Abstract
Postnatal expression of Na+ channels and development of somatic excitability were studied in dorsal horn neurones of rat using patch-clamp recordings from spinal cord slices in combination with the ‘entire soma isolation’ method.
The amplitude of Na+ current in the intact neurone in the slice increased with postnatal development (days 0-39) with a mean rate of 83 pA day−1.
The Na+ current in the neuronal soma did not increase with age and the soma, separated from the axon, was not able to fire spikes at any stage of development studied.
It is concluded that the postnatal development of the spinal dorsal horn neurone is accompanied by intensive expression of Na+ channels in the axonal but not somatic membrane. The estimated minimum density of Na+ channels in the axon initial segment is ≈160 channels μm−2.
Voltage-gated Na+ channels play a major role in the generation and propagation of action potentials in central neurones. Their properties and distribution over the excitable membrane are essential factors that determine the shape of the individual action potential as well as the pattern of complex firing behaviour. It has been shown that most Na+ channels are located in the axon hillock and axon initial segment, whereas their density in the soma is considerably lower (Catterall, 1981; Boudier et al. 1985; Wollner & Catterall, 1986; Angelides et al. 1988; Safronov et al. 1997). Increased density of Na+ channels in the axonal membrane lowers the threshold at which action potentials occur, making the axon initial segment the favourable site for action potential initiation (Coombs et al. 1957a,b; Stuart & Sakmann, 1994; Safronov et al. 1997).
A number of studies have indicated that the process of neuronal maturation is accompanied by an increase in total Na+ conductance (Spitzer, 1979; O'Dowd et al. 1988; Huguenard et al. 1988; McCobb et al. 1990). It has also been shown that complex forms of electrophysiological activity appear with the growth and morphological differentiation of neurites possessing active ionic conductances (Gruol & Franklin, 1987). In addition, molecular biology studies have revealed a striking change in the expression of different Na+ channel mRNAs in the rat brain occurring within the first month of postnatal development (Beckh et al. 1989). Unfortunately, still very little is known about the time course and spatial pattern of Na+ channel expression in the intact neurone during the period when most developmental changes in membrane excitability occur. Such a problem, however, can be solved by applying the ‘entire soma isolation’ (ESI) method, which was recently developed for studying the distribution of Na+ and K+ channels in intact neurones (Safronov et al. 1997; Wolff et al. 1998). The ESI method has allowed an estimation of the partial contributions of somatic and axonal channels to the total Na+ current of a whole neurone. It was shown that only 1/7 of all Na+ channels in a spinal dorsal horn neurone are present in the soma, whereas the majority are located in the axon initial segment. In this study the ESI method was further employed to examine the changes in proportions of somatic and axonal Na+ currents in dorsal horn neurones during postnatal development.
Our previous ESI experiments performed using 2- to 9-day-old rats (in most cases 3- to 6-day-old rats) have also revealed that the soma of dorsal horn neurones (with no axon) is not able to generate action potentials, showing only passive and local responses (Safronov et al. 1997; Wolff et al. 1998). The concept of a non-excitable soma, however, does not account for changes in neuronal excitability in the early stages of postnatal development. For example, the possibility cannot be excluded that at birth the neurones with poorly developed axons and dendrites generate simple somatic spikes. On the other hand, a possible increase in the density of Na+ channels in the somatic membrane during cell maturation might finally enable the soma to generate spikes. Therefore, further investigation of somatic excitability at different stages of postnatal development is required.
In this paper, ESI experiments were performed on spinal neurones to study the expression of Na+ channels and changes in soma excitability during the first postnatal weeks (days 0-39). It was found that the total Na+ current of a neurone progressively increases due to expression of new Na+ channels in the axonal but not the somatic membrane. The isolated somata of neurones did not generate action potentials at any stage of development studied (days 0, 3-7, 15, 21, 32 and 39). Intact dorsal horn neurones from birth to maturation therefore appear to generate action potentials of axonal origin.
METHODS
Preparation
Experiments were performed using the patch-clamp technique (Hamill et al. 1981) on 150 or 200 μm thick slices (Edwards et al. 1989) prepared from the lumbar enlargement (L3-L6) of the spinal cord of 0- (several hours after birth) to 39-day-old rats. Rats were rapidly decapitated and the spinal cords were carefully cut out. This procedure was approved by the local veterinary authority (Regierungspräsidium Giessen) and is in full accordance with German guidelines. The slices were prepared and kept according to a description given by Takahashi (1990). The study was performed on dorsal horn neurones with a soma diameter of 8-12 μm (soma size did not change noticeably with age) visually identified in laminae I-III. The neurones were distinguished from glial cells on the basis of either their ability to generate action potentials or the amplitude of the voltage-activated Na+ current, according to a procedure described previously (Safronov et al. 1997). It should be noted that the number of surviving neurones suitable for patch-clamp investigation decreased with the age of the animal, becoming very low for rats older than 30 days.
Solutions
The preparation solution, also used for maintaining the slices, contained (mM): NaCl, 115; KCl, 5.6; CaCl2, 2; MgCl2, 1; glucose, 11; NaH2PO4, 1; NaHCO3, 25 (pH 7.4 when bubbled with 95 % O2-5 % CO2). In order to reduce synaptic activity in neurones, the slices in the experimental chamber were perfused with low-Ca2+, high-Mg2+ solution which had the same composition as preparation solution except the concentrations of CaCl2 and MgCl2 were 0.1 and 5 mM, respectively. The solution used for filling the pipettes contained (mM): NaCl, 5; KCl, 144.4; MgCl2, 1; EGTA, 3; Hepes, 10 (adjusted to pH 7.3 using NaOH giving a final concentration of 10 mM). All experiments were carried out at a room temperature of 21-24°C.
Current recordings
Patch pipettes were pulled from borosilicate glass tubes (GC 150, Clark Electromedical Instruments, Pangbourne, UK). The pipettes were fire polished directly before the experiments and had a resistance of 2-7 MΩ. A List EPC-7 patch-clamp amplifier was used in all voltage- and current-clamp experiments. The effective corner frequency of the low-pass filter was 3 kHz. The frequency of digitization was 10 kHz. The data were stored and analysed with commercially available software (pCLAMP version 5.5.1, Axon Instruments). Transients and leakage currents were digitally subtracted using records with negative pulses. Offset potentials were nulled directly before formation of a seal. Junction potentials (∼3 mV) and voltage errors due to resistance in series were not corrected.
The method of ‘entire soma isolation’
A detailed description of the ‘entire soma isolation’ (ESI) method has been given elsewhere (Safronov et al. 1997). Briefly, in whole-cell recording mode, the entire soma of the neurone was isolated from the slice by slowly withdrawing the recording pipette. The isolated structure was classified as a ‘soma’ if it had lost all its processes during isolation. The isolated structure was classified as a ‘soma+axon’ complex (not used in the present analysis of somatic excitability) if it contained one process and preserved more than 90 % of the original Na+ current recorded in the slice before isolation. The good physiological state of the isolated structures was confirmed by a decrease in membrane leakage conductance, by stable or even improved membrane resting potentials and by the ability of ‘soma+axon’ complexes to generate action potentials.
The present study is based on recordings from 84 intact neurones in the slice and 72 isolated ‘somata’. All numerical values are given as means ± standard error of the mean (s.e.m.). The numbers obtained by fitting the data points using a linear least-squares procedure are given as means ± standard error (s.e.).
The input resistances were calculated from changes in the membrane current evoked by hyperpolarizing 50 ms voltage pulses from -80 to -120 mV in voltage-clamp mode. In intact neurones in the slice the input resistances decreased with the age of the animal from 4.8 ± 0.7 GΩ (n = 17 neurones) at birth to 0.6 ± 0.1 GΩ (n = 9) at day 39. The resting potentials of intact neurones at day 39 were between -70 and -80 mV (-76.5 ± 1.1 mV, n = 9). At birth the resting potentials of the neurones were less stable, indicating that the process of neuronal development was probably accompanied by an increase in the resting membrane conductance selective for K+ (equilibrium potential for K+ was -83 mV under present experimental conditions). In isolated ‘somata’ the input resistances were 6.7 ± 1.4 GΩ (n = 7) at birth and 6.0 ± 2.4 GΩ (n = 7) at day 39. Similar input resistances of isolated ‘somata’ at days 0, 21 and 39 resulted in almost equal passive membrane hyperpolarizations produced by an injection of -10 pA current pulses (see Fig. 2B).
Figure 2. Change in neuronal and somatic excitability during postnatal development.
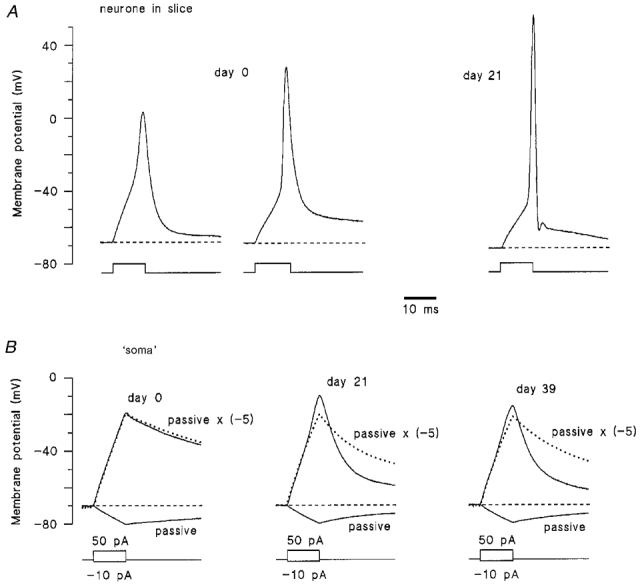
A, left-hand traces, two types of action potential recorded in different 0-day-old neurones. Right-hand trace, a typical action potential recorded from an adult (21-day-old) dorsal horn neurone. The action potentials were elicited by suprathreshold 10 ms pulses of depolarizing current. B, responses of the isolated ‘somata’ (ages indicated above the traces) to 10 ms current pulses of -10 (passive, continuous downward curve) and +50 pA (continuous upward curve). Dotted lines show the calculated passive membrane responses to +50 pA current pulses, obtained as passive response to a -10 pA current pulse multiplied by a factor of -5 and fitted with two exponentials, one for the rising phase of the response and another for its decay. The resting potentials in the isolated ‘somata’ were kept near -70 mV by injecting in- or outward steady-state currents.
RESULTS
Postnatal development is accompanied by an increase in Na+ current in the whole neurone but not in its soma
In order to study the postnatal expression of Na+ channels, we performed ESI experiments on dorsal horn neurones of 0-, 3- to 7-, 15-, 21-, 32- and 39-day-old rats. The whole-cell Na+ currents in intact neurones in the slice were compared with those recorded from their isolated ‘somata’ (Fig. 1A). The currents were activated by depolarizing 50 ms voltage pulses each following a 50 ms prepulse to -120 mV. Maximum Na+ currents, usually observed at potentials of between -30 and -10 mV, were measured in both the intact neurone and its isolated ‘soma’. The amplitude of Na+ current in the intact neurone progressively increased from 1.65 ± 0.27 nA (n = 7) after birth (day 0) to 5.44 ± 0.59 nA (n = 7) at day 39 (Fig. 1B). The data points for the intact neurones in the slice were best fitted with a linear function with a steepness factor of 83 ± 15 pA day−1. (Without allowance for insufficient space clamp of neuronal membrane, this value corresponds to an additional expression of about 100 active Na+ channels per day, assuming a single-channel amplitude of 0.85 pA at -20 mV: the fast Na+ channel as described in Safronov et al. 1997).
Figure 1. Postnatal expression of Na+ currents in the dorsal horn neurone.
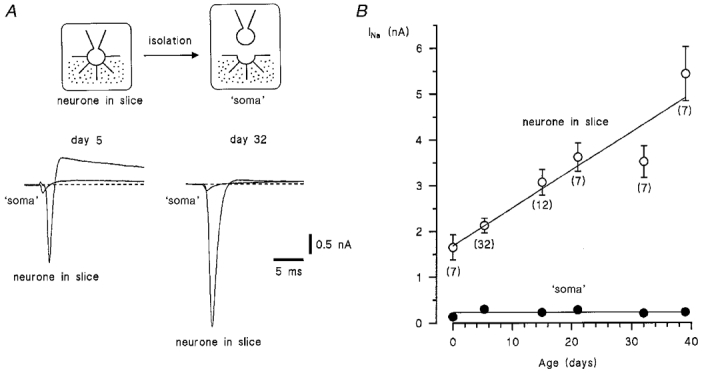
A, Na+ currents recorded from intact neurones in the slice and from their isolated ‘somata’ at postnatal days 5 and 32. Holding potential was -80 mV. The currents were activated by voltage pulses to -30 to -10 mV following 50 ms prepulses to -120 mV. B, amplitude of Na+ currents in intact neurones (○) and in their isolated ‘somata’ (•). Error bars show s.e.m. where it exceeds the symbol size; n values are given in parentheses.
In contrast to that in the intact neurone, Na+ current (INa) in the isolated ‘soma’ remained relatively constant during the first 39 days of postnatal development. The data points for the ‘soma’ could be well fitted with a straight line, INa= 231 pA (s.e. of the fit was 24 pA) (Fig. 1B, filled circles).
It is interesting to note that the proportion of somatic Na+ current related to the total Na+ current recorded from the neurone decreased with the age from 14.0 % at day 3-7 to 4.2 % at day 39.
In the present study no K+ channel blocker was used and, therefore, some overlap of inward Na+ and fast outward K+ currents could not be excluded. In order to estimate the contribution of fast K+ current, we compared the mean peak Na+ current in the isolated ‘somata’ of 3- to 7-day-old animals from this study with that from our previous work (Safronov et al. 1997; 2- to 9-day-old rats), where outward K+ currents were suppressed by external 20 mM tetraethylammonium and by replacement of internal K+ with Cs+. The mean currents in ‘somata’ were 299 ± 29 pA (n = 32) and 306 ± 27 pA (n = 52), respectively, indicating a negligible reduction in the peak Na+ current amplitude due to activation of the fast K+ conductance at the potentials studied.
Excitability of the soma
Our voltage-clamp measurements indicate that the Na+ current in the intact neurone increases during development, whereas that in the ‘soma’ remains almost unchanged. In current-clamp experiments, we compared the excitability of both the neurone and its isolated ‘soma’ at different stages of development. At birth, two types of action potential were usually seen in intact neurones (Fig. 2A, day 0). The first type has a slow rising phase and an overshoot of 0 to +15 mV. The second type rose more rapidly and had a larger overshoot of +20 to +35 mV. From a total of 17 intact neurones investigated in the slice at birth, 12 showed slow and 5 showed more rapid action potentials. Action potentials in neurones from older animals were shorter, displayed clearly larger overshoots to about +50 mV (Fig. 2A, day 21) and were often followed by a pronounced after-hyperpolarization or after-depolarization.
In contrast to those of intact neurones, the responses of isolated ‘somata’ were mostly passive and did not change considerably during development (Fig. 2B). In order to analyse responses of the ‘somata’, they were stimulated with hyperpolarizing (-10 pA) and depolarizing (+50 pA) current pulses of 10 ms duration. Hyperpolarizing current pulses were supposed to evoke only a passive membrane response (Safronov et al. 1997). By multiplying this trace by a factor of -5, we calculated the passive membrane response which would correspond to a depolarizing current pulse of +50 pA (Fig. 2B, dotted line). It can be clearly seen that the rising phase of the original ‘soma’ responses to +50 pA pulses (Fig. 2B, continuous upward curves) consisted of passive responses with only small local depolarizations and that the ‘somata’ were not able to generate action potentials at any of the stages of development investigated (days 0, 3-7, 15, 21, 32 and 39). The decay of the original ‘soma’ response became faster with age and showed more deflection from the passive membrane response in the hyperpolarized direction, indicating development of repolarizing, probably, voltage-gated potassium conductances. Injection of longer (up to 500 ms) current pulses into the isolated ‘soma’ did not result in appearance of action potentials (data not shown).
DISCUSSION
Increase in axonal but not somatic Na+ current during development
The present experiments show that over the first 39 days of postnatal development the amplitude of the Na+ current in the whole dorsal horn neurone increases, whereas that in its soma remains constant. In general, such an increase can be explained by additional Na+ channel expression in the axonal and/or dendritic membrane. A number of experimental observations, however, support the idea that the additional Na+ channel expression occurs mostly in the axonal membrane. In our study of young animals (up to day 9), the isolated ‘soma+axon’ complexes (in which the isolated part of the axon was longer than 25-30 μm) preserved 90-100 % of the original Na+ current recorded from the intact neurone before isolation (Safronov et al. 1997). This indicated a negligible contribution of the dendritic Na+ channels to the whole-cell Na+ current measured in a neurone with a patch-clamp electrode placed on its soma. In addition, several studies performed on other central neurones have shown that the density of Na+ channels in the dendrites usually does not exceed that in the soma (Huguenard et al. 1989; Stuart & Sakmann, 1994). Taking into account the fact that the amplitude of Na+ current in the isolated ‘soma’, and therefore the low channel density in the somatic membrane (1 channel μm−2, Safronov et al. 1997), did not increase with age, one can assume that the density of dendritic channels in dorsal horn neurones also remains low (not higher than 1 channel μm−2) during the first month of postnatal development. In this case an increase in the amplitude of Na+ current in the whole spinal neurone is unlikely to result from a dramatic increase in the density of dendritic channels. Thus, although we have so far failed to isolate the soma with a sufficiently long part of the axon at later developmental stages, the arguements mentioned above allow us to assume that the increase in the amplitude of the whole-cell Na+ current results from an additional expression of axonal rather than dendritic channels.
Estimation of Na+ channel density in the axon initial segment
Present experiments allowed us to make an estimation of the mean density of Na+ channels in the axon initial segment in the dorsal horn neurone. Our previous ESI experiments have shown that the majority of Na+ channels are distributed within the first 25 μm of the axon (Wolff et al. 1998). Such an estimate of the length of the axon initial segment in the dorsal horn neurone is in good agreement with mean values of 21-25 μm obtained in an anatomical study of pyramidal neurones of cat visual cortex (Farinas & DeFelipe, 1991). The diameter of non-myelinated axons of dorsal horn neurones from lamina II of adult cat spinal cord ranged from 0.1 to 2 μm (Ralston, 1968). Based on our observations made in spinal cord slices and in isolated ‘soma+axon’ complexes, the diameter of the axon initial segment in dorsal horn neurones from young rat can be estimated as 0.5 μm.
The amplitude of the axonal Na+ current calculated as the difference between Na+ current measured in the intact neurone and that in its isolated ‘soma’ was 5.2 nA at day 39 (see Fig. 1B). Assuming a single Na+ channel current of 0.85 pA at -20 mV (the fast Na+ channel, Safronov et al. 1997), and an axon initial segment length of 25 μm and diameter of 0.5 μm, one can calculate the mean density of Na+ channels to be ∼160 channels μm−2. Since saturation of Na+ current in the whole neurone was not reached at day 39 (Fig. 1B), this estimate should be considered as a lower limit of Na+ channel density. The density of Na+ channels in the axon initial segment is, therefore, two orders of magnitude higher than that in the soma (1 channel μm−2, Safronov et al. 1997). It is interesting to note that the estimated density of Na+ channels in the axon initial segment is comparable with that reported for non-myelinated axon (200 channels μm−2, Pellegrino et al. 1984), but is about 10 times lower than the Na+ channel density in the node of Ranvier (1000- 2000 channels μm−2, Vogel & Schwarz, 1995; see also Waxman, 1995).
Development of somatic excitability
It has previously been found that the soma of spinal dorsal horn neurones in the newborn rat is not able to generate action potentials and that it seems to play a complex role in cell excitability (Safronov et al. 1997; Wolff et al. 1998). Here we show further evidence that the properties of the soma do not change during the first 5 weeks of postnatal development when a dramatic increase in neuronal Na+ conductance and most changes in expression of Na+ channel mRNAs occur (Spitzer, 1979; O'Dowd et al. 1988; Huguenard et al. 1988; Beckh et al. 1989; McCobb et al. 1990). The somatic membrane has a low density of Na+ channels, which does not essentially increase with age. In this respect, dorsal horn neurones differ from rat neocortical neurones (Huguenard et al. 1989) and pyramidal neurones from rat sensorimotor cortex (Alzheimer et al. 1993) where an increse in the density of somatic Na+ channels with development was reported.
In conclusion, the postnatal development of the dorsal horn neurone is accompanied by an increase in the amplitude of axonal but not somatic Na+ current. The soma of the neurone is not able to generate action potentials. The increase in axonal Na+ conductance influences the firing properties of the neurones increasing the amplitude and rising phase of the spike.
Acknowledgments
We would like to thank Dr M. E. Bräu for helpful discussions and critically reading the manuscript, Mrs B. Agari and Mr O. Becker for excellent technical assistance. The work was supported by the Deutsche Forschungsgemeinschaft (DFG Vo188/16).
References
- Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. Journal of Neuroscience. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelides KJ, Elmer LW, Loftus D, Elson E. Distribution and lateral mobility of voltage-dependent sodium channels in neurons. Journal of Cell Biology. 1988;106:1911–1925. doi: 10.1083/jcb.106.6.1911. 10.1083/jcb.106.6.1911 (Published erratum appears in Journal of Cell Biology, 1989, vol. 108, preceding p. 2001.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckh S, Noda M, Lubbert H, Numa S. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO Journal. 1989;8:3611–3616. doi: 10.1002/j.1460-2075.1989.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudier JA, Berwald-Netter Y, Dellmann HD, Boudier JL, Couraud F, Koulakoff A, Cau P. Ultrastructural visualization of Na+-channel associated [125I]alpha-scorpion toxin binding sites on fetal mouse nerve cells in culture. Brain Research. 1985;352:137–142. doi: 10.1016/0165-3806(85)90097-5. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Localization of sodium channels in cultured neural cells. Journal of Neuroscience. 1981;1:777–783. doi: 10.1523/JNEUROSCI.01-07-00777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. The interpretation of spike potentials of motoneurones. The Journal of Physiology. 1957a;139:198–231. doi: 10.1113/jphysiol.1957.sp005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. The generation of impulses in motoneurones. The Journal of Physiology. 1957b;139:232–249. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Farinas I, Defelipe J. Patterns of synaptic input on corticocortical and corticothalamic cells in the cat visual cortex. II. The axon initial segment. Journal of Comparative Neurology. 1991;304:70–77. doi: 10.1002/cne.903040106. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Franklin CL. Morphological and physiological differentiation of Purkinje neurons in cultures of rat cerebellum. Journal of Neuroscience. 1987;7:1271–1293. doi: 10.1523/JNEUROSCI.07-05-01271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Hamill OP, Prince DA. Developmental changes in Na+ conductances in rat neocortical neurons: appearance of a slowly inactivating component. Journal of Neurophysiology. 1988;59:778–795. doi: 10.1152/jn.1988.59.3.778. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Hamill OP, Prince DA. Sodium channels in dendrites of rat cortical pyramidal neurons. Proceedings of the National Academy of Sciences of the USA. 1989;86:2473–2477. doi: 10.1073/pnas.86.7.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccobb DP, Best PM, Beam KG. The differentiation of excitability in embryonic chick limb motoneurons. Journal of Neuroscience. 1990;10:2974–2984. doi: 10.1523/JNEUROSCI.10-09-02974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'dowd DK, Ribera AB, Spitzer NC. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. Journal of Neuroscience. 1988;8:792–805. doi: 10.1523/JNEUROSCI.08-03-00792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino RG, Spencer PS, Ritchie JM. Sodium channels in the axolemma of unmyelinated axons: a new estimate. Brain Research. 1984;305:357–360. doi: 10.1016/0006-8993(84)90442-6. [DOI] [PubMed] [Google Scholar]
- Ralston HJ. The fine structure of neurons in the dorsal horn of the cat spinal cord. Journal of Comparative Neurology. 1968;132:275–302. doi: 10.1002/cne.901320205. [DOI] [PubMed] [Google Scholar]
- Safronov BV, Wolff M, Vogel W. Functional distribution of three types of Na+ channel on soma and processes of dorsal horn neurones of rat spinal cord. The Journal of Physiology. 1997;503:371–385. doi: 10.1111/j.1469-7793.1997.371bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. Ion channels in development. Annual Review of Neuroscience. 1979;2:363–397. doi: 10.1146/annurev.ne.02.030179.002051. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. The Journal of Physiology. 1990;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W, Schwarz JR. Voltage-clamp studies in axons: Macroscopic and single-channel currents. In: Waxman SG, Kocsis JD, Stys PK, editors. The Axon. New York, Oxford: Oxford University Press; 1995. pp. 257–280. [Google Scholar]
- Waxman SG. Voltage-gated ion channels in axons: Localization, function, and development. In: Waxman SG, Kocsis JD, Stys PK, editors. The Axon. New York, Oxford: Oxford University Press; 1995. pp. 218–243. [Google Scholar]
- Wolff M, Vogel W, Safronov BV. Uneven distribution of K+ channels in soma, axon and dendrites of rat spinal neurones: functional role of the soma in generation of action potentials. The Journal of Physiology. 1998;509:767–776. doi: 10.1111/j.1469-7793.1998.767bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner DA, Catterall WA. Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proceedings of the National Academy of Sciences of the USA. 1986;83:8424–8428. doi: 10.1073/pnas.83.21.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]


