Abstract
The tachykinin substance P was recovered from the commissural subdivision of the nucleus tractus solitarii (cNTS) using in vivo microdialysis during activation of cardiorespiratory and skeletal muscle receptors in thirteen chloralose-anaesthetized cats.
Tetanic muscle contraction was evoked by stimulating L7-S1 ventral roots (n = 7). Electrically induced muscle contraction increased mean arterial pressure (MAP) by 55 ± 10 mmHg and heart rate by 29 ± 6 beats min−1. During contraction the dialysate concentration increased 154 % above resting control levels (from 0·217 ± 0·009 to 0·546 ± 0·023 fmol (100 μl)−1, control vs. contraction, P < 0·05).
Loss of cardiorespiratory input following disruption of the carotid sinus and vagus nerves significantly blunted, but did not abolish, the increase in substance P during muscle contraction (from 0·247 ± 0·022 to 0·351 ± 0·021 fmol (100 μl)−1, control vs. contraction, P < 0·05). Approximately 44 % of the substance P release during contraction was independent of cardiorespiratory input transmitted by carotid sinus and vagus nerves.
To determine the contribution of cardiorespiratory related neural input on substance P release, an intravascular balloon positioned in the thoracic aorta was inflated to increase arterial pressure (n = 6). Balloon inflation increased MAP by 50 ± 5 mmHg and substance P increased from 0·251 ± 0·025 to 0·343 ± 0·028 fmol (100 μl)−1 (control vs. balloon inflation, P < 0·05). This increase was completely abolished following interruption of vagal and carotid sinus nerves (from 0·301 ± 0·012 to 0·311 ± 0·014 fmol (100 μl)−1, control vs. balloon inflation). This finding shows that neural input from cardiorespiratory receptors (primarily arterial baroreceptors) accounted for 37 % of the total substance P release during muscle contraction.
The findings from this study demonstrate that activation of skeletal muscle receptors and cardiorespiratory receptors (predominantly arterial baroreceptors) increases the extraneuronal concentration of substance P in the cNTS. Because substance P release was not completely abolished during muscle contraction following disruption of carotid sinus and vagus nerves it is proposed that: (1) afferent projections from contraction-sensitive skeletal muscle receptors may release substance P in the NTS; (2) neural input from muscle receptors activates substance P-containing neurones within the NTS; and (3) convergence of afferent input from skeletal muscle receptors and arterial baroreceptors onto substance P-containing neurones in the cNTS facilitates the release of substance P. The role of tachykininergic modulation of cardiorespiratory input is discussed.
The nucleus tractus solitarii (NTS) is a medullary region involved in central transmission and processing of peripheral viscerosensory input (Spyer, 1994). Neural input from peripheral cardiorespiratory receptors (arterial baroreceptors, cardiac and pulmonary receptors, peripheral chemoreceptors) and supra-bulbar regions (hypothalamus, parabrachial nucleus, sensorimotor cortex) synapse in the NTS (Jordan et al. 1988; Felder & Mifflin, 1988; Ba-M'Hamed et al. 1996). In addition, neuroanatomical (Kalia et al. 1981; Nyberg & Blomqvist, 1984; Menetrey & Basbaum, 1987) and electrophysiological (Person, 1989; McMahon et al. 1992; Toney & Mifflin, 1994) studies have shown that somatic afferent fibres also project to and alter the discharge properties of NTS neurones. These findings suggest that the NTS may be a principal relay site for viscerosomatic sensory input. However, NTS neurones also contain an abundance of neuropeptides, including neuropeptide Y, angiotensin II, vasopressin, opioids, bradykinin, substance P and related tachykinins (Maley & Elde, 1982; Van Giersbergen & De Jong, 1992; Lawrence & Jarrott, 1996). Therefore, the NTS may perform integrative functions that are controlled in a highly complex and refined manner.
The role of the tachykinin substance P in central cardiovascular regulation in the NTS has recently been an area of increasing interest. To date, there are several lines of evidence demonstrating that substance P may be involved in mediating central autonomic neurotransmission. First, biochemical and immunohistochemical evidence has demonstrated that the NTS contains substance P nerve fibres, terminals and receptors (Maley & Elde, 1982; Van Giersbergen et al. 1992; Sykes et al. 1994). Second, substance P immunoreactivity has been localized in the nodose and petrosal ganglia that contain cell bodies for primary baroreceptor, chemoreceptor and cardiopulmonary receptor afferent fibres (Helke et al. 1980, 1984). Third, in vivo microdialysis has shown that substance P is released from neurones in the NTS in response to electrical stimulation of aortic baroreceptor afferent fibres and activation of peripheral chemoreceptors by hypoxia (Lindefors et al. 1986; Morilak et al. 1988; Srinivasan et al. 1991). Fourth, localized microinjection of substance P in the NTS has been reported to evoke hypotension and bradycardia in rats (Hall et al. 1989; Chan et al. 1990). And finally, blockade of tachykinin receptors in the NTS attenuates the cardiovascular responses evoked by cardiac vagal receptors (Paton, 1998). Because some of these cardiovascular responses are similar to those evoked by the arterial baroreceptor reflex, it has been suggested that substance P may be the neurotransmitter for arterial baroreceptor afferent fibres (Gillis et al. 1981). However, it has also been suggested that substance P is not involved in modulating the arterial baroreceptor reflex (Feldman, 1995; Massari et al. 1998). Thus, although there is evidence that substance P is present and released by NTS neurones there remains debate as to whether substance P exerts a tonic modulatory action on central pathways involved in reflex autonomic control.
Despite the lack of agreement over the action of substance P on central cardiovascular regulation, it has been reported that substance P is involved in the transmission of nociceptive and non-nociceptive stimuli in spinal pathways. For example, electrically induced contraction of the triceps surae releases substance P in the dorsal horn of the lower lumbar-upper sacral spinal cord (Wilson et al. 1993a,b). Selective blockade of substance P receptors in the spinal cord has also been reported to blunt the cardiovascular responses evoked by muscle contraction (Hill et al 1992; Wilson et al 1992). These findings suggest that substance P mediates the transmission of contraction-related afferent input in the dorsal horn of the spinal cord. Because these afferent fibres project to cardiovascular-related regions in the brainstem, including the NTS (Kalia et al. 1981; Nyberg & Blomqvist, 1984; Menetry & Basbaum, 1987), it is important to determine if neural input from contraction-sensitive skeletal muscle afferent fibres is a source of tachykininergic input. Furthermore, knowledge of the origin of substance P-releasing (SPergic) inputs to the NTS may provide insight into the peptidergic mechanisms in the lower brainstem that mediate the cardiovascular responses during muscular exercise.
Two objectives are proposed in this study: (1) to determine if activation of skeletal muscle afferent fibres releases substance P in the NTS; and (2) to determine if the release of substance P is secondary to activation of cardiorespiratory receptors (arterial baroreceptors, cardiac receptors, pulmonary stretch receptors). We hypothesized that skeletal muscle contraction increases the extraneuronal concentration of substance P in the NTS independent of neural input transmitted by the carotid sinus and vagus nerves. A preliminary report of these findings has been published (Potts et al. 1997).
METHODS
General animal preparation
Experimental protocols were reviewed and approved by the Institutional Animal Care and Research Advisory Committee at The University of Texas Southwestern Medical Centre in compliance with the Animal Welfare Act and in accordance with Public Health Service Policy on Humane Care and Use of Laboratory Animals. Thirteen cats (2.5-4.0 kg) of either sex were studied. Induction of anaesthesia was accomplished by inhalation of halothane (2-3 % halothane in oxygen) using a gas anaesthesia machine. The brachial artery and vein were cannulated for measurement of arterial blood pressure and for access to the venous circulation for supplemental anaesthesia and fluids. Surgical anaesthesia was maintained by a solution of α-chloralose (80 mg kg−1, i.v.) and urethane (200 ml kg−1, i.v.). The need for supplemental anaesthesia was determined by the presence of a corneal reflex, pedal reflex, or gradual increases in heart rate and blood pressure. When supplemental anaesthesia was required, a solution of α-chloralose (15 mg kg−1) and urethane (75 mg kg−1) was administered intravenously. A tracheotomy was performed and the cat was mechanically ventilated (Model 661, Harvard Apparatus). Arterial blood gases and pH were measured every 30-45 min by an automated blood gas analyser (Model ABL-3, Radiometer) and maintained within normal ranges (arterial PO2, 80-100 mmHg; arterial PCO2, 35-45 mmHg; pH 7.3-7.4). If necessary, 100 % oxygen was supplemented to maintain Pa,O2 above 80 mmHg. Rectal temperature was continuously monitored throughout each experiment and was maintained between 37 and 38°C by a water-perfused heating pad and a near-infrared heat lamp.
Activation of skeletal muscle receptors
Contraction-sensitive skeletal muscle receptors were activated by electrically induced contraction of the triceps surae in seven animals. Briefly, a laminectomy was performed to expose spinal cord from the L5 to S2 level, and the cat was placed in a spinal unit (David Kopf Instruments, Tujunga, CA, USA). The spinal dura was opened longitudinally and the L7 and S1 spinal roots were identified. The dorsal and ventral roots of L7 and S1 were carefully dissected, and the ventral roots sectioned and positioned over bipolar platinum stimulating electrodes. The stimulating electrodes were covered in a pool of warmed mineral oil (37°C) and connected to a stimulator (Model S88, Grass Instrument Co.) via a stimulation isolation unit (Model SIU5C, Grass). A mid-line dorsal incision on the lower limb was made and the skin deflected to expose the triceps surae. The calcaneal tendon was transected and attached to a force transducer (Model F10, Grass) to measure the level of tension development. When alternating contractions of the left and right hindlimbs were performed, the calcaneal tendon from both hindlimbs was transected and attached to separate force transducers. The pelvis was stabilized in the spinal unit, and the patellar tendon was attached to a steel post. Bone wax was applied to the calcaneal tendon to minimize bleeding, and the triceps surae was kept moist by applying a gauze pad soaked in warmed (37°C) mineral oil.
Activation of cardiorespiratory receptors
Cardiorespiratory receptors (primarily arterial baroreceptors) were activated by inflation of a balloon catheter (5F, Fogarty, Baxter Healthcare, Santa Ana, CA, USA) positioned in the thoracic aorta in six animals. Graded inflation of the balloon was used to increase arterial pressure to the same level as produced by muscle contraction. The goal of this procedure was to determine the contribution of carotid sinus and vagal afferent input on substance P release in the NTS.
Denervation of cardiorespiratory input to the NTS
The destruction of the carotid sinus and vagus nerves was performed to eliminate afferent input from cardiorespiratory receptors. This was accomplished by exposing the carotid sinus regions and placing a suture around the internal carotid artery (ICA), occipital artery (OA) and carotid sinus nerve (CSN) distal to the carotid bifurcation bilaterally. Ligation of this suture effectively crushed the carotid sinus nerve and eliminated afferent input from carotid baroreceptors. A second suture was placed around the cervical vagi. This suture was ligated and the nerves sectioned to eliminate cardiac, pulmonary and aortic vagal inputs. Together, these two procedures eliminated neural input to the NTS carried by the vagus and carotid sinus nerves. The effectiveness of this procedure was confirmed when the reflex tachycardia produced by bilateral carotid occlusion or the reflex bradycardia produced by aortic balloon inflation was reduced by 90 % of the control response.
Recovery of substance P from the NTS using microdialysis
A limited occipital craniotomy was performed to expose the dorsal surface of the brainstem, and the cerebellum was retracted 2-3 mm rostrally to expose the dorsal surface of the medulla using a customized retractor. A microdialysis probe (model CMA 10, Bioanalytic Systems Inc., West Lafayette, IN, USA) with a 1 mm membrane (0.5 mm o.d.) was stereotaxically positioned in the commissural subdivision of the NTS ipsilateral to the contracting hindlimb. The insertion co-ordinates were made at the level of the calamius scriptoris, 1.5 mm lateral to the mid-line, 1.5 mm ventral of the dorsal surface of the medulla. When both hindlimbs were contracted the probe was randomly placed into either the left or right quadrant of the NTS. The probe was continuously perfused at 3 μl min−1 with a physiological salt solution containing 0.2 % bovine serum albumin, 0.1 % bacitracin and the following ions (mM): K+, 6.2; Cl−, 134; Ca2+, 2.4; Na+, 150; H2PO4−, 1.3; HCO3−, 13; Mg2+, 1.3) buffered to pH 7.4. This solution was prepared fresh before each experiment. The molecular mass cut-off of the dialysis membrane was 10 kDa.
Prior to the experiment, the microdialysis probe was perfused at 3 μl min−1 with a protein solution (1 % polypeptide and 1 % sodium azide in 0.9 % saline solution) for 12 h to saturate the protein binding sites on the dialysis membrane prior to in vivo use. We have found that this procedure improves the in vivo recovery of substance P (J. Potts, unpublished personal observations). During the actual experiment, dialysate samples were collected directly on ice and immediately frozen in liquid nitrogen. The samples were then stored at -80°C until analysed for substance P-like immunoreactivity (SP-LI).
Measurement of substance P-like immunoreactivity by radioimmunoassay (RIA)
SP-LI was measured by a RIA procedure that was developed in our laboratory (Wilson et al. 1993a,b). Briefly, removable polystyrene microplate wells were coated with 1 μg purified protein A (Purified Recomb Protein A, binding 1.3 mg rabbit IgG per mg) in 100 μl of 0.2 mol l−1 sodium borate (pH 9.0). Following a 24 h incubation period at 4°C, wells were washed 3 times with a RIA buffer (0.05 mol l−1 sodium phosphate buffer, pH 7.4). Fifty microlitres of purified substance P antiserum, raised in rabbits and purified in-house, was diluted in RIA buffer (1:131 000) and then added. The wells were again washed 3 times with RIA buffer after 8 h of incubation at 4°C. The remaining protein binding sites were saturated by incubating the wells with 3 % bovine serum albumin in phosphate-buffered saline at 4°C overnight. After washing 3 times with RIA buffer, standards in triplicate and samples (60 μl) were added to the wells and incubated for 24 h at 4°C. 125I-labelled [Tyr8]substance P-(1-11) was added to the standards and samples, which were then incubated for 24 h at 4°C. Wells were then washed 3 times with RIA buffer, separated and counted for 10 min in a 20-well gamma counter, using a cubic spline algorithm for data processing.
The detection limit of this assay has been shown to be 0.07 fmol (100 μl)−1 and the mean ±s.d. for the IC50 is 0.98 ± 0.12 fmol (100 μl)−1. Previous studies from our laboratory have successfully used this assay to measure the extraneuronal concentration of substance P recovered from the spinal cord (Wilson et al. 1993a,b). All assays were performed in a double-blind fashion.
Experimental protocols
Activation of skeletal muscle receptors
Following completion of surgery and placement of the dialysis probe, timed control collections of dialysate (20 min each) were obtained over 120 min. Two protocols were used to evoke substance P release: (1) alternating bilateral contraction of the left and right triceps surae; and (2) unilateral contraction of the left or right hindlimb. Alternating hindlimb contraction (1 min) was performed in five animals over 20 min. In comparison, unilateral contraction (45 s on-45 s off) of either the left or right hindlimb for 20 min was performed in two animals. Twenty minutes of muscle contraction was necessary because a total volume of 60 μl was required for the RIA. Since analysis of the dialysis samples showed that there was no difference in the SP-LI obtained from these two contraction protocols, the results were pooled and analysed together. During muscle contraction, the dialysate was continuously collected on ice and immediately frozen in liquid nitrogen for later analysis. Immediately following the first muscle contraction, 3 × 20 min recovery samples were collected.
Following the recovery period, denervation of cardiorespiratory receptors was performed. The effectiveness of this procedure was tested as previously outlined. A post-denervation period (120 min) ensued during which time sequential 20 min samples of dialysate were collected. Hindlimb muscle contraction was then repeated and followed by 60 min of recovery. The microdialysis probe was then perfused with a solution of KCl (150 mM) for 20 min, and finally with a 2 % Evans Blue solution to determine the exact location of the probe as well as the zone of perfusion.
Activation of cardiorespiratory receptors
In six animals, the above protocol was repeated with the exception that inflation of the aortic balloon catheter was performed instead of hindlimb muscle contraction. Partial inflation of the aortic balloon was performed over 20 min (45 s inflation-45 s deflation), and the increase in arterial pressure was matched to the increase in blood pressure produced by electrically induced muscle contraction. Microdialysis samples were again collected during balloon inflation for measurement of SP-LI before and after denervation.
Histology
To terminate each experiment a solution of KCl (150 mM) was administered intravenously while the animal remained deeply anaesthetized. The brainstem was recovered and post-fixed in 10 % formalin for at least 48 h. The tissue was blocked using Tissue-tek OCT compound (Miles) and mounted on a frozen cryostat chuck. Transverse sections (40-50 μm thick) were cut serially using a cryostat (model 2800 Frigocut-E, Cambridge Instruments) at -20°C and mounted on plus-coated slides. The tissue was stained with 0.1 % Cresyl Violet, coverslipped and standard histological techniques used to determine the neuroanatomical location of the probe. The perfusion area was verified by the distribution of the Evans Blue dye.
Data analysis
Peak changes in mean arterial presure (MAP), heart rate and developed muscle tension during electrically induced muscle contraction were measured during control (30 s prior to contraction), at the peak response, and at 2, 10 and 20 min of muscle contraction in intact and denervated cats. Data were sampled and digitized at 100 Hz and stored for later analysis. All signals were also recorded on an ink recorder (model 2800S, Gould) and to VHS tape by a videotape multiplex adaptor (model 4000A, Vetter).
The haemodynamic data were subjected to a two-way analysis of variance (ANOVA) with one repeated measure. When a treatment effect was found, comparisons were made using a Student- Newman-Keuls multiple comparison test. The effect of skeletal muscle contraction and balloon inflation on the extraneuronal concentration of substance P, as well as the effect of barodenervation on substance P release, was determined by Student's paired t test. All analyses were performed using a commercially available computer software package (SAS Institute, Cary, NC, USA). P < 0.05 was considered statistically significant. Unless otherwise stated, all results are presented as the mean ± standard error of the mean (s.e.m.).
RESULTS
Haemodynamic responses to muscle contraction and balloon inflation
The cardiovascular responses evoked by contraction of the triceps surae from intact and denervated cats are shown in Fig. 1. Tetanic contraction of the triceps surae significantly increased MAP and heart rate (P < 0.05). The peak increase in MAP (169 ± 11 vs. 161 ± 9 mmHg, intact vs. denervated) occurred during the first or second minute of muscle contraction, whereas the maximal heart rate response (177 ± 9 vs. 182 ± 7 beats min−1, intact vs. denervated) occurred after 10 min of contraction. Denervation did not significantly affect baseline MAP and heart rate values nor did it affect the peak response evoked by muscle contraction. MAP was significantly increased through 15 min of contraction, while heart rate remained significantly elevated above control over the entire 20 min contraction period. Ventral root stimulation significantly increased the level of tension developed by the triceps surae (P < 0.05). However, there was no significant difference in the peak level of muscle tension following denervation (7.5 ± 0.6 vs. 8.8 ± 0.3 kg before and after denervation, respectively). There was also no significant difference in the mean levels of tension development over 20 min of muscle contraction.
Figure 1. Time course of the changes in arterial blood pressure (ABP), heart rate (HR) and developed muscle tension during electrically induced muscle contraction of the triceps surae.
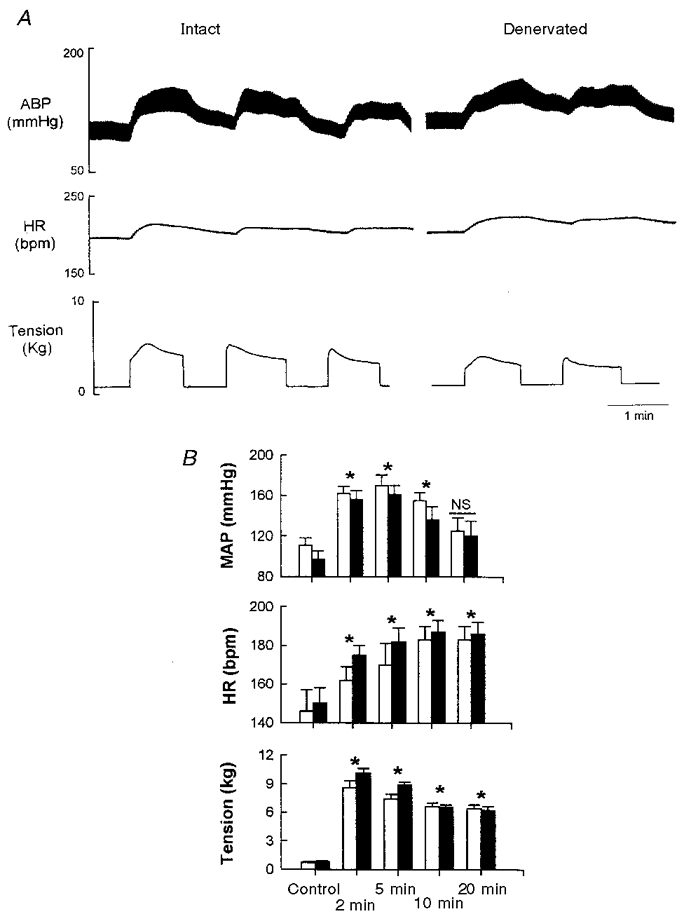
A is an example of a raw trace showing contraction-induced changes in ABP, HR and developed muscle tension in intact and denervated animals. B represents the average pre-contraction values and the contraction-induced changes in mean arterial pressure (MAP), HR and tension at 2 min, 5 min, 10 min and 20 min in intact (□) and denervated (▪) animals (n = 7). Note that denervation did not affect the response of any variable to contraction at any time point. Data are means ±s.e.m.* Significantly different from control (P < 0.05); NS, no significant difference from control (P > 0.05).
An example of the reflex bradycardia evoked by inflation of the aortic balloon before and after denervation is illustrated in Fig. 2. Partial occlusion of the thoracic aorta increased arterial pressure and produced a rapid and sustained bradycardia. A 45 s inflation-deflation duty cycle was used to increase arterial pressure to a level that was equivalent to the pressor response evoked by muscle contraction (ΔMAP 55 ± 10 mmHg). The increase in MAP by balloon inflation (ΔMAP, 50 ± 5 mmHg) was accompanied by reflex bradycardia, which was completely abolished following denervation (Fig. 2B).
Figure 2. Time course of the responses in arterial blood pressure (ABP) and heart rate (HR) to partial mechanical occlusion of the thoracic aorta by balloon inflation.
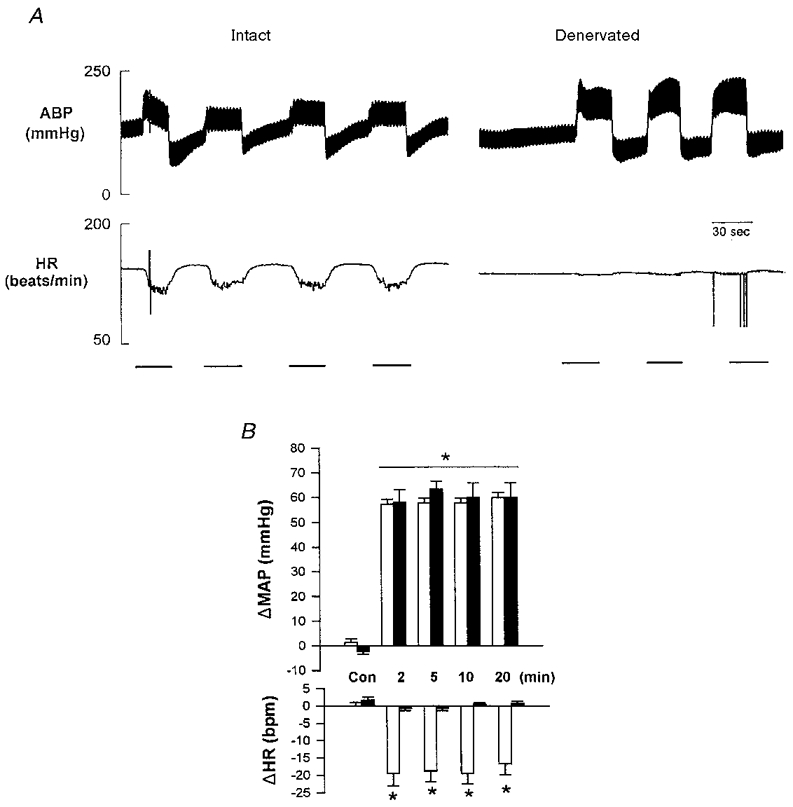
A is an example of a raw trace showing reflex bradycardia evoked by repeated balloon inflation. The bar indicates the period of inflation. Note that denervation completely abolished the reflex bradycardia to balloon inflation. B represents the mean changes in mean arterial pressure (MAP) and HR in intact (□) and denervated (▪) animals. n = 6 for intact and n = 3 for denervated. * Significant difference from control (P < 0.05).
Effect of muscle contraction and balloon inflation on SP-LI recovered from the NTS
The changes in SP-LI following stereotaxic placement of the microdialysis probe are shown in Fig. 3 and Table 1. Following the initial insertion of the probe, SP-LI was significantly elevated. However, the levels of SP-LI progressively decreased over time and attained steady-state values approximately 80 min post-insertion (see Fig. 3A).
Figure 3. Substance P-like immunoreactivity (SP-LI) recovered from the nucleus tractus solitarii (NTS).
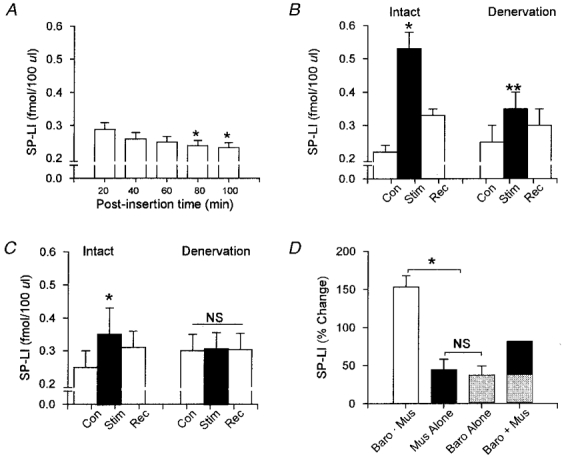
A shows the time course of the changes in SP-LI during 5 × 20 min timed collections immediately following insertion of the microdialysis probe. Steady-state values of SP-LI were obtained 80 min post insertion. SP-LI recovered during electrically induced muscle contraction (B) and during balloon inflation (panel C) are shown in intact (Intact) and denervated (Denervation) animals. □, SP-LI recovered over 20 min control (Con) and recovery (Rec) periods. ▪, SP-LI recovered over 20 min periods of muscle contraction or balloon inflation (Stim). D shows the percentage change in SP-LI during the following conditions: (1) muscle contraction in intact animals (Baro. Mus); (2) muscle contraction following denervation (Mus alone); (3) balloon inflation in intact animals (Baro alone); and (4) summation of Baro alone and Mus alone (Baro + Mus). Data represent means ±s.e.m.* Significantly different from control (P < 0.05); ** Significant difference between intact and denervated (P < 0.05); NS, no significant difference from control.
Table 1.
Baseline values and mean changes in SP-LI evoked by activation of skeletal muscle receptors via electrically induced muscle contraction and by activation of arterial baroreceptors via mechanical elevation of arterial blood pressure
| Skeletal muscle contraction (fmol (100 μl)−1) | Aortic balloon inflation (fmol (100 μl)−1) | |
|---|---|---|
| Neurally intact | ||
| n | 7 | 6 |
| Baseline | 0.217 ± 0.009 | 0.251 ± 0.025 |
| Stimulation | 0.546 ± 0.023* | 0.343 ± 0.028* |
| Recovery | 0.315 ± 0.012 | 0.307 ± 0.023 |
| Cardiorespiratory receptor denervation | ||
| n | 4 | 3 |
| Baseline | 0.247 ± 0.022 | 0.301 ± 0.012 |
| Stimulation | 0.351 ± 0.021*† | 0.311 ± 0.014 |
| Recovery | 0.284 ± 0.019 | 0.308 ± 0.012 |
| KCl perfusion | ||
| n | 5 | |
| Baseline | 0.283 ± 0.015 | |
| 20 min perfusion | 1.632 ± 0.128* | |
Values represent the mean ± S. E. M. Data represent the mean SP-LI recovered over a 20 min collection period.
Significantly different from baseline (P < 0.05).
Significant difference between intact and denervated states (P < 0.05).
The average changes in SP-LI prior to muscle contraction (Con), during electrically induced muscle contraction (Stim), and recovery (Rec) in intact (Intact) and denervated (Denervation) animals are shown in Fig. 3B and Table 1. Electrically induced muscle contraction increased SP-LI approximately 150 % in neurally intact animals from 0.217 ± 0.009 to 0.546 ± 0.023 fmol (100 μl)−1 (P < 0.05). SP-LI decreased significantly over the first 20 min of recovery. However, a period of 60 min was required for SP-LI to return to pre-contraction levels (data not shown).
Carotid sinus and vagus nerves were sectioned to eliminate neural input primarily from arterial baroreceptors. The effectiveness of this procedure was confirmed by the absence of reflex tachycardia to bilateral carotid occlusion or reflex bradycardia to aortic balloon inflation (data not shown). Prior to contraction, SP-LI was similar to the levels recovered from neurally intact animals (0.247 ± 0.022 vs. 0.217 ± 0.009 fmol (100 μl)−1, denervated vs. intact). Following denervation, muscle contraction increased SP-LI 44 % above pre-contraction control levels (0.247 ± 0.022 vs. 0.351 ± 0.021 fmol (100 μl)−1, P < 0.05). However, the magnitude of this increase was significantly less than in the neurally intact state (44 %vs. 150 %, respectively, P < 0.05). SP-LI partially returned towards control levels over the first 20 min of recovery and attained baseline values within 60 min.
The changes in SP-LI produced by activation of cardiorespiratory receptors are shown in Fig. 3C. Basal release of SP-LI was comparable to the control levels from animals used for muscle contraction (0.217 ± 0.009 vs. 0.251 ± 0.025 fmol (100 μl)−1, contraction vs. balloon inflation respectively). Cardiorespiratory receptors were activated by aortic balloon inflation. Using this procedure, MAP was increased from 128 ± 6 mmHg to 189 ± 9 mmHg (P < 0.05). The magnitude of this pressor response was similar to that produced by muscle contraction (ΔMAP 55 ± 10 vs. 50 ± 5 mmHg, muscle contraction vs. balloon inflation). Activation of cardiorespiratory receptors significantly increased SP-LI by 37 %, from 0.251 ± 0.025 to 0.343 ± 0.028 fmol (100 μl)−1 (P < 0.05). SP-LI returned towards control levels over the first 20 min of recovery. Following denervation, balloon inflation failed to increase SP-LI (0.301 ± 0.012 vs. 0.311 ± 0.014 fmol (100 μl)−1, intact vs. denervated).
Summation of the changes in SP-LI is shown in Fig. 3D. For this analysis, SP-LI was normalized to pre-stimulation levels. When carotid sinus and vagus nerves were intact, muscle contraction activated both skeletal muscle receptors and cardiorespiratory receptors secondary to the reflex increase in blood pressure. When both reflexes were activated in this fashion (Baro and Mus), SP-LI was increased 154 ± 12 % above control (P < 0.05). However, when skeletal muscle receptors were activated in denervated animals (Mus alone), the increase in SP-LI was only 44 ± 10 %. This was not due to residual input from cardiorespiratory receptors because denervation completely abolished the increase in SP-LI (see Fig. 3C). Likewise, the increase in SP-LI produced by aortic balloon inflation (Baro alone) was similar to that produced by muscle contraction (37 ± 9 %vs. 44 ± 10 %, Baro alone vs. Mus alone). Summation of the changes in SP-LI evoked by muscle receptors and cardiorespiratory receptors was less than the increase in SP-LI produced when both reflexes were activated simultaneously in the neurally intact state (81 %vs. 154 %, Baro alone + Mus alone vs. Baro and Mus, respectively).
At the termination of the protocol, perfusion of the dialysis probe with KCl (150 mM) produced a robust increase in SP-LI (0.283 ± 0.015 vs. 1.632 ± 0.128 fmol (100 μl)−1, baseline vs. KCl, P < 0.05).
Area perfused by microdialysis probe
A composite representation of the perfusion area from thirteen cats (7 animals for muscle contraction and 6 animals for balloon inflation) is shown in Fig. 4. Two representative transverse sections of the lower brainstem are shown. The sections were stained with 0.1 % Cresyl Violet to localize NTS cell bodies. The right side of each section was outlined to show more clearly the region that was stained when the dialysis probe was perfused with Evans Blue dye (shaded area). Staining was found in the cNTS extending 1.5 mm caudal of the obex, with the most densely stained regions 0.5-1.0 mm caudal of the calamis scriptoris. In addition, dye staining extended medially and laterally in a 1 mm radius from the point of probe insertion (1.5 mm lateral to the mid-line). Limited staining was found in the dorsal column nuclei (i.e. cuneate and gracilis nucleus).
Figure 4. Representative transverse sections of the lower brainstem showing the perfusion region of the microdialysis probe.
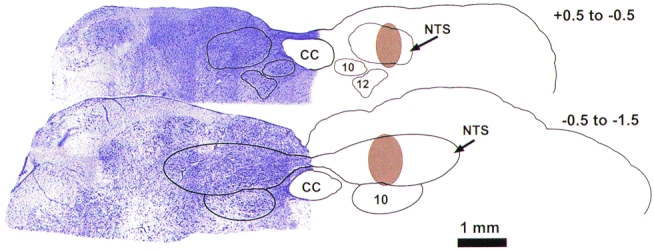
Illustrated are two representative sections located between 0.5 and -0.5 mm and between -0.5 and -1.5 mm of the calamis scriptoris. Shaded brown areas illustrate the regions stained by perfusing the probe with 2 % Evans Blue dye for 20 min. The left side of each section shows Cresyl Violet (0.1 %) staining. CC, central canal; NTS, nucleus tractus solitarii; 10, dorsal motor nucleus; 12, hypoglossal nucleus.
DISCUSSION
Activation of skeletal muscle receptors by muscle contraction elicits a co-ordinated series of cardiovascular and respiratory responses that include increases in heart rate, blood pressure and ventilation as well as a redistribution of blood flow away from non-active vascular beds and viscera and towards contracting skeletal muscle (Mitchell et al. 1983). Previous studies have demonstrated that release of substance P in the dorsal horn of the lower lumbar-upper sacral spinal cord is responsible, in part, for mediating the cardiovascular responses evoked by muscle contraction (Hill et al. 1992; Wilson et al. 1992, 1993a,b). The purpose of the present study was to determine if activation of skeletal muscle afferent fibres that project to the cNTS release substance P when activated by muscle contraction. In this regard, the results show for the first time that mechanically and metabolically sensitive skeletal muscle receptors increase the extraneuronal concentration of substance P in a cardiovascular-related region of the NTS in the absence of synaptic input from cardiorespiratory receptors.
Previous studies have documented the role of substance P in mediating transmission of skeletal muscle afferent activity in the dorsal horn of the spinal cord. It was reported that skeletal muscle contraction releases substance P in a dose-dependent manner (Wilson et al. 1992, 1993). The stimulus for this release was activation of thinly myelinated group III (predominantly mechanically sensitive) and unmyelinated group IV (predominantly metabolically sensitive) afferent fibres (Kaufman et al. 1983). The role for substance P in transmission of skeletal muscle afferent activity has been demonstrated by intrathecal injection of a NK-1 receptor antagonist (Hill et al. 1992) and microinjection of a substance P receptor antagonist directly into the dorsal horn (Wilson et al. 1992). Blockade of substance P receptors in the spinal cord attenuated the reflex cardiovascular responses produced by muscle contraction. Therefore, substance P modulates neurotransmission of Aλ and C fibre inputs in the dorsal horn of the spinal cord. However, the role for substance P as a neuromodulator or neurotransmitter in brain stem circuitry remains controversial.
It has been reported that substance P modulates the baroreceptor-related neural input in the NTS. Intracerebroventricular administration (Chan et al. 1990; Appedrot et al. 1993), localized microinjections (Hall et al. 1989) or microdialysis (Chan et al. 1995) of substance P into the NTS has been reported to alter the sensitivity of the arterial baroreflex. Others, however, have reported that substance P does not alter the baroreceptor reflex. Feldman (1995) microinjected substance P into the NTS and found that substance P evoked bradycardia and hypotension in the acutely anaesthetized rat. This supports previous studies showing that exogenous substance P excites cardiovascular-related neurones in the NTS (Morin-Surun et al. 1984; Hall et al. 1989). It was suggested that this effect was not mediated by a change in the arterial baroreflex because the reflex heart rate response to intravenous phenylephrine and sodium nitroprusside was not altered following blockade of NK1 receptors. However, SPergic input to cardiovascular regulatory circuits that controlled central sympathoinhibitory pathways appeared to be altered. Whether these substance P-sensitive sympathoinhibitory pathways also received primary baroreceptor input was not determined in this study.
Paton et al (1998) recently investigated the potential role of NK1 receptors in integrating cardiac vagal inputs in the NTS of the mouse. It was reported that administration of CP-99,994 (a specific NK1 receptor antagonist) attenuated the reflex bradycardia and depressor response when cardiac vagal receptors were activated by either injection of veratridine or bradykinin into the aortic root or the left ventricle. Moreover, when CP-99,994 was applied directly to the NTS it reversibly reduced the peak firing frequency of NTS neurones that received cardiac vagal input. Interestingly, blockade of NK1 receptors had no effect on the cardiovascular responses evoked by either the pulmonary chemoreflex or peripheral chemoreceptor input. Therefore, this study demonstrates a functional role for SPergic transmission in the NTS in the integration of vagal inputs from the heart. Because substance P affected cardiac vagal input but not afferent input from pulmonary chemoreceptors, peripheral chemoreceptors or arterial baroreceptors, this study suggests that chemical encoding of visceral inputs is a mechanism that contributes to the integrative function of the NTS.
Massari et al. (1998) also reported that substance P appeared to mediate only a modest portion of the total sensory input conveyed by carotid baroreceptors in the dorsolateral subnucleus of the NTS. While only 15 % of the total number of transganglionically labelled carotid sinus nerve afferents were immunoreactive for substance P, they found that the vast majority of substance P-labelled terminals in the NTS originated from sources other than the carotid sinus nerve. Therefore, we propose that a portion of these immunoreactive substance P terminals may have originated from Aλ and C fibre afferent fibres from skeletal muscle receptors that project to the NTS. This supports our finding that a portion of the substance P recovered from the NTS during muscle contraction was independent of baroreceptor input.
To our knowledge only one other study has examined the effect of skeletal muscle receptors on substance P release in the medulla. Williams et al. (1994) measured SP-LI in the ventrolateral medulla (VLM) and the periaqueductal grey (PAG) during isometric contraction of the hindlimb in anaesthetized cats. They found that muscle contraction increased SP-LI in some regions of the VLM but not in other regions such as the PAG and the NTS. However, since arterial baroreceptors were intact it was not possible to determine if the release of substance P was due to activation of skeletal muscle receptors or to the secondary activation of arterial baroreceptors. Furthermore, the sensitivity of their substance P antibody probe (10 μM to 1 nM range, approximately 10−8 M) was less than the sensitivity of the RIA used in the present study (10−12 M). Therefore, changes in SP-LI that were evoked by muscle contraction may not have been detected.
The present study found that in the absence of neural input from cardiorespiratory receptors, the release of substance P was only 33 % of the measured release when muscle contraction was performed in neurally intact cats (see Fig. 3C). From this observation it may be concluded, therefore, that 66 % of the substance P release was due to input from cardiorespiratory receptors. However, this was not the case because activation of cardiorespiratory receptors by balloon inflation increased SP-LI by only 37 %. Therefore, it is proposed that convergence of neural input from skeletal muscle receptors and cardiorespiratory receptors may facilitate the release of substance P in the NTS. In support of this hypothesis, we reported that summation of the changes in SP-LI by skeletal muscle receptors and cardiorespiratory receptors alone was 80 % compared with 150 % when skeletal muscle receptors and cardiorespiratory receptors were activated simultaneously.
Substance P may have been released from intrinsic SPergic neurones that were synaptically driven by converging neural input from skeletal muscle receptors and arterial baroreceptors. Alternatively, substance P may have been released directly from skeletal muscle afferent fibres and arterial baroreceptors that projected to the cNTS. If the latter were the case then the contribution of each afferent population to substance P release should have been additive. However, this was not supported by our results. Therefore, it is more likely that convergence of neural input onto NTS neurones may have facilitated the release of substance P. If this mechanism were valid, then these intrinsic neurones must release substance P locally within the NTS. However, since a portion of these neurones project to other regions of the medulla, such as the parabrachial nucleus and the ventrolateral medulla (Lowey & Burton, 1978; Riche et al. 1990), their contribution to the local release of substance P remains unknown. This apparent paradox requires further investigation.
Previously it has been shown that substance P exerts its effect on cardiovascular reflexes by altering the firing properties, or excitability, of neuronal pools. In this regard, substance P has been reported to exert both excitatory and inhibitory effects on neurones in different regions of the brain. For example, Olpe et al. (1987) found that ionophoretically applied substance P depressed the spontaneous firing rate of sensory neurones from the olfactory bulb using both in vivo and in vitro preparations. The depressant effect of substance P appeared to be mediated by GABA, since its effect was completely abolished by bath-applied bicuculline. Similarly, Perez et al. (1992) demonstrated that substance P induced a dose-dependent depression of baro- and chemosensory transmission in the NTS. Since the depressant action of substance P was inhibited by the GABAA receptor antagonist bicuculline, it seems possible that the substance P-induced depression of baro- and chemosensory transmission in the NTS is mediated indirectly via a GABAergic mechanism. For this hypothesis to be valid there must be GABAergic inhibitory mechanisms operating in the NTS. In this regard, a role for GABA in the modulation of cardiovascular reflexes at the level of the NTS has already been established (Sved & Sved, 1990). Although the relationship between substance P and GABA in the NTS requires further investigation, it represents a mechanism to explain the apparent inhibition of the carotid-cardiac baroreflex by muscle contraction (McWilliam et al. 1991).
Limitations
A potential limitation with the design of this study was the extended period of time that the microdialysis probe remained in the NTS (i.e. 8 h on average). This prolonged exposure of the dialysis probe to the interstitial fluid may have resulted in the coating of the dialysis membrane with collagen or other large proteins, which thereby may have reduced the ability of smaller peptides to diffuse across the membrane and be recovered. However, we do not feel that accumulation of interstitial protein adversely affected our results for the following reasons. First, each probe was perfused for 12 h with a protein solution (1 % polypeptide and 1 % sodium azide in 0.9 % saline solution) prior to the experiment. This was designed to minimize the non-specific binding of protein to the microdialysis membrane when the probe was in vivo. Second, at the end of each experiment the dialysis probe was perfused with 150 mM KCl. This was done to demonstrate that neuronal depolarization was the primary factor in mediating the release of substance P, and that its release was not due to non-specific effects such as mechanical disruption of neurones in the general vicinity of the probe. In addition, due to the extended length of the protocol, KCl perfusion was performed to demonstrate that the preparation remained viable over the duration of the experiment. Third, since the baseline level of substance P at the end of the experiment was similar to that measured 8-10 h earlier, this provided further evidence that the permeability of the probe membrane was not impaired in such a fashion as to decrease substance P recovery. Finally, a new microdialysis probe was used for each experiment to minimize deterioration of the dialysis membrane.
Time-dependent factors, such as depletion of the neuronal stores of substance P, may have contributed to the blunted release over the course of the experiment. To address this possibility we repeated the experimental protocol in one animal with the carotid sinus and vagus nerves intact. In this control experiment, two muscle contractions (20 min each) were performed separated by a 2 h recovery period. The initial muscle contraction generated a maximal tension of 8.5 kg and increased substance P 153 % above the pre-contraction level. The second contraction increased hindlimb tension 8.3 kg and increased substance P by 168 %. The pressor response during each muscle contraction was similar in magnitude. Finally, perfusion of the dialysis probe with KCl at the termination of the experiment increased substance P to 400 % above the pre-perfusion level. These results were consistent with those reported in the present study. Therefore, this control experiment demonstrates that substance P release was not adversely affected by time-related factors.
The purpose of denervating the carotid sinus and vagus nerves was to remove afferent input from arterial (aortic and carotid sinus) baroreceptors. However, the denervation procedure also eliminated afferent input from all vagally mediated receptors including cardiac receptors, pulmonary stretch receptors and gustatory receptors. It is unlikely that input from gustatory afferent fibres contributed to the substance P release since gustatory afferent fibres synapse in the rostral regions of the NTS that were not perfused by the dialysis probe (Bradley et al. 1996). This was confirmed by the distribution of Evans Blue dye in the NTS (see Fig. 4).
Due to the close proximity of neurones in the dorsal column nuclei (DCN) it is possibly that these cells were activated during muscle contraction and may have released substance P that was recovered by the dialysis probe. However, we feel that this is unlikely for the following reasons. First, although there is a high concentration of SPergic fibres and terminals in the intermediate and commissural subdivisions of the NTS, there are few SPergic terminals, fibres or receptors in the DCN (Shults et al. 1984). Second, localized microinjection of substance P into the NTS, but not the DCN, has been reported to evoke cardiovascular responses (Feldman, 1995). This suggests that substance P receptors may not be present in the DCN at functional levels. Therefore, it would seem unlikely that substance P was endogenously released in the DCN. Additionally, there was sparse staining of Evans Blue dye in the DCN (see Fig. 4). This suggests that the extracellular space sampled by the dialysis probe was limited to the cNTS.
Finally, it is difficult to determine whether the model of muscular exercise used in this study may have activated putative nociceptive fibres. It is well known that thinly myelinated Aλ and unmyelinated C fibres are polymodal in nature and are activated by mechanical, chemical, thermal, and nociceptive stimuli (Mense & Stahnke, 1983; Kaufman et al. 1983). Thus, the relative contribution of each fibre will depend on all of these modalities. However, there is evidence showing that Aλ and C fibres contribute to the cardiovascular responses evoked by muscle contraction. First, anodal blockade of the large myelinated muscle afferent fibres (type I and II) did not alter the magnitude of the reflex cardiovascular responses evoked by muscle contraction (McCloskey & Mitchell, 1972). Second, graded levels of muscle contraction (from 1 to 8 kg) have been shown to release substance P in the dorsal horn of the lower lumbar spinal cord in a dose-dependent fashion (Wilson et al. 1993b). Finally, Adreani et al. (1997) recorded discharge patterns from single fibre Aλ and C fibres during stimulation of mesencephalic locomotor region in the decerebrate cat. They reported that these afferent fibres were activated using levels of hindlimb tension development that were considerably lower than the levels used in the present study (< 1 kg). Therefore, a very mild level of hindlimb muscle contraction is sufficient to activate Aλ and C fibres in a dose-dependent fashion.
Functional implications
There is functional evidence supporting the notion that afferent input from contraction-sensitive skeletal muscle receptors alters the arterial baroreceptor reflex. McWilliam et al. (1991) reported that electrically induced contraction of the triceps surae resulted in a 40 % attenuation of the reflex-evoked bradycardia when carotid sinus pressure was increased in the decerebrate cat. Recently, we have also reported that sensory inputs from skeletal muscle receptors interact with carotid baroreceptors in a manner that determines the cardiovascular response evoked during exercise (Potts et al. 1998; Potts & Li, 1998; Potts & Mitchell, 1998). For example, the sympathoexcitatory response evoked by the carotid baroreflex and the skeletal muscle reflex is mutually inhibitory (Potts et al. 1998). Second, the nature of this interaction (i.e. inhibitory vs. facilitatory) can be modulated by the level of afferent baroreceptor input (Potts & Li, 1998). Third, contraction-induced activation of skeletal muscle receptors reset the carotid baroreflex to higher blood pressure (Potts & Mitchell, 1998). Together, these studies demonstrate a functional interaction between these two reflex pathways; however, the central mechanisms that mediate this interaction remain unknown.
In conjunction with the findings from the present study, it is proposed that substance P release in the NTS during muscle contraction may modify the processing of primary baroreceptor input. This may take the form of either inhibition or excitation of barosensitive NTS neurones. If substance P inhibits the transmission of excitatory cardiorespiratory input, or excites inhibitory circuits in the NTS as reported by Perez et al. (1992), this could provide a mechanism to explain the changes in arterial baroreflex function reported during muscular exercise (Melcher & Donald, 1981; McWilliam et al. 1991; Potts et al. 1993, 1998; Papelier et al. 1994; Potts & Mitchell, 1998). This hypothesis is currently under investigation.
Conclusion
In conclusion, the present study found that neural input from skeletal muscle receptors and cardiorespiratory receptors releases substance P in the NTS. Approximately 40 % of substance P release was mediated by cardiorespiratory receptors (primarily arterial baroreceptors), while 37 % was released by skeletal muscle receptors. When contraction-sensitive skeletal muscle receptors were activated simultaneously with cardiorespiratory receptors the increase in SP-LI was greater then the simple sum of the responses evoked by each reflex (150 %vs. 77 %, combined activation vs. simple summation). This finding suggests that neural input from skeletal muscle receptors may converge with input from cardiorespiratory receptors and facilitate the release of substance P in the NTS. Knowledge of the effect of substance P on the excitability of NTS neurones may provide insight into the neurochemical basis for alterations in the arterial baroreceptor reflex that have been reported during muscular exercise.
Acknowledgments
The authors would like to thank Mr James Jones and Mr Julius Lamar Jr for their expert technical assistance. We would also like to thank Ms Margaret Robledo for histological staining of NTS tissue, as well as Mr Mike Davis and Dr Mary Garry for their expert assistance with image processing of the NTS sections. This work was supported by National Heart, Lung and Blood Program Project Grant HL-06296, and the Lawson and Rogers Lacy Research Fund in Cardiovascular Disease.
References
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. Journal of Applied Physiology. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Appedrot E, Pharow L, Brattstrom A. Effects of central substance P on baroreflex regulation. Neuropeptides. 1993;25:77–81. doi: 10.1016/0143-4179(93)90073-j. 10.1016/0143-4179(93)90073-J. [DOI] [PubMed] [Google Scholar]
- Ba-M'Hamed S, Roy JC, Bennis M, Poulain P, Sequeira H. Corticospinal collaterals to medullary cardiovascular nuclei in the rat: An anterograde and a retrograde double-labeling study. Journal für Hirnforschung. 1996;37:367–375. [PubMed] [Google Scholar]
- Bradley RM, King MS, Wang L, Shu X. Neurotransmitter and neuromodulator activity in the gustatory zone of the nucleus tractus solitarius. Chemical Senses. 1996;21:377–385. doi: 10.1093/chemse/21.3.377. [DOI] [PubMed] [Google Scholar]
- Chan JYH, Barnes CD, Chan SHH. Tonic enhancement of the sensitivity of baroreceptor reflex response by endogenous substance P in the rat. Regulatory Peptides. 1990;29:199–213. doi: 10.1016/0167-0115(90)90083-9. [DOI] [PubMed] [Google Scholar]
- Chan JYH, Tsou M-Y, Len W-B, Lee T-Y, Chan SHH. Participation of noradrenergic neurotransmission in the enhancement of baroreceptor reflex response by substance P at the nucleus tractus solitarii of the rat: a reverse microdialysis study. Journal of Neurochemistry. 1995;64:2644–2652. doi: 10.1046/j.1471-4159.1995.64062644.x. [DOI] [PubMed] [Google Scholar]
- Felder RB, Mifflin SW. Modulation of carotid sinus afferent input to nucleus tractus solitarius by parabrachial nucleus stimulation. Circulation Research. 1988;63:35–49. doi: 10.1161/01.res.63.1.35. [DOI] [PubMed] [Google Scholar]
- Feldman PD. Neurokinin receptor mediation of the vasodepressor effects of substance P in the nucleus of the tractus solitarius. Journal of Pharmacological and Experimental Therapeutics. 1995;273:617–623. [PubMed] [Google Scholar]
- Gillis RA, Helke CJ, Hamilton BL, Norman WP, Jacobowitz DM. Evidence that substance P is a neurotransmitter of baro- and chemoreceptor afferents in nucleus tractus solitarius. Brain Research. 1981;181:476–481. doi: 10.1016/0006-8993(80)90633-2. [DOI] [PubMed] [Google Scholar]
- Hall ME, Miley FB, Stewart JM. Cardiovascular effects of substance P peptides in the nucleus of the solitary tract. Brain Research. 1989;497:280–290. doi: 10.1016/0006-8993(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Helke CJ, O'donohue TL, Jacobowitz DM. Demonstration of substance P in aortic nerve afferent fibers by combined use of fluorescent retrograde neuronal labeling and immunocytochemistry. Peptides. 1980;1:1–9. doi: 10.1016/0196-9781(80)90015-7. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Shults CW, Chase TN, O'donohue TL. Autoradiographic localization of substance P receptors in rat medulla: effect of vagotomy and nodose ganglionectomy. Neuroscience. 1984;12:215–223. doi: 10.1016/0306-4522(84)90148-9. [DOI] [PubMed] [Google Scholar]
- Hill JM, Pickar JG, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static contraction by an NK-1 receptor antagonist. Journal of Applied Physiology. 1992;73:1389–1395. doi: 10.1152/jappl.1992.73.4.1389. [DOI] [PubMed] [Google Scholar]
- Jordan D, Mifflin SW, Spyer KM. Hypothalamic inhibition of neurones in the nucleus tractus solitarius of the cat is GABA mediated. The Journal of Physiology. 1988;399:389–404. doi: 10.1113/jphysiol.1988.sp017087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M, Mei SS, Kao FF. Central projections from ergoreceptors (C fibers) in muscle involved in cardiopulmonary responses to static exercise. Circulation Research. 1981;48(suppl. 1):I48–62. [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. Journal of Applied Physiology. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Progress in Neurobiology. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Yamamoto Y, Pantaleo T, Lagercrantz H, Brodin E, Ungerstedt U. In vivo release of substance P in the nucleus tractus solitarii increases during hypoxia. Neuroscience Letters. 1986;69:94–97. doi: 10.1016/0304-3940(86)90421-0. [DOI] [PubMed] [Google Scholar]
- Lowey AD, Burton H. Nuclei of the solitary tract: efferent projections to the lower brainstem and spinal cord of the cat. Journal of Comparative Neurology. 1978;181:421–450. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- Mccloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. The Journal of Physiology. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SE, Mcwilliam PN, Robertson J, Kaye JC. Inhibition of carotid sinus baroreceptor neurones in the nucleus tractus solitarius of the anaesthetized cat by electrical stimulation of hindlimb afferent fibres. The Journal of Physiology. 1992;452:224P. [Google Scholar]
- McWilliam PN, Yang T, Chen LX. Changes in the baroreceptor reflex at the start of muscle contraction in the decerebrate cat. The Journal of Physiology. 1991;436:549–558. doi: 10.1113/jphysiol.1991.sp018566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley B, Elde R. Immunohistochemical localization of putative neurotransmitters within the feline nucleus tractus solitarii. Neuroscience. 1982;7:2469–2490. doi: 10.1016/0306-4522(82)90208-1. [DOI] [PubMed] [Google Scholar]
- Massari VJ, Shirahata M, Johnson TA, Lauenstein J-M, Gatti PJ. Substance P immunoreactive nerve terminals in the dorsolateral nucleus of the tractus solitarius: roles in the baroreceptor reflex. Brain Research. 1998;785:329–340. doi: 10.1016/s0006-8993(97)01335-8. [DOI] [PubMed] [Google Scholar]
- Melcher A, Donald DE. Maintained ability of carotid sinus baroreflex to regulate arterial pressure during exercise. American Journal of Physiology. 1981;241:H838–849. doi: 10.1152/ajpheart.1981.241.6.H838. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: A possible substrate for somatovisceral and viscerovisceral reflex activation. Journal of Comparative Neurology. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. The Journal of Physiology. 1983;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW, Felder RB. Synaptic mechanisms regulating cardiovascular afferent inputs to nucleus tractus solitarius. American Journal of Physiology. 1990;259:H653–661. doi: 10.1152/ajpheart.1990.259.3.H653. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annual Review of Physiology. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Morris M, Chalmers J. Release of substance P in the nucleus tractus solitarius measured by in vivo microdialysis: response to stimulation of the rabbit aortic depressor nerves in rabbit. Neuroscience Letters. 1988;94:131–137. doi: 10.1016/0304-3940(88)90283-2. [DOI] [PubMed] [Google Scholar]
- Morin-Surun MP, Jordan D, Champagnat J, Spyer KM, Denavit-Saubie M. Excitatory effects of iontophoretically applied substance P on neurons in the nucleus tractus solitarius of the cat: lack of interaction with opiates and opioids. Brain Research. 1984;307:388–392. doi: 10.1016/0006-8993(84)90502-x. [DOI] [PubMed] [Google Scholar]
- Nyberg G, Blomqvist A. The central projection of muscle afferent fibres to the lower medulla and upper spinal cord: An anatomical study in the cat with transganglionic transport method. Journal of Comparative Neurology. 1984;230:99–109. doi: 10.1002/cne.902300109. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Heid J, Bittiger H, Steinmann MW. Substance P depresses neuronal activity in the rat olfactory bulb in vitro and in vivo: possible mediation via m-aminobutyric acid release. Brain Research. 1987;412:269–274. doi: 10.1016/0006-8993(87)91133-4. [DOI] [PubMed] [Google Scholar]
- Papelier Y, Escourrou P, Gauthier P, Rowell LB. Carotid baroreflex control of blood pressure and heart rate in man during dynamic exercise. Journal of Applied Physiology. 1994;77:502–507. doi: 10.1152/jappl.1994.77.2.502. [DOI] [PubMed] [Google Scholar]
- Paton JFR. Importance of neurokinin-1 receptors in the nucleus tractus solitarii of mice for the integration of cardiac vagal inputs. European Journal of Neuroscience. 1998;10:2261–2275. doi: 10.1046/j.1460-9568.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- Perez H, Ruiz S, Inostroza H, Perretta M. Substance P depresses bioelectrical responses evoked in the nucleus tractus solitarii: interaction with γ-aminobutyric acid-ergic neurons. European Journal of Pharmacology. 1992;213:435–437. doi: 10.1016/0014-2999(92)90633-f. [DOI] [PubMed] [Google Scholar]
- Person RJ. Somatic and vagal afferent convergence on solitary tract neurons in cat: electrophysiological characteristics. Neuroscience. 1989;30:283–295. doi: 10.1016/0306-4522(89)90254-6. [DOI] [PubMed] [Google Scholar]
- Potts JT, Fuchs I, Li J, Leshnower B, Mitchell JH. Baroreceptor-independent release of substance P in the nucleus tractus solitarius: role of skeletal muscle afferents. FASEB Journal. 1997;11:A47. [Google Scholar]
- Potts JT, Hand GA, Li J, Mitchell JH. Central interaction between carotid baroreceptors and skeletal muscle receptors inhibits sympathoexcitation. Journal of Applied Physiology. 1998;84:1158–1165. doi: 10.1152/jappl.1998.84.4.1158. [DOI] [PubMed] [Google Scholar]
- Potts JT, Li J. Interaction between carotid baroreflex and exercise pressor reflex depends on baroreceptor afferent input. American Journal of Physiology. 1998;274:H1841–1847. doi: 10.1152/ajpheart.1998.274.5.H1841. [DOI] [PubMed] [Google Scholar]
- Potts JT, Mitchell JH. Rapid resetting of carotid baroreceptor reflex by afferent input from skeletal muscle receptors. American Journal of Physiology. 1998;275:H2000–2008. doi: 10.1152/ajpheart.1998.275.6.H2000. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. American Journal of Physiology. 1993;265:H1928–1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Riche D, de Pommery J, Menetrey D. Neuropeptides and catecholamines in efferent projections of the nuclei of the solitary tract in the rat. Journal of Comparative Neurology. 1990;293:399–424. doi: 10.1002/cne.902930306. [DOI] [PubMed] [Google Scholar]
- Shults CW, Quirion R, Chronwall B, Chase TN, O'donohue TL. A comparison of the anatomical distribution of substance P and substance P receptors in the rat central nervous system. Peptides. 1984;5:1097–1128. doi: 10.1016/0196-9781(84)90177-3. [DOI] [PubMed] [Google Scholar]
- Spyer KM. Central nervous mechanisms contributing to cardiovascular control. The Journal of Physiology. 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Goiny M, Pantaleo T, Lagercrantz H, Brodin E, Runold M, Yamamoto Y. Enhanced in vivo release of substance P in the nucleus tractus solitarii during hypoxia in the rabbit: role of peripheral input. Brain Research. 1991;546:211–216. doi: 10.1016/0006-8993(91)91483-h. [DOI] [PubMed] [Google Scholar]
- Sved AF, Sved JC. Endogenous GABA acts on GABAB receptors in nucleus tractus solitarius to increase blood pressure. Brain Research. 1990;526:235–241. doi: 10.1016/0006-8993(90)91227-8. [DOI] [PubMed] [Google Scholar]
- Sykes RR, Spyer KM, Izzo PN. Central distribution of substance P, calcitonin gene-related peptide and 5-hydroxytryptamine in vagal sensory afferents in the rat dorsal medulla. Neuroscience. 1994;59:195–210. doi: 10.1016/0306-4522(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Toney GM, Mifflin SW. Time-dependent inhibition of hindlimb somatic afferent input to nucleus tractus solitarius. Journal of Neurophysiology. 1994;72:63–71. doi: 10.1152/jn.1994.72.1.63. [DOI] [PubMed] [Google Scholar]
- Van Giersbergen PLM, Palkovits M, De Jong W. Involvement of neurotransmitters in the nucleus tractus solitarii in cardiovascular regulation. Physiological Reviews. 1992;72:789–824. doi: 10.1152/physrev.1992.72.3.789. [DOI] [PubMed] [Google Scholar]
- Williams CA, Brien PL, Nichols PL, Gopalan P. Detection of immunoreactive substance P-like substances from cat brainstem sites during fatiguing isometric contractions. Neuropeptides. 1994;26:319–327. doi: 10.1016/0143-4179(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Wilson LB, Fuchs IE, Matsukawa K, Mitchell JH, Wall PT. Substance P release in the spinal cord during the exercise pressor reflex in anaesthetized cats. The Journal of Physiology. 1993a;460:79–90. doi: 10.1113/jphysiol.1993.sp019460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Fuchs IE, Mitchell JH. Effects of graded muscle contractions on spinal cord substance P release, arterial blood pressure, and heart rate. Circulation Research. 1993b;73:1024–1031. doi: 10.1161/01.res.73.6.1024. [DOI] [PubMed] [Google Scholar]
- Wilson LB, Wall PT, Matsukawa K, Mitchell JH. Effect of spinal microinjections of an antagonist to substance P or somatostatin on the exercise pressor reflex. Circulation Research. 1992;70:213–222. doi: 10.1161/01.res.70.2.213. [DOI] [PubMed] [Google Scholar]


