Abstract
Apolipoprotein E- (apoE) deficient (E−/−) mice develop severe hyperlipidemia and diffuse atherosclerosis. Low-dose expression of a human apoE3 transgene in macrophages of apoE-deficient mice (E−/−hTgE+/0), which results in about 5% of wild-type apoE plasma levels, did not correct hyperlipidemia but significantly reduced the extent of atherosclerotic lesions. To investigate the contribution of apoE to reverse cholesterol transport, we compared plasmas of wild-type (E+/+), E−/−, and E−/−hTgE+/0 mice for the appearance of apoE-containing lipoproteins by electrophoresis and their capacity to take up and esterify 3H-labeled cholesterol from radiolabeled fibroblasts or J774 macrophages. Wild-type plasma displayed lipoproteins containing apoE that were the size of high density lipoprotein and that had either electrophoretic α or γ mobilities. Similar particles were also present in E−/−hTgE+/0 plasma. Depending on incubation time, E−/− plasma released 48–74% less 3H-labeled cholesterol from fibroblasts than E+/+ plasma, whereas cholesterol efflux into E−/−hTgE+/0 plasma was only 11–25% lower than into E+/+ plasma. E−/−hTgE+/0 plasma also released 10% more 3H-labeled cholesterol from radiolabeled J774 macrophages than E−/− plasma. E+/+ and E−/−hTgE+/0 plasma each esterified significantly more cell-derived 3H-labeled cholesterol than E−/− plasma. Moreover, E−/− plasma accumulated much smaller proportions of fibroblast-derived 3H-labeled cholesterol in fractions with electrophoretic γ and α mobility than E+/+ and E−/−hTgE+/0 plasma. Thus, low-dose expression of apoE in macrophages nearly restored the cholesterol efflux capacity of apoE-deficient plasma through the formation of apoE-containing particles, which efficiently take up cell-derived cholesterol, and through the increase of cholesterol esterification activity. Thus, macrophage-derived apoE may protect against atherosclerosis by increasing cholesterol efflux from arterial wall cells.
Keywords: reverse cholesterol transport, high density lipoprotein, subclasses, lecithin:cholesterol acyltransferase, atherosclerosis
Apolipoprotein E (apoE) plays a pivotal role in transport of plasma lipids. Predominantly synthesized by the liver, apoE is a structural component of chylomicron remnants, very low density lipoproteins, intermediate density lipoproteins, and some subpopulations of high density lipoproteins (HDLs) (1–3). As a ligand of at least two hepatic apoE receptors, apoE mediates the removal of cholesterol-rich lipoproteins from the circulation (4). ApoE deficiency in both humans and mice results in massive remnant hyperlipidemia and early onset of diffuse atherosclerosis (5–9).
Macrophages also produce apoE, although to a much lesser extent than hepatocytes, and contribute significantly to the initiation and progression of atherosclerosis (reviewed in 10, 11). In fact, atherosclerosis can be inhibited by transplantation of bone marrow, which contains stem cells for macrophage generation, from apoE-producing mice into apoE-deficient mice and by selective expression of a human apoE transgene in macrophages of apoE-deficient mice (12–14). Conversely, implantation of bone marrow from apoE-deficient mice promotes atherosclerosis in wild-type mice (15). Since neither expression of a human apoE transgene in macrophages of apoE-deficient mice nor transplantation of apoE-deficient macrophage stem cells into wild-type mice causes significant changes in plasma lipid levels (14, 15), antiatherogenic effects of macrophage-derived apoE appears to be independent of hypolipidemic effects.
One possible explanation for the antiatherogenicity of macrophage-derived apoE is its contribution to cholesterol efflux and reverse cholesterol transport (1, 2, 10, 11). In vitro, lipid loading of macrophages stimulates the synthesis and secretion of apoE, a process that facilitates cholesterol efflux from these cells (16–22). Moreover, we and Krimbou et al. have demonstrated in human and murine plasmas a lipoprotein that contains apoE (γ-LpE) as its only protein constituent and that releases cholesterol from cells (2, 3, 23–25).
To investigate further the contribution of macrophage-derived apoE to reverse cholesterol transport, we compared the cholesterol efflux capacities of plasmas from wild-type mice, completely apoE-deficient mice (E−/−), and from apoE-deficient mice hemizygous for expression of a human apoE3 transgene in macrophages (E−/−hTgE+/0). Because the metabolic fate of macrophage-derived apoE is unknown, we also investigated the appearance of apoE-containing lipoproteins on electrophoretic gels and their ability to take up radiolabeled cholesterol from cells.
MATERIALS AND METHODS
Animals and Sample Acquisition.
Wild-type C57BL/6J mice (E+/+) were purchased from Harlan (Borchen, Germany). E−/− and E−/−hTgE+/0 mice were described previously (8, 14, 26). E−/− mice were originally generated in C57BL/6J × 129/J hybrids by gene targeting. Two pairs of mice were provided by Nobuyo Maeda (University of North Carolina, Chapel Hill, NC) and used for breeding. Selective expression of human apoE in macrophages was achieved by transgenic expression of an apoE3 gene construct containing the sheep visna virus long terminal repeat. Transgenic mice homozygous for this gene construct were crossed with E−/− mice to obtain E+/−hTgE+/0 mice. The E+/−hTgE+/0 progeny was then bred back to the E−/− mice to obtain the E−/−hTgE+/0 mice which were the subject of this study. The genotype of the mice was assessed by Southern blotting, and the lipid profiles and amounts of atherosclerosis were analyzed (14). Subsequently the E−/−hTgE+/0 mice were bred with E−/− mice to obtain a more homogeneous genetic background.
The experiments described here were performed in six independent series. Mice were fed a regular chow diet (4RF21, Mucedola, Milan, Italy). E−/− and E−/−hTgE+/0 mice were selected on the basis of similar levels of total plasma cholesterol (Table 1). At 14–16 weeks of age, nonfasting mice were anesthetized and blood was collected by cardiac or aortic puncture. Streptokinase was used as the anticoagulant (final concentration 1000 units/ml). Plasma samples were obtained by centrifugation (2000 rpm, 15 min, 4°C). In each series of experiments plasma samples from three to six mice with identical genotypes were pooled to produce several aliquots of 500 μl. The aliquots were encoded and kept at −80°C until analyzed. The experiments were performed on blinded samples, and the code was broken after analysis of the data.
Table 1.
Effect of genotype on plasma lipid levels (mg/dl)
| Wild type | E−/− | E−/−hTgE+/0 | |
| Cholesterol | 83 ± 31 | 450 ± 93 | 405 ± 71 |
| Triglycerides | 72 ± 40 | 215 ± 71 | 243 ± 52 |
Lipid levels for each genotype were measured in five pools of plasma from three to four mice each. Values are mean ± SD.
Quantification of Lipids, Protein, and Apolipoproteins.
Plasma concentrations of cholesterol and triglycerides were measured enzymatically with kits from Boehringer Mannheim. The plasma concentration of human apoE was determined by sandwich enzyme immuoassay (24).
Demonstration of Lipoproteins by Agarose Gel Electrophoresis and Nondenaturing Two-Dimensional Electrophoresis.
Aliquots of plasma (1 μl) were fractionated by electrophoresis in precast agarose gels (Ciba Corning). Lipoproteins were stained for neutral lipids by Fat Red 7B according to the manufacturer′s instructions. Alternatively, proteins were electroblotted onto nitrocellulose membranes so that the lipoproteins could be detected by their apolipoprotein content. To detect apoA-I- and apoB-containing lipoproteins, the membranes were incubated with sheep antibodies against human apoA-I or apoB (Boehringer Mannheim). Antigen–antibody complexes were then detected with a rabbit anti-sheep IgG antiserum conjugated to horseradish peroxidase (Dako) and with 4-chloronaphthol as the chromogen. To detect apoE-containing lipoproteins, we used IgG of either a sheep antiserum against human apoE (WAK Chemie, Bad Homburg, Germany) or a rabbit antiserum against mouse apoE (kindly provided by Yadong Huang, Gladstone Institute of Cardiovascular Disease, San Francisco, CA). To avoid cross-reactions of secondary antibodies with mouse IgG, which would have interfered with the detection of γ-LpE (23–25), we biotinylated anti-apoE antibodies as recommended by the manufacturer of the protein biotinylation kit (Sigma). The apoE–antibody complexes were visualized with a streptavidin-biotinylated horseradish peroxidase complex (Amersham) and a chemiluminescent blotting substrate (Boehringer Mannheim). The chemiluminescence reaction was recorded by photoimaging with the BAS1500 (Fuji, Japan).
ApoE-containing lipoproteins were also demonstrated by nondenaturing two-dimensional polyacrylamide gradient gel electrophoresis (2D-PAGGE) of mouse plasma followed by anti-apoE immunoblotting, principally as described previously (23–25). Briefly, in the first dimension, 40 μl of plasma was separated by electrophoresis at 4°C in a 0.75% agarose gel. Agarose gel strips containing the separated lipoproteins were then transferred to a 3–20% polyacrylamide gradient gel. Separation in the second dimension was performed at 40 mA at 10°C. The proteins separated in the PAGGE gel were electroblotted onto a nitrocellulose membrane. ApoE-containing lipoproteins were detected with biotinylated antibodies against either human apoE or mouse apoE as described above.
Determination of 3H-Labeled Cholesterol Efflux from Cultured Cells into Mouse Plasma or Lipoproteins.
Normal human skin fibroblasts (3 × 105; 5–10 passages old) from two donors were cultured in DMEM containing 10% fetal calf serum in either 3.5-cm dishes or in 2.5-cm wells of 12-well plates. Alternatively, 4 × 105 J774 macrophages were cultivated in 3.5-cm dishes. At about 70–80% confluence, cells were labeled in the presence of fetal calf serum with 0.5 mCi (for fibroblasts in 3.5-cm dishes) or 0.1 mCi (for fibroblasts in 2.5-cm wells or J774 macrophages in 3.5-cm dishes) [1,2-3H]-cholesterol (51.7 Ci/mmol, New England Nuclear) for 72 h at 37°C. After six washes with PBS (pH 7.4), the specific radioactivity was 5.0 ± 1.2 × 106 cpm/mg protein (fibroblasts) or 1.2 ± 0.2 × 106 cpm/mg protein (J774 cells).
To measure cholesterol efflux capacity and esterification activity of total plasma, radiolabeled fibroblasts in 2.5-cm wells were incubated for 1 min or for 1 h with plasma diluted in DMEM to a final concentration of 4% (vol:vol) (25). Radiolabeled J774 macrophages were incubated with 4% plasma for either 1 min or 1, 2, 4, 8, or 12 h. After incubation, the medium was removed, and cell debris was pelleted by centrifugation at 30,000 rpm for 15 min at 4°C. After removal of the supernatant radioactivity in a 50-μl aliquot was determined directly by scintillation spectrometry. Lipids from a 375-μl aliquot were extracted with chloroform:methanol (2:1, vol:vol) for 72 h, and unesterified cholesterol (UC) and cholesteryl esters (CEs) were separated by TLC in silica gel plates (Merck, Darmstadt, Germany) using hexane:ether (6:4, vol:vol) as the mobile phase. Cells were lysed with 1.5 ml of 0.5 M NaOH, and their lipids were extracted with hexane:isopropanol (3:2, vol:vol). The associated radioactivity was counted by scintillation spectrometry. Fractional cholesterol efflux was calculated as cpmmedium/(cpmmedium + cpmcells) × 100%. Fractional esterification rate was calculated as cpmCE/(cpmCE + cpmUC) × 100%.
To measure the uptake of cell-derived 3H-labeled UC into lipoprotein subfractions, radiolabeled fibroblasts of 3.5-cm dishes were incubated for 1 min with 0.5 ml of mouse plasma (2, 23–25). After incubation, the medium was removed, and cell debris was sedimented by centrifugation (30,000 rpm, 15 min, 4°C). Thereafter, 40-μl aliquots of plasma were separated by agarose gel electrophoresis under the conditions described above for 2D-PAGGE (2, 23–25). Each gel lane was cut into 0.5-cm segments and transferred to a vial containing 5 ml of scintillation buffer (Instant Scint Gel PL, Packard Instruments BV, Groningen, The Netherlands). This buffer completely solubilized the agarose gel so that the radioactivity of 3H-labeled cholesterol could be measured directly.
Alternatively, radiolabeled and nonradiolabeled aliquots of an identical specimen were separated in parallel by 2D-PAGGE as described above. After separation, the half of the PAGGE gel with the radiolabeled sample was stored at 4°C. The other half of the gel with the nonradiolabeled sample was electroblotted onto a nitrocellulose membrane to identify apoE-containing lipoproteins. The immunoblot was then used as a template to localize the corresponding lipoproteins in the radiolabeled half of the gel. The gel slices containing these lipoproteins were cut out and incubated with chloroform:methanol (2:1, vol:vol) for 72 h to extract their lipids. After evaporation of the organic solvent, lipids were solubilized in 5 ml of scintillation buffer and the radioactivity was measured by scintillation spectrometry (23–25).
Statistical Analysis.
Because of the considerable interassay variation in cholesterol efflux experiments (27), some results were expressed as percentages of the results obtained with E−/− plasma. The statistical significance of differences in fractional cholesterol efflux capacities and cholesterol esterification rates among the various plasmas was determined by t tests or ANOVA as indicated. All calculations were done with the Statistical Package for the Social Sciences. Data are reported as the mean ± SD.
RESULTS
Effect of Macrophage ApoE on Plasma Levels of Lipids, Lipoproteins, and ApoE.
As reported previously (8, 9, 14), E−/− and E−/−hTgE+/0 mice had severe mixed hyperlipidemia (Table 1) which was associated with an accumulation of remnant lipoproteins and a decrease in the concentration of HDL (not shown, see ref. 14), the predominant lipoprotein class in wild-type mice. The plasma concentration of human apoE in E−/−hTgE+/0 mice was 1.5–4.4 μg/ml. This level is less than 7% and 10% of the serum apoE levels in wild-type mice and humans, respectively (14, 24, 28). Neither the cholesterol and triglyceride concentrations nor lipoprotein profiles of plasma differed significantly between the E−/− and E−/−hTgE+/0 mice.
Electrophoretic Appearance of Lipoproteins.
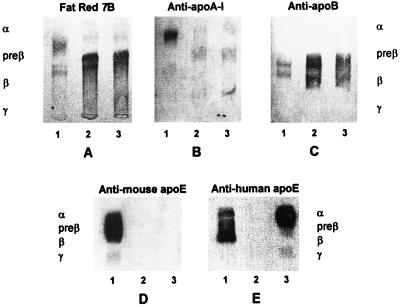
To investigate the effects of macrophage-derived apoE on the distribution of apolipoproteins among lipoproteins, plasma from each mouse strain was separated by agarose gel electrophoresis (Fig. 1). As expected, lipid staining of the gel identified the majority of lipoproteins in plasma of wild-type mice with electrophoretic α mobility and only a minority with β mobility. The α-migrating lipoprotein contained most of the anti-apoA-I and anti-apoE immunoreactive material. Minor proportions of apoA-I and apoE were found in pre-β-migrating fractions. Small proportions of apoE were also detected in a γ-migrating fraction. It is noteworthy that mouse apoE was detected by antibodies against either mouse apoE or human apoE (Figs. 1D and 1E, respectively). ApoB was detected in two bands that had β- and pre-β mobilities (Fig. 1C). In both types of apoE-deficient plasma, the majority of lipoproteins migrated as a broad β-band (Fig. 1A) which reacted with anti-apoA-I- and anti-apoB- antibodies (Fig. 1 B and C). Only a small proportion of apoA-I was found in a fraction with α mobility. Expression of apoE in macrophages did not change the electrophoretic appearance of apoA-I and apoB-containing lipoproteins. As in normal mouse plasma, the vast majority of human apoE in E−/−hTgE+/0 plasma was immunodetected in an α-migrating fraction and a minority in γ- and pre-β-migrating fractions (Fig. 1E).
Figure 1.
Electrophoretic demonstration of lipoproteins in plasmas of wild-type, E−/−, and E−/−hTgE+/0 mice. Aliquots (1 μl) of plasma were separated by electrophoresis in agarose gels. Lipoproteins were then demonstrated by staining with Fat Red 7B (A) or by immunoblotting with antibodies against human apoA-I (B), human apoB (C), murine apoE (D), or human apoE (E). Lane 1 contains E+/+ plasma; lane 2, E−/− plasma, and lane 3, E−/−hTgE+/0 plasma. Anti-human apoE antibodies cross-react with mouse apoE, whereas anti-mouse apoE antibodies do not cross-react with human apoE. In both E−/− (lane 2) and E−/−hTgE+/0 plasma (lane 3), broad β-migrating remnants immunoreacted with anti-apoA-I and anti-apoB antibodies (B and C) but not with anti-apoE antibodies. In E−/−hTgE+/0 plasma, apoE was present in lipoproteins that have electrophoretic α, pre-β, or γ mobility.
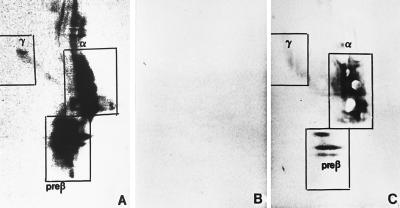
To further differentiate apoE-containing lipoproteins, plasma was separated by 2D-PAGGE. In E+/+ plasma, anti-apoE immunoblotting identified apoE-containing particles with γ mobility (i.e., γ-LpE), pre-β mobility (i.e., pre-β-LpE), and α mobility (i.e., α-LpE) (Fig. 2A). The latter two were inhomogenous with respect to size and staining intensity and hence appeared to consist of heterogenous particles. As expected, no apoE-containing particles were found in E−/− plasma (Fig. 2B). However, particles with the electrophoretic properties of γ-LpE, pre-β-LpE, and α-LpE were immunodetectable in plasma of E−/−hTgE+/0 mice (Fig. 2C).
Figure 2.
Nondenaturing 2D-PAGGE of apoE-containing lipoproteins in plasma from E+/+ (A), E−/− (B), and E−/−hTgE+/0 mice (C). For nondenaturing 2D-PAGGE agarose gel electrophoresis was followed by nondenaturing polyacrylamide gradient gel electrophoresis. After electroblotting to nitrocellulose membranes, apoE-containing lipoproteins were detected with biotinylated antibodies against either murine (A and B) or human apoE (C) and streptavidin-horseradish peroxidase. The boxes indicate the position of the various particles and also the regions that were removed in the cholesterol efflux experiments depicted in Fig. 5.
Effect of Macrophage-Derived ApoE on Cholesterol Efflux and Cholesterol Esterification Capacities of Mouse Plasma.
Depending on incubation time, E+/+ plasma released 48% (1 h) to 74% (1 min) more 3H-labeled cholesterol from fibroblasts than E−/− plasmas (Table 2) Compared with E−/− plasma, macrophage-specific expression of apoE in E−/−hTgE+/0 mice increased cholesterol efflux capacity by 37% (1 h) to 49% (1 min) (Table 2, both P < 0.001, t test). As a consequence, cholesterol efflux from fibroblasts into E−/− plasma was only 11% (1 h) to 25% (1 min) lower than that of E+/+ mouse plasma (not significant, Table 2).
Table 2.
Effect of genotype on cholesterol efflux and esterification capacities of murine plasmas
| E+/+ (N=7) | E−/− (N=12) | E−/−hTgE+/0(N=7) | E+/+ vs. E−/− | E−/− vs. E−/−hTgE+/0 | E+/+ vs. E−/−hTgE+/0 | |
|---|---|---|---|---|---|---|
| Cholesterol efflux (1-min incubation) | 174 ± 43 | 100 ± 10 | 149 ± 20 | <0.001 | 0.008 | 0.231 |
| Cholesterol efflux (1-h incubation) | 148 ± 15 | 100 ± 8 | 137 ± 18 | <0.001 | 0.004 | 0.244 |
| Cholesterol esterification (1-h incubation) | 187 ± 42 | 100 ± 7 | 164 ± 26 | <0.001 | <0.001 | 0.143 |
Plasma from several mice per genotype were pooled. In every indicated series of experiments, 4% dilutions of these plasmas were incubated for either 1 min or 1 h with [3H]cholesterol-labeled fibroblasts. Lipids were extracted from both the medium and the cells to count the radioactivity to calculate fractional cholesterol efflux rates by cpm medium/(cpmmedium+cpmcells) × 100%. Lipids of the medium were also separated by TLC to separate UC and CE and to calculate the fractional esterification rate by cpmce/(cpmce + cpmuc) × 100%. N gives the number of experiments per sample. Data were obtained from four independent series of experiments. Because of the high interassay variation, the data of all series were summarized as percentage of the fractional efflux or esterification rates which were obtained with E−/− plasma in the respective series. P values for genotype-related differences were calculated using an unpaired t test.
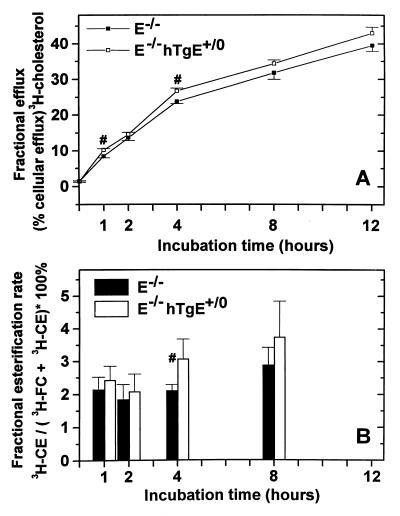
Cholesterol efflux from J774 macrophages during 1-min incubation with plasmas from E+/+, E−/−, and E−/−hTgE+/0 mice did not differ significantly. Paradoxically, 1 h and longer incubations of normolipidemic plasmas from wild-type mice with J774 macrophages led to significantly less cholesterol efflux than incubations with hyperlipidemic plasmas of E−/− or E−/−hTgE+/0 mice. Cholesterol efflux from J774 macrophages into E−/−hTgE+/0 plasma was consistently higher (mean 11%, range 5–20%) higher than into E−/− plasma during incubations of 1–12 h (P < 0.0001 for the entire curve as tested by ANOVA; P < 0.01 for 1-h and 4-h incubations as tested by t test) (Fig. 3A).
Figure 3.
Cholesterol efflux from murine J774 macrophages (A) and esterification of cell-derived cholesterol (B) by plasma from E−/− and E−/−hTgE+/0 mice. Diluted (4%) mouse plasma was incubated for increasing times with 3H-cholesterol-labeled J774 macrophages. To determine cholesterol efflux capacity, radioactivity in the medium was measured directly, and radioactivity in the cells was measured after lysis of cells with NaOH and lipid extraction. The fractional cholesterol efflux rate was calculated as cpmmedium/(cpmmedium + cpmcells) × 100% (A). To determine the esterification activity, lipids were extracted from the medium and separated by TLC to measure the radioactivity of UC and CE, and the fractional esterification rate was calculated as cpmCE/(cpmCE + cpmUC) × 100% (B). Symbols and bars represent mean values and SDs of a triplicate cholesterol efflux experiment (A) and a quintuplicate esterification experiment (B). Statistical analysis of pooled data by ANOVA showed that both the cholesterol efflux (P < 0.0001) and cholesterol esterification capacities (P = 0.005) of E−/− and transgenic E−/−hTgE+/0 mouse plasmas differed significantly. In addition symbols (#) indicate specific time points at which the cholesterol efflux and esterification capacities of E−/− and E−/−hTgE+/0 mice differed significantly upon t test (P < 0.01).
During a 1-h incubation with radiolabeled fibroblasts, E−/− plasma esterified significantly 47% and 39% less cell-derived 3H-labeled UC than plasma from wild-type or E−/−hTgE+/0 mice, respectively (Table 2). The difference between E+/+ and E−/−hTgE+/0 plasma (mean 23%) was not statistically significant. After 1- to 12-h incubations, 7–25% more cholesterol derived from J774 macrophages was esterified by E−/−hTgE+/0 plasma than by E−/− plasma (P = 0.005 for the entire curve as tested by ANOVA; P < 0.01 for the 4-h incubation as tested by t test) (Fig. 3B).
Effect of Macrophage-Derived ApoE on the Uptake of Cell-Derived Cholesterol by Lipoproteins.
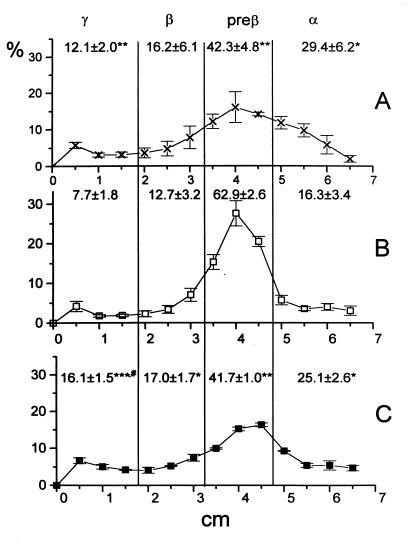
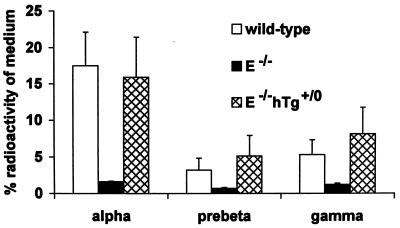
To identify the lipoproteins responsible for the defective uptake of cell-derived 3H-labeled cholesterol by E−/− plasma and for the reversal of this defect in E−/−hTgE+/0 plasma, plasmas were incubated for 1 min with 3H-cholesterol-labeled fibroblasts and then separated by agarose gel electrophoresis (Fig. 4) or 2D-PAGGE (Fig. 5). The γ- and α-fractions of E−/− plasma contained significantly less radioactivity than those from E+/+ or E−/−hTgE+/0 plasma. Compared with E+/+ plasma, E−/−hTgE+/0 plasma took up a significantly greater proportion by its γ-migrating fraction (Fig. 4). Uptake of cell-derived 3H-labeled cholesterol by the various lipoproteins was also analyzed by 2D-PAGGE to remove background radioactivity in the γ-fraction (likely from residual cell debris) and to quantify the uptake of cell-derived 3H-labeled cholesterol by the small pre-β-migrating apoE particle (see Fig. 2) which also was otherwise contaminated with background radioactivity (from pre-β-migrating remnants) (see Figs. 1 and 4). γ-LpE, pre-β-LpE, and α-LpE fractions of E+/+ and E−/−hTgE+/0 plasmas did not contain significantly different pools of radiolabeled cholesterol in the medium (Fig. 5). Compared with E−/− plasma, E+/+ and E−/−hTgE+/0 plasmas contained five to eight times more cell-derived 3H-labeled cholesterol in the γ-LpE, pre-β-LpE, and α-LpE fractions (Fig. 5).
Figure 4.
Uptake of cell-derived 3H-labeled cholesterol by various lipoproteins of apoE-plasma from wild-type (A), E−/− (B), and E−/−hTgE+/0 (C) mice. Mouse plasma was incubated for 1 min with 3H-cholesterol-labeled normal human fibroblasts. Lipoproteins from aliquots of plasma (40 μl) were then electrophoresed in 0.75% agarose gels. After electrophoresis, each lane of the gel was cut into 5-mm segments. Each segment was dissolved in scintillation buffer, and the radioactivity was determined by liquid scintillation spectrometry. The data summarize results of three experiments per genotype and represent the percentage of radioactivity in the α-, pre-β-, β-, and γ-migrating fractions of the gel. The statistical significance of differences between E−/− (∗) and wild-type mice (#) was estimated by t test. ∗ #, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Figure 5.
Uptake of cell-derived 3H-labeled cholesterol by apoE-containing lipoproteins in plasma from wild-type E+/+, E−/−, and E−/−hTgE+/0 mice. Mouse plasma was incubated for 1 min with 3H-cholesterol-labeled normal human fibroblasts. Lipoproteins from aliquots of plasma (40 μl) were then separated by 2D-PAGGE in parallel with a nonradiolabeled sample. Anti-apoE-immunoblots of 2D-electropherograms of E+/+ plasma were used to localize lipoproteins (see Fig. 3). The area containing the liporoteins were removed from the native gels, their lipids were extracted, and the radioactivity was counted. For further details, see Materials and Methods. The bars represent the percentage of radioactivity in 40 μl of medium released into the various lipoproteins and reflect mean data of two independent experiments. The percentages do not add to 100% because some lipoproteins were not removed from the gel or did not migrate into the gel (e.g., remnants) and because of incomplete recovery.
DISCUSSION
Several animal studies have pointed to an anti-atherogenic role of macrophage-derived apoE which is independent of the hypolipidemic effects of apoE (see Introduction and refs. 10, 11, 14, 15, 29). Because of apoE’s presence in certain HDL subclasses that release cholesterol from cells, several investigators have hypothesized that its antiatherogenic effects derive from its contribution to the reverse transport of excess cholesterol from arterial wall cells to the liver (1, 2, 10, 11, 16–25). To test this hypothesis, we compared plasma from wild-type mice (E+/+), apoE-deficient mice (E−/−), and apoE-deficient mice expressing human apoE expression in macrophages (E−/−hTgE+/0) for lipoprotein composition and for the ability to release 3H-labeled cholesterol from radiolabeled fibroblasts and J774 macrophages.
The small amounts of apoE in plasma of E−/−hTgE+/0 mice were predominantly found in particles with electrophoretic α mobility and, to a lesser extent, in particles with either γ or pre-β mobility. By size, these particles correspond to HDL.
Cholesterol efflux from fibroblasts into E−/− plasma was 48–74% lower than into wild-type plasma. Although producing less than 7% of the apoE levels in wild-type plasma (14, 28), expression of human apoE in macrophages of E−/−hTgE+/0 mice nearly corrected this efflux deficit. Cholesterol efflux from J774 macrophages into E−/−hTgE+/0 plasma was also significantly higher than into E−/− plasma. However, the difference in cholesterol efflux capacities of E−/− and E−/−hTgE+/0 plasmas was smaller in the presence of J774 macrophages than in the presence of fibroblasts (Table 2 and Fig. 3A).
The cholesterol efflux capacity of total plasma results from complex interactions between several components in plasma and cells (reviewed in refs. 11, 30, 31). Thus, incubation of radiolabeled cells with unlabeled plasma leads to an unspecific exchange of isotopic cholesterol molecules in cell membranes and nonisotopic cholesterol molecules in lipoproteins. Esterification of cholesterol by lecithin:cholesterol acyltransferase (LCAT) in lipid-rich, α-migrating HDL modifies this unproductive bidirectional equilibration process into slow and unsaturable net cholesterol efflux (32–34). Accordingly, regression analyses have identified levels of HDL cholesterol, apoA-I, and LCAT activity as important determinants of plasma cholesterol efflux capacity (27). In addition, lipid-free apolipoproteins and lipid-poor HDL subclasses, such as pre-β-migrating HDL, promote fast, saturable, and LCAT-independent cholesterol efflux (31, 32, 35–39).
The relative contributions of the two different types depend on the kind of cells used in efflux experiments (31–39). From studies of reverse cholesterol transport in plasma from patients with apoA-I deficiency or subjects with different apoE phenotypes and in plasma from apoA-I- or apoE-deficient mice, we have concluded that apoE is another important determinant of cholesterol efflux capacity (2, 23–25, 40). However, deficiency of either apoA-I or apoE and differences in apoE phenotype are associated with considerable changes in all lipoprotein classes, which may affect cholesterol efflux capacity independently of apoE. Since low-dose expression of apoE in macrophages of E−/−hTgE+/0 mice did not significantly alter the lipoprotein profile in apoE-deficient mouse plasma (14), our present results provide strong evidence that apoE affects cholesterol efflux directly rather than indirectly (e.g., through modulation of the lipoprotein composition in plasma). In conjunction with previous work, our studies point to three mechanisms by which apoE may regulate the ability of plasma to take up cholesterol from cells.
First, α-migrating HDL with apoE are more potent acceptors of cell-derived cholesterol than α-migrating HDL without apoE. In our experiments, the largest proportion of macrophage-derived apoE was associated with α-migrating HDL (Figs. 1 and 2). The absence of apoE from this fraction in E−/− plasma was associated with a dramatic decrease in the uptake of cell-derived 3H-labeled cholesterol from fibroblasts into α-migrating HDL. The presence of apoE in α-migrating HDL of E−/−hTgE+/0 mice nearly corrected this deficit (Figs. 4 and 5). These data are in agreement with those reported by Basu et al. (16, 17) and Hayek et al. (40). These authors showed that the capacity of apoE-depleted HDL of men or HDL of apoE-deficient mice to promote cholesterol efflux from mouse peritoneal macrophages is decreased and can be restored to normal by the addition of apoE to HDL.
Second, γ-LpE is important for the ability of plasma to promote cholesterol release from cells. In both E+/+ and E−/−hTgE+/0 plasma there is an apoE-containing lipoprotein with an electrophoretic mobility resembling that of γ-LpE from human plasma (Figs. 1 and 2) (2, 3, 23, 24). The absence of this fraction in E−/− plasma was associated with a significantly reduced proportion of cell-derived 3H-labeled cholesterol taken up by this fraction. We considered γ-LpE like pre-β1-HDL to be an initial and fast acceptor of fibroblast-derived cholesterol. Thus, it is important to note that cholesterol efflux from fibroblasts into E−/− plasma was reduced to a greater extent during a 1-min incubation than during a 1-h incubation (−28% versus E−/−hTgE+/0 plasma and −32% versus E+/+ plasma) (Table 2). The importance of the cell type for the contribution of fast cholesterol efflux onto lipid-poor acceptors (11, 31) is indicated by our observation that in the presence of J774 macrophages 1-min cholesterol efflux into E−/− plasma and E−/−hTgE+/0 plasma did not differ significantly (Fig. 3A).
Third, ApoE increases the esterification of cell-derived cholesterol by plasma. Compared with apoE-containing plasma from both E+/+ and E−/−hTgE+/0 mice, E−/−plasma had 40–45% less ability to esterify fibroblast-derived cholesterol and 10–25% less ability to esterify J774 macrophage-derived cholesterol (Table 2 and Fig. 3B). Possible reasons for this reduction include the lack of apoE as a LCAT activator (41, 42) and the absence of lipoproteins that are important for effective cholesterol esterification. In any case, the decreased ability of E−/− plasma to esterify cell-derived cholesterol appears to be causally linked to decreased cholesterol efflux, because esterification of cell-derived cholesterol by LCAT is a prerequisite for net cholesterol efflux into lipid-rich, mature HDL such as the α-migrating apoE-containing particle present in both E+/+ and E−/−hTgE+/0 plasma. Otherwise, the flux of cholesterol between plasma membranes and lipoproteins would be bi-directional and unproductive (11, 30–34).
In conclusion, apoE-containing lipoproteins appear to play an important role in regulating cholesterol efflux from cells into plasma. Even low plasma levels of apoE produced by expression of the apoE gene in macrophages restored to normal cholesterol efflux capacity of apoE-deficient plasma. This may explain why even low-level expression of the human apoE transgene in apoE-deficient mice inhibits the progression of atherosclerosis (14).
Acknowledgments
We thank Dr. Yadong Huang, Gladstone Institute of Cardiovascular Disease, San Francisco, CA, for providing us with anti-mouse apoE antiserum and Stephen Ordazy and Gary Howard for editorial assistance. This project was supported by Grant Ec116,3-2 from the Deutsche Forschungsgemeinschaft (to A.v.E).
ABBREVIATIONS
- 2D-PAGGE
two-dimensional nondenaturing polyacrylamide gradient gel electrophoresis
- apo
apolipoprotein
- CE
cholesteryl esters
- E+/+
murine apoE producing wild-type mice without human apoE transgene
- E−/−
apoE-deficient mice without human apoE transgene
- E−/−hTgE+/0
apoE-deficient mice hemizygous for expression of a human apoE3 transgene in macrophages
- HDL
high density lipoprotein
- LCAT
lecithin:cholesterol acyltransferase
- LpE
apoE-containing lipoprotein
- UC
unesterified cholesterol
References
- 1.Mahley R W. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, von Eckardstein A, Wu S, Maeda, Assmann G. Proc Natl Acad Sci USA. 1994;91:1834–1838. doi: 10.1073/pnas.91.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krimbou J L, Tremblay M, Davignon J, Cohn J S. J Lipid Res. 1997;38:35–48. [PubMed] [Google Scholar]
- 4.Ishibashi S, Herz J, Maeda N, Goldstein J L, Brown M S. Proc Natl Acad Sci USA. 1994;91:4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghiselli E, Schaefer J, Gascon P, Brewer H B., Jr Science. 1981;214:1239–1241. doi: 10.1126/science.6795720. [DOI] [PubMed] [Google Scholar]
- 6.Mabuchi H, Itoh H, Takeda M, Kajinami K, Wakasugi T, Koizumi J, Takeda R, Asagami C. Metabolism. 1989;38:115–119. doi: 10.1016/0026-0495(89)90249-7. [DOI] [PubMed] [Google Scholar]
- 7.Feussner G, Dobmeyer J, Grone H J, Lohmer S, Wohlfeil S. Am J Hum Genet. 1996;58:281–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S H, Reddick R L, Piedrahita J A, Maeda N. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 9.Plump A S, Smith J D, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft J G, Rubin E M, Breslow J L. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 10.Mazzone T. Curr Opin Lipidol. 1996;7:303–310. doi: 10.1097/00041433-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 11.von Eckardstein A. Curr Opin Lipidol. 1996;7:311–321. doi: 10.1097/00041433-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Linton M F, Atkinson J B, Fazio S. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 13.Boisvert W A, Spangenberg J, Curtiss L K. J Clin Invest. 1995;96:1118–1124. doi: 10.1172/JCI118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellosta S, Mahley R W, Sanan D A, Newland D L, Taylor J M, Pitas R E. J Clin Invest. 1995;96:2170–2179. doi: 10.1172/JCI118271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazio S, Babaev V R, Murray A B, Hasty A H, Carter K J, Gleaves L A, Atkinson J B, Linton M F. Proc Natl Acad Sci USA. 1997;94:4647–4652. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu S K, Ho Y, Brown M S, Bilheimer D W, Anderson R G W, Goldstein J L. J Biol Chem. 1982;257:9788–9795. [PubMed] [Google Scholar]
- 17.Basu S, Goldstein J L, Brown M S. Science. 1983;219:871–873. doi: 10.1126/science.6823554. [DOI] [PubMed] [Google Scholar]
- 18.Dory L. J Lipid Res. 1989;30:809–816. [PubMed] [Google Scholar]
- 19.Mazzone T, Reardon C. J Lipid Res. 1994;35:1345–1353. [PubMed] [Google Scholar]
- 20.Kruth H S, Skarlatos S I, Gaynor P M, Gamble W. J Biol Chem. 1994;269:24511–24518. [PubMed] [Google Scholar]
- 21.Zhang W Y, Gaynor P M, Kruth H S. J Biol Chem. 1996;271:28641–28646. doi: 10.1074/jbc.271.45.28641. [DOI] [PubMed] [Google Scholar]
- 22.Cullen, P., Cignarella, A., Brennhausen, B., Mohr, S., Assmann, G. & von Eckardstein, A. (1998) J. Clin. Invest., in press. [DOI] [PMC free article] [PubMed]
- 23.von Eckardstein A, Huang Y, Wu S, Funke H, Noseda G, Assmann G. Arterioscler Thromb Vasc Biol. 1995;15:691–703. doi: 10.1161/01.atv.15.5.691. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, von Eckardstein A, Wu S, Assmann G. J Clin Invest. 1995;96:2693–2701. doi: 10.1172/JCI118336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Langer C, Raabe M, Wiesenhütter B, Wu S, Seedorf U, Maeda N, Assmann G, von Eckardstein A. Arterioscler Thromb Vasc Biol. 1997;17:2010–2019. doi: 10.1161/01.atv.17.10.2010. [DOI] [PubMed] [Google Scholar]
- 26.Piedrahita J A, Zhang S H, Hagamann J, Oliver P M, Maeda N. Proc Natl Acad Sci USA. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de la Llera Moya M, Atger V, Paul J L, Fournier N, Moatti N, Giral P, Friday K E, Rothblat G H. Arterioscler Thromb. 1994;14:1056–1065. doi: 10.1161/01.atv.14.7.1056. [DOI] [PubMed] [Google Scholar]
- 28.Lusis A J, Taylor B A, Quon D, Zollman S, LeBoeuf R. J Biol Chem. 1987;262:7594–7604. [PubMed] [Google Scholar]
- 29.Shimano H, Ohsuga J, Shimada M, Namba Y, Gotoda T, Harada Y, Katsuki M, Yazaki Y, Yamada N. J Clin Invest. 1995;95:469–476. doi: 10.1172/JCI117687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fielding C J, Fielding P E. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 31.Oram J F, Yokoyama S. J Lipid Res. 1996;37:2473–2491. [PubMed] [Google Scholar]
- 32.Kawano M, Miida T, Fielding C J, Fielding P E. Biochemistry. 1993;32:5025–5028. doi: 10.1021/bi00070a008. [DOI] [PubMed] [Google Scholar]
- 33.Czarnecka H, Yokoyama S. J Biol Chem. 1996;266:2023–2028. doi: 10.1074/jbc.271.4.2023. [DOI] [PubMed] [Google Scholar]
- 34.Czarnecka H, Yokoyama S. Biochemistry. 1995;34:4385–4392. doi: 10.1021/bi00013a030. [DOI] [PubMed] [Google Scholar]
- 35.Hara H, Yokoyama S. J Biol Chem. 1991;266:3080–3086. [PubMed] [Google Scholar]
- 36.Yancey P G, Bielicki J K, Johnson W J, Lund-Katz S, Palgunachari M N, Anantharamaiah G M, Segrest J P, Phillips M C, Rothblat G H. Biochemistry. 1995;34:7955–7965. doi: 10.1021/bi00024a021. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Yokoyama S. J Biol Chem. 1995;269:26216–26223. doi: 10.1074/jbc.270.44.26216. [DOI] [PubMed] [Google Scholar]
- 38.Castro G R, Fielding C J. Biochemistry. 1988;27:25–29. doi: 10.1021/bi00401a005. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y, von Eckardstein A, Assmann G. Arterioscler Thromb. 1993;13:445–458. doi: 10.1161/01.atv.13.3.445. [DOI] [PubMed] [Google Scholar]
- 40.Hayek T, Oiknine J, Brook J G, Aviram M. Biochem Biophys Res Commun. 1994;205:1072–1078. doi: 10.1006/bbrc.1994.2775. [DOI] [PubMed] [Google Scholar]
- 41.Steinmetz A, Kaffarnik H, Utermann G. Eur J Biochem. 1985;152:747–51. doi: 10.1111/j.1432-1033.1985.tb09256.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen C H, Albers J J. Biochim Biophys Acta. 1985;836:279–285. doi: 10.1016/0005-2760(85)90131-6. [DOI] [PubMed] [Google Scholar]