgrounds (Shiells & Falk, 1999). A possible target of protein kinase C action is mGluR6, which possesses consensus phosphorylation sites (Nakajima et al. 1993). BAPTA also induced increases in dark current, indicating an increase in GMP-activated conductance mediated by a fall in intracellular Ca2+. Inward current shifts and rebounds on flash responses have also been observed in rods on equilibration with BAPTA (Matthews et al. 1985), reflecting similarities between the mGluR6-linked system expressed in ‘on’ bipolar cells and phototransduction.
The ‘on’ bipolar cell response in dark-adapted retina normally contains higher frequency components than that of photoreceptors (Ashmore & Falk, 1982), but there appears to be no further contribution to increased time resolution by the ‘on’ bipolar cell when desensitized by increased Ca2+ levels or by dim background light. The improvement in time resolution with backgrounds observed at the ganglion cell level, or psychophysically, appears to reside in the photoreceptors.
The question therefore arises as to where light adaptation of the visual system occurs. The rise in increment threshold of ‘on’ ganglion cells in cat retina by background light was orders of magnitude greater than that displayed by photoreceptors (Sakmann & Filion, 1972). This is paralleled by a 3000-fold elevation in human visual threshold following light adaptation of the rod system, whilst at the same time the reduction in gain in phototransduction is only some 5-fold (Thomas & Lamb, 1998). The b-wave of the electroretinogram (ERG), which derives from the population of ‘on’ bipolar cell responses (Falk & Shiells, 1995), displays the same shift of adaptive characteristics as ‘on’ ganglion cells (Dowling & Ripps, 1977) and human subjective visual changes. These observations have given rise to the concept of adaptation of the rod pool (Rushton, 1965) or network adaptation due to the release of some ‘desensitizing substance’, possibly K+, accumulating in the inner layer of the retina (Dowling & Ripps, 1977). However, there is no evidence for a rise in K+ of the correct magnitude in the inner retina.
The results presented here point to a light-induced rise in intracellular Ca2+ in ‘on’ bipolar cells as the ‘desensitizing substance’. A Ca2+-induced reduction in the voltage gain of synaptic transmission could be achieved by reducing the biochemical gain of the ‘on’ bipolar cell cGMP cascade, which would effectively couple mGluRs to the control of a smaller number of cGMP-activated channels (Shiells & Falk, 1994). Ca2+-dependent reduction in the cGMP-gated conductance would also reduce voltage gain. Thus there are two gain control systems in series in the retina: a high-gain control at the level of ‘on’ bipolar cells where rod signals are pooled, and a lower-sensitivity system in rods. Together, they give rise to the wide operating range of the scotopic visual system (Fain, 1977; Baylor 1987).
Acknowledgments
We would like to thank The Wellcome Trust for financial support and Professor Masahiro Yamada and Mr Hajime Hirasawa for participation in some experiments and technical advice.
References
- Aguilar M, Stiles WS. Saturation of the rod mechanism of the retina at high levels of stimulation. Optica Acta. 1954;1:59–65. [Google Scholar]
- Ashmore JF, Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. The Journal of Physiology. 1980;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF, Falk G. An analysis of voltage noise in rod bipolar cells of the dogfish retina. The Journal of Physiology. 1982;332:273–297. doi: 10.1113/jphysiol.1982.sp014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA. Photoreceptor signals and vision. Investigative Ophthalmology and Visual Science. 1987;28:34–49. [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau K-Y. The membrane current of single rod outer segments. The Journal of Physiology. 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- de la Villa P, Kurahashi T, Kaneko A. L-Glutamate-induced responses and cGMP-activated channels in three subtypes of retinal bipolar cells dissociated from the cat. Journal of Neuroscience. 1995;15:3571–3582. doi: 10.1523/JNEUROSCI.15-05-03571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K, Djupsund K, Reuter T, Vaisanen I. Adaptation to light fluctuations in the frog retina. Neuroscience Research. 1991;15:S175–184. [PubMed] [Google Scholar]
- Dowling JE, Ripps H. Visual adaptation in the retina of the skate. Journal of General Physiology. 1970;56:491–520. doi: 10.1085/jgp.56.4.491. 10.1085/jgp.56.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Ripps H. The proximal negative response and visual adaptation in the skate retina. Journal of General Physiology. 1977;69:57–74. doi: 10.1085/jgp.69.1.57. 10.1085/jgp.69.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Shapley RM. Adaptation and dynamics of cat retinal ganglion cells. The Journal of Physiology. 1973;233:271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL. The threshold signal of photoreceptors. In: Barlow HB, Fatt P, editors. Vertebrate Photoreception. Academic Press; 1977. pp. 305–323. [Google Scholar]
- Falk G. Signal transmission from rods to bipolar and horizontal cells: a synthesis. In: Osborne N, Chader J, editors. Progress in Retinal Research. Vol. 8. Pergamon Press; 1989. pp. 255–279. [Google Scholar]
- Falk G, Shiells RA. On-bipolar cells, visual sensitivity and the b-wave. In: Robbins JG, Djamgoz MBA, Taylor A, editors. Basic and Clinical Perspectives in Vision Research. New York: Plenum Press; 1995. pp. 95–102. [Google Scholar]
- Green DG, Dowling JE, Siegel IM, Ripps H. Retinal mechanisms of visual adaptation in the skate. Journal of General Physiology. 1975;65:483–502. doi: 10.1085/jgp.65.4.483. 10.1085/jgp.65.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR, Torre V, Lamb TD. Effects on the photoresponse of calcium buffers and cGMP incorporated into the cytoplasm of retinal rods. Nature. 1985;313:582–585. doi: 10.1038/313582a0. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. Journal of Biological Chemistry. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochimica et Biophysica Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Rushton WAH. Visual adaptation. Proceedings of the Royal Society B. 1965;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Filion M. Light adaptation of the late receptor potential in the cat retina. In: Arden GB, editor. Advances in Experimental Medicine and Biology, The Visual System. Vol. 24. Plenum Press; 1972. pp. 87–93. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proceedings of the Royal Society B. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Properties of the cGMP-activated channel of retinal on-bipolar cells. Proceedings of the Royal Society B. 1992;247:21–25. doi: 10.1098/rspb.1992.0004. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Responses of rod bipolar cells isolated from dogfish retinal slices to concentration-jumps of glutamate. Visual Neuroscience. 1994;11:1175–1183. doi: 10.1017/s0952523800006970. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. A rise in intracellular Ca2+ underlies light adaptation in dogfish retinal On-bipolar cells. The Journal of Physiology. 1998;509.P:54P. doi: 10.1111/j.1469-7793.1999.343ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Ca2+ activation of protein kinase C (PKC) initiates light adaptation in dogfish retinal ON-bipolar cells. The Journal of Physiology. 1999;515.P:34P. doi: 10.1111/j.1469-7793.1999.343ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G, Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981;294:592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Thomas MM, Lamb TD. Kinetics of post-bleach recovery of maximal response and amplification constant in human rod photoreceptors. The Journal of Physiology. 1998;504.P:30P. [Google Scholar]
- Yamada M, Sasa T, Hirasawa H, Shiells RA. Light stimulates an increase in intracellular Ca2+ in carp retinal On-bipolar cells. The Journal of Physiology. 1998;509.P:66P. [Google Scholar]


