Abstract
Knowledge of the size and orientation of the hand is essential if it is to be moved accurately in space. We used two psychophysical methods to determine whether the perceived size of a body part changes when its sensory input is changed: first, the selection of scaled drawings which matched the apparent size of a body part, and second, a motor task in which the subject drew the body part to depict its perceived size.
Complete anaesthesia of the thumb (with a digital nerve block) significantly increased its perceived size by 60–70% when assessed with both psychophysical methods. During this anaesthesia, the perceived size of the adjacent index finger or digits on the contralateral side was unaltered. However, the size of the unanaesthetized lips increased (by ∼50%).
Marked sensory loss for the lips (produced by topical anaesthetics) significantly increased their perceived size when assessed with both methods of measurement. There was a small increase in apparent size of the thumb.
To determine whether changes in perceived size could also be produced by an elevation of peripheral inputs, innocuous electrical stimulation of the digital nerves and also painful cooling of the digit were used. Both procedures produced small but significant increases in perceived size of the stimulated part.
The results highlight lability in the perceived size of parts of the body and how this affects motor output. The data may reveal perceptual consequences of acute changes in central somatosensory maps, changes which are known to occur with deafferentation.
To move the tip of the thumb accurately requires knowledge not only of relative muscle lengths and joint angles (for review see McCloskey, 1978; Gandevia, 1996), but also information about the dimensions of body segments. Indeed, without knowledge of the size of body segments, information related to joint angles is unable to specify uniquely the location of an extremity in space. Skill in normal movements, especially those involving the hand, presumably relies on sensory information received by cortical somatosensory areas before and during the movements (Porter & Lemon, 1993). However, in adult non-human primates and other experimental animals, cortical ‘representations’ of the digits are not fixed but change when amputation or anaesthesia removes the sensory input from them. This adaptation has a rapid phase beginning within minutes (Kelahan & Doetsch, 1984; Calford & Tweedale, 1988, 1991a) and a longer phase developing over weeks and months (e.g. Merzenich et al. 1983, 1984; see also Rasmusson et al. 1992; Zarzecki et al. 1993; for review see Kaas, 1991). Initially, in both flying foxes and monkeys, cells in the primary somatosensory cortex which represent the ‘lost’ digit respond to a larger area of cutaneous input, including that from more proximal skin and even skin on the adjacent fingers (Calford & Tweedale, 1988, 1991a). Acute changes may also occur at thalamic (Rasmusson et al. 1993) and possibly at cuneate levels (Dostrovsky et al. 1976; Pettit & Schwark, 1993; Northgrave & Rasmusson, 1996). Studies in human subjects have also revealed that the behaviour of the sensorimotor cortex is also not fixed. Human motor cortical ‘maps’ assessed with transcranial magnetic stimulation change acutely with local anaesthesia (Brasil-Neto et al. 1993; Kew et al. 1994). These studies have focused on apparent changes to motor and sensory representations produced by local anaesthesia, nerve section or amputation, but the perceptual implications of the altered sensory inputs have rarely been considered.
Some recent studies have indicated the extent to which sensory maps may change under extreme circumstances. When sensory input from the whole arm was removed years previously by an extensive dorsal rhizotomy in monkeys, the cortical representations of the hand and face reorganized over years with the areas usually devoted to the arm map being ‘invaded’ by inputs derived from the chin (Pons et al. 1991). After long-standing amputation of the hand, some patients sometimes mistakenly localize stimuli on the face to the ‘phantom’ hand (Ramachandran et al. 1992; Halligan et al. 1993), a phenomenon which has been taken, along with other evidence, to indicate the potential reorganization of the human somatosensory cortex following nerve injury (see Yang et al. 1994; Elbert et al. 1994). However, the subjective responses to stimulation of cutaneous nerve fascicles innervating the hand are preserved after amputation (Schady et al. 1994).
The present studies were designed to determine whether perceptual disturbances develop when the afferent input from a body part, usually the thumb, is acutely disturbed. Disturbances included both acute decreases in input produced by local anaesthesia and increases produced by innocuous electrical stimulation or painful cooling of the digits. We devised simple psychophysical techniques (selection by the subject of matching templates of body parts, and drawing by the subject of body parts to depict their size) and showed that the perceived size of a body part can change immediately its sensory input is altered. Some results have been published in abstract form (Gandevia, 1994; Glasby & Gandevia, 1995)
METHODS
Studies were performed on adult male and female subjects who were healthy without any apparent neurological disorders (age range, 18-42 years). For all studies the subject was comfortably seated with the ‘test’ hand resting on a smooth table. In most studies an opaque platform over the hand prevented vision of it. In studies in which drawings were performed, the subjects were also blindfolded. Informed consent was obtained and the local ethics committee approved the procedures. The work conformed with the Declaration of Helsinki.
Studies of thumb anaesthesia
In two separate studies, one thumb was completely anaesthetized. Anaesthesia was produced by injection of lignocaine (lidocaine) (1.5 % without adrenaline) approximately midway along the proximal phalanx (2-4 ml). Injections were begun with the needle (25 gauge) at the likely depth of the main digital nerves. The block was considered complete when light touch and painful sensations were abolished. A loose band or piece of tape was usually positioned at the base of the digit to prolong anaesthesia. There was no evidence of spread of the anaesthesia outside the test thumb so that the adjacent digit, proximal regions of the palm and dorsum of the hand were clinically and subjectively unaffected. Once the thumb was anaesthetized, no discomfort or pain was reported in the thumb or elsewhere.
In the first study with six subjects, perceived size was estimated by selection of a simple two-dimensional outline or ‘template’ of the digit which best matched its ‘size’. Templates of thumbs of different sizes (with near vertical orientations) were randomly arranged on sheets (Fig. 1). The templates of each part were made from scans of one-line drawings of the body part. The range of magnifications of the templates was 3.6-fold. Subjects were asked to ‘select the template which best matches how big your thumb feels or the perceived size of your thumb’. Subjects gave their responses within 10-15 s and were discouraged from spending long periods to select between two templates of similar size. Eight estimates were made prior to anaesthesia, when anaesthesia was clinically complete, during partial recovery, and finally when sensation had returned to normal (2-3 h after the onset of anaesthesia). In the same study subjects estimated the perceived size of the index finger adjacent to the anaesthetized thumb and the index finger and thumb on the contralateral (unanaesthetized) side with similar sets of templates. In two subjects this study was repeated but saline rather than local anaesthetic was injected around the digital nerves of the thumb.
Figure 1. Method of template matching.

A sample set of templates for the thumb is shown. The template sheet included thumbs with near vertical orientations but of a wide range of sizes. These drawings were arranged on a sheet with each identified by a small letter. The subject nominated a drawing which best matched the perceived size of their thumb and the process was repeated with a different sheet in which the same drawings were rearranged. A similar procedure was used to estimate the perceived size of other digits and the lips. The horizontal calibration bar (lower right) represents 10 mm.
In the second study in ten subjects, none of whom had participated in the first study, the perceived size of the hand was assessed during anaesthesia of the right thumb with a method which required the motor system to signify the perceived size. Blindfolded subjects drew the perceived outline of their right hand six times on sheets placed on the platform above it using their left hand. A single line was drawn beginning at the radial side of the wrist. In addition, the perceived size of the lips was also investigated. Lips were drawn as a line around the perceived edges of the vermilion. Drawings were made in random order before and during anaesthesia. Subjects were asked to ‘draw an outline which represents the size of the body part’ and they were reminded ‘to concentrate on how big the body part feels’. Outlines of the whole hand, individual digits and the lips were digitized and areas calculated.
Anaesthesia of the lips
In eleven subjects the perceived size of the lips and left and right thumbs was estimated with the ‘template’ method. Twelve scaled templates were selected for the three body parts before and after partial anaesthesia of the lips. In separate studies the ‘drawing’ method was also used (also 11 subjects): the left hand drew the right one and vice versa, and then the subject used the preferred hand to draw the perceived outline of the lips. Six drawings of each part were made before and after anaesthesia. Anaesthesia of the lips was induced with topical application of Emla cream on the outer keratinized part of the lips (5 %, containing lignocaine and prilocaine; Astra) and lignocaine cream (5 %) on the inner membranous portion. This produced marked subjective numbness of the lips and loss of responses to painful stimuli but not complete clinical anaesthesia. The discomfort of multiple injections into the lips and the confounding effect of pain (see Results) make it impractical to achieve complete anaesthesia of the lips.
Increases in background afferent input from the digit
In three additional studies the afferent input from one digit was increased. In the first two studies, electrical stimulation of digital nerves was used, and in the third, the digit was cooled to mildly painful levels. For electrical stimulation, soft flexible self-adhesive electrodes were cut (6-8 mm width) and placed around the thumb, index and middle fingers with the cathode around the proximal phalanx and the anode around the second phalanx. Although only one digit was stimulated electrically, electrodes were placed around three digits so that the initial conditions were the same for each. Wide flexible electrodes were used and cut from electrosurgical patient plates (no. 1180; 3M). We did this because conventional narrow ‘ring’ electrodes sometimes produce a strong local sensation of constriction at the site of placement and subjects then find it difficult to concentrate on the apparent size of the whole digit during testing. Trains of stimuli at 75 Hz (pulse width, 0.5 ms; train duration about 1-2 s) were delivered three times with about 5 s between trains. The frequency was chosen because large diameter axons can follow it, and it is well above background firing rates but below maximal peak rates. The intensity was adjusted so that the stimuli produced an innocuous feeling of tingling and paraesthesiae radiating to the tip of the digit. The stimulus intensity was at or just below 1.5 times sensory threshold for a single stimulus. During stimulation of the digital nerves subjects were unable to detect light stroking of the digit and when they touched surfaces with the stimulated digit they reported that it then felt ‘numb’. The template method was used to estimate perceived size during, and either immediately before or after, the trains of stimuli. Thus, each trial was done as a pair, with a control and an experimental estimate, with the order of estimates being randomized. Ten pairs of estimates were made. In one study the perceived size of the thumb, index and middle finger was measured when electrical stimuli were applied to the index finger, and in the other, the perceived size of the thumb, index and lips was measured with stimuli applied to the thumb (each n= 11).
In a separate study, the right thumb was cooled to produce a mildly painful, cold sensation localized to the digit (n= 11). The template-matching method was used to obtain estimates of the perceived size of both thumbs, the right index and the lips (with the order being randomized). Six control estimates were made and then the right thumb was placed up to the interphalangeal joint in a mixture of ice and water with the left thumb placed similarly in water at close to skin temperature (about 30°C) for 1-2 min. Subjects wore thin latex gloves. Ten judgements of size were made once subjects reported a painful sensation in the right thumb. There was no spread of the cooling as judged by recording the temperature on the dorsum of the hand just proximal to the metacarpophalangeal joint. The absolute skin temperatures would be sufficient to activate cold-sensitive C fibres (e.g. Campero et al. 1996). However, cooling did not prevent detection of simple cutaneous stimuli so that the results are unlikely to reflect conduction block in cutaneous afferents.
Statistical analysis
Control and experimental conditions were compared with Student's paired or unpaired t tests (both two-tailed) for group or individual data, respectively. For analysis of group data the mean of individual subjects’ data was used. An analysis of variance was used for the study in which the sizes of all digits and the lips were measured before and during anaesthesia. Mean changes in perceived area are given for the group of subjects in each study. The number of subjects who showed a significant change is usually also given. Statistical significance was set at the 5 % level.
RESULTS
The thumb appeared to grow in size following complete anaesthesia of its digital nerves (Figs 2 and 3). Within seconds of anaesthesia, many subjects spontaneously reported that the thumb felt larger. There was no discomfort reported for the thumb and no evidence that anaesthesia had developed outside the territory of the thumb's digital nerves. Based on subjects’ reports and their drawings it seems that the whole thumb is perceived to grow. The effect was not restricted to the proximal or distal part and involved an increase in the thumb's length and width. The illusion of enlarged size did not require the subject to view the thumb or to touch it with sentient parts of the body - such strategies were minimized by the methods used. The ‘growth’ of the thumb was documented with two methods, template matching and line drawing. Different groups of subjects were used for these two studies. As this illusion occurred when subjects drew around the hand (Figs 2B and 3), the motor system must take account of the changes in perceived body size evoked by thumb anaesthesia. With both methods of measurement, the mean increase in perceived area of the thumb was about 60-70 %. The changes were statistically significant for both groups of subjects (P < 0.001) and for most individual subjects. In no subject did perceived size of the thumb decrease when it was anaesthetized. In a control study using the usual template method, injection of the same volume of saline as for the digital nerve blocks produced no change in perceived size of the thumb.
Figure 2. Changes in perceived size of an anaesthetized thumb measured with two methods.
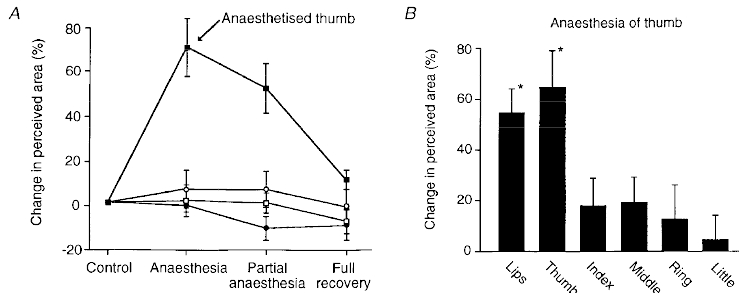
A, changes in perceived size of thumbs and index fingers during complete anaesthesia of the left thumb. Anaesthesia was produced by injection of lignocaine around the digital nerves 1 cm distal to the metacarpophalangeal joint. Hands rested palm downwards and vision was excluded. Estimates were made by selection of line-drawing templates which best matched the perceived size of the body part (see Methods). Data from 6 subjects (means ±s.e.m.). Full recovery occurred 2-3 h after the onset of anaesthesia. ▪, left thumb; □, right thumb; •, left index finger; ○, right index finger. B, changes in perceived size of digits on the right hand and of the lips during anaesthesia of the right thumb assessed with the drawing method. Subjects drew around the perceived outline of the body part (see Methods). Means ±s.e.m., 10 subjects. *P < 0.01.
Figure 3. Line drawings of the thumb and lips before and after anaesthesia of the thumb.
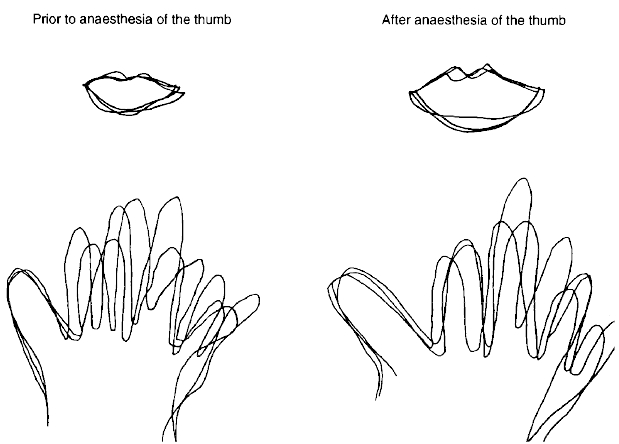
Drawings from one subject of the outline of the lips and the right hand before (left) and after (right) anaesthesia of the right thumb. Three typical traces are superimposed. The size of both the anaesthetized thumb and the (unanaesthetized) lips have increased after local anaesthesia.
When the subjects drew an outline of the hand and its digits during thumb anaesthesia, we examined whether there were perceptual changes involving the whole hand and individual digits. The most obvious change in the drawings was the increase in size of the thumb. While there was a tendency to draw the entire hand slightly larger (by about 10 %), this was not statistically significant for the group. Based on group data, the change in perceived size occurred for the anaesthetized thumb but not for the adjacent, unanaesthetized index finger, or other fingers on the same hand (Fig. 2B). In the study with matching templates, there was also no significant change in the perceived size of the index finger adjacent to the anaesthetized thumb. In addition, this study showed that the index finger and thumb on the side contralateral to the anaesthetized thumb did not change their perceived size. However, based on the drawings of an outline of the lips, their perceived size increased during unilateral thumb anaesthesia (by 55 % for the group, P < 0.01). This effect was also significant in eight out of ten subjects. Drawings of the lips before and during anaesthesia were symmetrical with no obvious tendency for the left or right sides to be distorted.
In complementary experiments, marked (but not complete) anaesthesia of the lips using topical anaesthetics significantly increased their perceived area. This increase occurred when tested with template matching (by an overall mean of 31 %, P < 0.01; also significant in 7 of 11 subjects) or with line drawing (by 60 %, P < 0.01; 9 of 11 subjects). Perceived area of both left and right thumbs increased slightly (by 5 %, P < 0.05) using the template method. This increase was present for six subjects for the left thumb and seven for the right one. With the drawing method the small increases in perceived size of the thumbs (by 4-5 %) were not significant for the group.
Given that removal of the usual sensory input from the thumb caused perceived enlargement of the thumb, we assessed whether an increased sensory input altered perceived size. The changes produced by stimulation of the thumb were smaller than those evoked by anaesthesia. In one study, digital nerves of the thumb were stimulated at an innocuous intensity which produced painless paraesthesiae referred to the tip of the thumb. This was designed to increase the input in large-diameter cutaneous (and joint) afferents. Perceived size of the stimulated digit increased (by 15 % for thumb, P < 0.01, Fig. 4A). This was evident in ten subjects, with one showing a decrease. Perceived size of the lips also increased (by 11 %; P < 0.05, evident in 8 of 11 subjects, Fig. 4A). In a separate study, stimulation of the index finger increased its perceived size (by 25 %, P < 0.01), without a consistent change in size of the adjacent thumb or middle finger.
Figure 4. Changes in perceived size of the digits when the input from them is increased.
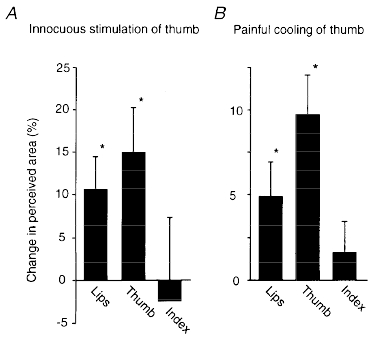
A, changes in perceived size of thumb and lips during artificial stimulation of the thumb (means ±s.e.m., 11 subjects). Stimuli were delivered through soft electrodes at about 1.5 times sensory threshold for single stimuli at 75 Hz for 1-2 s. Data were obtained with the template-matching method. B, changes in perceived size of the right thumb and lips during cooling of this thumb to a painful degree (means ±s.e.m., 11 subjects). Data were obtained with the template matching method. *P < 0.05.
To study the effect of an increase in input from small-diameter afferents the distal phalanx of the right thumb was cooled to painful levels by repeated immersion in a water/ice mixture for 1-2 min. The cooled thumb grew in perceived size (by an overall mean of 10 %; P < 0.05; evident in 8 of 11 subjects, Fig. 4B). There was a small but significant reduction in the perceived size of the contralateral thumb (by 4.7 %, P < 0.02). In addition, the lips increased in size during thumb cooling (by 5 % for the group, P < 0.02). For the lips, perceived size increased significantly in six subjects, decreased in one and there was no change in the remaining subjects. The apparent size of the right index finger did not change.
DISCUSSION
The main physiological observation in this study is that the perceived size of parts of the body can change rapidly when the afferent input from the part is altered. During anaesthesia of the thumb or lips subjects do not report them as ‘missing’ from the body, an effect which might occur had they simply monitored the amount and spatial distribution of peripheral input. Instead, subjects note a dramatic increase in perceived size when the part is anaesthetized. The results do more than confirm neurological opinion that a body schema remains when the sensory input is prevented from reaching the cerebral cortex following deafferentation, amputation or spinal cord transection (e.g. Head & Holmes, 1912; Melzack & Bromage, 1973).
The changes in perceived size were documented for anaesthesia of the thumb and lips using two psychophysical techniques. One involved matching apparent size to one of a set of templates and the other required drawing of the parts. The apparent enlargement of the thumb during anaesthesia of its digital nerves was of a similar magnitude when assessed by the two methods. The increase in (two-dimensional) area of the thumb would represent a doubling of its volume. Because subjects drew the anaesthetized thumb as larger, the perceptual distortion introduced by anaesthesia is able to disrupt motor performance. Smaller increases in perceived size were documented when the afferent input from the digit was increased, either by activation of its large-diameter afferents with non-painful electrical stimulation, or by activation of small-diameter afferents with painful cooling.
Although these types of perceptual change have not, to our knowledge, been measured previously, they are not necessarily surprising: many subjects reminded us that the lips, tongue and other parts of the face feel enlarged following dental anaesthesia. Furthermore, a well-known phenomenon, often depicted by cartoonists, is the growth in ‘size’ of the thumb when struck a painful blow by a hammer. The perceived increase is more dramatic than any actual increase produced by the blow.
A possible explanation for our findings is that the increases in perceived size with anaesthesia of the thumb (or the lips) are related to the unmasking of inputs to relevant primary somatosensory cortical cells after deafferentation. Acute removal of the afferent input from one digit (by anaesthesia) enlarges the size of receptive fields of cortical cells which represent skin areas adjacent to the site from which the input was removed (e.g. Calford & Tweedale, 1988, 1991a). This is likely to be associated with an increase in the background discharge not only of the cells which can normally respond to input from the anaesthetized part (and particularly its edges), but from a population of cells which receives a subliminal input from a wider area than just the anaesthetized part (Rasmusson et al. 1992). Perhaps these changes in a particular representation are interpreted as consistent with an increased size of the body part. One implication of this possibility is that illusory increases in size may not occur when a large part of the body is anaesthetized or removed because regions of somatosensory cortex devoted to the removed part become ‘silent’ (e.g. Merzenich et al. 1983; Li et al. 1994). This fits with the observation that, when the whole arm is anaesthetized, it is perceived as foreshortened and closer to the body (Melzack & Bromage, 1973). While short-term enlargements in the receptive fields of cortical neurones after deafferentation of a digit may contribute to the changes in perceived size, additional factors are likely to operate. Some receptive fields enlarge in the contralateral homologous cortex after acute deafferentation (Calford & Tweedale, 1990), but perceptual changes were not observed on the contralateral side during thumb anaesthesia.
Why did enhanced input in large- or small-diameter afferents increase the perceived size of the thumb, although to a smaller extent than anaesthesia? The input producing the changes in perceived size was substantial (either repetitive stimulation of the digital nerves or painful cooling of the whole thumb) and would rarely if ever have been encountered prior to these experiments. Interestingly, peripheral nerve stimulation can increase the size of receptive fields of neurones in the primary somatosensory cortex of the cat (Recanzone et al. 1990). Thus, intense or synchronous inputs may produce convergence onto cells and alter their behaviour in ways qualitatively similar, at least in terms of receptive field size, to that following focal deafferentation. Alternatively, or in addition, there may be an important contribution of subcortical interactions to the effects observed with peripheral stimulation.
An increased input in a class of C fibres which were cold sensitive and produced a sensation of pain also distorted the body image. The painful thumb felt larger than under control conditions. This result has implications for distortions of perception associated with small- and large-fibre inputs in states of acute and chronic pain. Some C fibre inputs act tonically to limit the receptive fields of primary somatosensory cortical neurones (Calford & Tweedale, 1991b) such that their removal with capsaicin expands receptive field size. However, other interactions between small- and large-fibre inputs from somatotopically related parts occur at subcortical sites, including the dorsal column nuclei (e.g. Pettit & Schwark, 1996; Dykes & Craig, 1998) and dorsal horn (e.g. Cook et al. 1987).
This study does not reveal the site or sites for the neural interactions leading to the changes in perceived size of the lips when the input from the thumb is altered. However, it could involve cortical and subcortical sites at which the inputs from the thumb and lips are anatomically close. Inputs from the thumb and lips are adjacent within the primary and secondary somatosensory areas of primates (e.g. Robinson & Burton, 1980; Cusick et al. 1989, their Fig. 3; Lin et al. 1994) and also in the thalamus (Jones & Friedman, 1982; cf. Loe et al. 1977). Indeed, based on extracellular recordings in the monkey, some cells in the second somatosensory area have convergent inputs from both thumb and lips (Robinson & Burton, 1980). Furthermore, some cells in the primary somatosensory area of the anaesthetized monkey respond to inputs from the thumb and the lips or chin (Calford, 1997). Input from the lips projects bilaterally to somatosensory areas (Lin et al. 1994) and this may explain why the perceptual distortions showed no obvious left-right asymmetry when the input from the lips (or thumb) altered. A limitation with many estimates of somatosensory ‘maps’ is that they are rarely based on intracellular recordings which are needed to define the full range of subthreshold peripheral inputs to a cell (e.g. Smits et al. 1991). In addition, peripheral and other inputs to the somatosensory cortex may act to restrict receptive field sizes (e.g. Calford & Tweedale, 1991b; Rasmusson et al. 1992).
Changes in the thumb input failed to alter significantly the perceived size of the adjacent index finger. One possibility is that the boundaries between the thumb and lip representations are functionally less ‘distinct’ than those between the thumb and index finger. There is some indirect evidence for this. Studies of syndactyly suggest that functionally independent digits have more localized cortical representations (e.g. Allard et al. 1991). Somatosensory area 3b for the human thumb is further from the index area, while non-thumb digits are represented closer together (Mogliner et al. 1993, their Fig. 1E). Because human manipulative skill depends so much on the simultaneous differential control of the index finger and thumb, both evolution and development may have required particularly distinct cortical representations for these digits. Furthermore, some kinaesthetic skills are more highly developed for the thumb than other digits (Kilbreath & Gandevia, 1993).
The present results highlight the lability of perceived sizes of body parts and indicate the need to include the sizes of body segments in analysis of kinaesthesia (Gandevia, 1996). Irrespective of the underlying mechanisms, our results provide new ways to expose the sensorimotor consequences of altered peripheral sensory inputs.
References
- Allard T, Clark SA, Jenkins WM, Merzenich MM. Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. Journal of Neurophysiology. 1991;6:1048–1058. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Solé J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Calford MB. Second Berlin Workshop on Cortical Plasticity: Mechanisms and Functional Significance. 1997. Dual hand and face representation in primary somatosensory cortex (area 3b) of Macaques: implications for mechanism of representational plasticity; p. 13. [Google Scholar]
- Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Interhemispheric transfer of plasticity in the cerebral cortex. Science. 1990;249:805–807. doi: 10.1126/science.2389146. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate expansion of receptive fields of neurons in area 3b of Macaque monkeys after digit denervation. Somatosensory and Motor Research. 1991a;8:249–260. doi: 10.3109/08990229109144748. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. C-fibres provide a source of masking inhibition to primary somatosensory cortex. Proceedings of the Royal Society of London B. 1991b;243:269–275. doi: 10.1098/rspb.1991.0041. [DOI] [PubMed] [Google Scholar]
- Campero M, Serra J, Ochoa JL. C-polymodal nociceptors activated by noxious low temperature in human skin. The Journal of Physiology. 1996;497:565–572. doi: 10.1113/jphysiol.1996.sp021789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Wall JT, Fellman DJ, Kaas JH. Somatotopic organization of the lateral sulcus of owl monkeys: area 3b, S-II, and a ventral somatosensory area. Journal of Comparative Neurology. 1989;282:169–190. doi: 10.1002/cne.902820203. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Millar J, Wall PD. The immediate shift of afferent drive of dorsal column nucleus cells following deafferentation: a comparison of acute and chronic deafferentation in gracile nucleus and spinal cord. Experimental Neurology. 1976;52:480–495. doi: 10.1016/0014-4886(76)90219-3. 10.1016/0014-4886(76)90219-3. [DOI] [PubMed] [Google Scholar]
- Dykes RW, Craig AD. Control of size and excitability of mechanosensory receptive fields in dorsal column nuclei by homolateral dorsal horn neurons. Journal of Neurophysiology. 1998;80:120–129. doi: 10.1152/jn.1998.80.1.120. [DOI] [PubMed] [Google Scholar]
- Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Largib W, Taub E. Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. NeuroReport. 1994;5:2593–2597. doi: 10.1097/00001756-199412000-00047. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Kinaesthetic illusions involving the hand which are not dependent on muscle afferents. Proceedings of the Australian Physiological and Pharmacological Society. 1994;25:31P. [Google Scholar]
- Gandevia SC. Kinesthesia: roles for afferent signals and motor commands. In: Smith J, editor. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems, Neural Control of Movement. New York: American Physiological Society, Oxford University Press; 1996. pp. 128–172. part I. [Google Scholar]
- Glasby CML, Gandevia SC. Afferent input from the human thumb changes the perceived size of the thumb and lips. Proceedings of the Australian Neuroscience Society. 1995;6:193. [Google Scholar]
- Halligan PW, Marshall JC, Wade DT, Davey J, Morrison D. Thumb in cheek? Sensory reorganization and perceptual plasticity after limb amputation. NeuroReport. 1993;4:233–236. doi: 10.1097/00001756-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain. 1912;34:102–254. [Google Scholar]
- Jones EG, Friedman DP. Projection pattern of functional components of thalamic ventrobasal complex on monkey somatosensory cortex. Journal of Neurophysiology. 1982;48:521–543. doi: 10.1152/jn.1982.48.2.521. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Plasticity of sensory and motor maps in adult mammals. Annual Review of Neuroscience. 1991;14:137–167. doi: 10.1146/annurev.ne.14.030191.001033. 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- Kelahan AM, Doetsch GS. Time-dependent changes in the functional organization of somatosensory cerebral cortex following digit amputation in adult raccoons. Somatosensory Research. 1984;2:49–81. [PubMed] [Google Scholar]
- Kew JM, Ridding MC, Rothwell JC, Passingham RE, Leigh PN, Sooriakumaran S, Frackowiak RSJ, Brooks DJ. Reorganization of cortical blood flow and transcranial magnetic stimulation maps in human subjects after upper limb amputation. Journal of Neurophysiology. 1994;72:2517–2524. doi: 10.1152/jn.1994.72.5.2517. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Neural and biomechanical specialization of human thumb muscles revealed by matching weights and grasping objects. The Journal of Physiology. 1993;472:537–556. doi: 10.1113/jphysiol.1993.sp019961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-X, Waters RS, Oladehim A, Johnson EF, McCandish CA, Dykes RW. Large unresponsive zones appear in cat somatosensory cortex immediately after ulnar nerve cut. Canadian Journal of the Neurological Sciences. 1994;21:233–247. doi: 10.1017/s0317167100041214. [DOI] [PubMed] [Google Scholar]
- Lin L-D, Murray GM, Sessle BJ. Functional properties of single neurons in the primate face primary somatosensory cortex. I. Relations with trained orofacial motor behaviors. Journal of Neurophysiology. 1994;71:2377–2390. doi: 10.1152/jn.1994.71.6.2377. [DOI] [PubMed] [Google Scholar]
- Loe PR, Whitsel BL, Dreyer DA, Metz CB. Body representation in ventrobasal thalamus of macaque: a single-unit analysis. Journal of Neurophysiology. 1977;40:1339–1355. doi: 10.1152/jn.1977.40.6.1339. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Kinesthetic sensibility. Physiological Reviews. 1978;58:763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- Melzack R, Bromage PR. Experimental phantom limbs. Experimental Neurology. 1973;39:261–269. doi: 10.1016/0014-4886(73)90228-8. 10.1016/0014-4886(73)90228-8. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983;10:639–665. doi: 10.1016/0306-4522(83)90208-7. 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynder MS, Shoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. Journal of Comparative Neurology. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Mogliner A, Grossman JAI, Ribary U, Joliot M, Volkmann J, Rapaport D, Beasley RW, Llinás RR. Somatosensory cortical plasticity in adult humans revealed by magnetoencephalography. Proceedings of the National Academy of Sciences of the USA. 1993;90:3593–3597. doi: 10.1073/pnas.90.8.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northgrave SA, Rasmusson DD. The immediate effects of peripheral deafferentation on neurons of the cuneate nucleus in raccoons. Somatosensory and Motor Research. 1996;13:103–113. doi: 10.3109/08990229609051398. [DOI] [PubMed] [Google Scholar]
- Pettit MJ, Schwark HD. Receptive field reorganization in dorsal column nuclei during temporary denervation. Science. 1993;262:2054–2056. doi: 10.1126/science.8266104. [DOI] [PubMed] [Google Scholar]
- Pettit MJ, Schwark HD. Capsaicin-induced rapid receptive field reorganization in cuneate neurons. Journal of Neurophysiology. 1996;75:1117–1125. doi: 10.1152/jn.1996.75.3.1117. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Clarendon Press; 1993. [Google Scholar]
- Ramachandran VS, Stewart M, Rogers-Ramachandran DC. Perceptual correlates of massive cortical reorganization. NeuroReport. 1992;3:583–586. doi: 10.1097/00001756-199207000-00009. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Louw DF, Northgrave SA. The immediate effects of peripheral denervation on inhibitory mechanisms in the somatosensory thalamus. Somatosensory and Motor Research. 1993;10:69–80. doi: 10.3109/08990229309028825. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Webster HH, Dykes RW. Neuronal response properties within subregions of raccoon somatosensory cortex 1 week after digit amputation. Somatosensory and Motor Research. 1992;9:279–289. doi: 10.3109/08990229209144777. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Allard TT, Jenkins WM, Merzenich MM. Receptive-field changes induced by peripheral nerve stimulation in SI of adult cats. Journal of Neurophysiology. 1990;63:1213–1225. doi: 10.1152/jn.1990.63.5.1213. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatotopographic organization in the second somatosensory area of M. fascicularis. Journal of Comparative Neurology. 1980;193:43–67. doi: 10.1002/cne.901920104. [DOI] [PubMed] [Google Scholar]
- Schady W, Braune S, Watson S, Torebjörk E, Schmidt R. Responsiveness of the somatosensory system after nerve injury and amputations in the human hand. Annals of Neurology. 1994;36:68–75. doi: 10.1002/ana.410360114. [DOI] [PubMed] [Google Scholar]
- Smits E, Gordon DC, Witte S, Rasmusson DD, Zarzecki P. Synaptic potentials evoked by convergent somatosensory and corticocortical inputs in raccoon somatosensory cortex: substrates for plasticity. Journal of Neurophysiology. 1991;66:688–695. doi: 10.1152/jn.1991.66.3.688. [DOI] [PubMed] [Google Scholar]
- Yang TT, Gallen CC, Ramachandran VS, Cobb S, Schwartz BJ, Bloom FE. Noninvasive detection of cerebral plasticity in adult human somatosensory cortex. NeuroReport. 1994;5:701–704. doi: 10.1097/00001756-199402000-00010. [DOI] [PubMed] [Google Scholar]
- Zarzecki P, Witte S, Smits E, Gordon DC, Kirchberger P, Rasmusson DD. Synaptic mechanisms of cortical representational plasticity: somatosensory and corticocortical EPSPs in reorganized raccoon SI cortex. Journal of Neurophysiology. 1993;69:1422–1432. doi: 10.1152/jn.1993.69.5.1422. [DOI] [PubMed] [Google Scholar]


