Abstract
Previous anatomical studies indicate that the nucleus of the solitary tract, pars centralis (NSTc) contains the neurones which receive vagal afferent input from the oesophagus. The purpose of the present study was to characterize the NSTc circuits in the medulla that may be responsible for oesophageal control of gastric motility.
Moderate balloon distension of the oesophagus of the rat (14–18 mmHg) provoked a significant reduction in gastric motility and tone recorded with strain gauges. This receptive relaxation effect was eliminated by bilateral lesions centred on the NSTc.
NSTc cells activated by oesophageal distension were labelled extracellularly and juxtacellularly with neurobiotin. NSTc neurones send axonal projections throughout the entire rostral-caudal extent of the dorsal motor nucleus of the vagus (DMN). These NSTc-DMN connections were confirmed by retrograde transport of neurobiotin from DMN to NSTc. NSTc neurones were observed with dendrites arborizing within the ependymal lining of the fourth ventricles. Thus, NSTc neurones may be in position to monitor blood-borne or ventricular agents and to alter the function of gastric-vago-vagal reflexes in response to these stimuli.
Neurophysiological recordings identified two subpopulations of DMN neurones which may be either activated or inhibited by oesophageal distension. Neurones excited by oesophageal distension were located mainly lateral and caudal in the DMN; neurones inhibited by oesophageal stimulation were located in medial and rostral DMN.
Our neurobiotin tracing results verified earlier studies showing that the NSTc projects to the intermediate reticular nucleus and the compact division of the nucleus ambiguus. Additionally, we found that the NSTc may be involved in reciprocal connections with the anterior, rostrolateral NST.
These results suggest that the gastric relaxation evoked by oesophageal distension is critically dependent on intact brainstem vago-vagal circuits. The NSTc, the recipient of oesophageal afferent projections from the vagus nerve, sends axons to the entire DMN, the source of parasympathetic control of the stomach. DMN neurones respond differentially to oesophageal distension, reinforcing the view that oesophageal afferents may provoke gastric relaxation by activating a vagal inhibitory pathway while simultaneously inhibiting a vagal excitatory pathway.
It has long been known that the oesophageal distension produced by swallowing elicits a powerful proximal gastric relaxation (Cannon & Leib, 1911). This reflex was termed the ‘receptive relaxation reflex’ and is an important mechanism which increases gastric volume and reduces intragastric pressure to ensure that swallowed food is efficiently transported to the stomach. Results from several laboratories (Abrahamsson & Jansson, 1969; Jansson, 1969; Miolan & Roman, 1984; Sengupta et al. 1989) have shown that this potent gastroinhibition results from stimulation of low threshold mechanoreceptive afferents of the vagus nerve, and that the reflex requires intact vagal connections between the brainstem, oesophagus and stomach. Recent tract-tracing techniques have shown that vagal afferents from the upper alimentary canal, including the oesophagus, are viscerotopically represented within the nucleus of the solitary tract. In particular, oesophageal vagal afferents were found to terminate specifically within the central subnucleus of the solitary tract (NSTc) (Fryscak et al. 1984; Bieger & Hopkins, 1987; Altschuler et al. 1989; Cunningham & Sawchenko, 1989).
Studies of the anatomical basis for the regulation of deglutition have shown a dense projection from the NSTc to the compact formation of the nucleus ambiguus (NA), the site containing vagal motor neurones projecting to the oesophagus and lower oesphageal sphincter (Doty, 1968; Jean, 1984; Bieger & Hopkins, 1987; Altschuler et al. 1989; Cunningham & Sawchenko, 1989). This visceral sensory-motor connection may utilize a number of different neurotransmitters including nitric oxide (Weidner et al. 1995; Krowicki et al. 1997), somatostatin (Cunningham & Sawchenko, 1989) and leu-enkephalin (Milner et al. 1995) to control different functional aspects of oesophageal and oesophageal sphincter function (Bieger & Hopkins, 1987; Bieger, 1993; Lu & Bieger, 1998). However, the relationship between the NSTc and the vagal motor neurones which regulate gastric functions has not been specifically described.
Vagal control over gastric motility is the result of a complex interplay between two competing and antagonistic efferent projections. Vagal augmentation of gastric motility and intramural pressure is mediated by the dorsal motor nucleus of the vagus (DMN) preganglionic parasympathetic neurones, which activate cholinergic enteric neurones (Gillis et al. 1989; Rogers et al. 1996). Neurophysiological data suggest that this excitatory efferent vagal innervation of the stomach is maintained by neurones contained primarily within the anterior and medial three-quarters of the DMN (Gillis et al. 1989; McCann & Rogers, 1994; Fogel et al. 1996). Vagal inhibition of gastric motility can occur as a consequence of the inhibition of this excitatory pathway (Abrahamsson & Jansson, 1969; Gillis et al. 1989; McCann & Rogers, 1994; Fogel et al. 1996; Rogers et al. 1996). Indeed, activation of gastric antral and intestinal mechanoreceptors does produce a brief relaxation of the stomach through such a mechanism (Keinke et al. 1984). However, activation of distension-sensitive oesophageal vagal afferents probably produces a much longer lasting and more potent gastroinhibition by also activating a non-adrenergic, non-cholinergic (NANC) inhibitory vagal projection to the stomach (Jansson, 1969; Rogers et al. 1996; Takahashi & Owyang, 1997).
Recent immunocytochemical and physiological studies suggest that the preganglionic vagal cell bodies of origin for this gastrointestinal NANC path may be found in the anterolateral and the extreme caudal division of the DMN (Rinaman et al. 1993; Krowicki et al. 1997; Zheng et al. 1998). Thus, the neural pathways responsible for gastroinhibitory control by the oesophagus should involve a significant projection from oesophageal distension-sensitive neurones in the NSTc to virtually all levels of the DMN. However, investigations to date using ‘blind’ (i.e. not physiologically directed) Phaseolus vulgaris lectoagglutinin tracing methods have revealed a dense input from the NSTc to the NA (Cunningham & Sawchenko, 1989), but only very modest, localized projections to the DMN.
The purpose of this investigation was to examine the brainstem circuits responsible for oesophageal control of gastric motility. We examined these circuits by combining extracellular neurophysiological recording methods with neurobiotin neural tracing techniques in order to reconstruct the brainstem projections of identified oesophageal distension-sensitive neurones.
METHODS
Surgical preparation
Eighteen male Long-Evans rats (300-550 g) were anaesthetized with urethane (ethyl carbamate, 1.5 g kg−1i.p.; Sigma Chemical Co.). This dose of urethane produced a deep plane of anaesthesia which persisted for 12 h or more as determined by the repeated failure of foot pad pinch to elicit a withdrawal. A tracheal catheter was placed to assist in the maintenance of a clear airway. In addition, a miniature strain gauge was sutured to the exterior of the gastric corpus to record changing gastric motility patterns as described elsewhere (Chen et al. 1997). Animals were then mounted in a stereotaxic frame. An oesophageal distension balloon cannula was placed in the thoracic oesophagus at the point where the cannula tip meets resistance at the diaphragmatic hiatus. The location was verified by direct inspection after the experiment.
Oesophageal distension
Oesophageal distension balloon catheters were constructed from 1.0 mm o.d., 0.5 mm i.d. silicone tubing connected with polyethylene tubing (PE 50) attached to a 3 ml syringe and a Statham P23 pressure transducer. The injection of 1.5-3.0 ml of air into the balloon expanded the stressed portion of the silicone tubing to a final o.d. of approximately 3 mm, an expanded volume of 0.1-0.4 ml, with a transmural oesophageal pressure increase of 14-18 mmHg. This range of distension pressures has been shown to activate the low-threshold vagal mechanoreceptors excited by the peristaltic oesophageal contractions of swallowing and not spinal nociceptors (Sengupta et al. 1989).
The effects of 1 min oesophageal balloon distensions on gastric contractility were then observed. Output from the strain gauge was digitized and stored using a PC-based data analysis system (RC Electronics, Santa Barbara, CA, USA). Motility area indices were generated for 100 s epochs of strain gauge data before and after the 60 s oesophageal distensions. This analysis was performed using the RC Electronics statistical cursor tool for calculating areas (in volt seconds) under the motility curves (Fig. 1).
Figure 1. Oesophageal distension reduces gastric motility.
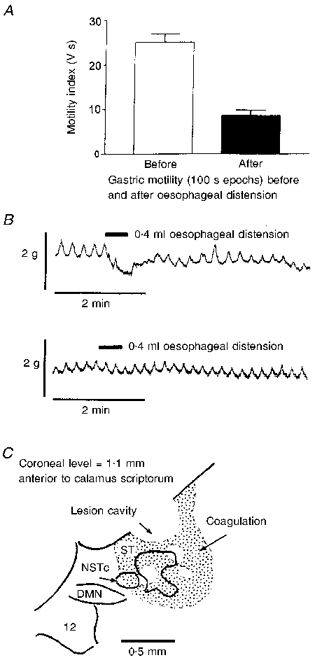
A, gastric motility recorded with an extraluminal strain gauge is significantly reduced (P < 0.0001) during and just after distension of the oesophagus. B, upper trace, receptive relaxation of the gastric corpus elicited by a 0.4 ml (17 mmHg) distension of the thoracic oesophagus with the silicone balloon catheter. Gastric motility was measured with a strain gauge sutured in parallel with the circular smooth muscle of the corpus. This figure demonstrates that the oesophageal stimuli used in the present study can provoke the receptive relaxation reflex. Lower trace, bilateral electrolytic lesions of the NSTc region were performed 30 min prior to oesophageal distension. Here, distension failed to evoke a relaxation response. C, outline drawing of a lesion site in the NSTc. ST, solitary tract; DMN, dorsal motor nucleus of the vagus nerve; 12, hypoglossal nucleus.
In eight cases, electrolytic lesions of the NSTc region were made bilaterally to determine if gastric relaxation effects produced by oesophageal distension were a function of intact brainstem vago-vagal reflex connections. This was done to evaluate the possibility that intramural neural pathways intrinsic to the oesophagus and stomach were responsible for the reflex, rather than a brainstem circuit centred on the NSTc (Schulze-Delriu, 1989). The dorsal surface of the brainstem was exposed as previously described (McCann & Rogers, 1992). With the lesion electrode held at a 26 deg angle from the vertical, the NSTc is located 1.1-1.5 mm anterior to the tip of calamus scriptorum, 0.9-1.1 mm medial to the mid-line and 0.4-0.5 mm ventral to the brainstem surface (Fryscak et al. 1984; Altschuler et al. 1989). Lesion currents of 1 μA (cathodal) were applied for 1 min to a tungsten recording-type microelectrode with an uninsulated tip diameter of 10 μm. These parameters were sufficient to destroy the NSTc. The effect of oesophageal distension on gastric motility was examined 30 min after the NSTc lesions (Fig. 1). At the end of the experiment, the anaesthetized preparations were transcardially perfused with saline followed by paraformaldehyde as described later under Neurobiotin histochemistry. Lesion sites were examined later using routine histological methods.
Extracellular recordings and tracer injection
Thirty-two male Long-Evans rats were anaesthetized, tracheal catheters placed and oesophageal cannulas installed as described above. A single-barrelled micropipette (tip diameter, 1 μm) was filled with 1 % neurobiotin (Vector Laboratories, Burlingame, CA, USA) in a 1 M NaCl solution and placed in a hydraulic microdrive (Kopf, Tujunga, CA, USA). The recording pipette was then directed toward the NSTc as described below. Extracellular electrical signals from the pipette were amplified and band-pass filtered with two AC-coupled amplifiers connected in series (two P15 amplifiers; total gain, 10000; band pass, 300-3000 Hz; Grass Instruments Co.). Amplified neural signals, oesophageal distension pressures and the timing of electrical vagal stimulation pulses (see Identification of DMN neurones, below) were displayed on storage oscilloscopes, and recorded on magnetic tape for subsequent analysis.
Neurophysiological identification of NSTc neurones
The pipette was advanced in 20 μm increments toward the NSTc while the oesophagus was periodically distended with the balloon (0.4 ml maximum inflations, 3-10 s in duration). NSTc neurones were neurophysiologically identified as responsive to oesophageal distension and their pattern of firing was characterized before and after oesophageal distension. In five cases, rats were also equipped with vagal electrodes to verify that NSTc neurones responsive to oesophageal distension were also orthodromically activated by vagal stimulation (0.5 ms, 0.4 mA, 0.5 Hz). Although the identification protocol required only a few minutes, identified NSTc neurones were sometimes recorded for 1 h or more.
In 18 cases, neurobiotin was microiontophoresed extracellularly by applying 200-1000 nA pulsed positive direct current (7 s on, 7 s off) for 10 min in order to provide an overall estimate of the extent of NSTc projections within the brainstem. In five cases, single NSTc neurones were labelled with neurobiotin using the ‘juxtacellular’ filling method described in detail by Pinault (1996). Briefly, NSTc neurones which responded to oesophageal distension were tested for responsiveness to low level (10-20 nA) positive current pulses (250-1000 ms applied at 0.5 Hz). If the cell responded to extracellular current injection, current pulses were applied for 30 min in order to fill the neurone with neurobiotin.
Identification of DMN neurones
In nine cases, DMN neurones were neurophysiologically identified by antidromic vagal stimulation using a vagal cuff electrode as described previously (McCann & Rogers, 1992). The application of twin pulses of current (300 μA, 0.5 ms, 15 ms interpulse delay) to the vagus produced two action potentials 15 ms apart at a constant post-stimulus latency. These identified DMN neurones were then examined for their responsiveness to oesophageal distension as described above. In five of these cases, neurobiotin was iontophoresed into the DMN (0.6-1.0 mm caudal to the tip of the calamus scriptorum) to examine further the possibility that the NSTc maintained direct projections with the caudal portions of the DMN.
Neurobiotin histochemistry
Injected neurobiotin was allowed a 4-12 h transport period. The rat, which remained deeply anaesthetized under the effects of urethane, was then transcardially perfused with saline, followed by 4 % paraformaldehyde in phosphate buffered saline (PBS; 124 mM NaCl, 26 mM NaHCO3, 2 mM KH2PO4 in distilled water, pH adjusted to 7.4). The brainstem was removed to a solution of 4 % paraformaldehyde and 20 % sucrose in PBS for a 6-12 h period. Brainstems were cut into 50-75 μm sections on a freezing microtome; sections were collected into PBS.
Preliminary studies used the standard avidin-peroxidase- diaminobenzidine (DAB)-cobalt chromogen method to demonstrate NSTc fibres and terminals (Rogers & McCann, 1993). Though very fine terminals were visible under the microscope in the wet, freshly mounted tissue, later exposure of the tissue to clearing and counterstaining agents destroyed the labelling of all but the more densely stained fibres. Use of the Vector SG diaminobenzidine replacement reagent (Vector Laboratories) appeared to solve this problem (Fig. 7).
Figure 7. Details of NSTc terminations in the anterior DMN.
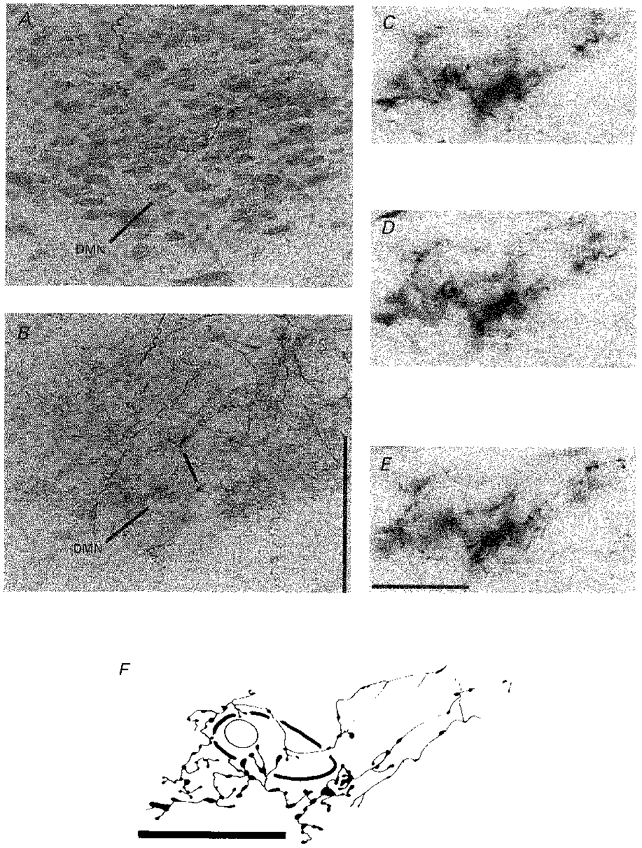
A, counterstained coronal section through the DMN adjacent to section B, DAB used as a peroxidase reactant. Note marked reduction in fine axonal detail compared with B.B, fine, dense plexus of NSTc fibre terminations in the DMN are clearly evident with the Vector SG reagent used as a peroxidase reactant. Asterisk defines the area enlarged in C-E. Scale bar, 200 μm. C-E, a dense cluster of NSTc axons apposed to a single DMN neurone using differential contrast optics. The outlines of the DMN cell body and nucleus are visible along with the neurobiotin-labelled terminal endings in this series of micrographs taken at different planes of section. Scale bar, 20 μm. F, a camera lucida drawing of the neurobiotin-labelled NSTc axons depicted in C-E. Scale bar, 20 μm.
Processed sections were then mounted on gelatin-coated slides and air dried. Once dry, the slides were counterstained with Nuclear Fast Red (Vector), rinsed in water and either air dried again or dehydrated in ascending alcohols. Sections were then cleared in Histoclear (National Diagnostics, Manville, NJ, USA) and a coverslip was secured with Entellan (Electron Microscopy Sciences Co., Fort Washington, PA, USA). Sections were viewed with a Nikon Microphot FXA microscope and images were generated using a Optronics CCD camera system coupled to a Sony video monitor and dye sublimation printer. Camera lucida drawings were made using a Leitz Dialux microscope equipped with a drawing tube.
Statistical analysis
Data are given as means ±s.e.m. and were compared using Student's paired t test or the sign test.
RESULTS
Oesophageal distension reduces gastric motility in the rat
Oesophageal balloon distension produced a significant reduction in gastric motility. The motility area index was 25.1 ± 1.8 V s before oesophageal distension and 8.5 ± 1.2 V s after distension (P < 0.0001, Student's t test for paired data). Gastric tone also declined (1.3 ± 0.4 g) during oesophageal distension (Fig. 1). Ablation of the NSTc region completely abolished the gastric relaxation originally elicited by oesophageal distension (P < 0.05; sign test; Fig. 1).
Oesophageal distension activates NSTc neurones
Oesophageal balloon distension strongly stimulated NST neurones that were later identified histologically as belonging to the NSTc subnucleus. In this study, 36 NSTc neurones were identified in 18 preparations (1 recording per side). In 34 cases, NSTc neurones responding to oesophageal distension were silent when not stimulated but were strongly activated by oesophageal distension. The average firing rate of NSTc neurones during inflation was 18 ± 5.4 spikes s−1 (Fig. 2). In the other two cases, NSTc neurones demonstrated resting activity and were potently stimulated by oesophageal distension. However, after the distension was relieved, these cells were completely inhibited for several seconds. Basal firing gradually recovered (Fig. 2). In five cases (10 recorded neurones), NSTc neurones identified with oesophageal distension were also tested for responsiveness to vagal stimulation. All NSTc neurones so tested were activated orthodromically by vagal stimulation. There was a 5.2 ± 2.2 ms latency for a conduction velocity (assuming a conduction path to the NST of 15 mm) of approximately 3 m s−1 (Fig. 3). This conduction velocity figure is consistent with that reported by Sengupta et al. (1989) for low threshold oesophageal vagal mechanoreceptors.
Figure 2. Recording of NSTc activity during oesophageal distension.
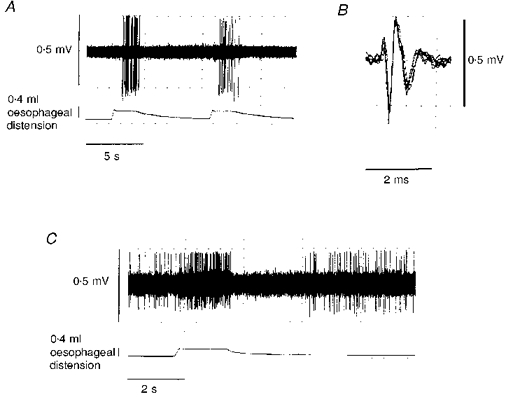
A, NSTc neurone responding to repeated oesophageal distension. Most of the NSTc cells recorded (44/46) responded in this manner. Spike amplitudes are distorted by the slow sampling rate (100 μs per sample per channel) necessary to demonstrate the entire spike train. B, superimposed spike records taken from the period of NSTc activation by oesophageal distension. This recording is made at a faster sampling rate (10 μs per sample) and shows the unitary nature of the NSTc extracellular potential. C, rarely (2/46), NSTc neurones demonstrated an on-off patterned response to oesophageal distension.
Figure 3. NSTc neurones respond to electrical vagal stimulation and oesophageal distension.
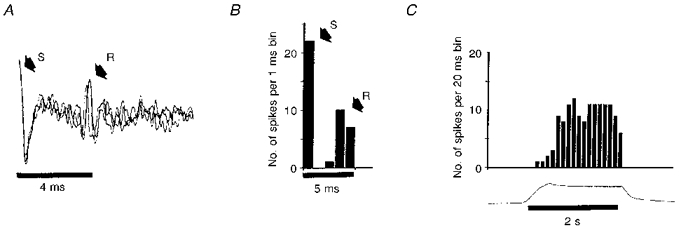
A, superimposed orthodromic NSTc neurone responses to three vagal stimuli. S, stimulus artifact; R, NSTc neurone response. Note slight inconsistency in the neuronal response latency. B, post-stimulus histogram illustrating the average NSTc response to vagal stimulation. S, number of stimulus pulses; R, number of NSTc responses. C, histogram illustrating the responses of the same NSTc neurone to oesophageal distension.
NSTc injection sites and neuronal morphology
All extracellular injection sites (n = 36) were completely contained within the NSTc. The dense core of the largest injection sites (Figs 4 and 5) did not exceed 50 μm in width. An average of 27 ± 3.8 cells were labelled with neurobiotin in the NSTc for each injection site. In many instances, NSTc neurones labelled with extracellular neurobiotin injections were observed with long primary dendrites extending dorsally and/or medially several hundred micrometres. These dendrites often arborized within the ependymal lining of the fourth ventricle (Fig. 5). Single NSTc cells labelled using the method of Pinault (1996) also exhibited long dendritic projections directed toward the fourth ventricle (Fig. 6).
Figure 4. Example of a ‘large’ extracellular neurobiotin injection site within the NSTc.
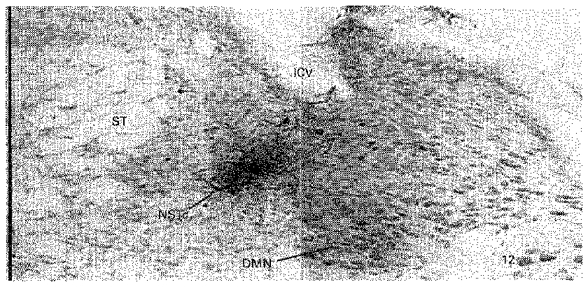
ST, solitary tract; ICV, inferior cerebellar vein; DMN, dorsal motor nucleus of the vagus nerve; 12, hypoglossal nucleus. Scale bar (far left), 0.5 mm.
Figure 5. Camera lucida drawing of the projection pattern obtained from a single extracellular injection of neurobiotin into the NSTc made under physiological guidance.
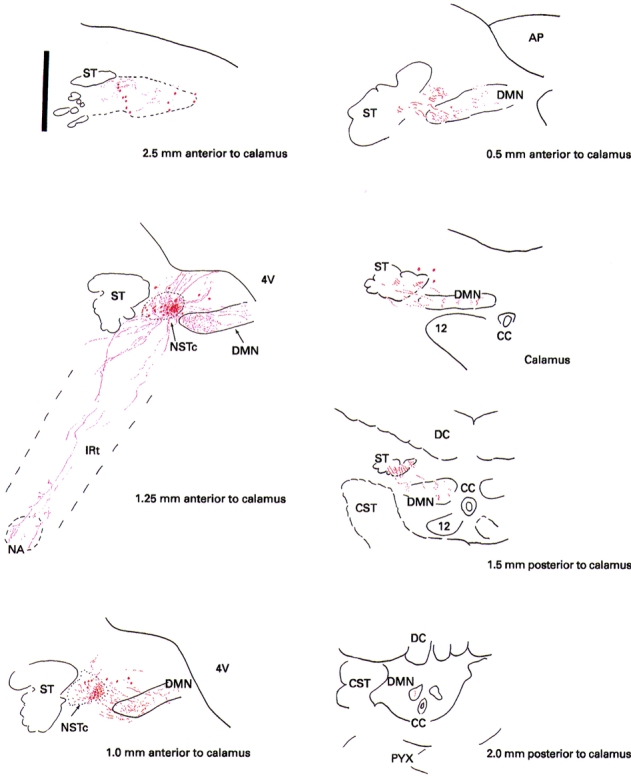
AP, area postrema; CC, central canal; CST, corticospinal tract/decussation; DC, dorsal columns; ST, solitary tract; 4V, fourth ventricle; NSTc, nucleus of the solitary tract, pars centralis; DMN, dorsal motor nucleus; IRt, intermediate reticular nucleus; NA, nucleus ambiguus; PYX, pyramidal tract; 12, hypoglossal nucleus. Scale bar (upper left), 0.5 mm.
Figure 6. Examples of single ‘juxtacellular’ neurobiotin-labelled cells in the NSTc.
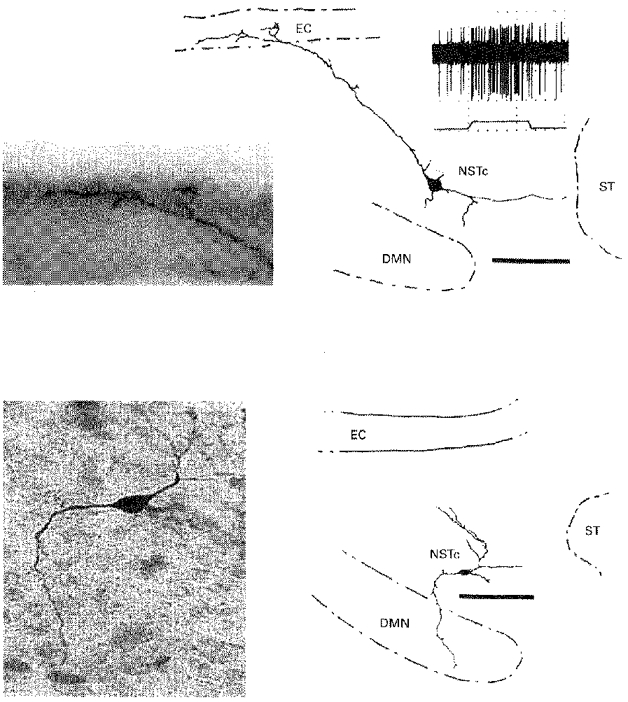
Top panels, neurobiotin-labelled cell, its response to oesophageal stimulation and details of its dendritic arborization in the ependymal layer. Duration of oesophageal distension, 5 s. Bottom panels, single neurobiotin-labelled cell with axon directed toward the DMN. Scale bars, 100 μm. DMN, dorsal motor nucleus of the vagus; EC, ependymal cell layer; NSTc, nucleus of the solitary tract, pars centralis; ST, solitary tract.
NSTc connections with the DMN
Neurones in the NSTc send axonal projections throughout the entire anterior-posterior extent of the DMN (Figs 5, 7 and 8). At anterior levels of the DMN (rostral to the area postrema) NSTc terminals are extremely dense and of a very fine calibre (Figs 5 and 7). Only under very high magnification (1000-fold) can the terminal branches of these NSTc axons in the DMN be resolved (Fig. 7). Terminations are most dense in the lateral division of the nucleus, yet NSTc terminations in the medial DMN are still prominent (Figs 5 and 7). Although terminal NSTc axons diminish in number at more caudal levels of the DMN, scattered NSTc terminals are readily observed in the DMN 1.5-2 mm posterior to the caudal tip of the calamus scriptorum (Figs 5 and 8). NSTc neurones labelled individually also demonstrated axonal projections to the DMN (Fig. 6).
Figure 8. Photomicrograph and matching camera lucida drawing of an NSTc axon traversing the caudal DMN 1.5 mm caudal to calamus scriptorum.
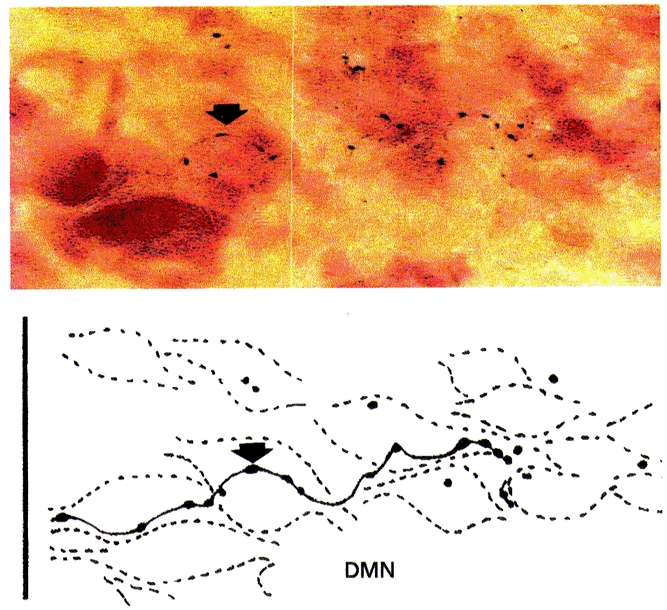
Arrows depict the same axonal swelling. Scale bar, 30 μm
In order to confirm the anatomical connections between NSTc and the caudal DMN, we made small iontophoretic deposits of neurobiotin in the DMN 0.6-1.0 mm caudal to the tip of the calamus. These injections revealed retrograde labelling of the neurones in the NSTc (Fig. 9). On average, 30 ± 10 NSTc cells were labelled as a consequence of each caudal DMN injection of neurobiotin. Scattered cells in the NST, pars medialis (NSTm) (not shown) were also labelled.
Figure 9. Verification that NSTc neurones project to the caudal DMN.
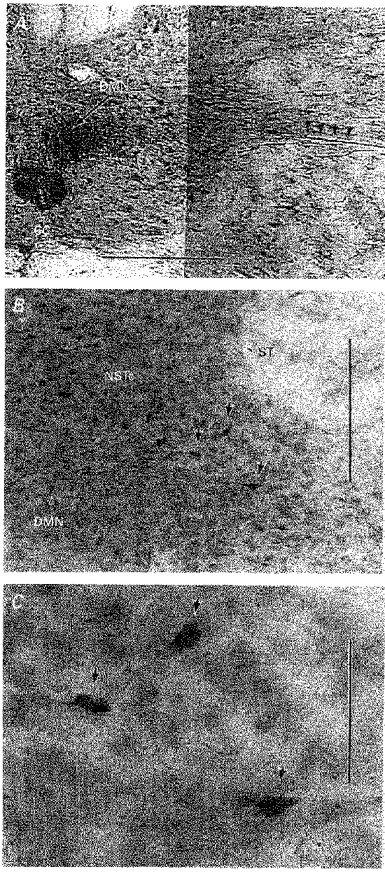
A, neurobiotin injection site in the DMN 1.0 mm caudal to calamus scriptorum. Small arrows point out neurobiotin-labelled axons in the vagal efferent root. Scale bar, 400 μm. B, neurobiotin filled NSTc neurones labelled following injection in A. Scale bar, 100 μm. Arrows point to cells seen in the enlargement. C, enlargement of the area containing labelled NSTc neurons seen in B. Scale bar, 35 μm.
NSTc terminations with the IRt and NA
The NSTc sends branched axonal projections to and through the intermediate reticular nucleus (IRt) en route to the NA (Figs 5 and 10). NSTc projections terminated in a varicose plexus in the anterior compact portion of the NA, i.e. the portion of the NA known to project to the oesophagus (Fryscak et al. 1984; Bieger & Hopkins, 1987; Cunningham & Sawchenko, 1989).
Figure 10. NSTc terminations in NA and IRt and within the NST.
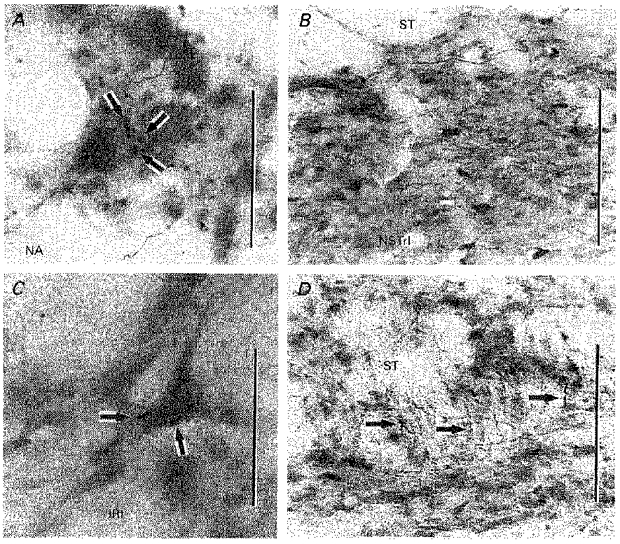
A, NSTc terminals apposed to neurones in the NA. Scale bar, 30 μm. B, neurons in the NSTrl labelled retrogradely following a neurobiotin injection in the NSTc. Scale bar, 100 μm. C, NSTc terminals in apposition to a neurone in the IRt. Scale bar, 30 μm. D, NSTc fibres descend through the brainstem along with the medial solitary tract. Selected fibres depicted by arrows. Scale bar, 75 μm.
Intrasolitary connections with the NSTc
Retrogradely labelled cells were found scattered just medial to the solitary tract in a loose column throughout the rostro-caudal extent of the solitary nucleus (Figs 5 and 10). The concentration of retrogradely labelled cells, as well as anterogradely labelled fibres, was greatest in the anterior rostrolateral NST (NSTrl). A few scattered retrogradely labelled cells were also found in the rostromedial solitary nucleus (NSTrm). In all cases, labelled projections from the NSTc remained ipsilateral to the side of the neurobiotin injection.
Oesophageal distension activates a subpopulation of caudal and lateral DMN neurones and inhibits a subpopulation of rostral and medial DMN neurones
Thirty-four DMN neurones were physiologically identified as responding to oesophageal distension. Recorded neurones demonstrated the typical slow, spontaneous firing rate (∼0.1-10.0 Hz) previously associated with DMN cells (Travagli et al. 1991, 1992; McCann & Rogers, 1992; Livingston & Berger, 1993; Travagli & Gillis, 1994). Of these 34 neurones, 21 were activated and 13 were inhibited in response to oesophageal distension (Fig. 11). DMN neurones activated by oesophageal distension had a basal firing rate of 2.5 ± 0.3 spikes s−1 which increased to 21 ± 7 spikes s−1 during oesophageal distension. These DMN neurones were localized to the caudal and lateral portions of the nucleus. Neurones inhibited by oesophageal distension had a basal firing rate of 2.9 ± 1.5 spikes s−1, which was reduced to 0.7 ± 0.4 spikes s−1 with oesophageal distension. These DMN cells were typically located in more anterior and medial portions of the DMN.
Figure 11. Responses of physiologically and histologically identified DMN neurones to oesophageal stimulation.
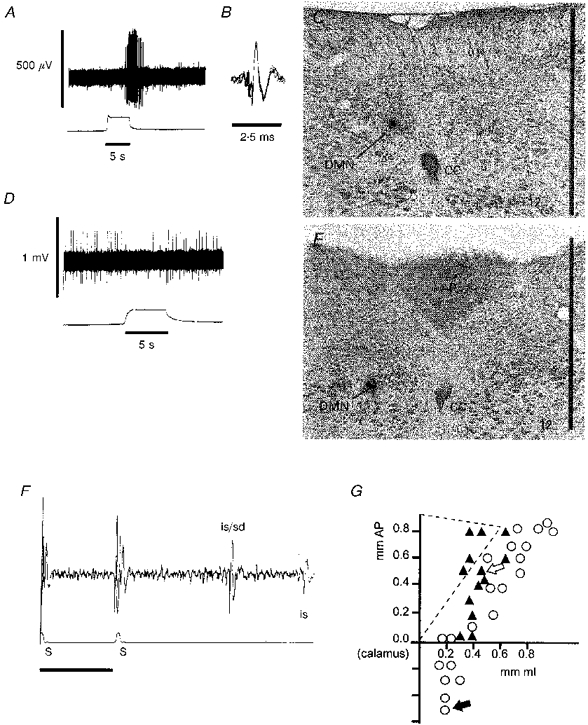
A, DMN response (upper trace) to oesophageal distension (lower trace). Spike amplitudes are distorted by the slow sampling rate (100 μs per sample per channel) necessary to capture the entire spike train. This DMN neurone was located in the caudal portion of the nucleus as shown by the neurobiotin injection in C and by the filled arrow in G. B, superimposed spike records taken from the period of DMN activation by oesophageal distension in A. This recording is made at a faster sampling rate (10 μs sample−1) and shows the unitary nature of the DMN extracellular potential. C, location of the cell recorded in A and B marked by an iontophoretic injection of neurobiotin. Scale bar, 1 mm. D, DMN neuroneal response to oesophageal distension. This neurone was located in the anterior and medial division of the nucleus as shown in E and by the open arrow in G.E, location of DMN neurone recorded in D. Scale bar, 1 mm. F, antidromic identification of the DMN unit shown in A and B. S, occurrence of vagal stimulation (500 μA, 0.7 ms) pulses along with the stimulus artifacts; is/sd, the combined initial segment and full soma-dendritic antidromic spike; is, second antidromic initial segment spike. The is-sd spike separation is commonly observed in DMN neurones due to the very large and prolonged after-spike hyperpolarization demonstrated by these cells. G, schematic map of the location of DMN neurones responsive to oesophageal distension horizontal. ○, the location of DMN neurones activated by oesophageal distension. ▴, the location of DMN neurones inhibited by oesophageal distension. Diagonal lines show the approximate boundary of the right area postrema. AP, anterior-posterior direction; ML, medial-lateral direction.
DISCUSSION
In the present study we have shown that (a) balloon distension of the thoracic oesophagus produces a reduction in motility and tone in the stomach of the rat; (b) NSTc neurones in the rat responding to modest oesophageal distension and labelled with neurobiotin project throughout the DMN; (c) oesophageal distension affects two distinct populations of DMN neurones in areas which receive input from the NSTc. One population of DMN cells is activated by oesophageal distension while another group is inhibited; (d) there are NSTc projections to the NA, IRt and to other medial subnuclei of the NST; and (e) labelled NSTc neurones possess long dendrites which arborize within the ependymal layer which forms the floor of the fourth ventricle.
Effects of oesophageal distension on gastric motility and tone in the rat
Cholinergic input to the stomach has long been associated with the regulation of motility and transport functions of the stomach while the NANC path regulates intragastric volume and compliance (Abrahamsson & Jansson, 1969; Takahashi & Owyang, 1997). These two parallel but complementary functions are experimentally separable. However, it is clear that factors which cause a reduction in tone often produce a suppression of motility as well (Abrahamsson & Jansson, 1969; Rogers & Hermann, 1987). It is clear from our observations that both motility and tone decline following oesophageal distension in the rat. It is likely, therefore, that the central oesophageal afferent pathway diverges to modulate gastric motility and volume by projecting to cells in the DMN responsible for controlling those functions.
NSTc responses to oesophageal distension
The neurophysiological mapping studies of Jean (1972, 1984) in the sheep were the first to associate the NSTc region with oesophageal function. Jean and his associates found that a dorsal medullary region (later identified through anatomical studies as the NSTc by Ross, 1981) and a ventral medullary region (later identified as the compact formation of the nucleus ambiguus; Gillis et al. 1989; Bieger, 1993) were both activated during autonomous swallowing behaviours and following electrical stimulation of the superior laryngeal nerve (Jean, 1972, 1984; Bieger, 1993). Jean's early model of swallowing behaviour focused on the critical involvement of these ‘medullary swallowing neurones’ in a central pattern generator which produced deglutition. Furthermore, it was thought that this central swallowing control mechanism could operate autonomously, without sensory feedback (Jean, 1972). It is clear, however, that the swallow program and the neurones involved in generating it are sensitive to oesophageal distension (Doty, 1968; Jean, 1984). The present study verifies the recent study of Lu & Bieger (1998) that, as in the sheep (Miolan & Roman, 1984), oesophageal distension produces a brisk and repeatable activation of NSTc neurones in the rat which is important for the production of deglutition.
Intramedullary connections of physiologically identified NSTc neurones
Though NSTc fibres and presumptive terminal endings could be found throughout the DMN, the input was most dense in the lateral portion of the nucleus. Recent investigations (Krowicki et al. 1997; Zheng et al. 1998) have shown that the lateral and extreme caudal divisions of the DMN contain a relatively large number of nitric oxide synthase (NOS) containing neurones which project to the fundus of the stomach. While the specific role for nitric oxide synthase in these preganglionic DMN neurones is still debatable, it is likely that these cells activate other NOS neurones in the enteric plexus of the fundus and corpus which, in turn, causes the NANC relaxation of gastric smooth muscle (Abrahamsson & Jansson, 1969; Travagli & Gillis, 1994; Berthoud, 1995; Krowicki, 1997; Takahashi & Owyang, 1997; Zheng et al. 1998).
Neurones in the DMN anterior to the calamus and in the medial half of the DMN also receive inputs from the NSTc. These neurones are probably responsible for excitatory cholinergic control of the stomach (Gillis et al. 1989; McCann & Rogers, 1992; Fogel et al. 1996; Rogers et al. 1996). Thus, the NSTc makes connections with DMN regions containing both the putative NANC inhibitory and cholinergic excitatory neurones. As a consequence, NSTc neurones may control both the excitatory and inhibitory ‘limbs’ of the receptive relaxation reflex.
Neurophysiological recordings from the present study show that DMN neurones may be either activated or inhibited by oesophageal distension. The responses of DMN neurones to this stimulus appear to correlate appropriately with the locations of putative NANC inhibitory and cholinergic excitatory neurones (Laughton & Powley, 1987; Gillis et al. 1989; Krowicki et al. 1997; Zheng et al. 1998). That is, neurones in the extreme caudal DMN and rostolateral portion of the DMN tend to be activated by oesophageal distension. These areas contain NOS and, when activated, cause gastroinhibition (Krowicki et al. 1997). In contrast, neurones in the anterior and medial DMN subdivision tend to be inhibited by oesophageal distension. This portion of the DMN is likely to be the site for cholionergic pathway neurones which, when activated, excite the stomach (Gillis et al. 1989; McCann & Rogers, 1992).
The relationship between the NSTc and the NA has been rigorously investigated using a variety of physiological and anatomical techniques (Beiger & Hopkins, 1987; Altschuler et al. 1989; Hayakawa et al. 1997). For example, Cunningham & Sawchenko (1989) used a combination of anterograde and retrograde tracing methods to demonstrate a substantial NSTc projection to the compact formation of the NA. Though moderate NSTc projections to the DMN were reported using the Phaseolus vulgaris tracing method, the authors concluded that the light terminal labelling in the DMN may have been a consequence of inadvertent labelling from cells outside the NSTc. Our results with iontophoretically applied neurobiotin verified the NSTc-NA projection and also revealed that neurones throughout the entire rostro-caudal length of the DMN may receive input from the NSTc. Retrogradely labelled NSTc neurones were observed following iontophoretic injections of neurobiotin into the caudal DMN, confirming the NSTc-DMN projection path.
NSTc communications with the IRt
Previous studies have demonstrated that NSTc axons pass through the IRt en route to the NA (Cunningham & Sawchenko, 1989). The present study shows that neurones in the IRt probably receive inputs from the NSTc as well. The IRt is thought to play a critical role in the programmed control of cranial motor neurones producing the oral and facial movements of ingestive behaviour (Travers et al. 1997). The oro-facial movements of ingestion are transiently halted at the initiation of a swallow (Doty, 1968) suggesting that a connection exists between brainstem areas programming deglutition and those which program ingestive movements such as licking and chewing (Travers et al. 1997). An NSTc-IRt connection may provide the basis for the co-ordination of ingestive with deglutitive behaviours.
NSTc terminations in the ventral IRt may also provide a basis for the observation that oesophageal distension elevates blood pressure and heart rate and is correlated with the onset of atrial fibrillation (Miller, 1982). Loomis et al. (1997) and Gieroba et al. (1995) have demonstrated that oesophageal distension evokes these cardiovascular changes in a path that requires intact vagal afferent and sympathetic efferent pathways. Furthermore, neurones in the ventrolateral region of the IRt surrounding the nucleus ambiguus (rostral ventrolateral medulla) are activated by oesophageal distension. Neurones in this region of the IRt are well known to be involved in the sympathetic control of vascular resistance and heart rate (Guyenet, 1991).
NSTc-NSTal interactions
It has long been understood that mechanical stimulation of the posterior oral cavity is sufficient to initiate the complex, but centrally programmed, events of deglutition (Harvey, 1628 cited by Doty, 1968; Jean, 1972, 1984; Miller, 1982; Bieger, 1993). Thus, buccopharyngeal contraction pressurizes the bolus and activates tactile receptors in the buccopharynx. This sensory event causes a significant ‘leading’ inhibition of the musculature of the pharyngeo-oesophageal junction, allowing the bolus to be deposited in the orad oesophagus. With the removal of the inhibitory influence from the buccopharyngeal receptors, the centrally programmed peristaltic movements of the bolus commences, under vagal control.
Just as sensory events from the buccopharynx can modulate the oesophageal phase of deglutition, oesophageal distension can potently effect the occurrence of successive buccopharyngeal swallows (Doty, 1968). These early observations on the mechanics of swallowing highlight the fact that the circuitry responsible for co-ordinating the buccopharyngeal and oesophageal phases of deglutition must be intimately connected. The NSTrl is a site which receives input from tactile sensors of the buccopharynx (Travers & Norgren, 1995). Thus, the reciprocal connections demonstrated in the present study between the NSTc and NSTrl may form, at least in part, the basis for the co-ordination of oesophageal and buccopharyngeal phases of the swallowing act.
NSTc neurones as chemosensors?
NSTc neurones possess long dendrites which branch and terminate in or near the ependymal lining of the fourth ventricle. This morphological feature has been reported for other medial NST cells which presumably form the sensory limb of other vago-vagal gastric control reflexes (Fogel et al. 1996; Rogers et al. 1996). Previous physiological and anatomical studies have shown that both the sensory (NST) and motor (DMN) components of vago-vagal reflexes probably lie outside the blood-brain barrier (Shapiro & Miselis, 1985; Gross et al. 1990; Broadwell & Sofroniew, 1993; Hernandez et al. 1994). This anatomical feature helps to explain how large peptide hormones released by the digestive tract can directly modulate the vago-vagal reflex control of digestive functions (Rogers et al. 1996; Chen et al. 1997).
This endocrine-neural communication mechanism has been well documented for caudal portions of the NST and DMN in the vicinity of the area postrema (Hernandez et al. 1994; Rogers et al. 1996; Chen et al. 1997). However, anterior divisions of the dorsal vagal complex, including the NSTc, have not been functionally associated with control by agents in the circulation or in the cerebrospinal fluid. Extensions of dendrites from oesophageal afferent NSTc neurones to the floor of the fourth ventricle suggest that they may be involved in sensing chemical agents in the CSF or the circulation. However, this function needs to be demonstrated physiologically.
Acknowledgments
This work was supported by a grant from the NIDDK (DK52142) to R. C. R. and G. E. H. Special thanks are due to Professors J. Bresnahan and M. Beattie of the Department of Neuroscience for the use of the Nikon microscope, Optronics CCD camera and Sony dye sublimation printer for this study.
References
- Abrahamsson H, Jansson G. Elicitation of reflex vagal relaxation of the stomach from pharynx and oesophagus in the cat. Acta Physiologica Scandinavica. 1969;77:172–178. doi: 10.1111/j.1748-1716.1969.tb04561.x. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Bieger D, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. Journal of Comparative Neurology. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Anatomical demonstration of vagal input to NADPH positive (nitrergic) neurones in rat fundic stomach. Journal of Comparative Neurology. 1995;358:428–439. doi: 10.1002/cne.903580309. [DOI] [PubMed] [Google Scholar]
- Bieger D. The brainstem oesophageal network pattern generator. Dysphagia. 1993;8:203–208. doi: 10.1007/BF01354539. [DOI] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tact in the medulla oblongata of the rat: The nucleus ambiguus. Journal of Comparative Neurology. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Sofroniew MV. Serum proteins bypass the blood brain barriers for extracellular entry into the central nervous system. Experimental Neurology. 1993;120:245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- Canon WB, Leib CW. The receptive relaxation of the stomach. American Journal of Physiology. 1911;29:267–273. [Google Scholar]
- Chen CH, Stephens RL, Rogers RC. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterology and Motility. 1997;9:109–116. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Sawchenko PE. A circumscribed projection from the nulcleus of the solitary tract to the nucleus ambiguus in the rat: Anatomical evidence for somatostatin-28- immunoreactive interneurones subserving reflex control of oesophageal motility. Journal of Neuroscience. 1989;9:1668–1682. doi: 10.1523/JNEUROSCI.09-05-01668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RW. Handbook of Physiology, Alimentary Canal. IV. Bethesda, MD, USA: American Physiological Society; 1968. Neural organization of deglutition; pp. 1861–1902. Chapt. 92. [Google Scholar]
- Fogel R, Zhang X, Renehan WE. Relationships between the morphology and function of gastric and intestinal distension sensitive neurones in the dorsal motor nucleus of the vagus. Journal of Comparative Neurology. 1996;364:78–91. doi: 10.1002/(SICI)1096-9861(19960101)364:1<78::AID-CNE7>3.0.CO;2-P. 10.1002/(SICI)1096-9861(19960101)364:1<78::AID-CNE7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fryscak T, Zenker W, Kantner D. Afferent and efferent innervation of the rat oesophagus. Anatomy and Embyrology. 1984;170:63–70. doi: 10.1007/BF00319459. [DOI] [PubMed] [Google Scholar]
- Gieroba ZJ, Messinger JP, Blessing WW. Abdominal vagal stimulation excites bulbospinal barosensitive neurons in the RVLM. Neuroscience. 1995;65:355–364. doi: 10.1016/0306-4522(94)00509-4. 10.1016/0306-4522(94)00509-4. [DOI] [PubMed] [Google Scholar]
- Gillis RA, Quest JA, Pagani FD, Norman WP. Control centers in the central nervous system for regulating gastrointestinal motility. In: Wood JD, editor. Handbook of Physiology, The Gastrointestinal System, Motility and Circulation. 6. Vol. 1. Bethesda, MD, USA: American Physiological Society; 1989. pp. pp.621–683. chap. 17. [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. American Journal of Physiology. 1990;259:R1131–1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Central noradrenergic neurons: the autonomic connection. Progress in Brain Research. 1991;88:365–380. doi: 10.1016/s0079-6123(08)63823-6. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zheng JQ, Yajima Y. Direct connections to esophageal motorneurons in the nucleus ambiguus from the nucleus of the solitary tract of the rat. Journal of Comparative Neurology. 1997;381:18–30. doi: 10.1002/(sici)1096-9861(19970428)381:1<18::aid-cne2>3.0.co;2-n. 10.1002/(SICI)1096-9861(19970428)381:1<18::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hernandez EJ, Whitcomb DJ, Vigna SR, Taylor IL. Saturable binding of circulating peptide yy in the dorsal vagal complex in rats. American The Journal of Physiology. 1994;266:G511–516. doi: 10.1152/ajpgi.1994.266.3.G511. [DOI] [PubMed] [Google Scholar]
- Jansson G. Vago-vagal reflex relaxation of the stomach in the cat. Acta Physiologica Scandinavica. 1969;75:245–252. doi: 10.1111/j.1748-1716.1969.tb04376.x. [DOI] [PubMed] [Google Scholar]
- Jean A. Localisation et activité des neurones deglutiteurs bulbaires. Journal de Physiologie. 1972;64:227–268. [PubMed] [Google Scholar]
- Jean A. Control of the central swallowing program by inputs from the peripheral receptors. Journal of the Autonomic Nervous System. 1984;10:225–233. doi: 10.1016/0165-1838(84)90017-1. 10.1016/0165-1838(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Keinke O, Schemann M, Ehrlein HJ. Mechanical factors regulating gastric emptying of viscous nutrient meals in dogs. Quarterly Journal of Experimental Physiology. 1984;69:781–795. doi: 10.1113/expphysiol.1984.sp002868. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of NO compounds upon gastric motor function. Journal of Comparative Neurology. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. 10.1002/(SICI)1096-9861(19970106)377:1<49::AID-CNE6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Laughton WB, Powley TL. Localization of efferent function in the dorsal motor nucleus of the vagus. American The Journal of Physiology. 1987;252:R13–25. doi: 10.1152/ajpregu.1987.252.1.R13. [DOI] [PubMed] [Google Scholar]
- Livingston CA, Berger AJ. Response of neurones in the dorsal motor nucleus of the vagus to thyrotropin-releasing hormone. Brain Research. 1993;621:97–105. doi: 10.1016/0006-8993(93)90302-4. 10.1016/0006-8993(93)90302-4. [DOI] [PubMed] [Google Scholar]
- Loomis CW, Yao D, Bieger D. Characterization of an esophageal-cardiovascular reflex in the rat. American Journal of Physiology. 1997;272:R1783–1791. doi: 10.1152/ajpregu.1997.272.6.R1783. [DOI] [PubMed] [Google Scholar]
- Lu WY, Bieger D. Vagovagal reflex motility patterns of the rat esophagus. American Journal of Physiology. 1998;274:R1425–1435. doi: 10.1152/ajpregu.1998.274.5.R1425. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. The Journal of Physiology. 1992;453:401–411. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Functional and chemical anatomy of a gastric vago-vagal reflex. In: Tache Y, Wingate DL, Burks TF, editors. Innervation of the Gut; Pathophysiological Implication. Boca Raton, FL, USA: CRC Press; 1994. pp. 81–92. [Google Scholar]
- Miller AJ. Deglutition. Physiological Reviews. 1982;67:129–182. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- Milner TA, Okada J, Pickel VM. Monosynaptic input from leu-enkephalin immunoreactive terminals to vagal motorneurones in the nucleus ambiguus: Comparisons with the dorsal motor nucleus of the vagus. Journal of Comparative Neurology. 1995;353:391–406. doi: 10.1002/cne.903530307. [DOI] [PubMed] [Google Scholar]
- Miolan JP, Roman C. The role of oesophageal and intestinal receptors in the control of gastric motility. Journal of the Autonomic Nervous System. 1984;10:235–241. doi: 10.1016/0165-1838(84)90018-3. 10.1016/0165-1838(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or neurobiotin. Journal of Neuroscience Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Verbalis JG, Striker EM, Hoffman GE. Distribution and neurochemical phenotypes of caudal medullary neurones activated to express cFOS following peripheral administration of cholecystokinin. Journal of Comparative Neurology. 1993;338:475–490. doi: 10.1002/cne.903380402. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. doi: 10.1016/0196-9781(87)90017-9. 10.1016/0196-9781(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McCann MJ. Intramedullary connections of the gastric region in the solitary nucleus; a neurobiotin histochemical tracing study in the rat. Journal of the Autonomic Nervous System. 1993;42:119–130. doi: 10.1016/0165-1838(93)90043-t. 10.1016/0165-1838(93)90043-T. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion: Modulation by central neural and peripheral endocrine factors. Neuroscience and Biobehavioral Reviews. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. 10.1016/0149-7634(95)00040-L. [DOI] [PubMed] [Google Scholar]
- Ross CA, Armstrong DM, Ruggiero DA, Pickel VM, Joh TH, Reis DJ. Adrenaline neurones in the rostral ventrolateral medulla innervate the thoracic spinal cord; A combined immunocytochemical and retrograde transport demonstration. Neuroscience Letters. 1981;25:257–262. doi: 10.1016/0304-3940(81)90401-8. 10.1016/0304-3940(81)90401-8. [DOI] [PubMed] [Google Scholar]
- Schulze-Delrieu K, Percy WH, Ren J, Shirazi SS, Von Derau K. Evidence for inhibition of opossum LES through intrinsic gastric nerves. American Journal of Physiology. 1989;256:G198–205. doi: 10.1152/ajpgi.1989.256.1.G198. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal oesophageal tension-sensitive afferent fibers in the opossum. Journal of Neurophysiology. 1989;61:1001–1010. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- Shapiro RE, Meselis RR. The central organization of the vagus nerve innervating the stomach of the rat. Journal of Comparative Neurology. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. The Journal of Physiology. 1997;504:479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA mediated synaptic currents in neurones of the rat dorsal motor nucleus of the vagus. American Journal of Physiology. 1991;260:G531–536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Vicini C. Effect of thyrotropin releasing hormone on neurones of the rat dorsal motor nucleus of the vagus. American Journal of Physiology. 1992;263:G508–517. doi: 10.1152/ajpgi.1992.263.4.G508. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA. Nitric oxide mediated excitatory effect on neurones of dorsal motor nucleus of the vagus. American The Journal of Physiology. 1994;266:G154–160. doi: 10.1152/ajpgi.1994.266.1.G154. [DOI] [PubMed] [Google Scholar]
- Travers JB, DiNardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neuroscience and Biobehavioral Reviews. 1997;21:631–647. doi: 10.1016/s0149-7634(96)00045-0. 10.1016/S0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of rat. Journal of Neurophysiology. 1995;73:2144–2162. doi: 10.1152/jn.1995.73.6.2144. [DOI] [PubMed] [Google Scholar]
- Weidner EB, Bao X, Altschuler SM. Localization of nitric oxide synthase in the brainstem neural circuit controlling oesophageal peristalsis in rats. Gastroenterology. 1995;108:367–375. doi: 10.1016/0016-5085(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Zheng ZL, Rogers RC, Travagli RA. Selective gastric projections of nitric oxide synthase containing vagal brainstem neurones. Neuroscience. 1998 doi: 10.1016/s0306-4522(98)00586-7. in the Press. [DOI] [PubMed] [Google Scholar]


