Abstract
As changes in core body temperature are generally associated with concomitant changes in sleep propensity, it is possible that the effects of hypnotic/soporific agents may be related to changes in thermoregulation. Therefore, to increase our knowledge of the mechanisms by which these agents exert their soporific effects, we compared the thermoregulatory and soporific effects of temazepam (20 mg per os (p.o.)) with those of melatonin (5 mg p.o.) when administered at 14.00 h to 20 young healthy adults (13 male, 7 female; age, 23·5 ± 0·4 years).
From 08.00 to 20.30 h, subjects lay in bed, and foot and rectal (Tc) temperatures were recorded. Sleep onset latency (SOL) was measured using 20 min multiple sleep latency tests, performed hourly from 11.00 to 20.00 h, during which time heart rate was recorded.
Compared with placebo, both melatonin and temazepam significantly reduced Tc (-0·17 ± 0·02 and -0·15 ± 0·03 °C, respectively) and SOL (by 4·8 ± 1·49 and 6·5 ± 1·62 min, respectively). Although both treatments significantly increased heat loss, only melatonin demonstrated cardiac effects. Importantly, there was a temporal relationship between minimum SOL and the maximum rate of decline in Tc for both melatonin (r = 0·48) and temazepam (r = 0·44).
A possible role of thermoregulation in sleep initiation is suggested by the similar temporal relationship between Tc and SOL for two different classes of soporific agents.
Changes in core body temperature are generally associated with changes in sleep propensity (Zully et al. 1981). For example, when thermoregulatory factors such as activity and food consumption are controlled, changes in sleep propensity are inversely correlated with the daily variation in core temperature (Lack & Lushington, 1996). In addition, there is some evidence to suggest that the soporific effects of agents such as the pineal hormone melatonin are associated with their thermoregulatory effects (Kräuchi et al. 1997a, b). Specifically, concomitant soporific and hypothermic effects have been observed in healthy young adults when oral melatonin has been administered during the day at doses greater than 1 mg (Cagnacci et al. 1992; Dollins et al. 1994; Zhdanova et al. 1995).
It may be the case, as has been suggested for melatonin (Dawson & Encel, 1993; Cagnacci et al. 1995), that the effects of other soporific agents are related to changes in thermoregulation. If the thermoregulatory system were to be involved in the soporific effect of hypnotic/soporific drugs, then such agents might be expected to display similar thermoregulatory effects. The benzodiazepines are a class of hypnotics/soporifics that, like melatonin, significantly reduce sleep onset latency (van der Kleijn, 1989). Currently, one of the most commonly prescribed and popular sleeping medications in Australia is the benzodiazepine temazepam (Gilbert, 1991). While preliminary evidence has suggested oral temperature may decrease following temazepam administration (Pleuvry et al. 1980), this has yet to be established under controlled conditions. Therefore, in an attempt to increase our knowledge of the mechanisms by which hypnotic/soporific agents exert their soporific effects, the present study compared the thermoregulatory and soporific effects of temazepam with those of melatonin.
METHODS
Subjects
Twenty young healthy subjects (13 male, 7 female) aged between 18 and 30 years (mean ± s.e.m., 23.5 ± 0.4 years) attended the laboratory on three non-consecutive occasions from 21.00 h to 21.00 h the following evening. Each session was separated by no more than 7 days, and all three sessions were completed between 6 and 21 days for all subjects. All female subjects were in the follicular stage of the menstrual cycle (4-14 days after menstruation begins) on the experimental days as a reduced effect of melatonin has been observed during the luteal phase of this cycle (Cagnacci et al. 1996). Potential subjects were screened for current medical conditions, sleep disorders and erratic sleep-wake schedules using a general health questionnaire and a 2 week sleep diary. In between each experimental session, subjects were instructed to maintain a regular sleep schedule, but could sleep when they desired. Subjects were excluded on the basis of existing medical illness or the use of drugs known to affect sleep or thermoregulation. Subjects gave written, informed consent to participate in the present study.
Experimental protocol
Subjects abstained from caffeine, alcohol and medications for 24 h prior to, and during, the experimental procedure. Upon arrival in the laboratory at 21.00 h, subjects were fitted with a conventional montage of polysomnographic (PSG) electrodes attached to the face and scalp and connected to a Medilog MPA-2 (Oxford Medical Limited, Oxton, UK). Temperature thermistors were placed at three body sites; left and right foot (YSI-4499E, Yellow Springs Instruments, OH, USA), and rectal (Steri-Probe 491B, Cincinnati Sub-Zero Products, Cincinnati, OH, USA) temperatures were recorded. Foot thermistors were placed on the arch of the foot sole and attached with Micropore surgical adhesive tape (3M, St Paul, MN, USA). Rectal thermistors were self-inserted 10 cm into the rectum. All thermistors were connected to a custom temperature system (Strawberry Tree, Sunnyvale, CA, USA) for sampling across 30 s intervals. In addition, standard ECG electrodes (Oxford disposable electrodes, Oxford Medical Limited) were attached to the right upper chest and left lower rib cage in order to measure heart rate. However, due to technical difficulties, ECG data were obtained from only 10 of the 20 subjects.
For the overnight sleep in the laboratory, subjects were allowed to self-select the time of lights out, which was no later than midnight for any subject. At 08.00 h, subjects were woken and ate a light breakfast before being instructed to lie quietly in bed. From 09.00 until 20.30 h (excluding multiple sleep latency tests), subjects lay in the supine position and were permitted to read or watch television. At 14.00 h, either 5 mg of melatonin, 20 mg of temazepam, or placebo (glucose) was administered orally in a double-blind, counterbalanced design. A light lunch containing no hot food was provided at 15.30 h. The study was approved by The Queen Elizabeth Hospital Ethics Committee and was performed according to the Declaration of Helsinki.
Measurement of objective sleep propensity
Sleep propensity was assessed hourly from 11.00 to 20.00 h using multiple sleep latency tests (MSLT; adapted from Carskadon & Dement, 1982). In an environment conducive to sleep (i.e. dark and quiet), subjects were instructed to lie still on their backs, close their eyes and attempt to fall asleep. The exact speech was repeated for each subject, every hour. Subjects were woken after they remained in stage 2 sleep for three consecutive, 30 s epochs (as measured via PSG). If the subject did not fall asleep after 20 min, the sleep latency was recorded as 20 min and the test was terminated.
Statistical analyses
Skin and rectal temperature data were averaged into 30 min bins and expressed relative to the time of drug administration in order to minimize random variability and to minimize interconditional differences in raw scores. Hourly sleep propensity was defined as the sleep onset latency to stage 1 (SOL; 3 consecutive 30 s epochs in stage 1 sleep). In addition, sleep onset latency to stage 2 (SOL2; 3 consecutive 30 s epochs in stage 2 sleep) was analysed.
Sex differences for all dependent measurements were analysed using a one-way between-groups (sex), two-way within-groups (condition and time) repeated-measures analysis of variance (ANOVA). As no sex differences were found for any measurement, the two sexes were collapsed into one group. Thus, the thermoregulatory and soporific effects of the placebo, melatonin and temazepam conditions reported in the Results section were compared using a two-way within-groups (condition and time) repeated-measures ANOVA. Data are expressed as means ± s.e.m.
The relationship between the core hypothermic effects of melatonin and temazepam was also examined. Each individual's maximum hypothermic response (placebo - treatment) to melatonin and temazepam administration was obtained and subjected to Pearson's r correlation analysis.
Heart rate was recorded only during each MSLT trial and the mean heart rate value for this period was employed for statistical analysis. Changes in heart rate between the three experimental conditions were examined using a two-way within-groups (condition and time) repeated-measures ANOVA. Data are expressed as means ± s.e.m.
As the 3 h period prior to administration (or baseline) was included in the above ANOVAs, the interaction effect was considered the most relevant. To determine times where significant differences between all three conditions occurred, planned means comparisons were conducted. For the baseline hours (11.00-14.00 h), both treatments were compared with placebo. Following administration, planned comparisons were conducted on each half-hour bin for rectal and foot temperatures. For heart rate and sleep onset latency (stages 1 and 2), planned comparisons were conducted on each hourly bin (15.00-20.00 h). For each of these time bins, both melatonin and temazepam treatments were compared separately with the placebo condition. In addition, melatonin was compared with temazepam.
Because of the large number of planned comparisons used, the significant P value for each comparison was reduced to 0.0025 to minimize the probability of committing a type I error. In addition, to account for possible violations in the covariance matrix resulting from large numbers of repeated measures, adjusted Greenhouse-Geisser (G-G) significance values were used to determine significance in the repeated-measures ANOVAs. Both G-G and standard P values are reported.
The temporal relationship between sleep onset latency and core temperature was also examined. The changes in core body temperature (placebo - treatment) and sleep onset latency (placebo - treatment), both relative to drug administration (14.00 h), were used for the analysis. Both the timing of absolute minimum core temperature and the maximum rate of decline (MROD) in core temperature were correlated with the timing of minimum sleep onset latency (maximum sleepiness) using Pearson's r correlation coefficient. The rate of core temperature change was obtained using the algorithm: (dt2 - dt1)/(t2- t1), where t is the time and dt is the data value at that time. Data are expressed as means ± s.e.m.
The intra-individual relationship between changing core temperature and sleep propensity was also examined. For each subject, the hour-to-hour (15.00-20.00 h) changes in core body temperature (placebo - treatment) were correlated with the hourly changes in sleep onset latency (placebo - treatment) using Pearson's r correlation coefficient. This yielded intra-individual correlation coefficients for each subject in each treatment condition. For each treatment, data are expressed as the mean of all the subjects’ correlation coefficients ± s.e.m.
RESULTS
Sleep onset latency
The mean changes in mean sleep onset latency to stages 1 (SOL) and 2 (SOL2) from 3 h prior to, to 6 h following administration (at 14.00 h) of melatonin, temazepam and placebo are illustrated in Fig. 1.
Figure 1. Changes in hourly sleep onset latency to stages 1 (A) and 2 (B) for placebo (^), melatonin (▪) and temazepam (▵) conditions from 11.00 to 20.00 h.
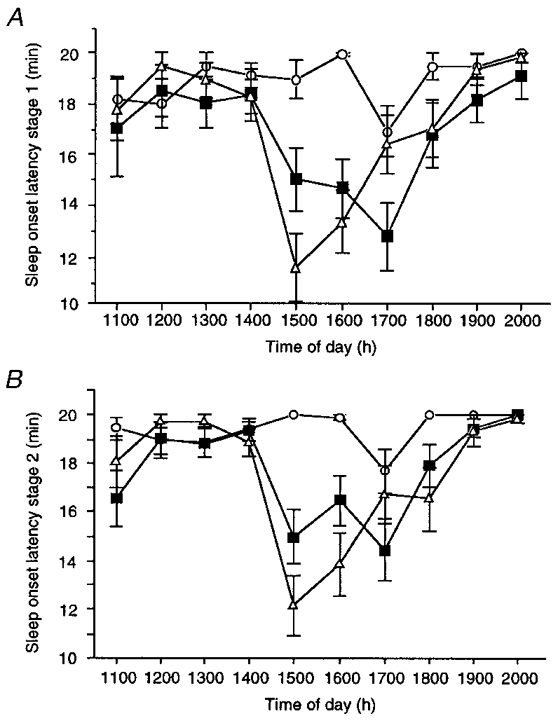
Values are raw sleep onset latencies following hourly multiple sleep latency tests and are means ± s.e.m. for 20 young adults.
For SOL, significant main effects were obtained for both ‘condition’ (F2,38= 6.6; P < 0.05 (G-G = 0.0061)) and ‘time’ (F9,171= 8.4; P < 0.05 (G-G = 0.0001)). In addition, a significant interaction effect (F18,342= 3.8; P < 0.05 (G-G = 0.0021)) was obtained. Planned comparisons revealed that, compared with the placebo trial, SOL was significantly shorter in both the melatonin and temazepam conditions for the first 2 h following drug administration (15.00-16.00 h). From 17.00 to 20.00 h, SOL was not significantly different between temazepam and placebo trials. However, SOL in the melatonin condition remained significantly shorter than placebo until 17.00 h. Except for 17.00 h, latencies to sleep onset were equivalent in the two treatment conditions (15.00-16.00 h and 18.00-20.00 h). At 17.00 h, SOL was significantly shorter in the melatonin condition compared with the temazepam trial. Relative to placebo, sleep onset latency to stage 1 was maximally reduced by 4.8 ± 1.49 min (at 16.00 h) and 6.5 ± 1.62 min (at 15.00 h) for melatonin and temazepam, respectively.
For SOL2, significant main effects for condition (F2,38= 13.3; P < 0.05 (G-G = 0.0001)) and time (F9,171= 11.4; P < 0.05 (G-G = 0.0001)) were obtained. As mentioned above for SOL, a significant interaction effect (F18,342= 5.9; P < 0.05 (G-G = 0.0001)) was also obtained for SOL2. For the melatonin condition, planned comparisons revealed that SOL2 was significantly shorter than placebo from 15.00 to 17.00 h. In the temazepam condition, SOL2 was significantly shorter than placebo from 15.00 to 18.00 h (but excluding 17.00 h). For the two treatment conditions, the latencies to stage 2 sleep across the entire testing period (11.00-20.00 h) were statistically equivalent. In addition, planned comparisons revealed that, prior to drug administration (11.00-14.00 h), SOL and SOL2 in both treatment conditions did not differ from placebo or each other. Relative to placebo, the latency to stage 2 sleep was smallest at 15.00 h in both conditions, with latencies reduced by 3.5 ± 1.2 and 7.1 ± 1.3 min for melatonin and temazepam, respectively.
Rectal core body temperature
The mean changes in core body temperature (Tc) for melatonin, temazepam and placebo conditions from 11.00 to 20.00 h are illustrated in Fig. 2. Data are expressed relative to the temperature at the time of drug administration (14.00 h). Significant main effects were obtained for both condition (F2,38= 6.8; P < 0.05 (G-G = 0.0002)) and time (F18,342= 65.7; P < 0.05 (G-G = 0.0001)). Importantly, a significant interaction effect (F36,684= 6.2; P < 0.05 (G-G = 0.0001)) was also obtained. Planned comparisons revealed that, from 14.30 to 20.00 h, core temperature in both temazepam and melatonin conditions remained significantly lower than in the placebo condition. While core temperature was not statistically different between the two treatments from 14.30 to 17.00 h, Tc was significantly higher in the temazepam condition than for melatonin at 17.30 h, and from 18.30 to 20.00 h. In addition, planned comparisons revealed that, prior to drug administration (11.00-14.00 h), rectal temperature in both treatment conditions did not differ from placebo or each other. Relative to placebo, core temperature reached a minimum of -0.17 ± 0.02 and -0.15 ± 0.03°C for the temazepam and melatonin conditions, respectively. In both these conditions, minimum Tc occurred 2 h following administration.
Figure 2. Changes in core body temperature for placebo (^), melatonin (▪) and temazepam (▵) conditions from 11.00 to 20.00 h.
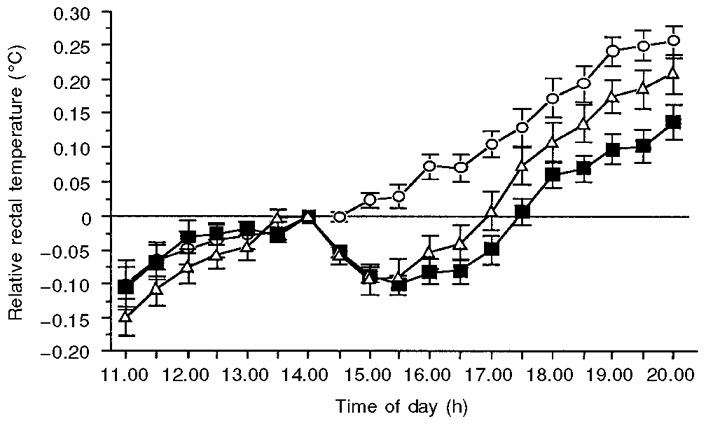
Data are expressed relative to the temperature at the time of drug administration (14.00 h), and values are means ± s.e.m. for 20 young adults. Following administration, core temperature in both treatment conditions remained significantly (P < 0.05) lower than in placebo until 20.00 h.
While there was a significant difference in core temperature between treatment conditions from 17.00 to 20.00 h, the rate at which core temperature returned to placebo levels appeared to be similar between melatonin and temazepam conditions. To determine whether this was the case, a post hoc repeated-measures ANOVA was performed. As expected, non-significant main effects were obtained for condition (F1,19= 1.24; P > 0.05) and time (F6,114= 1.7; P > 0.05). In addition, a non-significant interaction was obtained (F6,114= 0.64; P > 0.05).
For each subject, the maximal suppression of the normal elevation in core temperature (maximum hypothermic effect), regardless of when this effect occurred, was also recorded for both treatments. This was 0.26 ± 0.02°C for melatonin and 0.25 ± 0.03°C for temazepam.
When each individual's maximum hypothermic response to melatonin was correlated with their maximum decrease in core temperature following temazepam administration, a linear relationship was obtained with a significant (P < 0.05) Pearson's correlation coefficient of 0.58 (see Fig. 3).
Figure 3. Relationship between maximum core hypothermic effects following melatonin and temazepam administration.
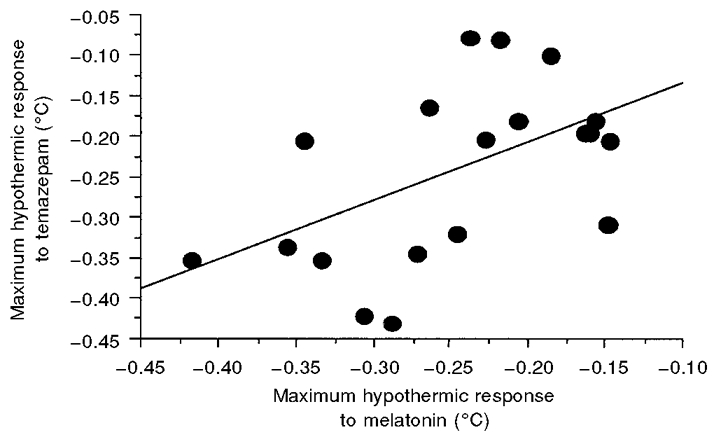
Values appear as each subject's relative maximum suppression of core body temperature (placebo - treatment) following both melatonin (x-axis) and temazepam (y-axis) conditions. A significant (P < 0.05) Pearson's r value of 0.58 was obtained.
Foot temperature
The mean changes in foot temperature following melatonin, temazepam and placebo administration (relative to the temperature at 14.00 h) are illustrated in Fig. 4. Although a non-significant main effect occurred for condition (F2,38= 2.4; P = 0.11 (G-G = 0.11)), a significant main effect for time (F18,342= 3.5; P < 0.05 (G-G = 0.043)) was obtained. In addition, a significant interaction effect (F36,684= 2.9; P < 0.05 (G-G = 0.008)) was obtained. Planned comparisons revealed that foot temperatures in both temazepam and melatonin conditions remained significantly elevated above those in the placebo trial from 15.00 to 20.00 h. Planned comparisons also revealed that foot temperatures were not statistically different between the melatonin and temazepam conditions for the entire testing period (11.00-20.00 h). In addition, planned comparisons revealed that, prior to drug administration (11.00-14.00 h), foot temperature in the two treatment conditions did not differ from placebo or each other. Following drug administration, relative foot temperature reached a maximum of 1.14 ± 0.26 and 1.61 ± 0.34°C for melatonin and temazepam, respectively. In both these treatment conditions, foot temperature maxima relative to placebo occurred at 16.00 h.
Figure 4. Changes in foot temperature following placebo (^), melatonin (▪) and temazepam (▵) administration.
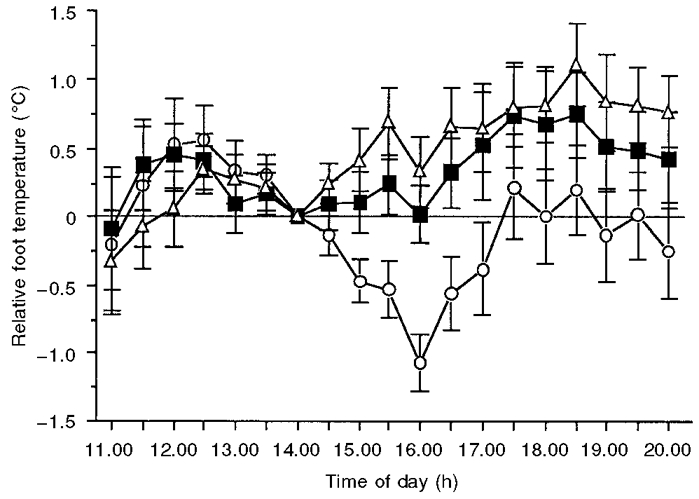
Data are expressed relative to the temperature at the time of treatment administration (14.00 h). Values are means ± s.e.m. for 20 young adults. Foot temperature following both melatonin and temazepam administration was significantly (P < 0.05) elevated above placebo until 20.00 h.
Heart rate
The mean changes in heart rate (relative to the rate at 14.00 h) following melatonin, temazepam and placebo administration are illustrated in Fig. 5. Although analysis of variance revealed no overall main effect for condition (F2,18= 1.9; P = 0.18 (G-G = 0.19)) on heart rate, a significant main effect for time (F9,81= 10.1; P < 0.05 (G-G = 0.0001)) was obtained. In addition, a significant interaction effect was obtained (F18,162= 15.3; P < 0.05 (G-G = 0.045)). Planned comparisons revealed that, from 15.00 to 17.00 h, mean heart rate following melatonin administration was significantly lower than in the placebo condition. During these 3 h, relative (placebo - melatonin) heart rate was reduced by a mean of 3.3 ± 1.2 beats min−1. In contrast, heart rate following temazepam administration was not significantly different from that in the placebo condition for the entire testing period (11.00-20.00 h). In addition, planned comparisons revealed that, prior to drug administration (11.00-14.00 h), heart rate in both treatment conditions did not differ from placebo or each other.
Figure 5. Changes in heart rate following placebo (^), melatonin (▪) and temazepam (▵) administration.
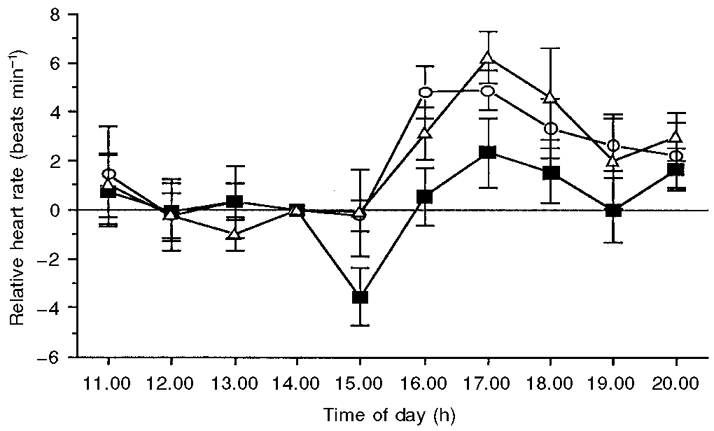
Data are expressed relative to the heart rate at the time of treatment administration (14.00 h). Values are means ± s.e.m. for 10 young adults. In the melatonin condition, heart rate was significantly (P < 0.05) below that seen in the placebo condition from 15.00 to 17.00 h.
The relationship between core temperature and sleep onset latency
The temporal relationship between sleep onset latency and core body temperature is displayed in Fig. 6 for both melatonin and temazepam conditions (Fig. 6A and B, respectively). For melatonin, a significant linear relationship (r = 0.44; P < 0.05) was obtained between the timing of minimum sleep onset latency and minimum core temperature. In the case of temazepam, this relationship was not quite significant (r = 0.39; P = 0.08). However, for both treatments, significant (P < 0.05) linear relationships were obtained between the timing of maximal sleepiness, and the timing of the MROD in core temperature. Specifically, correlation coefficients of 0.48 and 0.44 were obtained for melatonin and temazepam, respectively.
Figure 6. Temporal relationship between sleep onset latency (□) and core body temperature (•) for melatonin (A) and temazepam (B) conditions.
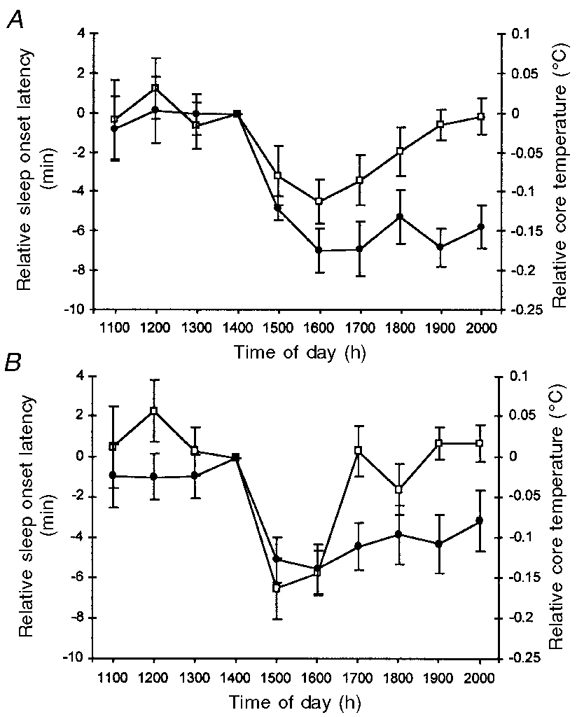
Responses are expressed relative to both the time of drug administration (14.00 h) and the placebo (placebo - treatment) condition. Values are expressed as means ± s.e.m. for 20 young adults. When the timing of minimum sleep onset latency was correlated with the timing of the maximum rate of core temperature decline, significant Pearson's r values (0.48 and 0.44, respectively) were obtained for both melatonin and temazepam conditions.
In addition, intra-individual correlations were calculated. Table 1 shows the intra-individual correlation for each subject in each treatment condition. The mean (± s.e.m.) intra-individual correlation for the melatonin condition was r = 0.41 ± 0.05, and for the temazepam condition was r = 0.40 ± 0.06.
Table 1.
Each subject's Pearson's correlation coefficient which illustrates the intra-individual relationship between changes in sleep propensity (sleep onset latency) and core body temperature between 15.00 and 20.00 h
| Subject | Melatonin | Temazepam |
|---|---|---|
| 01 | 0.45 | 0.43 |
| 02 | 0.21 | 0.48 |
| 03 | 0.43 | 0.41 |
| 04 | 0.89 | 0.70 |
| 05 | 0.20 | 0.17 |
| 06 | 0.22 | 0.10 |
| 07 | 0.15 | 0.14 |
| 08 | 0.33 | 0.46 |
| 09 | 0.37 | 0.24 |
| 10 | 0.35 | 0.29 |
| 11 | 0.69 | 0.67 |
| 12 | 0.07 | 0.01 |
| 13 | 0.55 | 0.53 |
| 14 | 0.49 | 0.46 |
| 15 | 0.45 | 0.40 |
| 16 | 0.32 | 0.24 |
| 17 | 0.37 | 0.29 |
| 18 | 0.55 | 0.66 |
| 19 | 0.53 | 0.46 |
| 20 | 0.55 | 0.81 |
| Mean ± S.E.M. | 0.41 ± 0.05 | 0.40 ± 0.06 |
DISCUSSION
The present study examined the relationship between core body temperature and sleep onset latency following administration of two different soporific agents: melatonin and temazepam. A temporal relationship was observed between minimum sleep latency and the MROD in core body temperature, with correlation coefficients of 0.48 and 0.44 for melatonin and temazepam, respectively. In addition, the two treatments displayed comparable soporific and hypothermic effects. Taken together, these observations highlight the role a declining core temperature may play in sleep initiation. The finding that temazepam administration increased foot temperature, while melatonin administration increased foot temperature and decreased heart rate, suggests that different thermoregulatory mechanisms may be employed by these agents to reduce core temperature.
Overall, the soporific effects of both melatonin and temazepam were consistent with those documented by previous researchers. Following temazepam administration, a significant decrease in sleep onset latency was observed relative to placebo, with a minimum sleep onset latency of 14.6 min occurring 1 h after administration. Although there appears to have been little documentation of the soporific effects of daytime temazepam administration in young healthy adults, significant reductions in sleep onset latency have been recorded in night-time studies employing both the elderly (van der Kleijn, 1989) and insomniacs (Ngen & Hassan, 1990) as subjects.
Relative to placebo, melatonin reduced sleep onset latency for the first 2 h, reaching maximal soporific efficacy (of approximately 5 min) 120 min after administration (see Fig. 6A). Similar time courses have been observed in previous daytime studies, with minimum sleep latencies typically occurring approximately 120 min (Dollins et al. 1994; Tzischinsky & Lavie, 1994) following administration. However, these studies have employed either subjective measures of sleep propensity (e.g. Stanford Sleepiness Scale: Dollins et al. 1994) or different objective (Dollins et al. 1994; Tzischinsky & Lavie, 1994) measures from that used in the present study (MSLT), making direct comparisons of the soporific efficacy of melatonin difficult. Still, a similar reduction in sleep onset latency (of 7 min) was observed in a previous study in our laboratory using an identical MSLT protocol (Reid et al. 1996).
Following melatonin administration, rectal core temperature paralleled the decline in sleep onset latency (see Fig. 6A), with maximal suppression of the normal elevation in core temperature (placebo - melatonin) reaching 0.17°C, 2 h following administration. However, because the time course of this response varied between individuals, the maximum hypothermic effect, regardless of the time this effect occurred, was also recorded. For melatonin, this mean effect was 0.26°C. Both of these measurements of hypothermic magnitude fit within the range observed by previous researchers. Typically, hypothermic effects in the order of 0.15-0.3°C following oral melatonin administration have been reported with doses ranging from 1.0 to 5.0 mg (Cagnacci et al. 1992; Zhdanova et al. 1995; Reid et al. 1996). Given the precision and reproducibility observed in these studies, it has been suggested that central thermoregulatory centres, such as the preoptic area of the anterior hypothalamus, are most likely to be involved in the regulation of core temperature following melatonin administration (Cagnacci et al. 1997). That is, only central nervous system centres could exert such fine control over temperature. However, because melatonin binding sites have been located in peripheral vasculature (Viswanathan et al. 1993), a direct action on thermoregulatory effector mechanisms, such as arteriovenous smooth muscle, cannot be discounted.
While the reduction in core body temperature following melatonin administration has been examined systematically, the hypothermic effects of other soporific agents, such as the benzodiazepines, have only been reported anecdotally. For example, Pleuvry et al. (1980) found that daytime administration of 40 mg temazepam reduced oral temperature by approximately 0.95°C compared with placebo. In the present study, 20 mg of temazepam significantly reduced rectal temperature by a mean of 0.15°C. Interestingly, Pleuvry et al. (1980) found that a 20 mg dose of temazepam had no effect on oral temperature. However, details in research design (subject activity not controlled) may have masked hypothermic effects of the order of 0.15°C. Alternatively, this difference may reflect the different recording sites (oral versus rectal) used.
According to current models of thermoregulation, reduced core body temperature results from either increased heat loss and/or decreased heat production. In the present study, the extent to which these two factors contributed to the core hypothermia following temazepam and melatonin administration was examined. Changes in foot temperature and heart rate were used as indirect measures of heat loss and heat production, respectively (Kräuchi & Wirz-Justice, 1994). Relative to placebo, both soporific agents significantly increased foot temperature (see Fig. 4). In addition, foot temperature in the two treatment conditions was not statistically different across the entire testing period. These results suggest that heat loss via the distal periphery may contribute significantly to the thermoregulatory effect of both melatonin and temazepam. While this is the first paper to demonstrate this for temazepam, several recent studies have documented a similar magnitude in heat loss following melatonin administration (Kräuchi et al. 1997a, b).
Although heat loss increased following both treatments, a significant decrease in heat production was observed only following melatonin administration. Specifically, heart rate was reduced by an average of 3.3 beats min−1 for 3 h following melatonin administration (see Fig. 5). These results suggest that both decreased heat production and increased heat loss may be responsible for the reduction in core temperature observed following melatonin administration. In contrast, temazepam displayed no significant cardiac effects relative to placebo. This finding supports those of Pleuvry et al. (1980) and suggests that heat production may not be involved in the hypothermic effect of temazepam when administered at 14.00 h. However, as there appears to be no documentation of significant cardiac effects following temazepam administration in the evening, it is possible that this effect may vary across the day.
In contrast to temazepam, significant decreases in heart rate have been previously documented following melatonin administration, and it appears that the magnitude of this effect may change across the day. Kräuchi et al. (1997b) reported a significant reduction in heart rate for 5 h when melatonin (5 mg p.o.) was administered at 18.00 h. However, when the same dose was administered at 13.00 h, there were no significant changes in heart rate. On the basis of these results, Kräuchi et al. (1997b) suggested that evening melatonin administration may advance the normal nocturnal decrease in heart rate. In the present study, melatonin (5 mg p.o.) administered at 14.00 h resulted in a significant reduction in heart rate for 3 h following administration. Taken together, the results of these previous studies and our own findings suggest that the responsiveness of the cardiac system to melatonin may be circadian in nature. However, it is not yet clear, due to insufficient administration points across the day, whether this is the case. It is possible, for example, that comparisons made across different hemispheres and seasons, and hence different photoperiods, may explain why our administration time (of 14.00 h) was close to the point where Kräuchi and colleagues (1997b) recorded no effect of melatonin (13.00 h). That is, in our study, administration (14.00 h) occurred approximately 7 h prior to sunset, whereas in the Kräuchi (1997b) study, administration (13.00 h) may have occurred 3-4 h before sunset. Therefore, future studies should aim to characterize how the heart rate response to melatonin changes over time, and perhaps control for the possible effect of different day lengths.
In addition, it is not yet clear whether the cardiovascular effects of melatonin result via a direct localized action or, as mentioned earlier, indirectly via thermoregulatory control centres in the hypothalamus. Moreover, it is uncertain whether the cardiac system normally has a functional role in the endogenous action of melatonin, or is merely an epiphenomenon of the large oral (5 mg) dose employed in this and other studies (i.e. Kräuchi et al. 1997a, b). Thus, future studies should also attempt to establish whether melatonin has a physiological role in cardiovascular regulation, including heart rate and changes in vascular tone associated with peripheral heat changes.
While the core hypothermic effects of melatonin and temazepam were quantitatively similar for the first 3 h following drug administration, core temperature following temazepam administration was significantly elevated above that seen in the melatonin condition for the remainder of the experiment. However, it is important to note that the rate at which core temperature returned to placebo levels was equivalent for the two treatment conditions during this time period (17.00-20.00 h; see Fig. 2). This most probably reflects the similar degree of heat loss between temazepam and melatonin at this time (see Fig. 4). It could be speculated that the difference in aforementioned core temperatures (from 17.00 to 20.00 h) may have resulted from the reduced heat production in the melatonin condition (seen in Fig. 5) from 15.00 to 17.00 h, thus resulting in the core temperature plateau seen (in the melatonin condition) during this time (see Fig. 2).
Another similarity between treatment conditions was the significant linear relationship found when the individual hypothermic effects were examined (r = 0.58). That is, individuals who demonstrated small hypothermic responses to melatonin administration also exhibited similar hypothermic effects following temazepam administration. Although there is a similarity in the measured hypothermic effects of temazepam and melatonin, it is not yet clear whether the two agents act via similar physiological mechanisms. One hypothesis is that the soporific effects of melatonin, like those of temazepam, may arise through occupation of the GABAA-benzodiazepine receptor complex (Dijk et al. 1995). On this basis, it is possible that the thermoregulatory effects of melatonin (and benzodiazepines) may also be mediated by an action at GABA receptors. However, in a recent study 10 mg of flumazenil (a central benzodiazepine antagonist) did not block either the hypothermic or the soporific effects of subsequent daytime administration of 3 mg melatonin (Nave et al. 1996). Thus, while it remains possible that both agents may act on peripheral benzodiazepine binding sites, a common central effect appears unlikely. Alternatively, it has been speculated that melatonin may influence chloride flux or other intracellular actions via a different mechanism (Nave et al. 1996).
In addition to sharing a common hypothermic effect, both treatments displayed a temporal association between minimum core temperature and minimum sleep onset latency (see Fig. 6). However, while a significant correlation coefficient of 0.44 was observed in the melatonin condition, a non-significant (P = 0.08) correlation coefficient of 0.39 was found in the temazepam condition. In contrast, a significant relationship between core temperature and sleep propensity was observed in both treatments when relative core temperature was decreasing most rapidly (i.e. from 14.00 to 16.00 h). That is, melatonin and temazepam displayed a significant correlation (r = 0.48 and 0.44, respectively) between the timing of minimum sleep onset latency and the timing of the MROD in core temperature. Similar findings have been documented by previous researchers investigating both normal nocturnal sleep (Campbell & Broughton, 1994) and daytime melatonin-induced sleepiness (Reid et al. 1996). Taken together, these findings suggest that the rate, rather than simply the absolute magnitude, of core temperature decline may be an important factor in sleep initiation.
However, because of the complexity of comparing two non-stationary time courses, the relationship between changes in core temperature and sleep onset latency was also calculated intra-individually (see Table 1). Moderate mean correlations for both melatonin and temazepam treatments (r = 0.41 ± 0.05 and 0.40 ± 0.06, respectively) were obtained and were of a similar magnitude to the inter-individual correlations discussed above. Interestingly, several researchers have suggested that the soporific effects of melatonin may be mediated by its thermoregulatory effects (Dawson & Encel, 1993; Cagnacci et al. 1995). Although the correlations in the present study support this speculation, a causal link between core temperature and sleep propensity cannot be inferred.
In conclusion, both core temperature and sleep propensity exhibit comparable changes after administration of two different classes of soporific agents. The temporal association between changes in core temperature and sleep propensity supports the suggestion that thermoregulatory effects (in particular the rate of core temperature decline) may mediate changes in sleepiness. If this is the case, it is possible that sleep-onset insomniacs may benefit from treatments that manipulate the thermoregulatory system directly, thus reducing adverse side effects resulting from chronic benzodiazepine use. However, as benzodiazepine use and dependence is more common in older individuals (Gilbert, 1991), it is important to determine whether the relationship between changing core temperature and sleep propensity observed in the present study may be generalized to include this age group.
Finally, while it appears that temazepam alters thermal homeostasis primarily by increasing heat loss, melatonin may both increase heat loss and decrease heat production when administered during the day. Future studies should aim to determine the precise changes in thermal balance induced by these and perhaps other substances known to affect sleep propensity. In this way, it will become clearer whether thermoregulatory effects are a general feature of soporific agents.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council (NH&MRC), and also from The Queen Elizabeth Hospital Research Foundation. Thanks to Nicole Lamond and Jo Pilgrim for technical assistance. Thanks also to Dr David Kennaway, Dr Kurt Lushington, Greg Roach and Eleanor Ahern.
References
- Cagnacci A, Elliott JA, Yen SSC. Melatonin: A major regulator of the circadian rhythm of core temperature in humans. Journal of Clinical Endocrinology and Metabolism. 1992;75:447–452. doi: 10.1210/jcem.75.2.1639946. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Kräuchi K, Wirz-Justice A, Volpe A. Homeostatic versus circadian effects of melatonin on core body temperature in humans. Journal of Biological Rhythms. 1997;12:509–517. doi: 10.1177/074873049701200604. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Soldani R, Laughlin GA, Yen SSC. Modification of core body temperature rhythm during the luteal menstrual phase: role of melatonin. Journal of Applied Physiology. 1996;80:25–29. doi: 10.1152/jappl.1996.80.1.25. 10.1063/1.362782. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Soldani R, Yen SSC. Hypothermic effect of melatonin and nocturnal core body temperature decline are reduced in aged women. Journal of Applied Physiology. 1995;78:314–317. doi: 10.1152/jappl.1995.78.1.314. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Broughton R. Rapid decline in body temperature before sleep: Fluffing the physiological pillow? Chronobiology International. 1994;11:126–131. doi: 10.3109/07420529409055899. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. The multiple sleep latency test: what does it measure? Sleep. 1982;5(suppl. 2):S67–72. doi: 10.1093/sleep/5.s2.s67. [DOI] [PubMed] [Google Scholar]
- Dawson D, Encel N. Melatonin and sleep in humans. Journal of Pineal Research. 1993;15:1–12. doi: 10.1111/j.1600-079x.1993.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Roth C, Landolt HP, Werth E, Aeppli M, Achermann P, Borbelly AA. Melatonin effect on daytime sleep in men: suppression of EEG low frequency activity and enhancement of spindle frequency. Neuroscience Letters. 1995;201:13–16. doi: 10.1016/0304-3940(95)12118-n. 10.1016/0304-3940(95)12118-N. [DOI] [PubMed] [Google Scholar]
- Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proceedings of the National Academy of Sciences of the USA. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A. A Programme to Alter Patterns of Benzodiazepine Use Amongst Residents of Aged Care Accommodation. Adelaide, South Australia: South Australian Health Commission Report, South Australian Health Commission Research Grants Committee; 1991. [Google Scholar]
- Kräuchi K, Cajochen C, Mori D, Graw P, Wirz-Justice A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. American Journal of Physiology. 1997a;272:R1189–1196. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Cajochen C, Wirz-Justice A. A relationship between heat loss and sleepiness: effects of postural change and melatonin administration. Journal of Applied Physiology. 1997b;83:134–139. doi: 10.1152/jappl.1997.83.1.134. [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin temperature under unmasking conditions in men. American Journal of Physiology. 1994;267:R819–829. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- Lack LC, Lushington K. The rhythms of human sleep propensity and core body temperature. Journal of Sleep Research. 1996;5:1–11. doi: 10.1046/j.1365-2869.1996.00005.x. 10.1046/j.1365-2869.1996.00005.x. [DOI] [PubMed] [Google Scholar]
- Nave R, Herer P, Haimov I, Shlitner A, Lavie P. Hypnotic and hypothermic effects of melatonin on daytime sleep in humans: lack of antagonism by flumazenil. Neuroscience Letters. 1996;214:123–126. doi: 10.1016/0304-3940(96)12899-8. 10.1016/0304-3940(96)12899-8. [DOI] [PubMed] [Google Scholar]
- Ngen CC, Hassan R. A double-blind placebo-controlled trial of zopiclone 7.5 mg and temazepam 20 mg in insomnia. International Clinical Psychopharmacology. 1990;5:165–171. doi: 10.1097/00004850-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Pleuvry BJ, Maddison SE, Odeh RB, Dodson ME. Respiratory and psychological effects of oral temazepam in volunteers. British Journal of Anaesthesiology. 1980;52:901–905. doi: 10.1093/bja/52.9.901. [DOI] [PubMed] [Google Scholar]
- Reid KJ, van den Heuvel CJ, Dawson D. Day-time melatonin administration: Effects on core temperature and sleep onset latency. Journal of Sleep Research. 1996;5:150–154. doi: 10.1046/j.1365-2869.1996.t01-1-00006.x. 10.1046/j.1365-2869.1996.t01-1-00006.x. [DOI] [PubMed] [Google Scholar]
- Tzischinsky O, Lavie P. Melatonin possesses time-dependent hypnotic effects. Sleep. 1994;17:638–645. doi: 10.1093/sleep/17.7.638. [DOI] [PubMed] [Google Scholar]
- van der Kleijn E. Effects of zopiclone and temazepam on sleep, behaviour, and mood during the day. European Journal of Clinical Pharmacology. 1989;36:247–251. doi: 10.1007/BF00558155. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Laitinen JT, Saavedra JM. Vascular melatonin receptors. Biological Signals. 1993;2:221–227. doi: 10.1159/000109495. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Wurtman RJ, Lynch HJ, Ives JR, Dollins AB, Morabito C, Matheson JK, Schomer DL. Sleep-inducing effects of low doses of melatonin ingested in the evening. Clinical Pharmacology and Therapeutics. 1995;57:552–558. doi: 10.1016/0009-9236(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Zully J, Weaver R, Aschoff J. The dependence of onset and duration of sleep on the circadian rhythm of core body temperature. Pflügers Archiv. 1981;391:314–318. doi: 10.1007/BF00581514. [DOI] [PubMed] [Google Scholar]


