Abstract
Using the perforated patch-clamp or whole-cell clamp technique, we investigated the contribution of Ca2+-activated K+ current (IK(Ca)) and non-selective cation currents (INSC) to the membrane potential in small pulmonary arterial smooth muscle cells of the rabbit.
The resting membrane potential (Vm) was -39·2 ± 0·9 mV (n = 72). It did not stay at a constant level, but hyperpolarized irregularly, showing spontaneous transient hyperpolarizations (STHPs). The mean frequency and amplitude of the STHPs was 5·6 ± 1·1 Hz and -7·7 ± 0·7 mV (n = 12), respectively. In the voltage-clamp mode, spontaneous transient outward currents (STOCs) were recorded with similar frequency and irregularity.
Intracellular application of BAPTA or extracellular application of TEA or charybdotoxin suppressed both the STHPs and STOCs. The depletion of intracellular Ca2+ stores by caffeine or ryanodine, and the removal of extracellular Ca2+ also abolished STHPs and STOCs.
Replacement of extracellular Na+ with NMDG+ caused hyperpolarization Vm of without affecting STHPs. Removal of extracellular Ca2+ induced a marked depolarization of Vm along with the disappearance of STHPs.
The ionic nature of the background inward current was identified. The permeability ratio of K+ : Cs+ : Na+ : Li+ was 1·7 : 1·3 : 1 : 0·9, indicating that it is a non-selective cation current (INSC). The reversal potential of this current in control conditions was calculated to be -13·9 mV. The current was blocked by millimolar concentrations of extracellular Ca2+ and Mg2+.
From these results, it was concluded that (i) hyperpolarizing currents are mainly contributed by Ca2+-activated K+ (KCa) channels, and thus STOCs result in transient membrane hyperpolarization, and (ii) depolarizing currents are carried through NSC channels.
Arterial tone is regulated by physiological and pathological changes, such as a decrease of O2 tension and an increase of CO2, H+ and K+, resulting in an autoregulation of local blood flow. Underlying mechanisms have been of great interest, and it has been proposed that the membrane potential of arterial smooth muscle cells plays a crucial role: depolarization of the membrane potential opens voltage-operated Ca2+ channels (VOCCs), increasing Ca2+entry, which leads to vasoconstriction (Post et al. 1992; Daut et al. 1994; Nelson & Quayle, 1995). In spite of such significance, the ion channel mechanism of resting membrane potential in vascular smooth muscle cells has not been investigated in detail.
Resting membrane potentials of arterial smooth muscle cells were reported to be in the range between -40 to approximately -60 mV, varying according to the tissues used by the authors, and the methods of measurement (Harder, 1984; Neild & Keef, 1985; Trieschmann & Isenberg, 1989; Nelson & Quayle, 1995; Nelson et al. 1995; Yuan, 1995). These values are much less negative than the theoretical K+ equilibrium potential (EK), suggesting that the resting membrane potential is not solely determined by K+ conductance, but Na+ conductance and/or Cl− conductance also contribute to some extent. Casteels et al. (1977) reported that the sodium : potassium permeability ratio (PNa/PK) is 0.22 in pulmonary artery, indicating the presence of Na+-conducting ion channels. However, the nature of the Na+-conducting channels of arterial smooth muscle cells still remains a question.
The contribution of K+ channels to the resting membrane potential has been widely investigated in many different types of cells. In cells whose resting membrane potential is around EK, such as cardiac ventricular myocytes, it is well known that inward rectifier K+ channels are responsible for providing high K+ conductance in the resting state. In arterial smooth muscle cells, however, inward rectifier K+ channels are apparently absent or very small in the normal extracellular K+ concentration, and so the input resistance of cells in the resting state is very high (e.g. in pulmonary arterial smooth muscle cells (PASMCs) it was found to be 18 GΩ by Evans et al. 1996 and 4.7 GΩ by Park et al. 1997). On the other hand, arterial smooth muscle cells have a high density of Ca2+-activated K+ (KCa) channels and voltage-activated K+ (KV) channels. Nelson et al. (1995) reported that a focal increase of Ca2+ concentration (Ca2+ sparks) occurs spontaneously in the cytosol of rat cerebral arterial smooth muscle cells, and this is responsible for the spontaneous transient outward currents (STOCs) which are carried through KCa channels. It was therefore suggested that the Ca2+-activated K+ current (IK(Ca)) is an important regulator of resting membrane potential in cerebral arterial smooth muscles, and thus important in regulating arterial tone. Although STOCs are found in various types of smooth muscles (Benham & Bolton, 1986; Nelson et al. 1995), it has not been directly investigated how the activation of the KCa channels by Ca2+ sparks affects resting membrane potential. In pulmonary arterial smooth muscle cells, the role of the KCa channels has been suggested as a target of redox modulation, and to play an important part in pulmonary vasoconstriction by hypoxia (Lee et al. 1994; Park et al. 1995a, b; Thuringer & Findlay, 1997). This hypothesis can be evaluated better when the role of the KCa channels in resting membrane potential is elucidated.
The aim of the present study was to investigate the contribution of non-selective cation currents (INSC) and IK(Ca) to the resting membrane potential in small PASMCs of the rabbit. In order to record membrane potential in physiological conditions preserving the intracellular environment of intact cells, we used the perforated patch-clamp technique (Horn & Marty, 1988). We found that: (1) hyperpolarizing currents are mainly contributed by KCa channels, and thus STOCs result in transient membrane hyperpolarization; and (2) depolarizing currents are carried through non-selective cation (NSC) channels, and their contribution to resting membrane potential is about two times greater than that of K+ selective currents.
METHODS
Cell preparation
Rabbits (1.0-2.0 kg) of either sex were anaesthetized with sodium pentobarbitone (50 mg kg−1) and injected with heparin (100 U kg−1) at the same time. The lungs were removed immediately and immersed in normal Tyrode solution. Small pulmonary arteries (outer diameter less than 400 μm), which are 3rd or 4th branches of the intralobar pulmonary arteries of a lower lobe on either side, were dissected out under the dissecting microscope and incubated at 37°C in nominally Ca2+-free normal Tyrode solution for 15 min. Then, the arteries were transferred to 3 ml of Ca2+-free normal Tyrode solution containing collagenase and elastase. After incubation for 30-50 min in enzyme-containing Ca2+-free Tyrode solution, the enzymes were washed out in enzyme-free, Ca2+-free Tyrode solution for 5 min. Then cells were isolated by gentle agitation with a fire-polished glass pipette in Kraft-Brühe (KB) medium. The isolated cells were also stored in KB medium at 4°C. The detailed procedures have been described previously (Park et al. 1995b).
Solutions and drugs
Solutions
Cells were transferred to an experimental chamber, mounted on the stage of an inverted microscope (IMT2, Olympus, Japan), and superfused with normal Tyrode solution at a temperature of 34-37°C. Normal Tyrode solution contained (mM): NaCl, 143; KCl, 5.4; NaH2PO4, 0.33; CaCl2, 1.8; MgCl2, 0.5; Hepes, 5; glucose 11; adjusted to pH 7.4 with NaOH. We substituted the NaCl in normal Tyrode solution with N-methyl-D-glucamine-Cl (NMDG-Cl) when making the low Na+ Tyrode solution (Figs 4 and 5E and F). The full substitution of the NaCl with NMDG-Cl in the normal Tyrode solution resulted in 0.33 mM Na+ concentration in 0.33 mM NaH2PO4. For the recording of perforated patches, nystatin at 200 μg ml−1 was added to the pipette solution containing (mM): KCl, 30; KOH, 118; Hepes, 10; MgCl2, 1; and methane sulphonate, 118; adjusted to pH 7.3 with KOH. The high Ca2+-buffer K+ internal solution for the patch electrode was of the following composition (mM): KCl, 20; potassium aspartate, 110; Hepes, 5; MgATP, 5; creatine phosphate, 5; BAPTA, 10; adjusted to pH 7.3 with KOH. For the recording of the non-selective cation currents (INSC), we used K+-free bath solution with low concentrations of divalent cations (divalent cation free or 0.5 mM Mg2+ only), and 148 mM CsCl, NaCl or NMDG-Cl pipette solution with high Ca2+ buffer (10 mM EGTA). The bath solution for recording the INSC contained only 148 mM NaCl, KCl, LiCl, CsCl, or NMDG-Cl with 5 mM Hepes and 11 mM glucose.
Figure 4. The effects of extracellular Na+ reduction on the membrane potential.
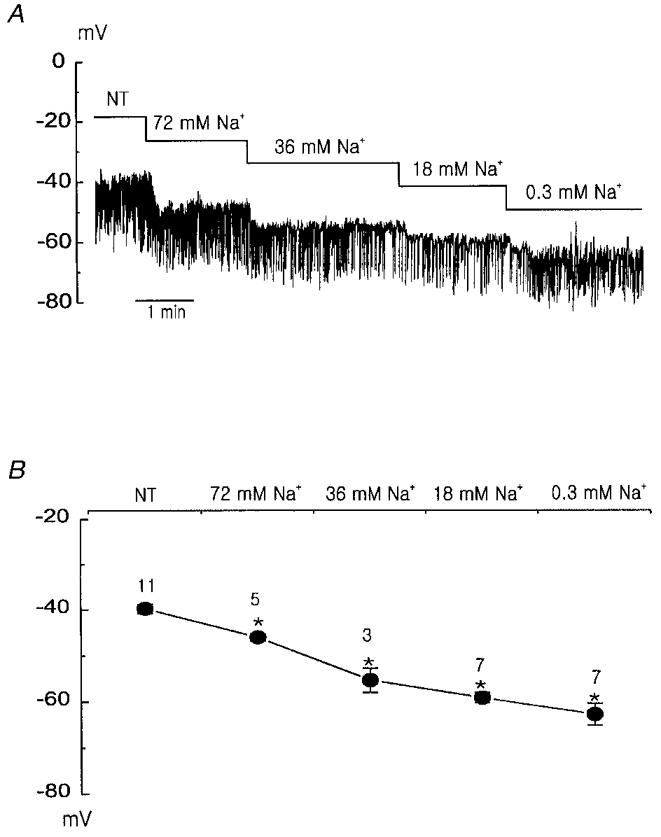
A, hyperpolarization of the membrane potential caused by a reduction in extracellular Na+ concentration. B, summary of the effects of extracellular Na+ reduction on the membrane potential. The numbers on the symbols represent the number of tested cells. * indicates that the mean values are significantly different (P < 0.05) from the value measured in 143 mM Na+ normal Tyrode (NT) solution.
Figure 5. Isolation of the background current.
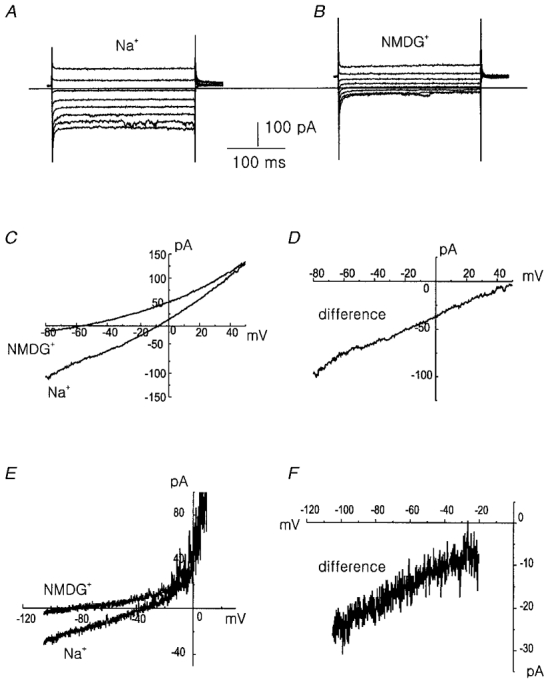
A, currents measured in 148 mM Na+ solution in response to voltage steps between -110 mV and +30 mV from the holding potential of 0 mV. B, same as A but for extracellular NMDG+ instead of Na+. The horizontal bar indicates zero current level. C, current-voltage relation in 148 mM Na+ and NMDG+ solution in response to voltage ramp. D, current-voltage relation obtained by subtracting current measured in Na+-free (NMDG+) solution from that in 148 mM Na+-containing solution. Traces A-D were recorded with CsCl pipette solution and divalent cation-free bath solution in the ruptured whole-cell mode. Similar results were obtained in 16 other cells. E, current-voltage relation measured in the normal Tyrode solution and NMDG+ Tyrode (0.3 mM Na+) solution in the perforated patch-clamp mode. F, current-voltage relation of the difference current between the normal Tyrode and NMDG+ Tyrode solution. Similar results in 3 other cells.
Drugs
K+ channels blockers such as charybdotoxin, TEA and 4-aminopyridine (4-AP) were used to block the IK(Ca) and the IK(V) and/or non-inactivating novel K+ current (IK(N)). Caffeine and ryanodine were used to deplete intracellular Ca2+ stores. Ryanodine was prepared as 10 mM stock solution in distilled water. In some experiments, Ca2+ chelator, the acetyl methyl ester form of BAPTA (BAPTA AM) which was previously dissolved in DMSO, was also used. All the chemicals except for BAPTA AM (which was from Research Biochemicals International Co.) were obtained from Sigma Chemical Co.
Electrophysiological recordings
Membrane currents and membrane potentials were recorded in nystatin-perforated patch or whole-cell configurations using an Axopatch-1D and Axopatch 200A amplifier (Axon Instruments). Data were stored on video tape with a pulse code modulator (Medical System, USA), and digitized with pCLAMP software 5.5 or 6.01 (Axon Instruments) at a sampling rate of 1-2 kHz and filtered at 0.5-5 kHz. The patch pipettes were pulled from borosilicate capillaries (Clark Electromedical Instruments, Pangbourne, UK) using a Narishige puller (PP-83, Japan). We used patch pipettes with a resistance of 2-4 MΩ when filled with the above pipette solutions. The junction potential between the pipette and standard bath (normal Tyrode) solution was 6 mV for the perforated patch solution, 7 mV for the K+ pipette solution, and 2 mV for the CsCl pipette solution (the pipette was negative to the bath, and a 3 M KCl agar bridge was used as a ground electrode). Membrane potentials presented in this paper were corrected for these junction potentials in each experimental condition. Step-pulse and ramp-pulse protocols and data acquisition were performed by a digital interface (Digidata 1200, Axon Instruments) coupled to an IBM-compatible microcomputer. The protocol of ramp pulses was that of ascending to a voltage between +60 and +80 mV from a holding potential of 0 mV followed by descending to a voltage of between -80 and -100 mV at a speed of Δ (±100 mV s−1) except for the trace in Fig. 5E, where the pulse protocol was stepped to -100 mV from a holding potential of -60 mV followed by ascending to +40 mV at a speed of Δ (+40 mV s−1). The current- voltage relations were determined while the pulse was descending from the positive potential to the negative potential except for the traces in Fig. 5E. The composite data are expressed as means ±s.e.m. (n), where n is the number of cells tested. Student's t test was used to compare values. A value of P < 0.05 was considered to be significant.
RESULTS
Recording of resting membrane potential and membrane currents
We recorded membrane potentials with the nystatin perforated-patch clamp technique. In current clamp mode, membrane potentials were not quiescent, but showed spontaneous hyperpolarizations as was shown in Fig. 1A. These spontaneous activities were also observed at room temperature (22-25°C). In the following we refer to the minimum negative level of potential as a resting membrane potential (Vm), and spontaneous transient hyperpolarization as STHP. Vm was -39.2 ± 0.9 mV (n = 72). STHPs were irregular in frequency and amplitude, and the maximum level of the STHPs was about -58 mV. The mean frequency and amplitude of the STHPs were 5.6 ± 1.1 Hz and -7.7 ± 0.7 mV (n = 12), respectively. When the cell was voltage clamped and applied with step depolarization to -46, -26, and -6 mV from the holding potential of -86 mV, current traces showed spontaneous transient outward currents (STOCs) superimposed on the slowly inactivating outward currents (Fig. 1D). The mean frequency and amplitude of the STOCs recorded at -31 mV were 5.8 ± 1.2 and 27.8 ± 3.2 mV (n = 10), respectively. The similar frequency and irregularity suggest a possibility that STHPs are attributable to STOCs. When membrane potential was hyperpolarized, the activity of STHPs or STOCs was usually decreased, but in some cells with high spontaneous activities, we could obtain the reversal potential. The reversal potentials for STHPs and that for STOCs were similar, and found at about -88 mV, which is near the calculated K+ equilibrium potential (-87.9 mV).
Figure 1. Recording of the resting membrane potential and membrane currents in the PASMCs.
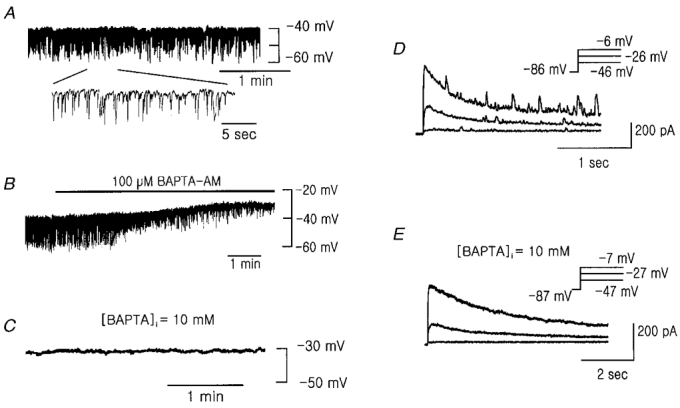
A, representative trace of membrane potential. B, effect of bath application of 100 μM BAPTA AM on the membrane potential. A and B were recorded in the perforated patch-clamp mode. C, membrane potential recorded in ruptured whole-cell clamp mode with strong Ca2+ buffer (10 mM BAPTA) in the pipette. D and E, membrane currents elicited by step depolarizations to the indicated voltage from a holding potential of -86 or -87 mV in perforated patch-clamp mode (D) and whole-cell clamp mode (E), respectively.
Since Ca2+-activated K+ current is known to produce STOCs (Benham & Bolton, 1986), we tested the effect of acetoxymethyl ester of BAPTA (BAPTA AM) on the membrane potential in order to determine the dependence of STHPs on intracellular Ca2+. It is known that BAPTA AM permeates the cellular membrane and the anionic form of BAPTA is released by the action of esterases within the cell, which then chelates intracellular Ca2+ (Nichol & Hutter, 1996). The bath application of 100 μM BAPTA AM not only inhibited STHPs, but also depolarized Vm from -38.8 ± 2.4 to -28.3 ± 1.0 mV (n = 4, Fig. 1B). When we recorded the membrane potential in the ruptured whole-cell mode using the pipette solution containing 10 mM BAPTA to lower intracellular Ca2+ concentration, Vm was -31.0 ± 2.1 mV (n = 12) and did not show STHPs (Fig. 1C). In the voltage-clamp mode with 10 mM BAPTA-containing pipette, membrane currents elicited by the same step depolarization protocol showed no STOCs but only showed slowly inactivating outward currents (Fig. 1E). These results indicate that intracellular Ca2+-dependent currents play an important role in STHPs and Vm in pulmonary arterial smooth muscle cells.
Regulation of membrane potential by IK(Ca)
In order to know whether the Ca2+-dependent current is the large conductance Ca2+-activated K+ current (maxi-K+ current, IK(Ca)), we tested the effect of TEA and charybdotoxin. TEA at either 1 or 2 mM reversibly inhibited the STHPs and slightly depolarized the resting membrane potential (Fig. 2A, left). Charybdotoxin (40 nM), a more selective blocker of IK(Ca) also showed a similar effect as TEA (Fig. 2A, right). When the cells were voltage clamped at -31 mV, the STOCs were recorded. Application of TEA or charybdotoxin to the bath solution inhibited the STOCs, in a manner similar to their effect on the STHPs (Fig. 2B). These results show that IK(Ca) is responsible for generation of the STHPs in the membrane potential and plays an important role in the regulation of resting membrane potential in pulmonary arterial smooth muscle cells.
Figure 2. Effects of KCa channel blockers on membrane potentials and membrane currents.
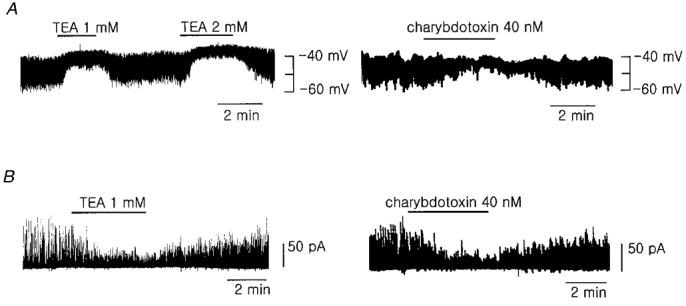
A, effect of TEA (left) and charybdotoxin (right) on the membrane potential. B, effect of TEA and charybdotoxin on the STOCs recorded at the membrane potential of -31 mV in voltage-clamp mode. Perforated patch-clamp mode was used in A and B. Similar results were obtained in 4-9 other cells.
Effect of [Ca2+]i and [Ca2+]o
The activities of IK(Ca) are greatly affected by the concentration of intracellular Ca2+ (Benham & Bolton, 1986; Thuringer & Findlay, 1997). Nelson et al. (1995) reported that focal and transient increases of Ca2+ concentration (Ca2+ sparks), which were released from the intracellular Ca2+ store, happened frequently in the subsarcolemmal space, and contributed to the hyperpolarization of the resting membrane potential and to the relaxation of the artery via the activation of IK(Ca). Since high activities of IK(Ca) in the resting PASMCs were recorded in this study (Fig. 1A, B and D and Fig. 2), we tested the effects of inhibition of the intracellular Ca2+ increase by depleting intracellular Ca2+ stores. We used high concentrations of caffeine and/or ryanodine to deplete intracellular Ca2+ stores. A high concentration of caffeine is known to deplete intracellular Ca2+ stores after a transient burst of Ca2+ release. Bath application of 10 mM caffeine induced transient hyperpolarization near to EK followed by sustained inhibition of the STHPs (Fig. 3A). In accordance with this result, a marked increase of outward current followed by sustained inhibition of STOCs was observed in the voltage-clamp mode when 10 mM caffeine was applied (Fig. 3B). The effect of caffeine was rapidly reversed after washing out the drug, but in the presence of ryanodine (4 μM), which prevents the recovery of intracellular Ca2+ stores from depletion, caffeine irreversibly inhibited the STHPs. It was not recovered even after the washout of the ryanodine and caffeine, and under this condition bath application of 1.5 mM TEA had little effect on the membrane potential (Fig. 3C). These results show that spontaneous transient intracellular Ca2+ increases, which are released from the intracellular Ca2+ store, take part in the regulation of resting membrane potential via the activation of IK(Ca) in the PASMCs.
Figure 3. Regulation of the membrane potential by caffeine, ryanodine, and .
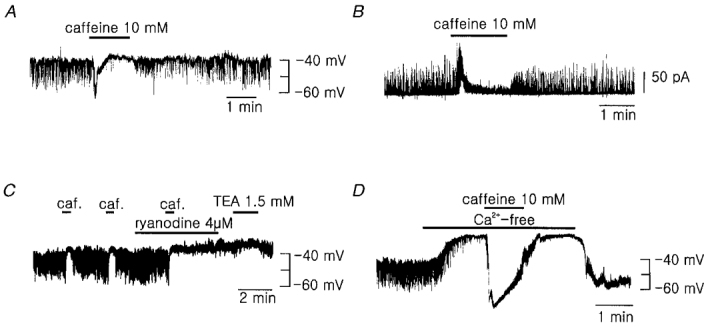
A and B, effect of bath application of 10 mM caffeine on the membrane potential (A) and membrane currents (B). The holding potential was -31 mV in trace B. C, effect of caffeine and ryanodine. TEA had little effect on the membrane potential while the STHPs were suppressed by the treatment with caffeine and ryanodine. D, effect of the extracellular Ca2+ removal (nominally Ca2+-free) on the membrane potential. Caffeine still evoked a marked hyperpolarization of the membrane potential while the STHPs were inhibited by the removal of the external Ca2+. Similar results were obtained in 3-7 other cells.
We also tested the effect of extracellular Ca2+ removal on the membrane potential in order to see whether sustained influx of Ca2+ from the extracellular space is required for transient Ca2+ release from Ca2+ stores. When we applied Ca2+-free Tyrode bath solution, the STHPs disappeared slowly, and Vm was markedly depolarized (from -40.5 ± 4.0 to -23.3 ± 3.1 mV, n = 6, Fig. 3D). In this condition the bath application of 10 mM caffeine still evoked marked hyperpolarization near to EK (Fig. 3D), which indicates that the intracellular Ca2+ store was not yet depleted. This result suggests that sustained influx of Ca2+ from the extracellular space is required for the triggering of spontaneous intracellular Ca2+ release (Ca2+-induced Ca2+ release, CICR), and thus for the activation of IK(Ca) and generation of the STHPs. One important finding noticed in this experiment is that the resting membrane potential was depolarized to a much greater extent by Ca2+ removal than by other changes which simply block IK(Ca). This result may imply that Ca2+ removal not only inhibits IK(Ca) but also increases some inward currents.
Na+ replacement
The resting membrane potential (-39.2 ± 0.9 mV) recorded in this study is much more positive than the theoretical equilibrium potential for the K+ ion (-87.9 mV, assuming [K+]i= 148 mM in this experimental condition). This implies that some other inward currents are participating in the regulation of resting membrane potential. We tested whether the membrane potential is regulated by the background cationic conductance. As the main extracellular cation was Na+, we substituted the extracellular Na+ with membrane-impermeable NMDG+. The resting membrane potential hyperpolarized progressively as Na+ concentration was decreased (Fig. 4A). However, the amplitude of STHPs was hardly affected. Changes of Vm by Na+ replacement are summarized in Fig. 4B. These results indicate that the background inward Na+ conductance is present in resting PASMCs, and it contributes to displace Vm from the EK to the more positive potential.
Background currents
Since the membrane potential was hyperpolarized by the replacement of extracellular Na+ with NMDG+, we aimed to identify the Na+-dependent background current. We used the whole-cell voltage-clamp technique with Cs+ or Na+ pipette solution and K+-free bath solution to minimize contamination by the K+ channel current. The holding potential was 0 mV in order to inactivate other remaining voltage-gated currents. Current traces recorded in response to 250 ms step pulses to potentials between -110 and +30 mV from a holding potential of 0 mV are shown in Fig. 5A. The background currents showed no time-dependent activation and inactivation. The treatment of 10 mM TEA, 4-AP, or nicardipine (up to 7 μM) had no effect on this background current (data not shown), indicating no contamination of K+ channel currents or Ca2+ channel currents. Replacement of extracellular Na+ by NMDG+ markedly reduced inward currents with no effect on the outward currents (Fig. 5B). Current-voltage relations were obtained from the currents recorded in response to the ramp pulse (from +60 mV to -80 mV for 1.4 s) in the presence and absence of Na+ (Fig. 5C) and the current-voltage relation of the difference current is shown in Fig. 5D. Replacement of Na+ in bath solution by NMDG+ induced a significant decrease of inward current and shift of the reversal potential towards more negative values. These results were obtained in divalent cation-free bath solutions in order to increase the amplitudes of background currents since they were markedly reduced by the application of extracellular Ca2+ and Mg2+ (Fig. 8). In order to see whether the background current is active in physiological conditions, we repeated the Na+ substitution experiment in perforated patch-clamp configuration in normal Tyrode bath solution containing 1.8 mM Ca2+ and 0.5 mM Mg2+. When we replaced Na+ by NMDG+, the decrease of inward current and negative shift of reversal potential were also observed (Fig. 5E and F), indicating the presence of inward Na+ background current in resting PASMCs under control conditions.
Figure 8. The blockade of the INSC by extracellular Ca2+ and Mg2+.
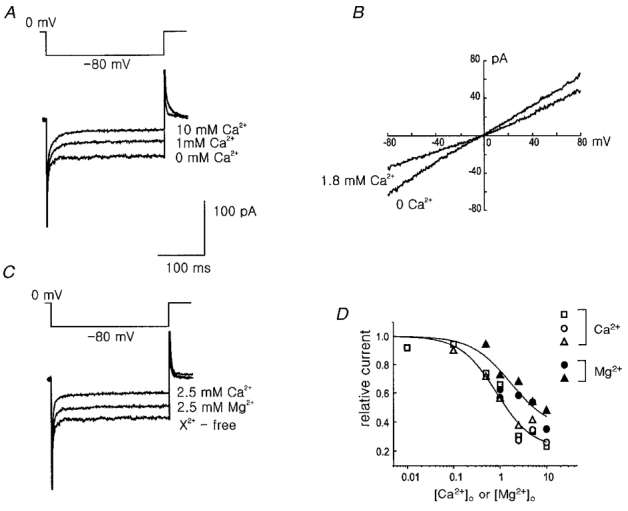
The concentrations of Ca2+ and Mg2+ are nominal. A, extracellular Ca2+ blocks the INSC in a dose-dependent manner. The currents were elicited by step voltage to -80 mV from the holding potential of 0 mV. B, current-voltage relations measured in the presence and absence of extracellular 1.8 mM Ca2+. C, blockade of the INSC by external Mg2+ and Ca2+. D, comparison of the blockade of the INSC at the various concentrations of extracellular Ca2+ and Mg2+. The current values were measured at -80 mV and expressed relative to the currents measured in the divalent cation-free bath solution. Open symbols indicate the blockade by Ca2+ and filled symbols by Mg2+. Each cell used is represented by a different shape of symbol. CsCl pipette solution was used for the traces in A and C and NaCl pipette solution was used for B.
Cation selectivity of the background currents
In order to determine the cation selectivity of the background current, we replaced extracellular Na+ by K+, Cs+, Li+, or NMDG+. The pipette solution contained 148 mM Cs+. When the same concentration of Cs+ was perfused in the bath solution, the current recorded in response to a ramp pulse showed an almost linear I-V relation with the reversal potential of 0 mV. Replacement of external cation with Na+ and Li+ reduced the inward current along with a shift of the reversal potential to a negative direction. Replacement by NMDG+ resulted in further reduction, and very little inward current remained (Fig. 6A), indicating that most of background inward current is cation current. To examine the permeability of K+ through the background cation channel, 10 mM TEA and 4-AP were applied to block the contribution of K+ channel currents (possibly due to residual non-inactivated IK(V) and/or the non-inactivating novel IK(N)). Replacement of K+ by Na+ in the presence of K+ channel blockers reduced the inward current and shifted the reversal potential in the negative direction, indicating a higher permeability of K+ than Na+ through background channels (Fig. 6B). Taken together, it can be concluded that the background current is carried by a NSC channel, and the permeability sequence is K+ > Cs+ > Na+ > Li+ >> NMDG+.
Figure 6. Cation selectivity of the background current.
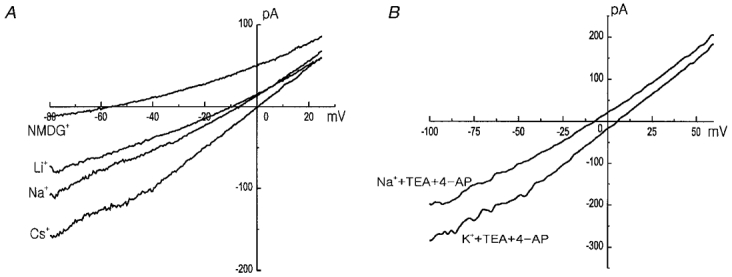
A, current-voltage relations measured in 148 mM CsCl, NaCl, LiCl, and NMDG-Cl bath solution. B, to obtain the K+ selectivity of the Na+-dependent background current, 10 mM TEA and 4-AP were added to the bath. CsCl pipette solution was used in both A and B.
The relative permeability was calculated using the following equation (see Hille, 1992) derived from the Goldman- Hodgkin-Katz equation for the bi-ionic condition:
| (1) |
where ΔErev is the change in the reversal potential, (Erev (X+) - Erev (Cs+)), and X+ is either K+, Na+, Li+ or NMDG+, F is the Faraday constant, R is the gas constant, and T is absolute temperature. The changes in the reversal potential of Na+/Cs+; K+/Cs+; Li+/Cs+; and NMDG+/Cs+ were -7.4 ± 0.2 (5); +7.3 ± 0.6 (3); -10.3 ± 1.8 (3); and -50.3 ± 1.8 (4) mV, respectively. The relative permeabilities (K+ : Cs+ : Na+ : Li+ : NMDG+) calculated from these values were 1.74 : 1.32 : 1 : 0.90 : 0.20.
From the relative permeability of PK/PNa 1.74, we calculated the theoretical reversal potential (Vrev) of the NSC channel in the experimental conditions we used for recording Vm ([K+]o= 5.4 mM, [K+]i= 148 mM, [Na+]o= 143 mM, [Na+]i assumed to be 0 mM, and T= 35°C) using the Goldman-Hodgkin-Katz voltage equation:
| (2) |
The Vrev of the NSC channel was calculated to be -13.9 mV.
In Fig. 7, we examined the contribution of the background NSC channels to the outward currents. When the pipette solution contained NMDG+ instead of Cs+, the outward currents were significantly reduced and the reversal potential was found to be at a more positive potential. The mean current-voltage relations obtained with Cs+ and NMDG+ (n = 3 for each) pipettes are shown in Fig. 7A, and the mean amplitudes of currents measured at ± 80 mV are shown in Fig. 7B. This result suggests that the NSC channel contributes not only to inward current but also to outward current.
Figure 7. The outward component of the INSC is cation dependent.
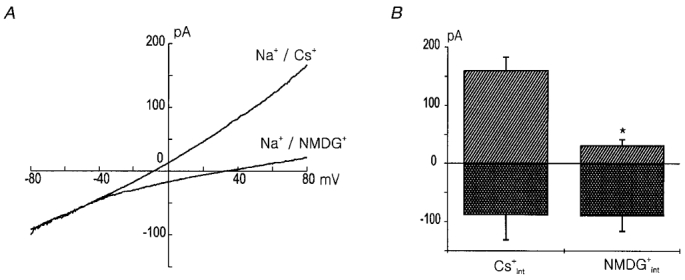
A, current-voltage relation measured in the NaCl bath solution with Cs+ and NMDG+ in the pipette. Note the difference in the amplitude of the outward currents. The current traces are means of the currents obtained from the 3 different cells for each condition. B, the amplitudes of the currents measured at a voltage of ± 80 mV in A are compared. * indicates that they are significantly different (P < 0.05).
Blockade of the INSC by external Ca2+ and Mg2+
It is well known that in many tissues background NSC channels are blocked by external divalent cations including Ca2+ and Mg2+ (Hagiwara et al. 1992; Votes et al. 1996; Mubagawa et al. 1997). In Fig. 8, we tested the effect of Ca2+ and Mg2+ on the background INSC recorded in the Na+ext/Cs+int (Fig. 8A, C and D) or Na+ext/Na+int (Fig. 8B) condition. Increase of external Ca2+ decreased background INSC in a dose-dependent manner (Fig. 8A), and the blockade was observed in the whole voltage range tested (Fig. 8B). Mg2+ also blocked the background INSC in a dose-dependent manner, but the blocking potency of Mg2+ was weaker than that of Ca2+ (Fig. 8C). Figure 8D summarizes the blockade of background INSC by extracellular Ca2+ and Mg2+ in three different cells. Concentrations for half block by Ca2+ and Mg2+ were about 0.8 mM and 2.5 mM, respectively. This result suggests that removal of external Ca2+ causes the increase of background INSC, thus contributing to the depolarization observed in Fig. 3D.
Relative contributions of KCa and NSC channels
In the present study, we found that the membrane potential of PASMCs lies in between the reversal potential of the INSC (Erev(NSC)) and that of the K+ current (Erev(K)). In Fig. 9, we summarized the membrane potential recordings obtained under different experimental conditions. The relative contribution of K+-specific current and INSC in each condition was calculated using the following equation:
| (3) |
The conductance ratio of NSC and K+ channels (GNSC : GK) was calculated as 1 : 0.52 at the resting potential, which indicates a significant role of INSC in the regulation of resting membrane potential. The K+-specific conductance increased up to about threefold when the STHPs were generated (GNSC : GK= 1 : 1.47), and it decreased in the presence of intracellular BAPTA (the membrane potential was depolarized to -29 mV), indicating that the KCa channels is mainly responsible for GK conductance. The removal of extracellular Ca2+ caused the more distinct depolarization to -23 mV, which can be interpreted as a decrease of KCa channel conductance and a increase of background GNSC at the same time. The substitution of extracellular Na+ with NMDG+ caused a remarkable hyperpolarization to -63 mV as it decreased the inward current carried through the background NSC channels.
Figure 9. The relative contribution of the NSC and K+ selective channels.
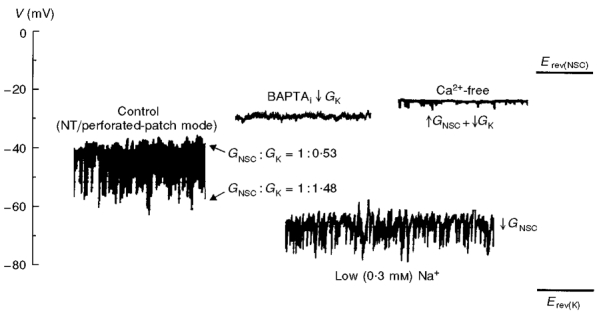
Erev(NSC) indicates the reversal potential of the background INSC and Erev(K) the reversal potential of the K+ channel, or the equilibrium potential for the K+ ion assuming the K+ selectivity of the K+ channels is complete. ↑GNSC indicates the increase of NSC channel conductance, ↓GNSC the decrease of NSC channel conductance, and ↓GK the decrease of K+ channel conductance. NT, normal Tyrode solution.
We have also considered the role of Cl− current in the membrane potential, since the presence of several types of Cl− channels were reported in vascular smooth muscle cells (Strobaek et al. 1996; Wang et al. 1997; Nelson et al. 1997). The membrane potentials and STHPs were not significantly affected by a chloride channel blocker (DIDS), or by changing internal Cl− concentration (from 30 to 7 mM or to 140 mM). Reduction of external Cl− concentration did not produce significant depolarizations (data not shown). We therefore concluded that the contribution of Cl− conductance to the membrane potential in the resting state is negligible. However, there is still a possibility that Cl− current contributes to the response to some stimuli, such as change in pressure or shear force (Nelson et al. 1997).
DISCUSSION
The aim of this study was to examine the role of different membrane currents in the regulation of resting membrane potential in small PASMCs of the rabbit. The results showed that conductance through the NSC and KCa channels are the major components that regulate the membrane potential in resting PASMCs.
Background INSC
Our results provide evidence for the presence of a background INSC in resting PASMCs and this background INSC plays a significant role in the regulation of resting membrane potential in PASMCs. The linear current-voltage relation, the permeability sequence (K+ > Cs+ > Na+ > Li+ >> NMDG+) and sensitivity for divalent cations (Fig. 8) resemble the properties of the background current described in rabbit sino-atrial node cells, cultured bovine pulmonary artery endothelial cells, and rat ventricular myocytes (Hagiwara et al. 1992; Votes et al. 1996; Mubagawa et al. 1997). Under physiological conditions this background INSC is very small because of the blockade by the extracellular divalent cations. But this small current can depolarize cells from EK, resulting in lower membrane potential. The role of INSC was also investigated in pacemaking cells of the heart (Hagiwara et al. 1992) or in pacemaking endocrine cells (GH3 cells in Simasko, 1994; rat lactrophs in Sankaranarayanan & Simako, 1996), and it was suggested that the background inward Na+ current is important in the generation of spontaneous action potentials by raising the resting membrane potential to the threshold for the activation of Ca2+ current, which is thought to be important in the secretion in these cells. The role of the background Na+ inward current (INSC) in PASMCs is not clear at present, but it is possible that it can mediate some cellular responses to the changes in extracellular or intracellular environment provided that it has a modulation site sensitive to those changes. Recently, ion channels are attracting attention as a sensor or effector of many cellular responses; O2-sensitive K+ channel in chemotransduction in the carotid body (Lopez-Barneo et al. 1988), O2-sensitive Ca2+ channel in hypoxic relaxation of arterial smooth muscle cell (Franco-Obregon et al. 1995), and ATP-sensitive K+ channel in the secretion of insulin in β-cells (Rorsman et al. 1991). In PASMCs, the role of the K+ channel in regulating membrane potential has been mainly considered as a mechanism of hypoxic response (Post et al. 1992; Park et al. 1995b, 1997; Yuan, 1995). But the result of the present study (GNSC : GK= 1 : 0.52) showed the important role of background INSC in the regulation of membrane potential, suggesting the possibility that the tiny modulation of INSC current may mediate many cellular responses to various stimuli. On the other hand, the relatively large background inward Na+ current implies that active Na+ extrusion mechanisms are operational in the resting PASMCs. Since most of the extrusion mechanisms such as the Na+ pump are active processes which require ATP, it is also possible that quite a high degree of increase in intracellular Na+ concentration would be caused by metabolic inhibition or hypoxia. Therefore, the effect of intracellular Na+ on cellular function needs to be examined as a possible mechanism of hypoxic response.
Activities of IK(Ca) and [Ca2+]i in resting PASMCs
The present study clearly demonstrates that the activity of IK(Ca) which is dependent on the intracellular Ca2+ store and extracellular Ca2+ contributes to resting membrane potential and to STHPs in PASMCs. Although IK(Ca) can be recorded in most types of smooth muscle cells, its role in membrane potential has not been well recognized until recently (Nelson et al. 1995; Gokina et al. 1996). Nelson et al. (1995) observed spontaneous and focal increases in Ca2+ concentration (Ca2+ sparks) in the cytosol of rat cerebral artery smooth muscle cells, and found that the Ca2+ sparks occurred more frequently in the subsarcolemmal space than in the deep cytosol. They suggested that Ca2+ sparks activate IK(Ca), resulting in STOCs and membrane hyperpolarization. Such information is not available yet in PASMCs, but the disappearance of STHPs following treatment with caffeine and ryanodine in the present study implies that similar Ca2+ sparks also occur and contribute to the regulation of membrane potential via the activation of IK(Ca) in the PASMCs.
It is very strange that STHPs in the resting state, however, have rarely been reported previously (Trieschmann & Isenberg, 1989), although STOCs can be recorded in most types of smooth muscle cells. At this moment we do not know the exact reasons for this discrepancy. Considering the fact that most studies investigating membrane potential were performed in tissue preparation using microelectrodes, we may postulate that cell to cell coupling may cause the difference. In that condition, it is probable that the random and spontaneous activities of IK(Ca) (reflected as STOCs in the voltage-clamp mode) in each PASMC cannot produce individual STHPs, but are integrated to a certain averaged level, resulting in a resting potential value in tissues more hyperpolarized than in single cells. The presence of cell coupling in rabbit pulmonary artery was suggested by Casteels et al. (1977) from the length constant of the tissue (1.48 mm) and the time constant of the membrane (182 ms).
Contributions of other channels
Although we have presented evidence that IK(Ca) provides a major K+-specific conductance regulating membrane potential of PASMCs in the resting state, many previous reports have focused on the role of voltage-dependent K+ (KV) channels (Post et al. 1995; Yuan, 1995). Actually, the electrophysiological characteristics of IK(V) (the range of membrane potential for the window current) and the effect of blocking IK(V) with 4-AP on the membrane potential suggests that this K+ current is also a very important regulator of resting membrane potential in PASMCs. Recently, a non-inactivating novel K+ current (IK(N)) was identified (Evans et al. 1996), which has a low threshold for activation and thus is very likely to be participating in the regulation of resting membrane potential. This novel K+ current was also blocked by millimolar concentrations of 4-AP. But in most experiments Evans et al. (1996) used a high concentration of Ca2+ buffer in the pipette solutions or loaded the cells with Ca2+ indicator fluorescence dyes such as fura-2 or indo-1, which themselves are strong Ca2+ buffers. Under such experimental conditions, IK(Ca) must be more suppressed than in intact cells, and it is possible that the role of other K+ currents might be somewhat overestimated. So we have tried to evaluate the role of 4-AP sensitive currents in the regulation of resting membrane potential with the perforated patch-clamp technique. 4-AP caused remarkable depolarization at concentrations above 5 mM but this was accompanied by significant inhibition of STHPs (data not shown). In these cells the STOCs were almost completely blocked by the 4-AP at a voltage of -40 mV (data not shown) in the voltage-clamp mode, suggesting that the effect of 4-AP is not selective and IK(Ca) is also inhibited. So, the evaluation of the relative roles of IK(V), IK(N) and IK(Ca) does not seem to be possible by using 4-AP, and more selective blockers are necessary.
Acknowledgments
This work was supported by grants from the Korea Science and Engineering Foundation (97-0403-1301-5) and the Basic Medical Research Fund from the Korea Research Foundation (1997).
References
- Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of rabbit. The Journal of Physiology. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R, Kitamura K, Kuriyama H, Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. The Journal of Physiology. 1977;271:41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J, Standen NB, Nelson MT. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. Journal of Cardiovascular Electrophysiology. 1994;5:154–181. doi: 10.1111/j.1540-8167.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Evans AM, Osipenko ON, Gurney AM. Properties of a novel K+ current that is active at resting potential in rabbit pulmonary artery smooth muscle cells. The Journal of Physiology. 1996;496:407–420. doi: 10.1113/jphysiol.1996.sp021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Obregon A, Urena J, Lopez-Barneo J. Oxygen-sensitive calcium channels in vascular smooth muscle and their possible role in hypoxic arterial relaxation. Proceedings of the National Academy of Sciences of the USA. 1995;92:4715–4719. doi: 10.1073/pnas.92.10.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokina NI, Wellman TD, Bevan RD, Walters CL, Penar PL, Bevan JA. Role of Ca2+ activated K+ channels in the regulation of membrane potential and tone of smooth muscle in human pial arteries. Circulation Research. 1996;79:881–886. doi: 10.1161/01.res.79.4.881. [DOI] [PubMed] [Google Scholar]
- Hagiwara N, Irisawa H, Kasanuki H, Hosoda S. Background current in sino-arterial node cells of the rabbit heart. The Journal of Physiology. 1992;448:53–72. doi: 10.1113/jphysiol.1992.sp019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circulation Research. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. MA, USA: Sinauer Associates, Inc.; 1992. [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. Journal of General Physiology. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Park MK, So IS, Earm YE. NADH and NAD modulate Ca2+-activated K+ channels in small pulmonary arterial smooth muscle cells of the rabbit. Pflügers Archiv. 1994;427:378–380. doi: 10.1007/BF00374548. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Mubagwa K, Stengl M, Flameng W. Extracellular divalent cations block a cation non-selective conductance unrelated to calcium channels in rat cardiac muscle. The Journal of Physiology. 1997;502:235–247. doi: 10.1111/j.1469-7793.1997.235bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild TO, Keef K. Measurements of the membrane potential of arterial smooth muscle in anesthetized animals and its relationship to changes in artery diameter. Microvascular Research. 1985;30:19–28. doi: 10.1016/0026-2862(85)90034-2. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium spark. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Conway MA, Knot HJ, Brayden JE. Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. The Journal of Physiology. 1997;502:259–264. doi: 10.1111/j.1469-7793.1997.259bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. American Journal of Physiology. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nichol JA, Hutter OF. Ca2+ loading reduces the tensile strength of sarcolemmal vesicles shed from rabbit muscle. The Journal of Physiology. 1996;493:199–209. doi: 10.1113/jphysiol.1996.sp021375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Bae YM, Lee SH, Ho W-K, Earm YE. Modulation of voltage-dependent K+ channel by redox potential in pulmonary and ear arterial smooth muscle cells of the rabbit. Pflügers Archiv. 1997;434:764–771. doi: 10.1007/s004240050463. [DOI] [PubMed] [Google Scholar]
- Park MK, Lee SH, Ho W-K, Earm YE. Redox agent as a link between hypoxia and the responses of ionic channels in rabbit pulmonary vascular smooth muscle. Experimental Physiology. 1995a;80:835–842. doi: 10.1113/expphysiol.1995.sp003891. [DOI] [PubMed] [Google Scholar]
- Park MK, Lee SH, Lee SJ, Ho W-K, Earm YE. Different modulation of Ca2+-activated K+ channels by the intracellular redox potential in pulmonary and ear arterial smooth muscle cells of the rabbit. Pflügers Archiv. 1995b;430:308–314. doi: 10.1007/BF00373904. [DOI] [PubMed] [Google Scholar]
- Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Circulation Research. 1995;77:131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- Post JM, Hume JR, Archer SL, Weir EK. Direct role of potassium channel inhibition in hypoxic pulmonary vasoconstriction. American Journal of Physiology. 1992;262:C882–890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Bokvist K, Ämmälä C, Arkhammar P, Berggren PO, Larsson O, Wahlander K. Activation by adrenaline of a low-conductance G protein-dependent K+ channel in mouse pancreatic B cells. Nature. 1991;349:77–79. doi: 10.1038/349077a0. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Simako SM. A role for a background sodium current in spontaneous action potentials and secretion from rat lactotrophs. American Journal of Physiology. 1996;271:C1927–1934. doi: 10.1152/ajpcell.1996.271.6.C1927. [DOI] [PubMed] [Google Scholar]
- Simasko SM. A background sodium conductance is necessary for spontaneous depolarizations in rat pituitary cell line GH3. American Journal of Physiology. 1994;266:C709–719. doi: 10.1152/ajpcell.1994.266.3.C709. [DOI] [PubMed] [Google Scholar]
- Strobaek D, Christophersen P, Dissing S, Olesen SP. ATP activates K+ and Cl− channels via purinoceptor-mediated release of Ca2+ in human coronary artery smooth muscle. American Journal of Physiology. 1996;271:C1463–1471. doi: 10.1152/ajpcell.1996.271.5.C1463. [DOI] [PubMed] [Google Scholar]
- Thuringer D, Findlay I. Contrasting effects of intracellular redox couples on the regulation of maxi-K channels in isolated myocytes from rabbit pulmonary artery. The Journal of Physiology. 1997;500:583–592. doi: 10.1113/jphysiol.1997.sp022044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieschmann U, Isenberg G. Ca2+-activated K+ channels contributes to the resting potential of vascular myocytes: Ca2+-sensitivity is increased by intracellular Mg2+ ions. Pflügers Archiv. 1989;414:S183–184. doi: 10.1007/BF00582296. [DOI] [PubMed] [Google Scholar]
- Votes T, Droogmans G, Nilius B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. The Journal of Physiology. 1996;497:95–107. doi: 10.1113/jphysiol.1996.sp021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang Y-X, Yu M, Kotlikoff MI. Ca2+-activated Cl− currents are activated by metabolic inhibition in rat pulmonary artery smooth muscle cells. American Journal of Physiology. 1997;273:C520–530. doi: 10.1152/ajpcell.1997.273.2.C520. [DOI] [PubMed] [Google Scholar]
- Yuan XJ. Voltage-gated K currents regulate resting membrane potential and [Ca]i in pulmonary arterial myocytes. Circulation Research. 1995;77:370–378. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]


