Abstract
The contributions of neurotransmitters and neuromodulators to the responses of the respiratory network to acute hypoxia were analysed in anaesthetized cats.
Samples of extracellular fluid were collected at 1–1.5 min time intervals by microdialysis in the medullary region of ventral respiratory group neurones and analysed for their content of glutamate, γ-aminobutyric acid (GABA), serotonin and adenosine by high performance liquid chromatography. Phrenic nerve activity was correlated with these measurements.
Levels of glutamate and GABA increased transiently during early periods of hypoxia, coinciding with augmented phrenic nerve activity and then fell below control during central apnoea. Serotonin and adenosine increased slowly and steadily with onset of hypoxic depression of phrenic nerve activity.
The possibility that serotonin contributes to hypoxic respiratory depression was tested by microinjecting the 5-HT-1A receptor agonist 8-OH-DPAT into the medullary region that is important for rhythmogenesis. Hypoxic activation of respiratory neurones and phrenic nerve activity were suppressed. Microinjections of NAN-190, a 5-HT-1A receptor blocker, enhanced hypoxic augmentation resulting in apneustic prolongation of inspiratory bursts.
The results reveal a temporal sequence in the release of neurotransmitters and neuromodulators and suggest a specific role for each of them in the sequential development of hypoxic respiratory disturbances.
The classical respiratory response to acute hypoxia consists of an initial augmentation of central respiratory activity followed by a secondary depression which progresses to complete respiratory arrest in hypoxic apnoea (Richter et al. 1991). A variety of mechanisms which generate or contribute to this biphasic respiratory response has been implicated. Hypoxic activation of arterial chemoreceptors increases excitatory synaptic drive of respiratory neurones under in vivo conditions (Lawson et al. 1989). However, augmentation of central respiratory activity also occurs in in vitro preparations in which chemoreceptor afferents are deleted (Völker et al. 1995; Ramirez et al. 1998). Hence, activation of medullary chemosensory neurones (Kawai et al. 1996) or direct stimulation of respiratory neurones (Völker et al. 1995) contribute to the increased respiratory response to hypoxia. Another factor leading to augmentation results from reduction of Na+-K+ pump activity leading to an increase in extracellular potassium levels and direct depolarization of axon terminals and postsynaptic neurones (Acker & Richter, 1985; Haddad & Donnelly, 1990; Trippenbach et al. 1990; Richter, 1996).
The processes which lead to persistent secondary depression of central respiratory activity and finally to hypoxic apnoea are, as yet, unclear. However, it appears that depression of neuronal excitability and synaptic interactions between bulbar respiratory neurones play an important role (Richter et al. 1991). Mechanisms underlying such disturbances may involve: (i) a generalized release and local accumulation of inhibitory neurotransmitters (Neubauer et al. 1990; Haddad & Jiang, 1992; Young et al. 1992; Katoh et al. 1997) and/or neuromodulators such as adenosine, catecholamines, serotonin and opioids (Runold et al. 1989; Neubauer et al. 1990; Moss et al. 1993; Bentue-Ferrer et al. 1994; Yan et al. 1995); (ii) activation of ATP-sensitive potassium (KATP) channels of respiratory neurones due to a decrease in intracellular ATP levels (Jiang & Haddad, 1991; Haddad & Jiang, 1993; Pierrefiche et al. 1996); and (iii) accumulation of metabolic byproducts such as adenosine (Schmidt et al. 1995), which augment KATP channel currents in postsynaptic neurones (Mironov et al. 1998) and depress the otherwise increased Ca2+ influx in cell bodies and axon terminals and consequently release of neurotransmitters.
A previous intracellular investigation (Richter et al. 1991) showed that, during hypoxia, there is an orderly temporal sequence of membrane potential changes in medullary respiratory neurones. The findings pointed to an involvement of neuromodulatory mechanisms that disrupt synaptic interactions between respiratory neurones. Since then, however, the identity, time course and functional consequences of such neuromodulatory processes within the respiratory network have not been further analysed. Therefore, in the present investigation we made sequential, short time scale measurements of glutamate (Glu), γ-aminobutyric acid (GABA), serotonin (5-HT) and adenosine (Ado) content in the extracellular fluid of the ventral medullary respiratory group (VRG) before, during and after acute hypoxic periods and correlated them with the changes in respiratory neuronal activities. All these neurochemicals have been shown to exert prominent effects on the central respiratory rhythm during normoxia (Bonham, 1995). In addition, we made complementary electrophysiological measurements to determine whether serotonin 5-HT-1A receptors (Lalley, 1994; Lalley et al. 1994; Richter et al. 1997) and increased potassium currents (Richter et al. 1991) in VRG neurones are causal for the transition from hypoxic augmentation to depression of the respiratory network.
Preliminary reports of some of these findings have been given in abstract form (Schmidt-Garcon et al. 1994).
METHODS
Anaesthesia and drugs
Experiments were performed on 34 adult cats of either sex (body weight, 2.5-4.5 kg). Animals were anaesthetized with sodium pentobarbitone (initial dose, 40 mg kg−1i.v.; thereafter 4-8 mg kg−1 h−1i.v.) to produce a steady level of anaesthesia. Supplementary doses of anaesthetic were given if mean arterial blood pressure increased above 110 mmHg, respiratory frequency increased above 25 min−1, irregular fluctuations in heart rate or phrenic nerve discharges occurred or there were nociceptive stimulus-evoked increases in phrenic nerve discharges. Atropine sulphate (0.1-0.2 mg kg−1i.v.) was given to reduce salivation and dexamethasone (0.2 mg kg−1i.m.) was administered to prevent brain oedema. Animals were paralysed with gallamine triethiodide (4-8 mg kg−1i.v. initial dose, 4-8 mg kg−1 h−1i.v. thereafter). In a few experiments, arterial blood pressure was stabilized during hypoxia by infusing adrenaline (40 μg ml−1) and glucose (27 mg ml−1) in Ringer solution. Animals were killed by i.v. injection of an overdose (100 mg kg−1) of sodium pentobarbitone that caused rapid and irreversible cardiac arrest.
Animal preparation
A femoral artery and both femoral veins were cannulated for monitoring arterial pressure and administering drugs. A cannula was inserted into the trachea below the larynx. Animals were paralysed and mechanically ventilated with a positive pressure pump. The inspired O2 and end-tidal CO2 were continuously monitored with a respiratory gas analyser (Datex, Helsinki, Finland). Under control conditions, animals were ventilated with oxygen-enriched air (70 % O2 by vol.) which was switched to room air 2 min before starting a hypoxia test. Ventilatory volume and rate were adjusted to maintain end-tidal CO2 at 24-34 mmHg. A wide pneumothorax was established bilaterally to abolish respiratory movements of the thorax to maintain microdialysis probes and the oxygen electrode in their proper positions in the caudal part of VRG, and to increase the stability of intracellular recordings from VRG neurones. Atelectasis was prevented by applying positive pressure (1-2 cmH2O) to the expiratory outflow. Body temperature was maintained at 36-38°C by external heating.
The animal was held rigidly in a stereotaxic head holder and spinal frame. Through a dorsal approach, the cervical vagus nerve trunks were severed and both phrenic nerves (C5 branches) were sectioned, their central ends desheathed and mounted on bipolar silver hook electrodes for recording. The dorsal surface of the medulla was exposed by occipital craniotomy after ventroflexing the head. The dural and arachnoidal membranes were removed and the cerebellum was gently retracted to facilitate placement of microdialysis probes and electrodes. Patches of pia were removed over sites where probes and electrodes were inserted. A horseshoe-shaped pressure foot was placed over the pool of respiratory neurones where micropipettes for intracellular recording were inserted.
Recording responses of respiratory neurones
Glass micropipettes filled with 2 M potassium methylsulphate were used to localize the depth of medullary respiratory neurones for correct placement of microdialysis probes and the oxygen electrode, and to record intracellularly from caudal VRG expiratory neurones. For intracellular recordings, expiratory neurones with membrane potentials of -50 mV or more negative values throughout the test protocols were selected. Measurements were made in current clamp mode (DC 10 000 Hz bandwidth) with DC electrometers equipped with bridge balance and capacity compensation (SEC-5L, npi electronic GmbH, Germany, or Dagan 8100, Minneapolis, MN, USA). Phrenic nerve activity was bandpass filtered (80-3000 Hz), amplified (× 2000-10 000) and monitored on an oscilloscope along with the membrane potential of expiratory neurones. Phrenic nerve activity and its rectified moving average (τ= 10-100 ms), along with the membrane potential of expiratory neurones, tracheal pressure, end-tidal partial pressure of carbon dioxide (PET,CO2) and arterial blood pressure were recorded on a thermal strip chart recorder (frequency response, DC 10 000 Hz; Gould TA2000, Cleveland, OH, USA) and stored on magnetic tape (DC 5000 Hz; Racal, Southampton, UK) for off-line analysis.
Measurement of neuromodulators, neurotransmitters and tPO2 in the VRG
In this paper we present only data from the first hypoxia test in each experiment. For analysis of the sequences of hypoxic events we required maximal time resolution to measure neurotransmitter and neuromodulator concentrations in samples of extracellular fluid, therefore only one type of neurotransmitter or neuromodulator per experiment was measured. Glutamate (Glu), γ-aminobutyric acid (GABA), serotonin (5-HT) or adenosine (Ado) were collected by microdialysis from the extracellular fluid of the VRG, 1-2 mm rostral to the location of caudal expiratory neurones, and measured by high performance liquid chromatography (HPLC). A microdialysis probe (0.5 mm diameter, 2 mm membrane length; Carnegie Medicin, Stockholm, Sweden) was inserted in the VRG on one side to collect samples from the extracellular space. The pial membrane was carefully removed at the place where probes were inserted in order to avoid tissue compression. The probes were slowly driven in 20-30 μm steps by a nanostepper to tissue layers that were approximately 1 mm ventral to the position where microelectrode recordings had revealed localization of respiratory neurones. This means that the middle of the dialysis membrane was right within the pool of respiratory neurones. Such careful positioning of the probes did not produce increases in phrenic nerve discharges indicating lack of major tissue damage. In addition, histological verification of probe positioning was performed in two experiments. The histological inspection revealed the probe tracks and positions of probe tips approximately 1 mm ventral to the ambigual nucleus, i.e. the VRG region. Otherwise, there was no obvious tissue damage.
After probes were carefully positioned in brain tissue, dialysis measurements of Ado and 5-HT levels revealed an exponential decay reaching a steady state within 60-70 min. We therefore started considering dialysis measurements only after 90 min of recovery from mechanical manipulations of the brain.
The dialysis probes were perfused with Ringer solution (containing (mM): 119 NaCl, 3.3 KCl, 1.3 CaCl2, 1.2 MgCl2, 0.5 Na2HPO4, pH 7.4 with 1 n HCl) by a CMA/100 microinjection pump (Carnegie Medicin) calibrated for Hamilton syringes. For adenosine measurements, the perfusion rate was 2 μl min−1, while the dialysate was sampled over a period of 1.5 min, or over a period of 1 min when the perfusion rate was 4 μl min−1. For 5-HT measurements, we used a 2 μl min−1 perfusion rate and 1.5 min collection time, while perfusion rates were 4 μl min−1 and sampling periods were 1 min for Glu and GABA measurements. Sampled probes were immediately frozen and stored at -20°C for off-line analysis. Concentrations of 5-HT, Glu, Ado or GABA in the dialysate were determined by reversed phase HPLC. The percentage of substance recovery was determined at room temperature (∼20°C) before and after each experiment. All measurements revealing a significant fall in these recovery values were rejected. Recovery values were 6.5 or 4.0 % for Ado (at a perfusion rate of 2 or 4 μl min−1), 9 % for 5-HT, 6 % for Glu and 7 % for GABA. Such in vitro recovery values were used in calculating the extracellular substance concentrations given in Results.
A unipolar oxygen electrode (20 μm tip diameter; Diamond General Corp., Ann Arbor, MI, USA) was inserted into the contralateral VRG to measure brain tissue oxygen tension (tPO2).
5-HT levels
To measure the concentration of 5-HT in the extracellular fluid of the VRG, the dialysis probes were perfused with Ringer solution at a rate of 2 μl min−1 and the perfusate was collected into separate vials every 1.5 min. A Brownlee C18 column (diameter, 2 mm) was used to separate different types of amines. The mobile phase (10 mM NaH2PO4, 0.01 mM sodium octylsulphate, 0.5 mM EDTA, pH 2.4, containing 14 % methanol) was pushed through the system by an Applied Biosystems (Foster City, CA, USA) 140B solvent delivery system at a velocity of 100 μl min−1. 5-HT was electrochemically detected (ESA Coulochem II Detector, Bischoff Chromatographie (Leonberg, Germany): E1 = 50 mV, R1 = 20 nA, E2 = 250 mV, R2 = 10 nA, Guard cell = 300 mV; where E is the electrode voltage and R is the current divided by the range) and identified by its characteristic retention time. The relation between the concentration in the dialysate and the concentration in the surrounding medium outside the probe (recovery) was estimated in vitro in a test-tube filled with Ringer solution containing a known concentration of 5-HT. The recovery was 9 % with a flow rate of 2 μl min−1. This recovery value was used in calculating the extracellular 5-HT concentrations presented in the Results.
Adenosine levels
To detect Ado levels, the perfusate was collected into separate vials every 1.5 min. The concentration of Ado was determined during HPLC by passing the perfusate through a Brownlee C18 column (1 mm × 25 cm) packed with Spherisorb (5 μm) (Brownlee Labs. of Applied Biosystems) to separate the purines. The mobile phase (0.1 M NaH2PO4, pH 6.1 in 10 % methanol) was pushed through the system by an Applied Biosystems 140B solvent delivery system at a velocity of 40-50 μl min−1. The absorbance at 254 nm was continuously measured by an Applied Biosystems 785A absorbance detector. Adenosine was identified by selective enzymatic degradation (Hagberg et al. 1987) and by its retention time. Recovery of Ado was between 4 and 6.4 %.
Glutamate and GABA levels
Dialysis probes were perfused at a rate of 4 μl min−1. The perfusate was collected into separate vials at 1 min intervals. To convert the amino acids to chinoids for electrochemical detection, the perfusate was reacted for 3 min with an equal volume of a solution containing 5.4 mg ortho-phthaldialdehyde in 100 μl methanol, 1 mg sodium borate, 6 μl mercaptane, at pH 9.3. The Brownlee C18 columns used for reversed phase HPLC were 2 mm × 25 cm. The mobile phases for the detection of either amino acid were passed through the analysis system at a velocity of 100 μl min−1. To detect Glu, the mobile phase consisted of 0.1 M sodium acetate, 0.1 M citric acid, 0.5 M EDTA, 8 % (v/v) acetonitrile, at pH 6.0. For the measurement of GABA, the mobile phase contained 0.02 M sodium acetate, 45 % (v/v) acetonitrile, at pH 5.0. Glu and GABA were electrochemically detected with an ESA Coulochem II Dectector (for Glu, E1 = 300 mV, E2 = 550 mV; GABA, E1 = 200 mV, E2 = 500 mV) and identified by their characteristic retention times. Recovery of glutamate and GABA was 6 and 7 %, respectively.
Microdialysis sampling protocol
Phrenic nerve activity and its moving average were recorded during collection of dialysate samples for neurochemical measurements in all experiments. Ten samples of dialysate were collected for measurement of each neurochemical during control periods of 15 min. Hypoxia was then induced by replacing inspired air with a gas mixture containg 5-8 % O2 by vol. in nitrogen until central apnoea occurred, as signalled by the absence of phrenic nerve activity. Hypoxic apnoea was maintained long enough to allow collection of two to four dialysis samples. The hypoxic gas mixture was then replaced by room air in which the O2 content was gradually increased from 21 to 70 % over a period of 1-2 min. Samples were collected for 12 to 45 min after terminating hypoxia.
Electrophysiological analysis of the contributions of 5-HT-1A receptors and potassium currents to hypoxic respiratory disturbances
To analyse the potential contributions of 5-HT-1A receptors and membrane potassium currents in the respiratory responses to hypoxia, a 5-HT-1A receptor agonist, an antagonist, or a potassium channel blocker was applied in the vicinity of pools of neurones in the VRG or administered intravenously, while monitoring effects of hypoxia on phrenic nerve activity, and in some experiments, on membrane potential of VRG expiratory neurones. The 5-HT-1A receptor agonist 8-OH-DPAT or the antagonist NAN-190 were administered intravenously in two experiments and microinjected into the pre-Bötzinger complex (PBC), a region of the VRG which is essential for respiratory rhythm (Smith et al. 1991; Schwarzacher et al. 1995; Ramirez et al. 1998) in two other experiments. The rationale of this protocol followed the finding that in the neonatal rhythmic slice preparation, injection of 8-OH-DPAT into the PBC depresses respiratory discharges (Johnson et al. 1996). In the present investigation, solutions of 8-OH-DPAT or NAN-190 (50 or 100 μm in Ringer solution) were injected into the PBC on one side under microscopic control with solenoid-controlled pressure pulses applied to micropipettes with tip diameters of 5-15 μm. The exact location of electrode tips in the PBC was verified histologically (in the same series of experiments as described by Ramirez et al. 1998). Quantities of chemicals were calculated by measuring the ejected volumes (20-25 nl) from the micropipette with a microscope that was equipped with a reticule for calibrating meniscus movements of intrapipette solution. Injection sites within the PBC were verified histologically. The pressure pipette tip, positioned at an effective site, was carefully cut near the surface of the medulla with fine scissors, then the medulla and upper cervical cord were removed and stored in paraformaldehyde for histological processing.
Statistics
Measurements of Ado, GABA, Glu and 5-HT content are expressed as means ± standard error of the mean. The statistical significance of differences in neurotransmitter and neuromodulator concentrations produced by hypoxia were determined by Student's paired t tests. Differences were taken to be significant when P≤ 0.05.
RESULTS
The extracellular space in the VRG of the medulla was microdialysed in 27 experiments to measure the concentrations of Glu (n= 6 cats; Fig. 2), GABA (n= 6; Fig. 3), 5-HT (n= 3; Fig. 4) or Ado (n= 12; Fig. 5) released during normoxic and hypoxic conditions. In seven additonal experiments, the functional contributions derived through activation of medullary 5-HT-1A receptors or membrane potassium channels to the hypoxic responses of respiratory neurones were analysed electrophysiologically (Figs 6 and 7).
Figure 2. Changes of glutamate levels during hypoxia.
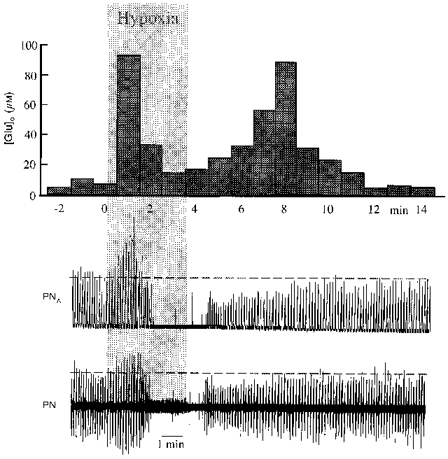
Increased glutamate concentrations in the extracellular space of the ventral respiratory region during and after hypoxic disturbances of respiratory activity. Hypoxia was induced by ventilation with 7 % O2 in N2 (grey area). The extracellular concentration of glutamate ([Glu]o) increased strongly with the onset of hypoxic augmentation and then fell towards control levels during hypoxic apnoea. [Glu]o revealed a second peak during reoxygenation while phrenic nerve activity recovered. Phrenic nerve activity is shown as original recording (PN) and its moving average (PNA).
Figure 3. Changes of GABA levels during hypoxia.
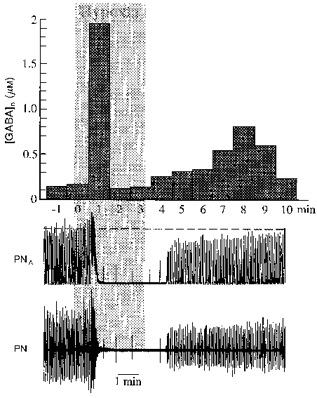
Accumulation of γ-aminobutyric acid in the ventral respiratory region during and after exposure to hypoxia (grey area). Hypoxia was induced by ventilation with 7 % O2 in N2. The extracellular concentration of GABA ([GABA]o) increased strongly with the onset of hypoxic augmentation and then fell to control levels during hypoxic apnoea. [GABA]o rose again during reoxygenation while phrenic nerve activity recovered. Phrenic nerve activity is shown as original recording (PN) and its moving average (PNA).
Figure 4. Hypoxic elevation of serotonin ([5-HT]o) levels in the extracellular fluid of the ventral respiratory region.
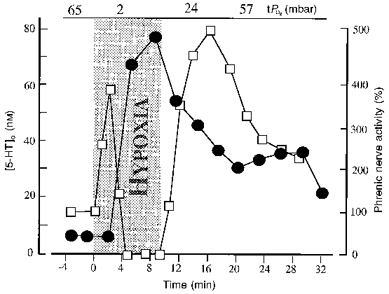
The increase in 5-HT concentration (•) coincided with secondary depression and hypoxic apnoea. The severity of hypoxia (grey area) was reduced from 6 to 10 % O2 by vol. in order to prolong the duration of the hypoxia test. Values of tissue oxygen pressure (tPO2) before, during and after hypoxia are shown. 5-HT levels remained elevated when hypoxia was terminated after 9 min. Relative changes of phrenic nerve activity (□; % of control) were calculated from its moving average.
Figure 5. Changes of adenosine levels during hypoxia.
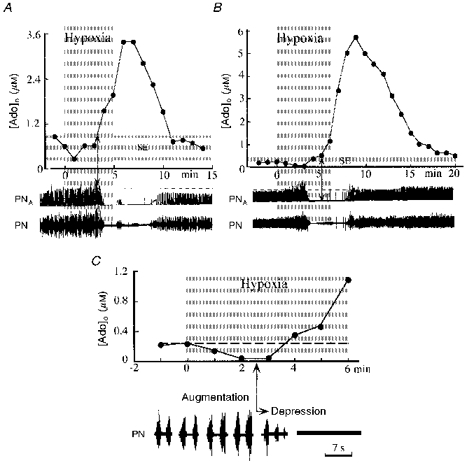
Hypoxic changes in extracellular concentrations of adenosine ([Ado]o) are shown in A and B, as measured in two different experiments. Hypoxic conditions were induced by ventilation with 7 % O2 in N2 (grey areas). Phrenic nerve activity is shown as original recordings (PN) and the moving averages of discharges (PNA). In A, onset of adenosine increase coincided with the beginning of phrenic nerve depression (arrow). B, in another experiment, however, phrenic nerve depression clearly preceded the increase in adenosine concentration. The initial part of B is shown in C at expanded time resolution. The area shaded in light grey (SE) indicates the width of the s.e.m.
Figure 6. Depression of expiratory neuronal discharges during activation of 5-HT-1A receptors.
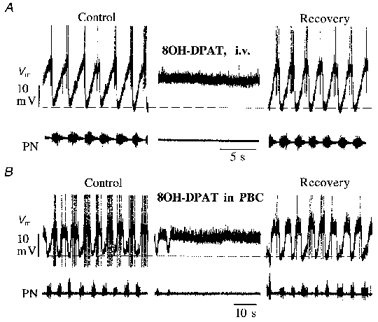
Results shown in A and B were obtained from two different animals. Recordings illustrate membrane potential (Vm) of expiratory neurones in the caudal ventral respiratory group and phrenic nerve activity (PN). A, apnoea was produced by i.v. injection of 8-OH-DPAT (20 μg kg−1). B, a similar apnoeic response was produced by injecting 8-OH-DPAT (0.23 nmol; 50 μm solution) into the ipsilateral pre-Bötzinger complex (PBC). Recovery was complete after 20 min in each test.
Figure 7. Modulation of hypoxic respiratory network disturbances by 5-HT-1A receptors in neurones of the pre-Bötzinger complex.
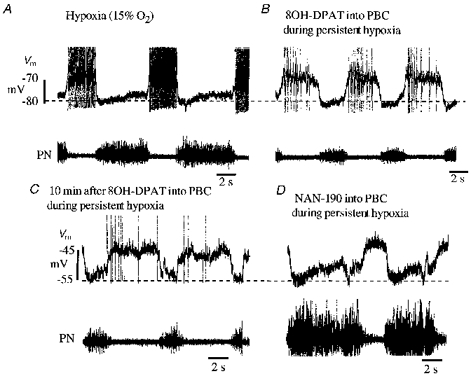
Records in each panel illustrate membrane potential (Vm) of an expiratory neurone in the caudal respiratory group and phrenic nerve activity (PN). Firstly, a typical apneustic pattern with intensified and prolonged phrenic nerve burst discharges was provoked by ventilating the animal with 15 % O2 by vol., as illustrated in A. The expiratory neurone started to discharge early during the expiratory interval and reached discharge frequencies that were higher than under control conditions when the animal was ventilated with oxygen-enriched air (not illustrated, compare B). Thereafter, the hypoxic augmentation of expiratory neuronal discharge and phrenic nerve activity was successfully reduced to normal by injection of the 5-HT-1A receptor agonist 8-OH-DPAT (0.23 nmol; 50 μm solution) into the ipsilateral pre-Bötzinger complex (PBC), as shown in B. The effect of 8-OH-DPAT on both neural activities lasted for several minutes as illustrated in C. Finally, injection of the 5-HT-1A receptor antagonist NAN-190 (0.45 nmol; 100 μm solution) again provoked massive augmentation of inspiratory burst discharges in phrenic nerve and pronounced inspiratory synaptic inhibition in the expiratory neurone as illustrated in D. Hypoxic conditions persisted throughout all tests.
Responses of the respiratory network to acute hypoxia
Animals were ventilated with oxygen-enriched air during control periods before hypoxic tests. Under such conditions, tissue oxygen tension (tPO2) ranged between 50-90 mbar (102 N m−2) as previously described (Acker & Richter, 1985). Shortly before starting hypoxia tests (2-3 min), artificial ventilation was changed to room air until tPO2 stabilized at constant levels, on average 23.6 mbar (range between 10.0 and 37 mbar; n= 5). Under such normoxic conditions phrenic nerve activity exhibited rhythmic burst discharges occurring at a frequency of 17 ± 6 min−1 (n= 5), each with a typical three-phased pattern of discharge consisting of an augmenting burst pattern during inspiration, a declining discharge pattern during post-inspiration and a silent period during stage 2 of expiration. Expiratory neurones exhibited an augmenting discharge pattern during the late expiratory phase and two distinct phases of synaptic inhibition during the inspiratory and post-inspiratory phases (Figs 6 and 7; for review, see Richter, 1996).
Severe systemic hypoxia produced by ventilation with 5-8 % O2 by vol. in N2 reduced tPO2 to less than 2.3 ± 0.9 mbar within 1-2 min. This low level was maintained throughout the period of hypoxic challenge (Fig. 1). After 0.5-3 min of hypoxic exposure, phrenic nerve burst activity increased significantly, signalling the initial period of hypoxic augmentation (Figs 1-5). During the next 2-4 min of hypoxia, augmentation of phrenic nerve activity turned into secondary hypoxic depression which proceeded to complete arrest of respiratory activity in central hypoxic apnoea (Fig. 1). Femoral arterial pressure increased initially from a control level of 107 ± 22 to 125 ± 15 mmHg, then fell to 65 ± 22 mmHg during hypoxic apnoea (n= 12). Stabilization of blood pressure with adrenaline infusion did not affect the hypoxic response of phrenic nerve activity.
Figure 1. Typical effects of acute hypoxia.
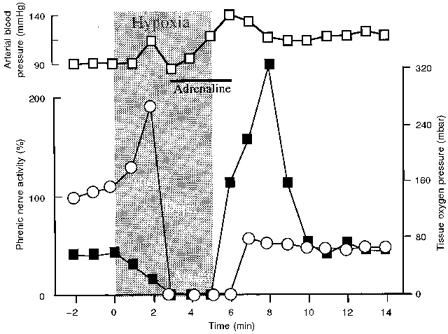
Typical effects of acute hypoxia (grey area: ventilation with 7 % O2 in N2) on phrenic nerve activity (○; expressed as % of control, where control activity is 100 %), arterial blood pressure (□) and tissue oxygen pressure (▪). Hypoxia produced an initial augmentation (Aug.) of phrenic nerve activity followed by depression (Depr.) which progressed to apnoea. These effects were consistent and independent of changes in arterial blood pressure, as shown in this example when the fall in arterial pressure was prevented by slow intra-arterial infusion of adrenaline.
Reoxygenation with oxygen-enriched air resulted in an overshoot of tPO2 to 102.9 ± 40 mbar (e.g. Fig. 1). Arterial blood pressure was usually elevated for several minutes during recovery. Phrenic nerve activity initially remained somewhat depressed, requiring more than 10 min to reach control levels.
Extracellular accumulation of neuromodulators and neurotransmitters in the VRG during hypoxia
HPLC measurements revealed that hypoxia induced local accumulation of both the neurotransmitters Glu and GABA and the neuromodulators 5-HT and Ado. The temporal patterns of accumulation were notably different for each of the neurotransmitters and neuromodulators. Increases in Glu and GABA concentrations were the first to be detected, followed by 5-HT, and after a significantly longer delay by Ado.
Glutamate levels
Hypoxic accumulation of Glu occurred in two temporal phases. From a control level of 9.5 ± 2.1 μm, there was an initial rapid accumulation of Glu which was detected in the first samples collected after 1 min of exposure to hypoxia. The initial increase in Glu concentration shown in Fig. 2 was the greatest measured in the six experiments. For all experiments, Glu accumulation reached maximal levels of 36.6 ± 12.7 μm that were significantly elevated above control. Phrenic nerve activity was increased during the period of elevated Glu concentration. Elevation of Glu concentration was, however, only transient and declined quickly to levels of 7.4 ± 2.5 μm as phrenic nerve activity became depressed and progressed to hypoxic apnoea. After 2-6 min of reoxygenation, Glu concentration once again increased to a secondary post-hypoxic peak of 81.3 ± 11.1 μm that coincided with restoration of stable levels of phrenic nerve activity. The extracellular Glu concentration returned to control levels 4-8 min thereafter. Thus, the initial increase in Glu concentration was temporally well correlated with the augmented phrenic nerve activity, while hypoxic blockade of synaptic interactions between neurones during hypoxic apnoea (Richter et al. 1991) was correlated with a fall of extracellular Glu concentration to or below control levels. However, there was a clear discrepancy between the high Glu concentration during reoxygenation and the return of phrenic nerve activity to control levels. The changes in Glu levels were also poorly correlated with changes in arterial blood pressure. Arterial blood pressure declined during hypoxia, but the elevations of Glu and phrenic nerve activity preceded the onset of arterial hypotension. Moreover, elevating blood pressure with infusions of adrenaline did not influence the patterns of extracellular Glu accumulation.
GABA levels
GABA levels measured in six experiments were smaller than those of Glu, both under control conditions and following exposure to acute hypoxia. GABA also increased in two phases (Fig. 3). Under control conditions, concentrations of GABA were 0.11 ± 0.01 μm. The first increases of GABA were detected 1.8 ± 0.21 min after the onset of hypoxia and reached maximal levels of 0.52 ± 0.29 μm which were significantly higher than the control concentrations of GABA. Similar to Glu accumulation, the initial increase coincided with the phase of respiratory augmentation, at a time when rhythm- and pattern-shaping synaptic interactions between neurones, including mutual inhibition, were maximal (Richter et al. 1991). Thereafter, GABA fell below control levels during hypoxic apnoea. After 1-3 min of reoxygenation there was a second increase in GABA levels to 0.39 ± 0.14 μm which, in some experiments (e.g. Fig. 3), coincided with the recovery of phrenic nerve activity.
5-HT levels
The extracellular 5-HT concentrations measured in three experiments started to increase after 3.2 ± 0.2 min of hypoxia, coincident with the transition from augmentation to depression of phrenic nerve activity. 5-HT rose from a mean control level of 7.7 μm to a maximum of 41.9 μm after 5.7 ± 1.4 min of hypoxia. 5-HT levels remained elevated for 8-32 min following reoxygenation, then declined gradually (Fig. 4).
Adenosine levels
Extracellular control levels of Ado were 0.39 ± 0.05 μm and did not change significantly during the hypoxic augmentation of phrenic nerve activity in 8 of 12 experiments. In four other experiments, Ado levels decreased slightly below control levels during initial exposure to hypoxia (see Fig. 5). After 3-5 min of hypoxia, Ado levels started to increase in synchrony with depression of phrenic nerve activity (Fig. 5). Accumulation of Ado continued throughout exposure to hypoxia, including the time of hypoxic apnoea. Due to the high probability of cardiovascular collapse during severe hypoxia tests lasting longer than 5 min, we did not test whether Ado would have peaked during sustained hypoxia. Ado levels continued to increase for the first 2-3 min of reoxygenation to reach maximal, significantly higher levels of 3.33 ± 0.9 μm. It is noteworthy that phrenic nerve activity reappeared and increased in intensity during this time of significantly elevated Ado levels. Afterwards, extracellular levels of Ado began to decline towards control levels that were reached 13-45 min after reoxygenation. All changes of Ado were independent of changes in systemic arterial pressure, whether or not they were compensated for by infusions of saline containing adrenaline.
Effects of activating 5-HT-1A receptors on hypoxic respiratory disturbances
Since elevation of 5-HT levels in the extracellular space of the VRG was highly correlated with the period of hypoxic depression, we investigated the possibility that activation of 5-HT-1A receptors of respiratory neurones (Lalley, 1994; Lalley et al. 1994) might contribute to hypoxic depression of respiration. Evidence which pointed to this possibility was that, under normoxic conditions, intravenous administration of 8-OH-DPAT produced apnoea, during which excitatory and inhibitory synaptic drives on medullary respiratory neurones were blocked (Fig. 6A). Microinjections of 8-OH-DPAT into the PBC, a specific region that is essential for normal respiratory network function, resulted in arrest of respiratory activity (Fig. 6B). These findings are consistent with previous experiments (Lalley et al. 1997) in which similar effects were evoked by stimulating nucleus raphe obscurus, a source of serotonergic neurones which project to VRG neurones. The stimulus-evoked inhibition of respiratory neurones was blocked by i.v. injection of NAN-190 (data not shown).
Three additional experiments provided direct evidence that 5-HT-1A receptor activation contributes to the transition from hypoxic augmentation to hypoxic depression of respiratory discharges. As seen in Fig. 7, prolonged moderate hypoxia (15 % O2 by vol.) increased the intensity of respiratory neuronal discharges and led to phrenic nerve burst discharges with prolonged apneustic patterns. Microinjection of 8-OH-DPAT (0.23 nmol) into the PBC altered the pattern of medullary expiratory neuronal discharges, reduced their discharge and decreased phrenic nerve activity, which now revealed augmented inspiratory bursts that were within the range of durations which occur during normoxia. In contrast to the effects of the 5-HT-1A receptor agonist, hypoxic disturbances of respiratory activity were potentiated by microinjecting the 5-HT-1A receptor blocker NAN-190 into the PBC (Fig. 7). Thus, blockade of 5-HT-1A receptors during hypoxia resulted in a greatly prolonged augmentation phase of the hypoxic response and dramatic enhancement of apneustic inspiratory activity patterns.
DISCUSSION
The principal finding of the present investigation is that Glu, GABA, 5-HT and Ado accumulate in the extracellular fluid of the VRG in different temporal sequences. Such hypoxic accumulation of neurotransmitters and neuromodulators in the VRG could be anticipated because hypoxia impairs uptake processes and catabolic enzymes in neurones and glial cells (Neubauer et al. 1990; Haddad & Jiang, 1993). Nonetheless, the distinctly different temporal patterns of accumulation of each of the substances point to state-dependent release, each chemical exerting specific functions in the development and progression of the respiratory responses to acute hypoxia. Our main conclusions are that: (1) fast, but transient rises of extracellular Glu and GABA result from enhanced synaptic interactions between neurones during the initial period of augmented respiratory activity and do not result from non-specific hypoxic release of neurotransmitters; (2) hypoxic augmentation is terminated by increased endogenous 5-HT levels, probably due to feedback from respiratory-driven raphe neurones, activating the highly expressed 5-HT-1A receptors and hence potassium currents in respiratory neurones, whereas; (3) extracellular Ado levels rise surprisingly late and thus contribute to sustained hypoxic depression of respiration. (4) Ado-mediated depression alone, however, cannot explain hypoxic apnoea. During prolonged hypoxic periods other neuromodulators, such as endogenous opioids, may contribute to the depression of respiratory activity.
Technical considerations
Using 500 μm probes for microdialysis raises the potential for tissue damage and disruption of network functions. We tried to minimize damage by removing arachnoidal and pial membranes and slowly advancing the probes ventrally with a motorized manipulator. Such careful positioning of the probes did not provoke significant increases in phrenic nerve discharges. In addition, histological verification of probe positioning did not reveal any major damage of the tissue. We therefore assumed that the respiratory neurones and network connections were not significantly lesioned.
After probes were carefully positioned in brain tissue, dialysis measurements of Ado and 5-HT levels revealed an exponential decay reaching a steady state within 60-70 min. We therefore started considering measurements after 90 min of recovery from mechanical manipulations of the brain.
Temporal patterns of neurotransmitter and neuromodulator accumulation and their possible contribution to hypoxic responses
We have invested much effort in achieving a fast time resolution in the HPLC analyses of the microdialysed extracellular space fluid. This allowed us to detect the rapid build-up of Glu and GABA concentrations, which were followed by declines during hypoxic depression and apnoea. This type of response could be expected for Glu, the principle excitatory neurotransmitter of the respiratory network (Bonham, 1995). The initial hypoxic facilitation of respiratory activity seems causal for accumulation of Glu, which was the first of the neurotransmitters measured to reach peak levels in the VRG, and occurred in parallel with the augmentation of phrenic nerve activity. The early facilitation of respiration during acute hypoxia is generally attributed to stimulation of arterial chemoreceptors projecting excitatory afferents to the nucleus of the solitary tract (NTS). This triggers Glu release in the NTS (Mizusawa et al. 1994) and increases excitatory drive by activating AMPA/kainate and NMDA receptors in respiratory neurones (Vardhan et al. 1993; Mizusawa et al. 1994). These neurones project excitatory inputs to VRG neurones (Lawson et al. 1989). Glu should have been released in addition by direct activation of VRG circuits, since early hypoxic increases in respiratory discharges also occur in chemo-denervated animals (Richter et al. 1991).
The initial increase in Glu was followed by a rapid decline which occurred in parallel with hypoxic depression of respiratory neuronal activity, indicating that Glu release was activity dependent rather than non-specific during the acute phase of hypoxia. The late elevation of Glu levels, however, might have been linked to hypoxic elevation of intracellular Ca2+ in axon terminals and non-specific release of Glu. Biphasic increases in Glu evoked by hypoxia have also been measured in the hippocampus (Katoh et al. 1997). However, it is noteworthy that in the present study Glu elevation was not accompanied by increased phrenic nerve activity. Such an increase in phrenic nerve activity may have been prevented because, at the same time, GABA levels revealed a late increase (see below).
Our measurements of GABA at fast time resolution also showed two phases of release, each being transient. Such changes are not in agreement with reports that GABA levels would persistently increase during hypoxia in other brain regions (Wood et al. 1968). In the respiratory network, mechanisms identical to those responsible for elevated Glu concentrations may provoke the patterned increases in extracellular GABA seen in the present investigation. Reciprocal inhibitory interconnections are characteristic of the respiratory network, that is, augmentation of one type of neuronal population is rhythmically followed by reinforced inhibition through an antagonistic group of neurones (Lawson et al. 1989; Smith et al. 1991; Richter, 1996). Thus, the decline of GABA from its first peak during hypoxic depression of respiratory activity can be related to depression of inhibitory synaptic interactions (Richter et al. 1991). During reoxygenation, GABA levels increased again. This second increase was not accompanied by correlated changes of phrenic nerve activity, which speaks against a direct action on respiratory neurones. On the other hand, it may serve to prevent excessive glutamate-evoked depolarization and excitotoxicity (Choi, 1988; Chow & Haddad, 1998).
Adenosine is known to depress the excitability of CNS neurones and to inhibit release of various neurotransmitters presynaptically (Coradetti et al. 1984; Schmidt et al. 1995). Our data are consistent with reports that adenosine concentrations are increased in other brain regions during hypoxia (e.g. Phillis et al. 1987). In the present investigation, however, we demonstrated that hypoxic release of adenosine occurs surprisingly late under in vivo conditions and cannot be responsible for onset of hypoxic depression of respiratory neurones. It rather seems to contribute to sustained protection of respiratory neurones during later and longer periods of hypoxia (Schmidt et al. 1995; Mironov et al. 1998). It is noteworthy that increases in Ado levels outlasted hypoxic periods, which were not associated with pronounced depression of phrenic nerve activity.
Increases in 5-HT concentrations occurred before elevation of Ado levels and coincided clearly with the beginning of hypoxic depression. This temporal correlation suggests that it contributes to termination of the augmenting phase. Rapid accumulation of 5-HT is not surprising, since raphe neurones are known to receive respiratory drive (Lindsey et al. 1994) and hence should be synaptically activated from the very beginning of hypoxic augmentation of the respiratory network. Such synaptic activation will lead to release of 5-HT at feedback appositions that are closely associated with medullary and spinal respiratory neurones (Connelly et al. 1989; Voss et al. 1990; Lindsay & Feldman, 1993). This conclusion is consistent with the finding that raphe neurones are strongly activated during hypoxia, as seen in increased c-fos expression (Erickson & Millhorn, 1994). Such increase in 5-HT release will be preferentially directed towards 5-HT-1A receptors which are abundantly expressed in respiratory neurones and depress their excitability by activation of potassium channels (Lalley, 1994; Lalley et al. 1994, 1997; Richter et al. 1997). There is probably also a direct hypoxic activation of raphe neurones, because 5-HT levels remained elevated during and shortly after hypoxic apnoea. Persistent activation of 5-HT-1A receptors may protect the respiratory network by curtailing influx of calcium ions into neurones and serve to protect against hypoxic impairment of brain structure and function (Shibata et al. 1992; Peruch et al. 1994).
Functional significance
Endogenous mechanisms involving Glu, GABA, 5-HT and Ado are rapidly mobilized during hypoxia and seem to play a major role in modulating respiratory network functions. Glu and GABA seem important for stabilizing enhanced respiratory network activity in order to reinforce gas exchange and possibly O2 uptake at the onset of hypoxia. If this manoeuvre remains unsuccessful, 5-HT and Ado play a critical role in protecting the respiratory network against excitotoxicity mediated by excessive glutamate release and toxic calcium influx into neurones, thereby preserving neuronal integrity and maintaining the impressive potential of respiratory rhythmicity to recover after prolonged hypoxic episodes.
Acknowledgments
This work was supported by the Sonderforschungsbereich 406.
References
- Acker H, Richter DW. Changes in potassium activity, calcium activity and oxygen tension in the extracellular space of inspiratory neurons within the NTS of cats. In: Bianchi AL, Denavit-Saubié M, editors. Neurogenesis of Central Respiratory Rhythm. Lancaster, UK: MTP Press Ltd.; 1985. pp. 183–186. [Google Scholar]
- Bentue-Ferrer D, Bellissant E, Decombe R, Allain H. Temporal profile of aminergic neurotransmitter release in striatal dialysates in rats with post-ischemic seizures. Experimental Brain Research. 1994;97:437–443. doi: 10.1007/BF00241537. [DOI] [PubMed] [Google Scholar]
- Bonham AC. Neurotransmitters in the CNS control of breathing. Respiration Physiology. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends in Neurosciences. 1988;11:466–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Chow E, Haddad GG. Differential effects of anoxia and glutamate on cultured neocortical neurons. Experimental Neurology. 1998;150:52–59. doi: 10.1006/exnr.1997.6764. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to ventral respiratory group in the rat? Neuroscience Letters. 1989;105:34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Coradetti R, Lo Conte G, Moroni F, Passani MB, Pepeu G. Adenosine decreases aspartate and glutamate release from rat hippocampal slices. European Journal of Pharmacology. 1984;104:19–26. doi: 10.1016/0014-2999(84)90364-9. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotonergic neurons of the rat brainstem. Journal of Comparative Neurology. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Donnelly DF. O2 deprivation induces a major depolarization in brain stem neurons in the adult but not in the neonatal rat. The Journal of Physiology. 1990;429:411–428. doi: 10.1113/jphysiol.1990.sp018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad GG, Jiang C. O2 deprivation in the central nervous system: on mechanisms of neuronal response, differential sensitivity and injury. Progress in Neurobiology. 1993;40:277–318. doi: 10.1016/0301-0082(93)90014-j. 10.1016/0301-0082(93)90014-J. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Andersson P, Lacarewicz J, Jacobson I, Butcher S, Sandberg M. Extracellular adenosine inosine, hypoxanthine and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. Journal of Neurochemistry. 1987;49:227–231. doi: 10.1111/j.1471-4159.1987.tb03419.x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Haddad GG. Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. Journal of Neurophysiology. 1991;66:103–111. doi: 10.1152/jn.1991.66.1.103. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. Journal of Applied Physiology. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- Katoh H, Sima K, Nawashiro H, Wada K, Chigasaki H. The effect of MK-801 on extracellular neuroactive amino acids in hippocampus after closed head injury followed by hypoxia in rats. Brain Research. 1997;758:153–162. doi: 10.1016/s0006-8993(97)00213-8. 10.1016/S0006-8993(97)00213-8. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Mückenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem-spinal cord preparation of the neonatal rat. The Journal of Physiology. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. The excitability and rhythm of medullary respiratory neurons in the cat are altered by the serotonin receptor agonist 5-methoxy-N,N-dimethyltryptamine. Brain Research. 1994;648:87–98. doi: 10.1016/0006-8993(94)91909-7. 10.1016/0006-8993(94)91909-7. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Benacka B, Bischoff AM, Richter DW. Nucleus raphe obscurus evokes 5-HT-1A receptor-mediated modulation of respiratory neurons. Brain Research. 1997;747:156–159. doi: 10.1016/s0006-8993(96)01233-4. 10.1016/S0006-8993(96)01233-4. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff A-M, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. The Journal of Physiology. 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- Lawson EE, Richter DW, Ballantyne D, Lalley PM. Peripheral chemoreceptor inputs to medullary inspiratory and postinspiratory neurons of cats. Pflügers Archiv. 1989;414:523–533. doi: 10.1007/BF00580987. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. The Journal of Physiology. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey BG, Segers LS, Morris KF, Hernandez YM, Saporta S, Shannon R. Distributed actions and dynamic associations in respiratory-related neuronal assemblies of the ventrolateral medulla and brain stem midline: evidence from spike train analysis. Journal of Neurophysiology. 1994;72:1830–1851. doi: 10.1152/jn.1994.72.4.1830. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Haller M, Richter DW. Hypoxia activates ATP-dependent potassium channels in inspiratory neurones of neonatal mice. The Journal of Physiology. 1998;509:755–766. doi: 10.1111/j.1469-7793.1998.755bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. The Journal of Physiology. 1994;478:55–66. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Surun MP, Gacel G, Champagnat J, Denavit-Saubié M, Roques BP. Pharmacological identification of delta and mu opiate receptors on bulbar respiratory neurons. European Journal of Pharmacology. 1984;98:241–247. doi: 10.1016/0014-2999(84)90595-8. [DOI] [PubMed] [Google Scholar]
- Moss IR, Scott SC, Inman JD. Hypoxia, sleep and respiration in relation to opioids in developing swine. Respiration Physiology. 1993;92:115–125. doi: 10.1016/0034-5687(93)90124-s. [DOI] [PubMed] [Google Scholar]
- Neubauer J, Melton JE, Edelman NH. Modulation of respiration during brain hypoxia. Journal of Applied Physiology. 1990;68:441–449. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- Peruch B, Backhauss C, Prehn JH, Krieglstein J. Protective effects of 5-HT-1A receptor agonists against neuronal damage demonstrated in vivo and in vitro. Journal of Neural Transmission. 1994;8:73–83. doi: 10.1007/BF02250918. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Walter GA, O'Regan MH, Stair RE. Increases in cerebral cortical perfusate adenosine and inosine concentrations during hypoxia and ischemia. Journal of Cerebral Blood Flow and Metabolism. 1987;7:679–686. doi: 10.1038/jcbfm.1987.122. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Bischoff AM, Richter DW. ATP-sensitive K+ channels are functional in expiratory neurones of normoxic cats. The Journal of Physiology. 1996;494:399–409. doi: 10.1113/jphysiol.1996.sp021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJA, Wilken B, Richter DW. The hypoxic response of neurones within the in vitro mammalian respiratory network. The Journal of Physiology. 1998;507:571–582. doi: 10.1111/j.1469-7793.1998.571bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher O, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. The Journal of Physiology. 1998;507:895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW. Neural regulation of respiration: rhythmogenesis and afferent control. In: Gregor R, Windhorst U, editors. Comprehensive Human Physiology: From Cellular Mechanisms to Integration. Vol. 2. Heidelberg: Springer-Verlag; 1996. pp. 2079–2095. [Google Scholar]
- Richter DW, Bischoff AM, Anders K, Bellingham M, Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. The Journal of Physiology. 1991;443:231–256. doi: 10.1113/jphysiol.1991.sp018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Lalley PM, Pierrefiche O, Haji A, Bischoff AM, Wilken B, Hanefeld F. Intracellular signal pathways controlling respiratory neurons. Respiration Physiology. 1997;110:113–123. doi: 10.1016/s0034-5687(97)00077-7. [DOI] [PubMed] [Google Scholar]
- Runold M, Lagercrantz H, Prabhakar NR, Friedholm BB. Role of adenosine in hypoxic ventilatory depression. The Journal of Physiology. 1989;67:541–546. doi: 10.1152/jappl.1989.67.2.541. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bellingham MC, Richter DW. Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. The Journal of Physiology. 1995;483:769–781. doi: 10.1113/jphysiol.1995.sp020621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Garcon P, Nagel H, Richter DW. Extracellular GABA concentration changes during hypoxia within the ventral respiratory group of the cat. In: Elsner N, Breer H, editors. Proceedings of the 22nd Göttinger Neurobiology Conference, Stuttgart. Stuttgart: Thieme Verlag; 1994. p. 899. [Google Scholar]
- Schmidt P, Nagel H, Richter DW. Role of adenosine in the hypoxic response of central respiratory activity. In: Elsner N, Breer H, editors. Proceedings of the 21st Göttinger Neurobiology Conference, Stuttgart. Stuttgart: Thieme Verlag; 1993. p. 593. [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Boetzinger complex in the cat. Journal of Neurophysiology. 1995;73:1452–1459. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Shibata S, Kagami-Ishi Y, Tominaga K, Kodama K, Ueki S, Watanabe S. Ischemia-induced impairment of 2-deoxyglucose uptake and CA1 field potentials in rat hippocampal slices: protection of 5-HT1A receptor agonists and 5-HT2 receptor antagonists. European Journal of Pharmacology. 1992;229:21–29. doi: 10.1016/0014-2999(92)90281-8. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Boetzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippenbach T, Richter DW, Acker H. Hypoxia and ion activities within the brain stem of newborn rabbits. Journal of Applied Physiology. 1990;68:2494–2503. doi: 10.1152/jappl.1990.68.6.2494. [DOI] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commisural nucleus of the NTS mediate carotid chemoreceptor responses. American Journal of Physiology. 1993;264:R41–50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- Völker A, Ballanyi K, Richter DW. Anoxic disturbance of the isolated respiratory network of neonatal rats. Experimental Brain Research. 1995;103:9–19. doi: 10.1007/BF00241960. [DOI] [PubMed] [Google Scholar]
- Voss MD, DeCastro D, Lipski J, Pilowski PM, Jiang C. Serotonin immunoreactive boutons form close apposition with respiratory neurons of dorsal respiratory group in the cat. Journal of Comparative Neurology. 1990;295:208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- Wood JD, Watson WJ, Drucker AJ. The effect of hypoxia on brain gamma-aminobutyric acid levels. Journal of Neurochemistry. 1968;15:603–608. doi: 10.1111/j.1471-4159.1968.tb08959.x. [DOI] [PubMed] [Google Scholar]
- Yan S, Laferriere A, Zhang C, Moss IR. Microdialyzed adenosine in nucleus tractus solitarii and ventilatory response to hypoxia in piglets. Journal of Applied Physiology. 1995;79:405–410. doi: 10.1152/jappl.1995.79.2.405. [DOI] [PubMed] [Google Scholar]
- Young RS, During MJ, Aquila WJ, Tendler D, Levy E. Hypoxia increases extracellular concentrations of excitatory and inhibitory neurotransmitters in subsequently induced seizure: in vivo microdialysis study in the rabbit. Experimental Neurology. 1992;117:204–209. doi: 10.1016/0014-4886(92)90128-d. [DOI] [PubMed] [Google Scholar]


