Abstract
Experimental investigations in animals have highlighted the role of early reduced calorie and protein nutrition on fetal cardiovascular development, and the occurrence of a transition from a low fetal arterial blood pressure in late gestation to a high arterial blood pressure postnatally. These observations may explain the correlation between health, including appropriate nutrition, in pregnant women and the outcome of their pregnancies. Emphasis has been placed on low birth weight infants who have an increased risk of developing cardiovascular diseases, including hypertension, coronary heart disease and stroke in adulthood. Vascular pathology in adults is not always associated with low birth weight and animal experiments indicate that substantial changes in cardiovascular and endocrine function can result from maternal or fetal undernutrition without impairing fetal growth. Experimental investigation on organogenesis shows the pivotal role of adequate protein availability as well as total caloric intake. Amino acid metabolism in the feto-maternal unit appears to have a key influence on the development of organs involved in chronic degenerative disease in the adult. Experimental investigation has also highlighted the role of carbohydrate metabolism and its effect on the fetus in this respect. Either restriction of protein intake or diabetes in pregnant rats has intergenerational effects at least on the endocrine pancreas and the brain. Further investigation is needed to clarify the mechanisms involved and lead to a new understanding of the importance of nutrition during pregnancy. This will provide an important approach to the primary prevention of diabetes and chronic degenerative diseases.
The developing mammal needs to establish a degree of autonomy during fetal life in order to achieve independent survival after birth. It therefore develops homeostatic mechanisms necessary to guarantee its existence. But it passes through critical periods when it may be influenced by aspects of the intrauterine environment dependent on maternal nutrition and metabolism. These have been clearly demonstrated for implantation, organogenesis and parturition, all of which are influenced by maternal health, including nutritional intake. But apart from the striking effects on survival (e.g. in relation to implantation) or overt anatomical structure (organogenesis), recent evidence reveals that disturbances during critical periods can also affect homeostatic mechanisms. The effects may be subtle during development, but can nonetheless have long-lasting deleterious effects on health in adult life.
This review concentrates primarily on how nutritional intake during pregnancy affects cardiovascular (especially arterial blood pressure) and blood glucose homeostasis in the offspring. These two areas are increasingly studied in humans (Rich-Edwards et al. 1997; Barker, 1998) and, whilst superficially distinct, they share some common aetiological features. To date, most of the studies in humans have been epidemiological so that, whilst they identify phenomena, they do not give insight into mechanisms. Awareness of this has recently shifted emphasis to prospective studies of smaller groups of people and to animal studies, the latter providing the more direct approach to understanding the processes involved. We concentrate on these animal studies in this brief review. In some of them an isocaloric low protein diet has been used to explore the mechanisms by which protein metabolism affects developing organs. Other studies have used varying degrees of global reduction in nutrition. It is noteworthy that in some of these studies effects on homeostatic development were produced even in the absence of body growth restriction. It is therefore possible to envisage a spectrum of health problems in adult life deriving from the influence of the intrauterine environment and maternal/placental/fetal compensatory responses to a diet mildly to severely altered in its composition or its volume. It is also noteworthy that even if an isocaloric low protein diet does not affect birth weight significantly in the first generation it may reduce it in subsequent generations. These observations are discussed further below.
Cardiovascular development
It is well established in a variety of species that altered nutrition can have permanent effects if it occurs at a sensitive period during development (e.g. Winick & Noble, 1966; Osofsky, 1975; Stephens, 1980; Lechner et al. 1986; Snoeck et al. 1990; Lucas, 1991). The effects of a period of early undernutrition on cardiovascular function may not be manifest until a much later stage in life. Attempts to understand the underlying mechanisms have been made in a variety of animals, from rodents to sheep and non-human primates.
One of the major issues which emerges from recent work is that birth weight does not have to be significantly reduced for the physiological effects of reduced nutrition in pregnancy to be manifest. This raises the possibility that the placental or fetal compensatory mechanisms, which occur in response to reduced maternal nutrition, preserve normal fetal growth and hence birth weight, but have postnatal consequences which become important in later life. Thus the adoption of a biological ‘strategy’ needed for development in utero results in an organism in which the strategy for wellbeing in adulthood is not achieved.
In sheep global undernutrition in early pregnancy produces a reduction of the birth weight/placental weight ratio (De Barro et al. 1992) and in early to mid-gestation produces increased placental size (Faichney & White, 1987; McCrabb et al. 1991), whereas global undernutrition in late gestation only reduces fetal growth (Mellor & Murray, 1981). Early gestation undernutrition alters the distribution of placentome types in favour of those with a predominant fetal vascular pattern (Crowe et al. 1997). This pattern is reminiscent of the increased fetal vascularization reported in human, guinea-pig and sheep placentae at high altitude (Bacon et al. 1984; Scheffen et al. 1990; Burton et al. 1996; Krebs et al. 1997; Penninga & Longo, 1998) and some forms of intrauterine growth retardation (IUGR) (see Kingdom & Kaufmann, 1997), possibly occurring as a placental compensatory response.
Early undernutrition affects the development and responses of the hypothalamic-pituitary-adrenal (HPA) axis in late gestation. Both the pituitary response to an arginine vasopressin/corticotrophin releasing hormone (AVP/CRH) challenge and the adrenal cortical response to an adrenocorticotrophic hormone (ACTH) challenge are reduced (Hawkins et al. 1997, 1998). These fetuses have a lower basal plasma cortisol concentration, which may account for their lower arterial blood pressure (Fig. 1), as cortisol and other glucocorticoids are known to elevate arterial blood pressure (Tangalakis et al. 1992). Whether the suppression of basal cortisol concentration and of HPA axis responses is due to prior exposure of the fetus to cortisol is not known. A hypothesis has been proposed to relate the suppression of the HPA axis function to prior exposure of the fetus to elevated corticosteroids (Benediktsson et al. 1993; Edwards et al. 1993) and it is already known that dexamethasone administration suppresses HPA axis function in the sheep (Norman & Challis, 1987).
Figure 1. Basal mean arterial pressure (A) and ACTH response to administration of CRH + AVP (B) in control (□) and nutrient restricted (▪) fetuses (113-127 days gestation) and lambs (84 ± 4.4 days).
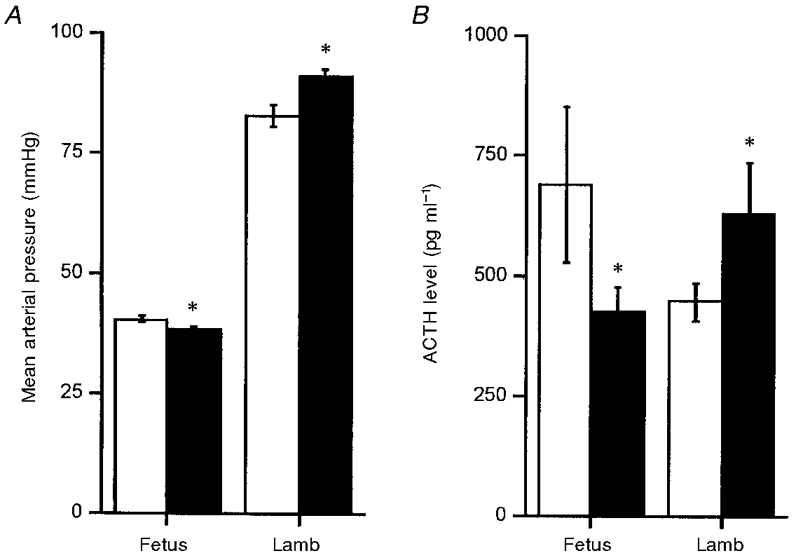
Values are means ±s.e.m. Arterial pressure is shown as the mean of measurements made over a 2 week period in the fetus, and as the mean of measurements made on a single day in the lamb. ACTH responses to CRH + AVP challenge were measured for 180 min after drug administration in the fetus, and for 60 min in the lamb; ACTH data are shown as the cumulative response over the first 15 min following drug administration, with samples taken every 5 min. Blood pressure data were compared by Student's unpaired t test, and ACTH data by two-way analysis of variance followed by Dunnett's post-hoc test (*P < 0.05). Fetal basal blood pressure, and ACTH responses to CRH + AVP were significantly reduced following maternal nutrient restriction. Postnatally, basal blood pressure and ACTH responses were significantly greater in lambs of undernourished mothers. Adapted from Hawkins et al. (1997, 1998).
Cardiovascular and HPA axis development are also affected postnatally. Lambs born after periconceptual undernutrition have higher arterial blood pressure and an exaggerated arterial blood pressure response to an HPA axis challenge (Hawkins et al. 1998). Their ACTH and cortisol responses are now also greater. The mechanism for this switching from blunted responses in utero to enhanced responses postnatally is not yet known.
The effects of undernutrition are manifest not only in terms of changes in reflex and endocrine control of the cardiovascular system, but also at the level of the local vasculature. Ozaki et al. (1998) found that the responses of small resistance arteries, especially to endothelium dependent vasodilator agonists, are altered in ewes and their fetuses subjected to an early gestation nutritional challenge. The causal role of such changes in arterial blood pressure development remains to be determined. Similar effects have also been reported in experimental diabetes and with a high fat diet in the rat (Gerber et al. 1997; Koukkou et al. 1997).
Besides undernutrition in the ewe, the removal of placental caruncles produces fetuses with lower mean arterial blood pressure, higher fetal heart rate and altered responses to an episode of acute hypoxia in late gestation (Robinson et al. 1983). Fetal heart rate and the rise in arterial blood pressure in acute hypoxia are greater in carunclectomized fetuses, suggesting greater chemoreflex or endocrine responses even though such fetuses are not necessarily smaller than controls.
These carunclectomized sheep fetuses, being hypoxaemic and hypoglycaemic have higher plasma levels of adrenaline and noradrenaline, suggesting greater sympathetic activity. Their higher levels of cortisol without increased ACTH and suppressed pituitary pro-opiomelanocortin (POMC) expression (Phillips et al. 1996) suggest that HPA axis feedback may have been altered. In another context, alteration of the HPA axis affects maturation of organ systems, and may even initiate parturition (see Carmichael et al. 1997 for discussion).
Studies in rats have shown that undernutrition in utero can lead to life-long elevation of blood pressure. For example, an isocaloric low protein diet (8%) during pregnancy reduces the fetal:placental weight ratio (Levy & Jackson, 1993), produces elevated arterial blood pressure in the offspring (Langley & Jackson, 1994), via changes in angiotensin converting enzyme activity (Langley-Evans & Jackson, 1995), and produces changes in glutathione metabolism and glucose tolerance as well as insulin secretion (Langley & Jackson, 1994; Langley-Evans & Jackson, 1995). In subsequent experiments it was shown that induction of hypertension by reduced maternal protein was abolished if cortisol synthesis was inhibited. This led to the hypothesis that maternal protein restriction programmes life-long changes in the fetal HPA axis, which in turn resets homeostatic mechanisms controlling blood pressure. Maternal protein restriction attenuates activity of placental 11β-HSD (hydroxysteroid dehydrogenase) type II and an alternative explanation is that fetal blood pressure is altered through increased exposure to maternal glucocorticoids (Edwards et al. 1993). In this species, placental 11β-HSD activity is correlated with birth weight and inversely correlated with placental weight (Bendiktsson et al. 1993), and inhibition of 11β-HSD with carbenoxolone in pregnancy produces offspring with a higher arterial blood pressure (Langley-Evans, 1997). A third possibility is that placental activity of the enzyme plays a crucial role in the development of the fetal adrenal and hence may determine patterns of glucocorticoid secretion throughout life. There may also be effects on the kidney (see below) and on vascular growth and metabolism.
The effects of severe total dietary restriction have also been examined in the rat. Woodall et al. (1996) reduced diet by 75% and reported elevated blood pressure. However, Holemans et al. (1998) found that severe restriction in late gestation did not produce hypertensive offspring. It appears that the timing of the insult in gestation is all-important, with insults occurring earlier having greater effects on cardiovascular development in the offspring.
The experiments on the rat appear to differ from those in the sheep in that cardiovascular and endocrine changes are associated with growth retardation. Methodological differences and the role of undernutrition versus isocaloric protein restriction have, however, to be noted. In the rat the level of reduction in maternal carbohydrate and/or protein intake has usually been based on requirements at the time of conception, and maintained throughout pregnancy. Because dietary requirement increased throughout pregnancy, this may constitute a challenge of increasing severity. Recently, in a series of studies in which intake was reduced by 30% of the appropriate daily requirement throughout gestation, only male pups were smaller than control, at least at the first generation. Nonetheless, they showed perturbed vascular development, particularly in arterial blood pressure and in responses of small vessels to vasodilator agonists (Ozaki et al. 1998) (Fig. 2). In addition, a recent report on the growth of the spontaneously hypertensive rat (SHR; Lewis et al. 1997; Fig. 3) shows that the pups of this strain are not smaller than normotensive pups at birth, although as fetuses they were smaller at days 16-20 of gestation. Their greater late gestation growth than controls is associated with a significantly larger placenta. Thus whatever the mechanisms for the earlier impaired growth, fetal growth in late gestation was accelerated by a greater placental mass. However, the latter has consequences for subsequent postnatal cardiovascular function. Spontaneously hypertensive rat pups had larger hearts and kidneys, which may be associated with the cardiovascular effects. Somewhat similar observations have been made in the rat using iron deficiency anaemia. This reduces birth weight and neonatal mean arterial blood pressure, but the pups become hypertensive relative to controls when postnatal ‘catch-up’ growth occurs and their heart size increases (Crowe et al. 1995).
Figure 2. Effects of a 30% restriction in global diet during pregnancy on the mean arterial pressure (A) and responses of small resistance arteries to the thromboxane A2 mimetic U46619 in vitro (B) of male offspring.
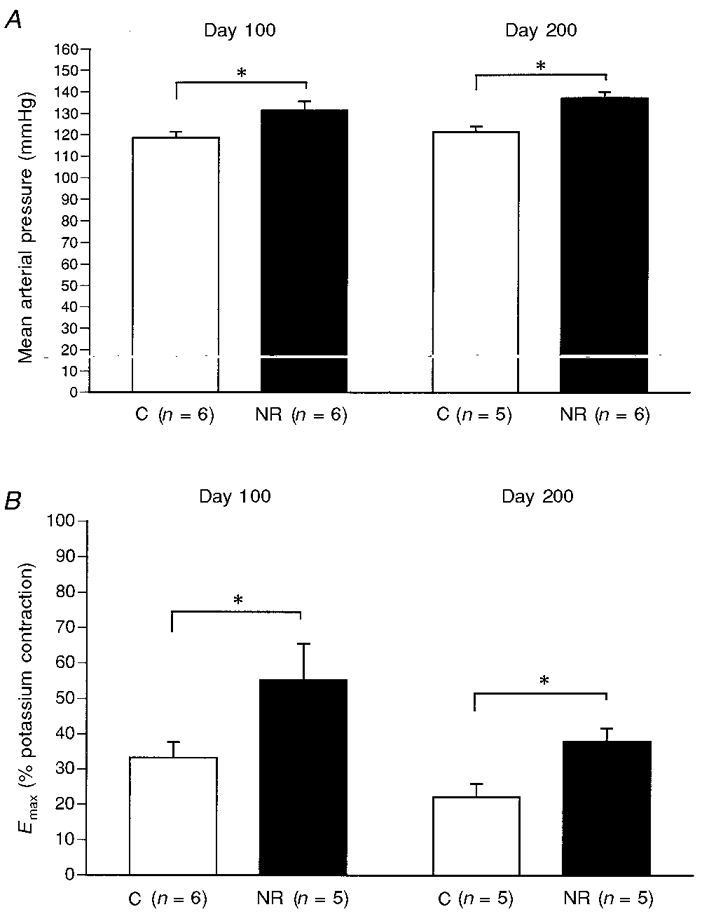
Offspring of nutritionally restriced dams (NR) show higher arterial blood pressure at both 100 and 200 days postnatally, compared with controls (C). In addition, small artery contractile responses, measured as maximal tension (Emax) to U46619 as a percentage of maximal K+-induced tension, are greater in NR than in C at both ages. All data are means ±s.e.m.*P < 0.05 NR vs. C by the Mann-Whitney U test. Data from Ozaki et al. (1998).
Figure 3. Weight (means ±s.d.) of the fetus (A) and placenta (B) in late gestation in spontaneously hypertensive rats (SHR; ▪) and normotensive Wistar-Kyoto controls (WKY; □).
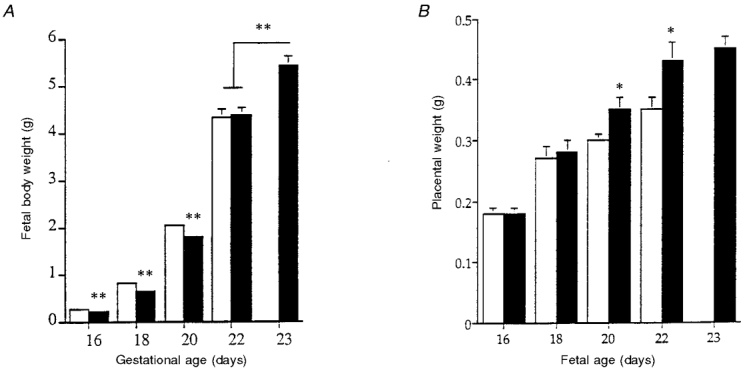
The lower fetal weight in mid-gestation in SHR is not seen in late gestation, and this effect is associated with greater placental weight. Note that gestation is slightly longer in SHR than WKY. * P less than 0.01 by unpaired t test; ** P less than 0.01 by ANOVA, SHR vs. WKY. Adapted from Lewis et al. (1997).
Blood pressures of SHR are permanently lowered if they are suckled by dams of normotensive strains for 2 weeks after birth. Milk of SHR differs from milk of other strains in protein and electrolyte concentrations (Di Nicolantonio, 1993; McCarty & Lee, 1994; McCarty & Fields-Okotcha, 1996). Other studies have shown that the intrauterine environment of the SHR is different, as the amniotic fluid has high osmolarity and sodium concentrations (Erkadius et al. 1994). Altering the intrauterine environment experimentally by inducing maternal diabetes also affects blood pressure in the SHR.
Finally, in the guinea-pig maternal undernutrition impairs placental development and fetal growth (Dwyer et al. 1992) and it has also been shown that unilateral ligation of the uterine artery during pregnancy indeed leads to reduced birth weight in the offspring and raised blood pressure after birth. The mechanism underlying this phenomenon is unknown but increased circulating catecholamines have been proposed (Persson & Jansson, 1992).
Endocrine pancreas
Besides the vasculature, the development of other key tissues may be affected in the offspring of ewes or dams having global nutritional restriction. However a specific limitation of protein in a normocaloric diet which prevents an energy constraint has been explored widely in pregnant animals and found to affect the offspring's islet cells (Snoeck et al. 1990), insulin sensitive tissues such as liver (Hales et al. 1996), muscle (Ozanne et al. 1996a,b) and adipose tissue (Shepherd et al. 1997) as well as the kidney (Merlet-Benichou et al. 1994) and brain (Resnick et al. 1979). With this experimental dietary approach particular mechanisms operating in the fetomaternal unit which are dependent upon specific nutritional intake of the mother might be explored. The later part of this review deals with the consequences for the offspring of dams receiving a moderate protein restriction in a normocaloric diet.
In vitro, fetal β cell differentiation, multiplication and insulin secretion are amplified more by an increase in essential amino acid than by an increase in glucose concentration (de Gasparo et al. 1978). This suggests a specific role for appropriate protein availability upon the development of fetal β cells, acting via changes in amino acid metabolism. This raises the possibility of abnormal features being acquired by the developing β cell which may lead to pathological events postnatally, as a result of a protein deficient diet of the dam.
With an isocaloric low protein (8%) diet during gestation the profile of amino acids is changed in maternal and fetal plasma as well as in amniotic fluid. The total essential and non-essential amino acid concentrations were not modified, nor were glucose and insulin levels (Reusens et al. 1995). More specifically, α-aminobutyric acid, phosphoserine, taurine and valine were reduced in maternal as well as in fetal plasma. The weight of the fetus was normal at 19.5 days but slightly lower at birth compared with the controls.
The endocrine pancreas was abnormal in that islet cell proliferation (not evenly distributed in the head and tail of the endocrine pancreas), islet cell size, pancreatic insulin content and islet vascular density were all reduced at birth (Snoeck et al. 1990). Furthermore the ontogeny of the endocrine pancreas of offspring from these dams was perturbed when the low protein diet was maintained during the suckling period. At each fetal and postnatal day analysed, the number of apoptotic cells in these islets was increased while the number of cells positive for insulin-like growth factor II (IGF-II), considered to be a survival factor which prevents apoptosis (Petrik et al. 1998), was decreased (Reusens et al. 1998). The function of these fetal β cells was affected with insulin secretion being diminished by 50%in vitro compared with controls (Dahri et al. 1994). With a normal diet postnatally, insulin secretion in vitro remained impaired when stimulated by arginine and leucine (Dahri et al. 1994). In vivo the adult offspring showed normal basal plasma glucose and insulin levels as well as a normal amino acid profile. During a glucose challenge, the insulin level was abnormally low in adult non-pregnant females and it remained low during pregnancy, associated with higher than normal plasma glucose levels (Table 1).
Table 1.
Effects of protein deficiency during pregnancy on the endocrine pancreas of the adult offspring
| Low diet maintained postnatally | Normal diet given postnatally |
|---|---|
| Islet cell mass ↓ | |
| Pancreatic insulin content ↓ | |
| β cell sensitivity to glucose and amino acids in vitro↓ | β cell sensitivity to amino acids in vitro↓ |
| Plasma insulin level ↓ | |
| Insulin response to oral glucose challenge ↓ | Insulin response to oral glucose only in female ↓ |
When the isocaloric low protein diet was maintained postnatally the adult offspring showed an abnormal amino acid profile which was associated with a reduced volume of the endocrine pancreas and pancreatic insulin content (Dahri et al. 1995). Islet blood vessel density (Dahri et al. 1993) as well as pancreatic and islet blood flow (Iglesias-Barreira et al. 1996) were also diminished. It was of particular interest that reduced activity of mitochondrial glycerophosphate dehydrogenase (mGPDH) was observed in these islets (Rasschaert et al. 1995). A similar reduction is observed in islet cells of human subjects with type 2 diabetes.
Special emphasis should be placed on the sensitivity of fetal islets to taurine, which is an indispensable amino acid during fetal and neonatal development in rats, cats and baboons (Sturman, 1993). Plasma taurine was significantly reduced in dams fed low protein in gestation, and in their fetuses. Insulin secretion by normal fetal islets was stimulated in vitro by taurine and, when added to the culture medium, it enhanced insulin secretion in response to other secretagogues (Cherif et al. 1996). Islets of fetuses from dams on a low protein diet did not secrete insulin in response to taurine and in the culture medium it did not restore a normal secretory response to other secretagogues. However, when taurine was added to the drinking water of rats on a low protein isocaloric diet, to re-establish plasma taurine levels in dams and fetuses, the insulin secretion in response to taurine and other secretagogues was restored to normal (Cherif et al. 1998). Thus, current observations point to the need for normal protein availability in the diet and identify taurine as a necessary amino acid for the normal functional development of fetal β cells.
A low protein isocaloric diet also has an intergenerational effect on birth weight and the endocrine pancreas (Hoet et al. 1997). This diet reduced the birth weight of pups and it reduced it further in subsequent generations, producing an increased number of growth restricted pups (Stewart, 1975). As noted above, the female offspring showed abnormally low plasma insulin and high plasma glucose levels in response to an oral glucose challenge when they were pregnant. Their pups had lower plasma insulin and lower insulin content and reduced volume density of the endocrine pancreas (Table 2). When the low protein intake was maintained postnatally as well as during gestation, the mother and the pups showed higher plasma glucose and lower plasma insulin levels than normal. Alterations of endocrine pancreas and its insulin content in these pups were even more marked than in the previous generation. Therefore chronic isocaloric protein deprivation, which lowered the feto-maternal levels of amino acids such as taurine, initiates intergenerational effects on glucose homeostasis and the development of the endocrine pancreas. A similar intergenerational effect of a protein restricted diet has already been observed on brain development and tryptophan metabolism (Resnick & Morgan, 1984).
Table 2.
Effects of protein deficiency during pregnancy on insulin glucose homeostasis in 1st and 2nd generation offspring
| First generation pregnant female offspring | Second generation late gestation fetus | |||
|---|---|---|---|---|
| Low protein postnatal diet | Normal postnatal diet | Low protein postnatal diet | Normal postnatal diet | |
| Plasma glucose | + | n.e. | + | n.e. |
| Plasma insulin | − | n.e. | − | − |
| Pancreatic insulin content | − | − | − | − |
| Insulin response to OGTT | − | — | — | — |
| Islet cell mass | — | — | − | − |
+, increase; −, decrease; n.e., no effect; —, not measured.
Insulin sensitive tissues
Many aspects of liver function, including cholesterol synthesis and fibrinogen production, are differentially expressed in the periportal and perivenous zones. The livers of pups born to mothers on an isocaloric low protein diet underwent changes in zonation and enzyme activity, including a reduction in glucokinase and an increase in phosphoenolpyruvate carboxykinase activity. These were not restored at adulthood even when the animals were nourished with a normal diet (Desai et al. 1995) and were responsible for changes in the regulation of hepatic glucose output (Ozanne et al. 1996a). Altered zonation is likely to be linked to other important changes in hepatic function, though these are as yet unknown. There are, however, indications that manipulations during gestation up-regulated cholesterol synthesis (Innis, 1983, 1985). The number of insulin receptors was increased in the liver, skeletal muscle (Ozanne et al. 1996a,b) and white fat adipocytes (Shepherd et al. 1997). In addition the adipocytes were smaller and did not show changes in GLUT-4 expression, although this was increased in the plasma membrane of skeletal muscle (Ozanne et al. 1996b). The adipocytes of adults had a greater glucose uptake and a higher phosphatidylinositol 3-kinase activity (Ozanne et al. 1997). Adipose tissue of offspring was also affected by global dietary restriction, which comprises low protein availability in the dams. In this instance white adipose tissue increased and brown adipose tissue decreased in the adult, possibly indicating lower sympathetic activity. Rats whose mothers had restricted food during the first 2 weeks of pregnancy indeed became obese, but depending on the strain and the diet used it was either the males or the females which were affected (Jones & Friedman, 1982; Anguita 1993).
Thus limited protein intake during gestation leads to alterations in glucose output by the liver as well as in the sensitivity of tissues to insulin. Glucose transporters in muscles and the expression of key components of insulin signalling pathways in adipocytes are also altered. In addition, studies have suggested that maternal dietary restriction during gestation and lactation and transient dietary protein restriction after weaning may permanently alter growth hormone secretion in offspring (Harel & Tannenbaum, 1995).
Kidney
In non-human primates and rats neonatal kidney weights were reduced by an isocaloric low protein diet given to the mother. Hence there appears to be a specific effect of protein limitation on the kidney (Cheek & Hill, 1975; Merlet-Benichou et al. 1994). In rats a severe low protein isocaloric diet during pregnancy is associated with low birth weight and a significant reduction in the number of mature glomeruli (Merlet-Benichou et al. 1994) with an increase in the number of immature glomeruli (Zeman, 1968) in the progeny. Kidney weight as well as the final number of mature glomeruli remained reduced at 14 days postnatally even when the pups were fed a normal diet postnatally. Therefore an isocaloric low protein diet during pregnancy leads to permanent changes in the kidney which are not reversible postnatally. The number of renal glomeruli is also reported to be reduced in infants who are malnourished in early life (Naeye, 1965; Hinchliffe et al. 1992) as well as in adults with hypertension (Hayman et al. 1939, Mackenzie et Brenner, 1995). Lastly, the number of nephrons in humans is known to be correlated with birth weight (Merlet-Benichou et al. 1993).
Brain
In the brain of offspring of dams exposed experimentally to an isocaloric low protein (8%) diet before and during pregnancy, there were anatomical and physiological alterations (Resnick et al. 1979). The distribution and levels of biogenic amines were changed and there were modifications of tryptophan metabolism. Whilst a normal diet postnatally restores some of these features, the changes in biogenic amines persist (Resnick et al. 1979). These alterations became even more severe when the same low protein isocaloric diet was given to subsequent generations (Resnick & Morgan, 1984). Thus for brain development a low protein isocaloric diet initiates an inter-generational effect similar to that shown for the endocrine pancreas. In addition, brain blood vessel density was reduced in pups born to dams on an isocaloric low protein diet (Reusens et al. 1997) and remained reduced when a normal diet was given postnatally. This contrasts with the restoration of blood vessel density in the endocrine pancreas, which was restored by giving a normal diet postnatally (Dahri et al. 1993). The mechanisms involved in the effects of nutrition on vasculogenesis in various tissues are not yet known.
Comparison of dietary restriction with maternal diabetes
Besides maternal nutritional deprivation, with protein reduction or global dietary reduction, maternal diabetes is also known to affect fetal tissue development in humans and experimental animals. Streptozotocin-induced diabetes in a mild form induced a higher birth weight than normal, an increased percentage of pancreatic endocrine tissue with β cell hyperplasia and an increase in the number of degranulated cells (Aerts et al. 1990). Hence the proliferative capacity of the islet was increased in vivo, and it remained elevated when cultured in a normal medium for 7 days (Reusens et al. 1984). In the gut, villous and microvillous surface area was increased in the duodenum, jejunum and ileum, and mucosal blood vessel density was also increased (Reusens et al. 1989). However when dams had severe diabetes, the fetal weight, islet size and β cell mass were decreased (Aerts et al. 1990) and atrophy of the fetal intestinal tract occurred (Reusens et al. 1989). In such maternal diabetes, induced experimentally by streptozotocin, the pups of a second generation showed a reduction in birth weight together with permanent changes in endocrine pancreatic structure and function (Aerts et al. 1990). Pups from moderately as well as severely diabetic dams became diabetic at adulthood. Pancreatic insulin depletion in the former and insulin resistance in the latter were causal in initiating the diabetes in the offspring postnatally (Holemans et al. 1991). There are in addition effects of maternal streptozotocin-induced diabetes on the responses of small resistance arteries in the pups (see Koukkou et al. 1997).
Both the experimental conditions of maternal protein restriction and diabetes stress the impact of maternal nutritional limitation or poor health in producing effects on the offspring. Several tissues are affected, including the vasculature, endocrine pancreas, insulin sensitive tissues, kidney and brain. Some of the pathological changes can develop after a delay and there are inter-generational effects. The effects may cause degenerative diseases in adults and they can occur without major changes in birth weight. Therefore birth weight may only be a poor proxy for intrauterine events.
Worldwide perspective
In considering sources of variation in fetal growth, the focus in this review has been on nutrition, with particular reference to the nutritional programming hypothesis. There are, however, many factors affecting fetal growth and size at birth: the fetal genotype, the maternal genotype, the mother's pre-pregnancy nutritional status, her metabolism and physiology, her diet during pregnancy, and the resultant hormonal and circulatory milieu which sustains fetal growth. At least as far as the protein restriction model is concerned, we have stressed the importance of the quantity and quality of amino acids available to the fetus. But for all the factors involved, there is a need to determine the specific mechanisms responsible for the alterations in fetal development.
Birth weight is a crude measure of fetal growth: babies of the same weight may, for example, be short and fat or long and thin, and may be markedly different in organ size and structure, physiology and metabolism. In humans, low birth weight is the result of multifactorial processes operating during pregnancy. It is confounded by the influence of ethnic origin, low socio-economic status and poor nutrition which may affect the mother and her offspring concomitantly. Data from developing countries indicate that in some areas of Asia, 20% of women have stunted growth with an adult height of below 1.45 m, and 65% have an adult body weight below 45 kg. More than 35% of mothers deliver infants with birth weights under 2.5 kg (Galloway & Anderson, 1994). Much of the situation stems from a poverty-related deficiency of protein and energy intake as well as of key micronutrients such as vitamin A, iodine and iron, from which women and children suffer disproportionately. Inadequate diets are also associated with infectious diseases which, when occurring during pregnancy, increase the energy, protein and micronutrient needs and are associated with low birth weight. In infants up to 5 years of age protein/energy malnutrition contributes 12.7% of the total burden of diseases. Malnutrition is also sex linked and severe malnutrition affects seven times more female than male infants in the developing world (World Development Report, 1993). Vitamin A, iodine or iron deficiency during pregnancy and early life each have a specific and immense impact on the offspring's health, contributing 11.7%, 7.2% and 14%, respectively, to the total disease burden throughout the developing world. Recently an epidemic of diabetes and cardiovascular disease in the younger age group has become apparent in these countries. In western countries, epidemiological surveys highlight the importance of low birth weight in increasing the risk of developing diabetes and cardiovascular disease in adulthood (Hales, 1996; Rich-Edwards et al. 1997). A study in 15 000 Swedish men and women provides by far the most convincing evidence of a true association between size at birth and mortality from ischaemic heart disease, and it strongly suggests that it is variation in fetal growth rate rather than size at birth that is important for aetiology (Leon et al. 1998). Low birth weight (< 2.5 kg) and poor early growth (< 8 kg at age 1 year) are associated with a high incidence (more than 45%) of diabetes, impaired glucose tolerance and/or myocardial infarction at 65 years of age. However, in twin pregnancies, the baby with the lower birth weight is more likely to develop type 2 diabetes than its twin, indicating that the intrauterine environment of the individual fetus is more important in the aetiology of this disease than genetics. Thinness at birth is also associated with insulin resistance later (Phillips et al. 1994).
Epidemiological studies of degenerative diseases should now focus on nutritional conditions across generations, to relate events occurring early in life to the time span for pathological conditions to appear. Recently the first studies on this topic have been published. Examination of maternal diet during pregnancy and health of the offspring suggests that in humans nutrition during pregnancy does indeed have long-term consequences for the health of the offspring. For example, in Aberdeen the blood pressures of men and women were found to be related to the balance of animal protein and carbohydrate in their mother's diet during late pregnancy (Campbell, 1996). This association did not depend on birth weight. The protein intake of the mother is also related to the offspring's glucose-insulin metabolism (Barker, 1998). Low protein intake was found to be associated with insulin resistance, though the association was weaker than that with low maternal body mass. Recently, it has been shown that people exposed to the Dutch famine of 1944-1945 in utero had higher plasma glucose and insulin concentrations after a standard glucose load, suggesting that their poor glucose tolerance was mainly determined by insulin resistance (Ravelli et al. 1998). The effects of famine were independent of size at birth. These findings provide direct evidence that undernutrition in utero is a key factor in the aetiology of non-insulin dependent diabetes mellitus, and show that the mother's dietary intake during pregnancy can program metabolism without altering size at birth.
It is thus clear from a range of human and animal studies that poor maternal health and/or nutritional deficiencies affect key tissues during their development, and can be responsible for pathological changes in the offspring. Specific protein malnutrition during human pregnancy and children's health has not been explored extensively although experimental observations suggest new approaches to establishing a correlation. In the human, neonatal hypercysteinemia and hypercysteinuria are associated with vascular damage in early life (McCully, 1969). Increased plasma levels of homocysteine have been found to be associated with increased risk of myocardial infarction in adulthood possibly due to enzymatic alterations associated with vitamin B6 or vitamin B12 activity. One may surmise that perturbed amino acid homeostasis during pregnancy may initiate fetal developmental abnormalities leading to pathological events in later life.
We suggest that extensive animal studies are now needed to discover specific mechanisms by which altered fetomaternal nutrition and amino acid metabolism lead to degenerative diseases in the offspring. Only when the causes in early life of these human diseases are established, will it be possible to devise ways for their primary prevention.
Acknowledgments
We would like to thank Professor C. Remacle and Dr B. Reusens for their helpful comments, and W. Rees for preparing the manuscript.
References
- Aerts L, Holemans K, Van Assche FA. Maternal diabetes during pregnancy: consequences for the offspring. Diabetes and Metabolism Review. 1990;16:147–197. doi: 10.1002/dmr.5610060303. [DOI] [PubMed] [Google Scholar]
- Anguita RM, Sigulem DM, Sawaya AL. Intrauterine food restriction is associated with obesity in young rats. Journal of Nutrition. 1993;123:1421–1428. doi: 10.1093/jn/123.8.1421. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CRW. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- Bacon BJ, Gilbert RD, Kaufmann P, Smith AD, Trevino RT, Longo LD. Placental anatomy and diffusing capacity in guinea pigs following long-term maternal hypoxia. Placenta. 1984;5:475–488. doi: 10.1016/s0143-4004(84)80002-8. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, Babies and Health in Later Life. 2. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- Burton G, Reshetnikova OS, Milovanov AP, Teleshova OV. Stereological evaluation of vascular adaptations in human placental villi to differing forms of hypoxic stress. Placenta. 1996;17:49–55. doi: 10.1016/s0143-4004(05)80643-5. [DOI] [PubMed] [Google Scholar]
- Campbell DM, Hall MH, Barker DJP, Cross J, Shiell AW, Godfrey KM. Diet in pregnancy and the offspring's blood pressure 40 years later. British Journal of Obstetrics and Gynaecology. 1996;103:273–280. doi: 10.1111/j.1471-0528.1996.tb09718.x. [DOI] [PubMed] [Google Scholar]
- Carmichael L, Sadowsky D, Olsen D, Challis J, Richardson B. Activation of the fetal hypothalamic-pituitary-adrenal axis with prolonged and graded hypoxaemia. Journal of the Society for Gynecologic Investigation. 1997;4:8–14. doi: 10.1016/S1071-5576(96)00039-1. [DOI] [PubMed] [Google Scholar]
- Cheek DB, Hill DE. Changes in somatic growth after placental insufficiency and maternal protein deprivation. In: Cheek DB, editor. Fetal and Postnatal Cellular Growth Hormones and Nutrition. New York: John Wiley & Sons; 1975. pp. 299–310. [Google Scholar]
- Cherif H, Reusens B, Ahn MT, Hoet JJ, Remacle C. Effect of taurine on the insulin secretion of islets of fetus from dams fed a low protein diet. Journal of Endocrinology. 1998;159:341–348. doi: 10.1677/joe.0.1590341. [DOI] [PubMed] [Google Scholar]
- Cherif H, Reusens B, Dahri S, Remacle C, Hoet JJ. Stimulatory effect of taurine on insulin secretion by fetal rat islets, cultured in vitro. Journal of Endocrinology. 1996;151:501–506. doi: 10.1677/joe.0.1510501. [DOI] [PubMed] [Google Scholar]
- Crowe C, Dandekar P, Fox M, Bennet L, Hanson MA. The effects of anaemia on heart, placental and body weight, and blood pressure in fetal and neonatal rats. The Journal of Physiology. 1995;488:515–519. doi: 10.1113/jphysiol.1995.sp020986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe C, Takla TY, Symonds M, Clarke L, Hanson MA. Villi density in the placentae of sheep that have been exposed to a mid-gestation nutritional insult. Proceedings of the Meeting of the Fetal and Neonatal Physiological Society, Santa Margherita Ligure. 1997 abstract no. 40. [Google Scholar]
- Dahri S, Cherif H, Reusens B, Remacle C, Hoet JJ. Effect of an isocaloric low protein diet during gestation in the rat on in vitro insulin secretion by islets of the offspring. Diabetologia. 1994;37(suppl. 1):A80. [Google Scholar]
- Dahri S, Reusens B, Remacle C, Hoet JJ. Nutritional influences on pancreatic development and potential links with non-insulin-dependent diabetes. Proceedings of the Nutrition Society. 1995;54:345–56. doi: 10.1079/pns19950003. [DOI] [PubMed] [Google Scholar]
- Dahri S, Snoeck A, Reusens B, Remacle C, Hoet JJ. Low protein diet during gestation in rats: its relevance to human non insulin dependent diabetes. The Journal of Physiology. 1993;467:292. [Google Scholar]
- De Barro TM, Owens JA, Earl CR, Robinson JS. Nutrition during early/mid pregnancy interacts with mating weight to affect placental weight in sheep. Australian Society for Reproductive Biology. 1992 Abs. [Google Scholar]
- De Gasparo M, Milner GR, Norris P, Milner RDG. Effect of glucose amino acid on fetal rat pancreatic growth and insulin secretion in vitro. Journal of Endocrinology. 1978;77:241–248. doi: 10.1677/joe.0.0770241. [DOI] [PubMed] [Google Scholar]
- Desai M, Crowther NJ, Ozanne SE, Lucas A, Hales CN. Adult glucose and lipid metabolism may be programmed during fetal life. Biochemical Society Transactions. 1995;23:331–335. doi: 10.1042/bst0230331. [DOI] [PubMed] [Google Scholar]
- Di Nicolantonio R. Altered electrolyte and taurine levels in milk from nursing hypertensive rats. Hypertension Research. 1993;16:179–184. [Google Scholar]
- Dwyer CM, Madgwick AJA, Crook AR, Stickland NC. The effect of maternal undernutrition on the growth and development of the guinea pig. Journal of Developmental Physiology. 1992;18:295–302. [PubMed] [Google Scholar]
- Edwards CRW, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355–357. doi: 10.1016/0140-6736(93)90148-a. 10.1016/0140-6736(93)90148-A. [DOI] [PubMed] [Google Scholar]
- Erkadius E, Di Iulio J, Lucente F, Bramich C, Morgan T, Di Nicolantonio R. The role of uterine factors in the development of hypertension in SHR. Clinical and Experimental Pharmacology and Physiology. 1994;21:239–242. doi: 10.1111/j.1440-1681.1994.tb02505.x. [DOI] [PubMed] [Google Scholar]
- Faichney GJ, White GA. Effects of maternal nutritional status on fetal and placental growth and on fetal urea synthesis in sheep. Australian Journal of Biological Sciences. 1987;40:365–377. doi: 10.1071/bi9870365. [DOI] [PubMed] [Google Scholar]
- Galloway R, Anderson M A. Vol. 11. WHO Ch. 1211 Geneva 27, Switzerland: SCN (Subcommitteee on Nutrition), News United Nations, Administrative Committee on Coordination; 1994. Prepregnancy nutritional status and its impact on birthweight; pp. 6–10. [PubMed] [Google Scholar]
- Gerber RT, Holemans K, Van Assche FA, Poston L. Fetal and Neonatal Physiology Symposium. Cambridge: 1997. Female offspring from pregnant diabetic rats demonstrate cardiovascular dysfunction. [Google Scholar]
- Hales CN. Fetal nutrition and adult diabetes. Scientific American, Science and Medicine. 1996;1:54–63. [Google Scholar]
- Hales CN, Desai M, Ozanne SE, Crowther NJ. Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. Biochemical Society Transactions. 1996;24:341–350. doi: 10.1042/bst0240341. [DOI] [PubMed] [Google Scholar]
- Harel Z, Tannenbaum GS. Long-term alterations in growth hormone and insulin secretion after temporary dietary protein restriction in early life in the rat. Pediatric Research. 1995;38:747–753. doi: 10.1203/00006450-199511000-00019. [DOI] [PubMed] [Google Scholar]
- Hawkins P, Crowe C, Calder NA, Saito T, Ozaki T, Stratford LL, Noakes DE, Hanson MA. Cardiovascular development in late gestation fetal sheep and young lambs following modest maternal nutrient restriction in early gestation. The Journal of Physiology. 1997;505.P:18. [Google Scholar]
- Hawkins P, Crowe C, Mcgarrigle HHG, Saito T, Ozaki T, Stratford LL, Noakes DE, Hanson MA. Effect of maternal nutrient restriction in early gestation on hypothalamic pituitary adrenal axis responses during acute hypoxaemia in late gestation fetal sheep. The Journal of Physiology. 1998;507.P:50. [Google Scholar]
- Hayman JM, Martin J, Miller M. Renal function and the number of glomeruli in the human kidney. Archives of Internal Medicine. 1939;64:69–83. [Google Scholar]
- Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. British Journal of Obstetrics and Gynaecology. 1992;99:296–301. doi: 10.1111/j.1471-0528.1992.tb13726.x. [DOI] [PubMed] [Google Scholar]
- Hoet JJ, Reusens B, Dahri S, El-Hajjaji H, Remacle C. 16th International Congress of Nutrition. Montreal, Canada: 1997. Protein malnutrition during pregnancy in the rat has an intergeneration effect on the endocrine pancreas; p. 70. PW 11.4. [Google Scholar]
- Holemans K, Aerts L, Van Assche FA. Evidence for an insulin resistance in the adult offspring of pregnant streptozotocin diabetic rats. Diabetologia. 1991;34:81–85. doi: 10.1007/BF00500377. [DOI] [PubMed] [Google Scholar]
- Holemans K, Gerber R, Meurrens K, Spitz B, Declerck F, Poston L, Van Assche FA. Maternal malnutrition in the rat affects vascular function but does not produce elevated blood pressure. British Journal of Nutrition. 1998 in the Press. [Google Scholar]
- Iglesias-Barreira V, Ahn MR, Reusens B, Remacle C, Hoet JJ. Pre- and postnatal low protein diet affect pancreatic islets blood flow and insulin release in adult rats. Endocrinology. 1996;137:3797–3801. doi: 10.1210/endo.137.9.8756549. 10.1210/en.137.9.3797. [DOI] [PubMed] [Google Scholar]
- Innis SM. Influence of the maternal cholestyramine treatment on cholesterol and bileacid metabolism in adult offspring. Journal of Nutrition. 1983;113:24264–2470. doi: 10.1093/jn/113.12.2464. [DOI] [PubMed] [Google Scholar]
- Innis SM. The role of diet during development on the regulation of adult cholesterol homeostasis. Canadian The Journal of Physiology and Pharmacology. 1985;63:557–564. doi: 10.1139/y85-095. [DOI] [PubMed] [Google Scholar]
- Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of ratsundernourished during pregnancy. Science. 1982;215:1518–1519. doi: 10.1126/science.7063860. [DOI] [PubMed] [Google Scholar]
- Kingdom JCP, Kaufmann P. Oxygen and placental villous development: originsof fetal hypoxia. Placenta. 1997;18:613–621. doi: 10.1016/s0143-4004(97)90000-x. 10.1016/S0143-4004(97)90000-X review. [DOI] [PubMed] [Google Scholar]
- Koukkou E, Lowry C, Poston L. The offspring of diabetic rats fed a high saturatedfat diet demonstrate abnormal vascular function. Journal of the Society for Gynecologic Investigation. 1997;4:115A. 10.1016/S1071-5576(97)00019-1. [Google Scholar]
- Krebs C, Longo LD, Leiser R. Term ovine placental vasculature comparison ofsea level and high altitude conditions by corrosion cast and histomorphometry. Placenta. 1997;18:43–51. doi: 10.1016/s0143-4004(97)90070-9. 10.1016/S0143-4004(97)90070-9. [DOI] [PubMed] [Google Scholar]
- Langley SC, Jackson AA. Increased systolic blood pressure in adult rats inducedby fetal exposure to maternal low protein diets. Clinical Science. 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Maternal carbonoxolene treatment lowers birthweight and induceshypertension in the offspring of rats fed on protein-replete diet. Clinical Science. 1997;93:423–429. doi: 10.1042/cs0930423. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure inrats with hypertension induced by fetal exposure to maternal low protein diets. Comparative Biochemistry and Physiology. 1995;A 110:223–228A. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- Lechner AJ, Winston CC, Bauman JE. Lung mechanics, cellularity and surfactantafter prenatal starvation in guinea pigs. Journal of Applied Physiology. 1986;60:1610–1614. doi: 10.1152/jappl.1986.60.5.1610. [DOI] [PubMed] [Google Scholar]
- Leon DA, Lithell HO, Vagero D, Koupilova I, Mohsen R, Berglund L, Lithell UB, Mckeigue PM. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: a cohort study of 15000 Swedish men and women born 1915-1929. British Medical Journal. 1998;4:241–245. doi: 10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L, Jackson AA. Modest restriction of dietary protein during pregnancy in therat: fetal and placental growth. Journal of Developmental Physiology. 1993;19:113–118. [PubMed] [Google Scholar]
- Lewis RM, Batchelor DC, Bassett NS, Johnston BM, Napier J, Skinner SJM. Perinatal growth disturbance in the spontaneously hypertensive rat. Pediatric Research. 1997;42:758–764. doi: 10.1203/00006450-199712000-00007. [DOI] [PubMed] [Google Scholar]
- Lucas A. Programming by early nutrition in man. In: Brock GR, Whelan J, editors. The Childhood Environment and Adult Disease. Chichester, UK: John Wiley & Sons; 1991. pp. 38–55. [Google Scholar]
- Mccarty R, Lee JH. Maternal influences in adult blood pressure of SHRs: a single-pup cross-fostering study. Physiology and Behavior. 1996;59:71–75. doi: 10.1016/0031-9384(95)02034-9. 10.1016/0031-9384(95)02034-9. [DOI] [PubMed] [Google Scholar]
- Mccarty R, Fields-Okotcha C. Timing of preweanling maternal effects on development of hypertension in SHR rats. Physiology and Behavior. 1994;55:839–844. doi: 10.1016/0031-9384(94)90069-8. 10.1016/0031-9384(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Mackenzie HS, Brenner BM. Fewer nephrons at birth. A missing link in the etiologic of essential hypertension. American Journal of Kidney Diseases. 1995;26:91–98. doi: 10.1016/0272-6386(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Mccrabb GJM, Egan AR, Hosking BJ. Maternal undernutrition during mid-pregnancy in sheep. Placental size and its relationship to calcium transfer during late pregnancy. British Journal of Nutrition. 1991;65:157–168. doi: 10.1079/bjn19910077. [DOI] [PubMed] [Google Scholar]
- Mccully KS. Vascular pathology of homocysteinemia. Implications for the pathogenesis of arteriosclerosis. American Journal of Pathology. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- Mellor DJ, Murray L. Effects of placental weight and maternal nutrition on the growth rates of individual fetuses in single and twin bearing ewes during late pregnancy. Research in Veterinary Science. 1981;30:198–204. [PubMed] [Google Scholar]
- Merlet-Benichou C, Gilbert T, Muffat-Joly M, Leliévre-pégorier M, Leroy B. Intrauterine growth retardation leads to permanent nephron deficit in the rat. Pediatric Nephrology. 1994;8:175–180. doi: 10.1007/BF00865473. [DOI] [PubMed] [Google Scholar]
- Merlet-Benichou C, Leroy B, Gilbert T, Leliévre-pégorier M. Retard de croissance intra utérin et déficit en néphrons. Médicine Science. 1993;6–7:777–780. [Google Scholar]
- Naeye RL. Malnutrition, probable cause of growth retardation. Archives of Pathology. 1965;79:264–291. [PubMed] [Google Scholar]
- Norman LJ, Challis J. Synergism between corticotropin-releasing factor and arginine vasopressin and adrenocorticotrophin release in vivo varies as function of gestational age in the ovine fetus. Endocrinology. 1987;120:1052–1058. doi: 10.1210/endo-120-3-1052. [DOI] [PubMed] [Google Scholar]
- Osofsky HJ. Relationships between nutrition during pregnancy and subsequent infantand child development. Obstetrical and Gynecological Survey. 1975;30:227–241. doi: 10.1097/00006254-197504000-00001. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hawkins P, Poston L, Hanson MA. Isolated systemic resistancevessel function in hypertensive male rat offspring of mild nutritionally restricted dams. The Journal of Physiology. 1998;513.P:118. [Google Scholar]
- Ozanne SE, Nave BT, Wang CL, Shepherd PR, Prins J, Smith GD. Poor fetal nutrition causes a long term change in expression of insulin signalling components in adipocytes. American Journal of Physiology. 1997;273:E46–51. doi: 10.1152/ajpendo.1997.273.1.E46. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Smith GD, Tikerpae J, Hales CN. Altered regulation of hepatic glucose output in the male offspring of protein malnourished rat dams. American Journal of Physiology. 1996a;270:E559–564. doi: 10.1152/ajpendo.1996.270.4.E559. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Wang CL, Coleman N, Smith GD. Altered muscle insulin sensitivity in the male offspring of protein malnourished rats. American Journal of Physiology. 1996b;271:E1128–1134. doi: 10.1152/ajpendo.1996.271.6.E1128. [DOI] [PubMed] [Google Scholar]
- Penninga L, Longo ID. Ovine placentome morphology: effect of high altitude, long-term hypoxia. Placenta. 1998;19:187–193. doi: 10.1016/s0143-4004(98)90008-x. [DOI] [PubMed] [Google Scholar]
- Persson E, Jansson T. Low birth weight is associated with elevated adult blood pressure in the chronically catheterised guinea pig. Acta Physiologica Scandinavica. 1992;145:195–196. doi: 10.1111/j.1748-1716.1992.tb09356.x. [DOI] [PubMed] [Google Scholar]
- Petrik J, Arany E, Mcdonald TJ, Hill DJ. Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin like growth factor II that may act as a survival factor. Endocrinology. 1998;139:2994–3004. doi: 10.1210/endo.139.6.6042. [DOI] [PubMed] [Google Scholar]
- Phillips DW, Barker DJP, Hales CN, Hirst S, Osmond C. Thinness at birth and insulin resistance. Diabetologia. 1994;37:150–154. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- Phillips DW, Simonetta G, Owens JA, Robinson JS, Clarke IJ, Mcmillen IC. Placental restriction alters the functional development of the pituitary-adrenal axis in the sheep fetus during late gestation. Pediatric Research. 1996;40:861–866. doi: 10.1203/00006450-199612000-00014. [DOI] [PubMed] [Google Scholar]
- Rasschaert J, Reusens B, Dahri S, Sener A, Remacle C, Hoet JJ, Malaisse WJ. Impaired activity of rat pancreatic islet mitochondrial glycerophosphate dehydrogenase in protein malnutrition. Endocrinology. 1995;136:2631–2634. doi: 10.1210/endo.136.6.7750486. [DOI] [PubMed] [Google Scholar]
- Ravelli ACJ, Van der Meulen JHP, Michels RPJ, Osmond C, Barker DJP, Hales CN, Bleker OP. Glucose tolerance after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Resnick O, Morgan PJ. Generational effect of protein malnutrition in the rat. Developmental Brain Research. 1984;15:219–227. doi: 10.1016/0165-3806(84)90099-3. [DOI] [PubMed] [Google Scholar]
- Resnick O, Miller M, Forbes W, Hall R, Kemper T, Bronzino J, Morgane PJ. Development protein malnutrition: influences on the central nervous system of the rat. Neuroscience and Behavioral Reviews. 1979;3:233–246. doi: 10.1016/0149-7634(79)90011-3. [DOI] [PubMed] [Google Scholar]
- Reusens B, Dahri S, Snoeck A, Bennis-Taleb N, Remacle C, Hoet JJ. Long term consequences of diabetes and its complications may have a fetal origin: experimental and epidemiological evidence. In: Cowett RM, editor. Nestlé Nutrition Workshop Series, Diabetes. Vol. 25. New York: Raven Press; 1995. pp. 187–198. [Google Scholar]
- Reusens B, Hill DJ, Petrik J, Remacle C, Hoet JJ. Balance of islet cell birth and death of fetal and neonatal rat is altered by a low protein diet through mechanisms which include cell cycle kinetics. Diabetologia. 1998;(suppl. 1):OP40. [Google Scholar]
- Reusens B, Remacle C, Hoet JJ. The development of the fetal rat intestine and its relation to maternal diabetes: effect of mild and severe maternal diabetes. Diabetes Research and Clinical Practice. 1989;6:213–219. doi: 10.1016/0168-8227(89)90031-4. [DOI] [PubMed] [Google Scholar]
- Reusens-Billen B, Remacle C, Daniline J, Hoet JJ. Cell proliferation in pancreatic islets of rat fetus and neonates from normal and diabetic mothers. An in vitro and in vivo study. Hormone and Metabolic Research. 1984;16:565–571. doi: 10.1055/s-2007-1014853. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards J, Stampfer MJ, Manson JEM, Rosner B, Hankinson SE, Colditz GA, Willett WC, Hennekens CH. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. British Medical Journal. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JS, Jones CT, Kingston EJ. Studies on experimental growth retardation in sheep. The effects of maternal hypoxaemia. Journal of Developmental Physiology. 1983;56:89–100. [PubMed] [Google Scholar]
- Scheffen I, Kaufmann P, Philippens L, Leiser R, Geisen C, Mottaghy K. Alterations in the fetal capillary bed in the guinea pig placenta following long-term hypoxia. Advances in Experimental Medicine and Biology. 1990;277:779–789. doi: 10.1007/978-1-4684-8181-5_89. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Crowther N, Desai M, Hales CN, Ozanne SE. Altered adipocyte properties in the offspring of protein malnourished rats. British Journal of Nutrition. 1997;78:121–129. doi: 10.1079/bjn19970124. [DOI] [PubMed] [Google Scholar]
- Snoeck A, Remacle C, Reusens B, Hoet JJ. Effects of low protein diet during pregnancy on the fetal rat endocrine pancreas. Biology of the Neonate. 1990;57:107–118. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- Stephens DN. Growth and development of dietary obesity in adulthood of rats which have been undernourished during development. British Journal of Nutrition. 1980;44:215–227. doi: 10.1079/bjn19800034. [DOI] [PubMed] [Google Scholar]
- Stewart RJ, Preele RF, Sheppart HG. Twelve generations of marginal protein deficiency. British Journal of Nutrition. 1975;33:233–253. doi: 10.1079/bjn19750027. [DOI] [PubMed] [Google Scholar]
- Sturman GA. Taurine in development. Physiological Reviews. 1993;73:119–147. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- Tangalakis K, Roberts FE, Wintour EM. The time-course of ACTH stimulation of cortisol synthesis by the immature ovine foetal adrenal gland. Journal of Steroid Biochemistry and Molecular Biology. 1992;42:527–532. doi: 10.1016/0960-0760(92)90266-l. [DOI] [PubMed] [Google Scholar]
- Winick M, Noble A. Cellular responses in rats during malnutrition at various ages. Journal of Nutrition. 1966;89:300–306. doi: 10.1093/jn/89.3.300. [DOI] [PubMed] [Google Scholar]
- Woodall SM, Breier BH, Johnston BM, Gluckman PD. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the somatotrophic axis and postnatal growth. Journal of Endocrinology. 1996;150:231–24. doi: 10.1677/joe.0.1500231. [DOI] [PubMed] [Google Scholar]
- World Development Report. Investing in Health. London: Published for the World Bank by Oxford University Press; 1993. pp. 72–81. [Google Scholar]
- Zeman J. Effect of maternal protein restriction on the kidney of newborn young of rats. Journal of Nutrition. 1968;94:111–116. doi: 10.1093/jn/94.2.111. [DOI] [PubMed] [Google Scholar]


