Abstract
The aim of this work was to investigate the role of sarcolemmal Ca2+-ATPase in rat ventricular myocytes. We have measured intracellular Ca2+ concentration ([Ca2+]i) using indo-1. The actions of the ATPase inhibitor carboxyeosin were studied.
Carboxyeosin increased resting [Ca2+]i and the magnitude of the systolic Ca2+ transient and slowed the rate of its relaxation by 5%.
Carboxyeosin increased the magnitude of the caffeine-evoked increase in [Ca2+]i and slowed its relaxation by 20%. Removal of extracellular Na+ slowed the rate constant by 80%. When Na+ was removed in a carboxyeosin-treated cell, the caffeine-evoked increase in [Ca2+]i did not decay.
Carboxyeosin increased the integral of the Na+-Ca2+ exchange current activated by caffeine. This is, in part, due to an increase in sarcoplasmic reticulum Ca2+ content.
When extracellular Na+ was removed, there was a transient increase in [Ca2+]i which then decayed. The rate of this decay was slowed by carboxyeosin by a factor of about four. The residual decay could be abolished with caffeine.
In the absence of extracellular Na+, increasing extracellular Ca2+ concentration ([Ca2+]o) elevated [Ca2+]i. In carboxyeosin-treated cells, [Ca2+]i was much more sensitive to [Ca2+]o.
These results demonstrate the role of a carboxyeosin-sensitive Ca2+-ATPase in the control of resting [Ca2+]i and the reduction in [Ca2+]i following an increase in [Ca2+]i.
In cardiac cells, as in many other cells, there is a large electrochemical gradient favouring Ca2+ entry into the cell from the extracellular fluid. Two sarcolemmal mechanisms are known to extrude Ca2+ from the cytoplasm: sarcolemmal Ca2+-ATPase and Na+-Ca2+ exchange. The role of Na+-Ca2+ exchange in Ca2+ homeostasis has been extensively characterized in cardiac muscle (Chapman & Tunstall, 1981; Allen et al. 1984; Crespo et al. 1990). In contrast, much less is known about Ca2+-ATPase. A sarcolemmal Ca2+-ATPase has been demonstrated in the heart (Caroni & Carafoli, 1981). It has been suggested that Ca2+-ATPase has a higher affinity and lower maximum velocity than Na+-Ca2+ exchange and may therefore be more important for control of resting [Ca2+]i than for removing larger Ca2+ loads produced by stimulation (DiPolo & Beaugé, 1979) although recent work has questioned this functional distinction (Lamont & Eisner, 1996).
Most previous work studying the role of Ca2+-ATPase in cardiac muscle has been performed under conditions that inhibit Na+-Ca2+ exchange to reveal any potential contribution of ATPase. Specifically, the following observations have led to the suggestion that something other than Na+-Ca2+ exchange, perhaps sarcolemmal Ca2+-ATPase, may have a significant functional role in Ca2+ homeostasis, at least under experimental conditions. (1) When caffeine is applied to release Ca2+ from the sarcoplasmic reticulum (SR), inhibition of Na+-Ca2+ exchange slows but does not abolish the decay of [Ca2+]i (O'Neill et al. 1991; Bassani et al. 1992; Negretti et al. 1993; Bassani et al. 1994). It has been suggested that the Na+-Ca2+ exchange-independent component of the decay of [Ca2+]i is due to a combination of sarcolemmal Ca2+-ATPase and mitochondrial sequestration. Separation of mitochondrial and sarcolemmal Ca2+-ATPase components has been achieved by inhibiting the former with uncouplers or the latter by elevating extracellular Ca2+ (Bassani et al. 1992; Negretti et al. 1993). However, these approaches are both rather non-specific. (2) Removal of extracellular Na+ produces an increase in [Ca2+]i as Ca2+ enters the cell via Na+-Ca2+ exchange. However, [Ca2+]i then falls to levels which are only slightly elevated above the control. This secondary relaxation of [Ca2+]i may reflect the activity of a sarcolemmal Ca2+-ATPase. (3) In the absence of extracellular Na+, [Ca2+]i can still be regulated and respond to changes in extracellular Ca2+ concentration (Lamont & Eisner, 1996) and, again, this may reflect the activity of a sarcolemmal Ca2+-ATPase.
Evidence for the role of sarcolemmal Ca2+-ATPase would be much stronger if there was a specific inhibitor which could be applied to dissect out its contribution. It has been shown that ATPase is specifically inhibited by carboxyeosin (Gatto & Milanik, 1993). In rabbit and ferret ventricular myocytes, carboxyeosin has been used to show that Ca2+-ATPase makes an appreciable contribution to the decay of [Ca2+]i during a caffeine-evoked response (Bassani et al. 1995).
Using carboxyeosin, in the present paper we show that sarcolemmal Ca2+-ATPase has significant effects on cell function even under physiological conditions. Furthermore it provides the major component of the secondary decay of [Ca2+]i following Na+ removal and is essential for [Ca2+]i homeostasis in Na+-free solutions.
METHODS
Cell isolation and fluorescence measurement
Ventricular myocytes were isolated from rats which had been killed by cervical dislocation and single ventricular myocytes were isolated as previously described (Eisner et al. 1989). Cells were loaded with the acetoxymethyl (AM) ester form of the fluorescent indicator indo-1 in order to measure the intracellular calcium concentration ([Ca2+]i) as described previously (O'Neill et al. 1990).
Carboxyeosin loading
Cells were loaded with 5- (and -6)-carboxyeosin diacetate (succinimidyl ester) from Molecular Probes. A 10 mM stock solution was made in DMSO. This stock solution was added to the control experimental solution (for composition see below) to a final concentration of 20 μM and the cells were loaded for 15 min. Cells were then superfused with carboxyeosin-free solution for a further 10 min to allow carboxyeosin de-esterification and to wash off the residual compound in the bath solution. As indicated in the text, in some experiments, recordings were made from the same cell before and after loading with carboxyeosin.
Membrane current measurement
Voltage clamp control was imposed with the perforated patch technique. The suction pipette was filled with (mM): 125 KCH3O3S, 20 KCl, 12 NaCl, 10 Hepes, 5 MgCl2, 0.1 K2EGTA, adjusted to pH 7.2 with KOH. Five microlitres of amphotericin B (stock 60 mg ml−1 in DMSO) was added to 1.25 ml of pipette solution. After forming a seal onto the cell membrane, the membrane potential was held at -80 mV. To compensate for the relatively high access resistance, a switch clamp (Axoclamp-2A, Axon Instruments) was used.
Experimental solutions
The control solution contained (mM): 134 NaCl, 4 KCl, 1 MgCl2, 10 Hepes, 11 glucose, 1 CaCl2, adjusted to pH 7.4 with NaOH. 4-Aminopyridine (5 mM) and BaCl2 (100 μM) were added to this solution to block K+ current in perforated patch clamp experiments. The Na+-free solution contained (mM): 130 LiCl, 10 Hepes, 11 glucose, 1 MgCl2, 4 KCl, 1 CaCl2, adjusted to pH 7.4 with KOH. In some experiments a Na+-free, Ca2+-free solution was used. In this case Ca2+ was removed and EGTA (1 mM) added. All experiments were performed at room temperature (23°C).
Statistics
Statistical comparisons were made using Student's paired t test. All data are expressed as means ±s.e.m. for n cells. The rate of decay of [Ca2+]i is generally analysed by fitting single exponentials to the indo-1 ratio. This should be regarded as an experimental convenience and not as meaning that the underlying processes are first order.
RESULTS
The effect of carboxyeosin on diastolic and systolic [Ca2+]i
The first series of experiments was designed to evaluate the role of sarcolemmal Ca2+-ATPase during electrically stimulated contractions. In Fig. 1A, a single rat cardiac myocyte was stimulated every 2 s and [Ca2+]i transients were recorded both under control conditions and after loading the cells with 20 μM carboxyeosin. Carboxyeosin increased both the diastolic and systolic [Ca2+]i. On average the amplitude of the systolic Ca2+ transient increased by 21 ± 2 % (n = 9; P < 0.01). Figure 1B shows normalized [Ca2+]i transients recorded before and after inhibition of the sarcolemmal Ca2+-ATPase by carboxyeosin. The rate constant of decay decreased significantly from 2.42 ± 0.61 to 2.30 ± 0.15 s−1. On average the rate constant in the presence of carboxyeosin was 95.0 ± 0.9 % of that in control (P < 0.05; n = 9).
Figure 1. The effect of carboxyeosin on the systolic Ca2+ transient.
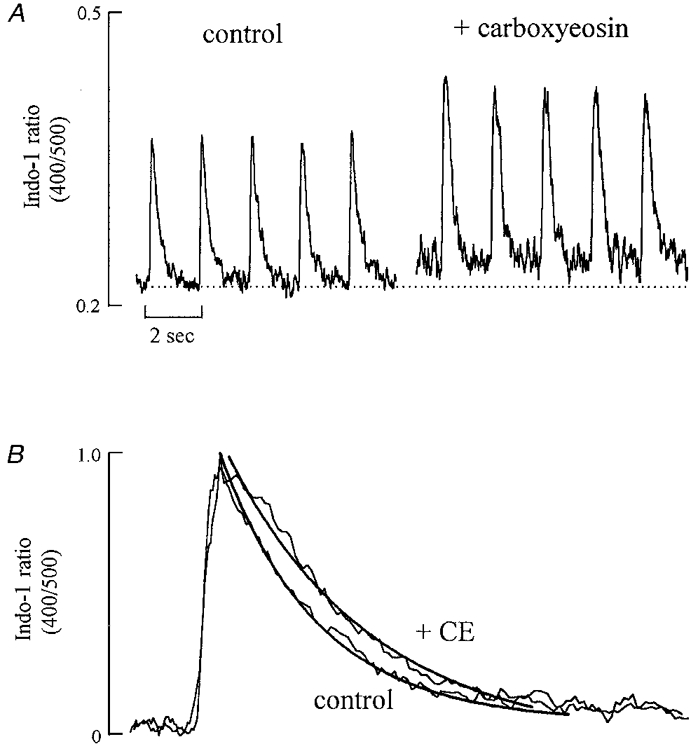
A, specimen records. The cell was stimulated at 0.5 Hz. The first 5 transients were obtained under control conditions. The cell was then exposed to 20 μM carboxyeosin for 15 min and, after removing carboxyeosin the remaining Ca2+ transients were obtained. The baseline is noiser after loading with carboxyeosin due to a loss of some of the indo-1 signal during the carboxyeosin (CE) loading period. B, normalized transients obtained from control and after CE loading. In each case 5 transients were averaged. The continuous curves through the data are single exponentials with rate constants of: control, 3.01 s−1; CE, 2.83 s−1.
The effect of carboxyeosin on the caffeine-induced calcium transient
The application of caffeine results in an increase in [Ca2+]i which decays over a few seconds (Smith et al. 1988). The rate constant of the decay of this response can be used as a measure of the rate of Ca2+ removal from the cytoplasm by processes other than the sarcoplasmic reticulum. To evaluate the fractional contribution of the sarcolemmal Ca2+-ATPase to Ca2+ removal during this response, we have measured the rate constant of decay of the caffeine-evoked increase in [Ca2+]i before and after inhibition of the sarcolemmal Ca2+-ATPase with carboxyeosin. Figure 2A shows that carboxyeosin (20 μM) increased the amplitude of the caffeine-induced [Ca2+]i transient. On average the magnitude of the caffeine-evoked response was increased by 27 ± 5 % of control (n = 9, P < 0.01). Figure 2B shows the caffeine-evoked responses normalized to the same peak magnitude. Carboxyeosin decreased the rate constant of [Ca2+]i decline from 0.31 ± 0.02 to 0.25 ± 0.01 s−1. On average the rate constant of decay in caffeine in carboxyeosin was 79.8 ± 0.1 % of that in control, a significant slowing (P < 0.05; n = 9).
Figure 2. The effect of carboxyeosin on the caffeine-evoked increase in [Ca2+]i.
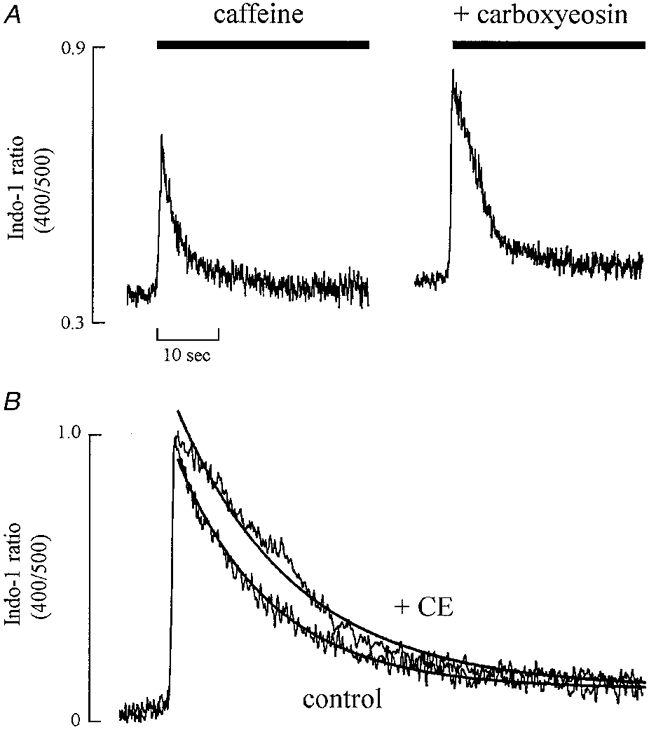
A, original data. Caffeine (10 mM) was applied for the periods shown by the bars. Until 5 s before the application of caffeine, the cell was stimulated at 0.5 Hz. Following the first caffeine-evoked response shown the cell was loaded with carboxyeosin (CE) before obtaining the second response. B, normalized records shown on an expanded time scale. The continuous curves through the data are single exponentials with rate constants of: control, 0.31 s−1; CE, 0.25 s−1.
If carboxyeosin only affected sarcolemmal Ca2+-ATPase, one might expect that it would produce the same absolute slowing of the systolic Ca2+ transient as that of the caffeine-evoked response. The experiments summarized in Table 1 were designed to investigate this directly. They show that carboxyeosin significantly (P < 0.05) slowed the rate constant of decay of the systolic Ca2+ transient by 0.13 s−1 whereas it slowed that of the caffeine-evoked response by a smaller amount (0.065 s−1). Assuming that it is legitimate to subtract rate constants, this suggests that, as well as affecting the sarcolemmal Ca2+-ATPase, carboxyeosin must be affecting some process which is involved in Ca2+ removal from the cytoplasm during the systolic Ca2+ transient but not in the caffeine-evoked response, most likely the SR Ca2+-ATPase. Another way to look at this is to measure the caffeine-sensitive component of Ca2+ removal: in other words the difference between the rate constant of decay of the systolic Ca2+ transient and the caffeine-evoked response. This had a value of 1.98 in the absence and 1.92 in the presence of carboxyeosin. The mean difference in these values (0.064) was significantly different from zero. The implications of this will be considered in Discussion.
Table 1.
The effects of carboxyeosin (CE) on the rate constants of decay of systolic and caffeine-activated Ca2+ transients
| Systolic Ca2+ transient | Caffeine Ca2+ transient | CE-sensitive component | Caffeine-sensitive component | |||||
|---|---|---|---|---|---|---|---|---|
| Control (A) | +CE (B) | Control (C) | +CE (D) | Systolic (E) (A − B) | Caffeine (F) (C − D) | Control (G) (A − C) | +CE (H) (B − D) | (I)(G − H) |
| 2.29 ± 0.18 | 2.17 ± 0.17 | 0.313 ± 0.017 | 0.248 ± 0.017 | 0.129 ± 0.027 | 0.065 ± 0.003 | 1.98 ± 0.18 | 1.92 ± 0.16 | 0.06 ± 0.02 |
All data show the rate constants of decay (in s−1) of the transients. Columns A–D show the rate constants derived directly from the data as indicated. Column E gives the result of subtracting the rate constant of decay of the systolic Ca2+ transient in CE from that in its absence. Column F is likewise obtained by subtracting the rate constant of the caffeine-evoked response in CE from that in control. Note that the calculated CE-sensitive component of the systolic decay is greater than that of the caffeine-evoked response (P < 0.05; Student's paired t test). Column G gives the result of subtracting the rate constant of the caffeine-evoked response from the control and column H gives the result of the same calculation in the presence of CE. Column I gives the difference of the caffeine-sensitive component in control and CE. Note that the calculated caffeine-sensitive component of relaxation is significantly less in CE than control (P < 0.05). n = 7 cells for all data.
The effects of carboxyeosin in Na+-free solutions
The effects of carboxyeosin were more prominent if the Na+-Ca2+ exchange was also inhibited. In the experiment illustrated in Fig. 3, following a control caffeine-evoked response, Na+-Ca2+ exchange was inhibited by removing extracellular Na+ (extracellular Ca2+ was also removed in order to prevent the large influx of Ca2+ which would otherwise have occurred). This resulted in a slowing of the decay. Finally, the cell was loaded with carboxyeosin and caffeine again applied in the Na+-free solution. Under these conditions relaxation did not occur. On average (in 7 cells), the rate of decay of the caffeine-evoked response in Na+-free solution was 19.9 ± 0.5 % of that in Na+-containing solution whereas the combination of Na+-free solution with carboxyeosin decreased the rate to 0.27 ± 0.09 %. This suggests that about 80 % of Ca2+ is removed by Na+-Ca2+ exchange and the remaining 20 % by sarcolemmal Ca2+-ATPase. This is consistent with the result in Fig. 2 in which carboxyeosin alone decreased the rate of [Ca2+]i decline during the caffeine-evoked response to 80 % of control.
Figure 3. Carboxyeosin (CE) has a greater effect in the absence of Na+-Ca2+ exchange.
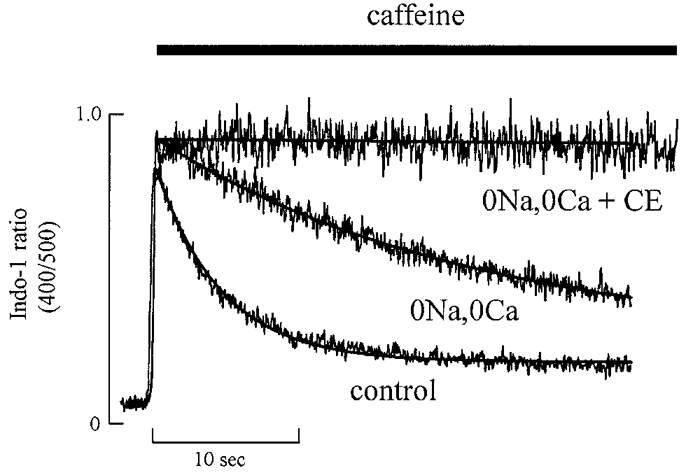
Three applications of caffeine (10 mM) (applied for the period shown by the bar) are shown superimposed. Caffeine was applied under the following conditions: control; 0 Na+, 0 Ca2+; 0 Na+, 0 Ca2++ CE. The continuous curves through the control and 0 Na+, 0 Ca2+ data are exponentials with rate constants of 0.36 and 0.079 s−1, respectively.
The effects of carboxyeosin on SR Ca2+ content
The experiments presented above show that both the systolic Ca2+ transient and the Ca2+ transient produced by caffeine are increased by carboxyeosin. One possible explanation for this observation is that the SR Ca2+ content has increased as a result of the measured rise in diastolic [Ca2+]i. In order to examine this possibility, we measured the SR Ca2+ content by measuring the integral of the Na+-Ca2+ exchange current during a caffeine-evoked response (Varro et al. 1993). The application of caffeine (Fig. 4) results in a transient increase in [Ca2+]i which is accompanied by a transient inward Na+-Ca2+ exchange current. Both the amplitude of the increase in [Ca2+]i and of the inward current are increased by exposure to carboxyeosin (Fig. 4). The integrated current is also increased. On average carboxyeosin increased the integrated net current from 87.6 ± 4.8 to 128 ± 7 pC (n = 8, P < 0.05). This increase is in part due to the fact that, whereas in control only 80 % of Ca2+ is removed by Na+-Ca2+ exchange (with the remainder being carried by the non-electrogenic Ca2+-ATPase), in the presence of carboxyeosin all the Ca2+ is transported by Na+-Ca2+ exchange. We can correct for this by dividing the control data by 0.8 to estimate the amount of charge which would have been transferred under control conditions if all the Ca2+ was removed via Na+-Ca2+ exchange. This gives a value of 109 ± 6 pC. This is still significantly (P < 0.05; Student's paired t test) less than the value measured in carboxyeosin. We therefore conclude that carboxyeosin increased the SR Ca2+ content.
Figure 4. The effect of carboxyeosin on the calcium transient and Na+-Ca2+ exchange current.
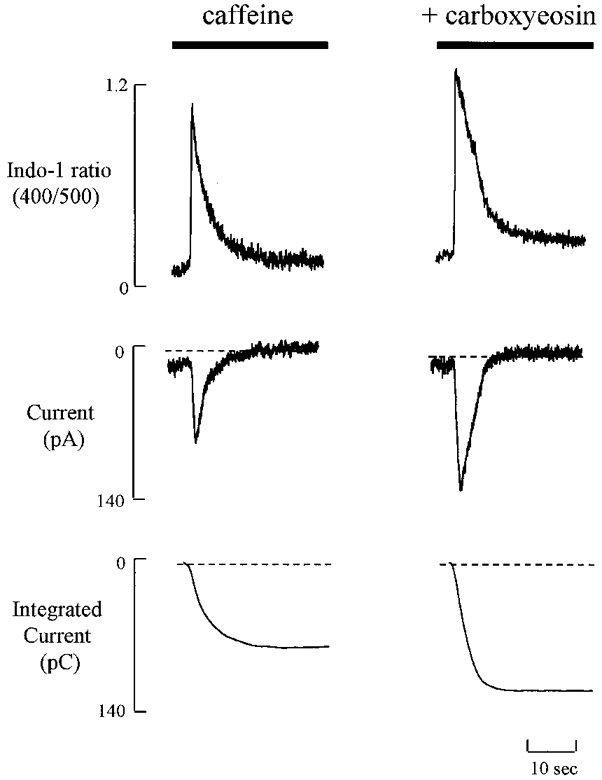
Caffeine (10 mM) was applied for the period shown by bars and the calcium transient and Na+-Ca2+ exchange current were simulataneously measured in perforated-patch clamped single rat ventricular myocyte. The membrane potential was held at -80 mV. The integrated currents before (control, left traces) and after (right traces) the cell was loaded with carboxyeosin were 83 and 119 pC, respectively.
Regulation of diastolic [Ca2+]i by sarcolemmal Ca2+-ATPase in the absence of Na+-Ca2+ exchange
The experiment in Fig. 1 showed that carboxyeosin increased diastolic [Ca2+]i. This suggests that sarcolemmal Ca2+-ATPase plays a role in regulating the resting level of [Ca2+]i. In subsequent experiments we looked directly at the role of Ca2+-ATPase by inhibiting Na+-Ca2+ exchange. This approach increases the importance of Ca2+-ATPase in Ca2+ homeostasis. In the experiment shown in Fig. 5A, extracellular Na+ was removed first under control conditions and then after loading with carboxyeosin. In agreement with previous work (Allen et al. 1984; Kim et al. 1987), the Na+ removal under control conditions displays an initial increase in [Ca2+]i which then decays to a lower level which is still greater than the resting level. The initial increase in [Ca2+]i is due to Ca2+ entry on ‘reverse’ Na+-Ca2+ exchange. However, the factors responsible for the subsequent decay of [Ca2+]i to and regulation at the steady-state level are uncertain, both mitochondria and sarcolemmal Ca2+-ATPase having been suggested to contribute (Allen et al. 1983, 1984; Chapman, 1986; Lamont & Eisner, 1996). We have therefore investigated the possibility that Ca2+-ATPase may play a role in determining both the relaxation of [Ca2+]i in Na+-free solution and the regulation of steady-state [Ca2+]i. Loading with carboxyeosin markedly slowed the rate of [Ca2+]i decay produced by Na+-free solution and resulted in a new steady-state [Ca2+]i level which was higher than that under control conditions. On average, carboxyeosin increased the half-time of [Ca2+]i decay from 124 ± 17 to 465 ± 36 s (n = 13, P < 0.05). This result implies that sarcolemmal Ca2+-ATPase contributes more than 70 % to [Ca2+]i removal showing that it is the main mechanism for Ca2+ removal when the Na+-Ca2+ exchanger is inactive. However, carboxyeosin did not completely abolish [Ca2+]i decay suggesting that other mechanisms also contribute to Ca2+ removal. We tested the possibility that the carboxyeosin-insensitive component of decay of [Ca2+]i results from uptake of Ca2+ into the SR. This was done by applying 10 mM caffeine at the same time as Na+ removal. As Fig. 5B shows, in the presence of caffeine, carboxyeosin almost abolished [Ca2+]i decay suggesting that part of the Ca2+ can be taken up by the SR while the remainder is removed by the sarcolemmal Ca2+-ATPase.
Figure 5. The effects of carboxyeosin on the Na+-withdrawal response.
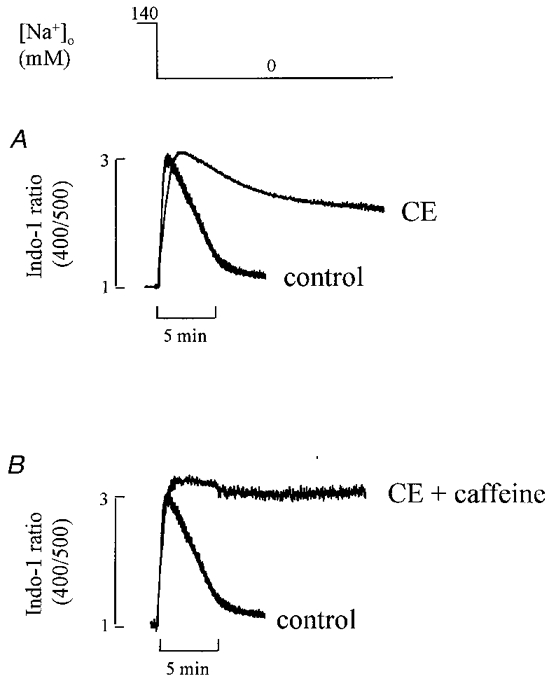
Extracellular Na+ was removed (replaced by Li+) for the period shown at the top. Before removing Na+, the cell was stimulated at 0.5 Hz. A, comparison of Na+ removal under control conditions with that in the presence of carboxyeosin (CE). B, comparison of Na+ removal under control conditions with that in the combined presence of carboxyeosin and caffeine (10 mM).
The effect of [Ca2+]o on [Ca2+]i in the absence of Na+-Ca2+ exchange
In the experiments described in the previous section, we examined the effects of carboxyeosin on the ability of the cell to recover from increases in [Ca2+]i produced by extracellular Na+ removal. In subsequent experiments, we looked at the regulation of steady-state [Ca2+]i in Na+-free solutions and, in particular, at the effects of changing [Ca2+]o. In the absence of extracellular Na+, the effect of changing [Ca2+]o on [Ca2+]i was tested in 11 rat ventricular myocytes before and after inhibition of the sarcolemmal Ca2+-ATPase. To prevent Ca2+ influx via Na+-Ca2+ exchange, cells were pre-exposed to Ca2+-free solution for 10-30 s before removing extracellular Na+ and Ca2+. The effects of varying extracellular Ca2+ in Na+-free solutions are shown in Fig. 6 which shows the change in [Ca2+]i when [Ca2+]o was raised to 0.3, 0.6 and 1 mM. Over this range of [Ca2+]o, the greater the [Ca2+]o, the greater [Ca2+]i. As [Ca2+]o was increased first to 0.3 mM and then to 0.6 mM, oscillations in [Ca2+]i developed due to release of Ca2+ from the SR. Increasing [Ca2+]o to lower levels produced no measurable increase in [Ca2+]i. This is demonstrated in Fig. 7A. In control (Na+-containing) solution changes in [Ca2+]o had smaller effects on [Ca2+]i (not shown; cf. Díaz et al. 1997). In a Na+-free solution, increasing [Ca2+]o from zero to low levels such as 0.1 mM had no measurable effect on [Ca2+]i. In 21 of 23 cells tested, no measurable increase in [Ca2+]i was produced by increasing [Ca2+]o from 0 to 0.1 mM. In contrast, after treatment with carboxyeosin, the same increase in [Ca2+]o produced a large increase in [Ca2+]i to a level larger than that of the normal systolic Ca2+ transient (Fig. 7A).
Figure 6. The effects of changing [Ca2+]o on [Ca2+]i in the absence of Na+-Ca2+ exchange.
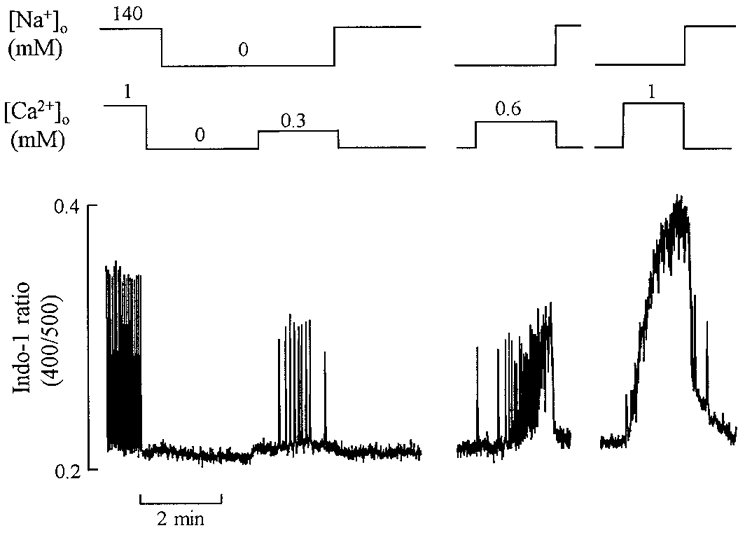
The cell was initially stimulated at 0.5 Hz. After stopping stimulation, extracellular Ca2+ was removed (1 mM EGTA) for 30 s and then extracellular Na+ removed (replaced by Li+) for 2 min. Following this, [Ca2+]o was increased to 0.3, 0.6 and 1 mM as shown. During the breaks in the record, extracellular Na+ and Ca2+ were removed as shown in the first part of the record.
Figure 7. The effects of a small increase in [Ca2+]o on [Ca2+]i in Na+-free solution in the presence and absence of carboxyeosin.
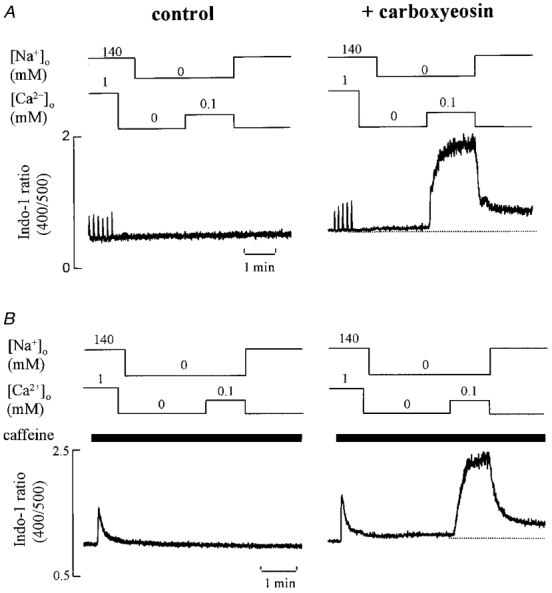
A, the cell was initially stimulated at 0.1 Hz. Extracellular Ca2+ and Na+ were removed. Extracellular Ca2+ was then increased from 0 to 0.1 mM for the period shown. In the right-hand panel the same protocol was repeated on this cell except that the cell had been loaded with carboxyeosin. B,the same protocol as in A except that caffeine (10 mM) was applied (for the period shown by the bars) immediately before removing extracellular Ca2+ in order to empty the SR Ca2+ store.
To test whether Ca2+ release from the SR is responsible for the increase in [Ca2+]i seen in Fig. 7A similar experiments were performed in the presence of 10 mM caffeine to deplete the SR. The result, illustrated in Fig. 7B, is identical to that in the absence of caffeine. It therefore appears that the changes in [Ca2+]i produced by changing [Ca2+]o do not involve significant contributions from the SR.
DISCUSSION
The experiments described in this paper allow quantification of the contribution of sarcolemmal Ca2+-ATPase to Ca2+ removal from the cytoplasm under a variety of conditions. We have examined the effects of carboxyeosin on the rate of decay of both the caffeine-evoked and the systolic Ca2+ transient, as well as on the regulation of diastolic or resting [Ca2+]i. For simplicity, we will consider the effects on the caffeine-evoked response first.
The effects of carboxyeosin on the caffeine-evoked increase in [Ca2+]i
Before considering the data, it is important to assess the specificity of carboxyeosin. At concentrations which inhibit sarcolemmal Ca2+-ATPase, carboxyeosin does not inhibit Na+-Ca2+ exchange (Gatto et al. 1994). Furthermore, although carboxyeosin may inhibit the Na+-K+ pump, its inhibition potency is lower than that for sarcolemmal Ca2+-ATPase (Skou & Esmann, 1981; Gatto & Milanik, 1993). It is therefore reasonable to assume that the effects of carboxyeosin on the rate of decay of the caffeine-evoked response are due to inhibition of sarcolemmal Ca2+-ATPase.
The present experiments show that carboxyeosin slows the rate of decay of the caffeine-evoked response by 20 % compared with control. This compares with a reduction of 80 % produced by removal of extracellular Na+. This is consistent with the hypothesis that the decay of the caffeine-evoked response can be entirely accounted for by two components: (1) a carboxyeosin-sensitive component, presumably sarcolemmal Ca2+-ATPase; (2) Na+-Ca2+ exchange. This is confirmed by the observation that the rate of decay of the caffeine-evoked response is decreased to less than 1 % in the combined presence of both carboxyeosin and a Na+-free solution. The fact that relaxation of [Ca2+]i is abolished in Na+-free solution containing carboxyeosin is inconsistent with a role for mitochondria in Ca2+ removal. This result differs from that found in ferret myocytes where, in order to completely inhibit the decay of [Ca2+]i, mitochondrial Ca2+ uptake had to be inhibited, as well as applying carboxyeosin and Na+-free solutions (Bassani et al. 1995). In summary, the present results suggest that, in rat ventricular myocytes, a carboxyeosin-sensitive sarcolemmal Ca2+-ATPase makes a contribution to Ca2+ removal from the cell which is equal to about 25 % of that made by Na+-Ca2+ exchange. The contribution of 80 % made by Na+-Ca2+ exchange to the decay of [Ca2+]i in rat ventricular myocytes is identical to that estimated by us in a previous study (Díaz et al. 1997), not very different from the ∼87 % reported by Bassani et al. (1994) but higher than the 66 % reported by Varro et al. (1993). Presumably the precise contribution depends on the exact experimental conditions.
As well as decreasing the rate of decay of the caffeine-evoked response, carboxyeosin produced a significant increase (27 %) in its magnitude. This suggests that it increases the SR Ca2+ content. This would be expected if carboxyeosin has inhibited one of the mechanisms of Ca2+ removal from the cell. This will have two effects on SR Ca2+ content. (1) It will increase resting [Ca2+]i (as shown directly in Fig. 1) thereby increasing the activation of SR Ca2+-ATPase and hence the SR Ca2+ content. (2) During the systolic Ca2+ transient, SR Ca2+-ATPase effectively competes with the combined effects of the sarcolemmal Na+-Ca2+ exchange and Ca2+-ATPase. Inhibition of the sarcolemmal Ca2+-ATPase will therefore result in a larger fraction of the Ca2+ being taken back into the SR thereby also increasing the SR Ca2+ content. In other experiments we measured the SR Ca2+ content directly by integrating the caffeine-evoked Na+-Ca2+ exchange current. This integral increased by 46 %. As mentioned above, part of this increase in integral is due to the fact that, whereas under control conditions only 80 % of the Ca2+ is removed from the cell by Na+-Ca2+ exchange (with the remainder being pumped by Ca2+-ATPase and not therefore producing current), in the presence of carboxyeosin all the Ca2+ is removed by Na+-Ca2+ exchange. However, even after this is corrected for, we still find a 17 % increase in SR Ca2+ content. This result suggests that, in the intact cell, sarcolemmal Ca2+-ATPase acts to decrease SR Ca2+ content.
The effects of carboxyeosin on the systolic Ca2+ transient
We found that carboxyeosin increased the magnitude of the systolic Ca2+ transient to 120 % of its control value. This presumably results from the increase in SR Ca2+ content suggested by the increase of the caffeine-evoked response. In ferret cells no significant effect was found on the magnitude of the systolic Ca2+ (Bassani et al. 1995). In addition carboxyeosin decreased the rate constant of decay of the Ca2+ transient by about 5 % similar to the effect in ferret cells (Bassani et al. 1995). In the ferret cells this slowing of the rate of decay was tentatively attributed to inhibition of SR Ca2+-ATPase by carboxyeosin. The present data allow us to distinguish the idea that the slowing results from (i) inhibition of the sarcolemmal Ca2+-ATPase versus (ii) an effect on the SR Ca2+-ATPase. The simplest explanation of this slowing of the decay is that it represents the contribution of the sarcolemmal Ca2+-ATPase to normal relaxation. In relation to Table 1, we have produced two arguments suggesting that the situation may be more complicated. (1) The slowing of the systolic Ca2+ transient produced by CE is more than would be expected from the contribution of the sarcolemmal Ca2+-ATPase as assessed by the effects of CE on the caffeine-evoked response. (2) The caffeine-sensitive component of decay of the systolic Ca2+ transient is affected by CE. The slowing of the caffeine-sensitive component could be due to either (i) a direct effect of carboxyeosin on the SR Ca2+-ATPase or (ii) an effect secondary to the increase in SR Ca2+ content. The present data do not allow us to discriminate between these alternatives. It should, however, also be noted that the above arguments depend on subtracting the rate constant of decay of the indo-1 ratio obtained in one condition from that in another. This is tantamount to assuming that Ca2+ is controlled by independent, first-order mechanisms (and also to ignoring any non-linearity between [Ca2+]i and the indo-1 ratio). As far as the use of carboxyeosin is concerned, it should be noted that any effect on SR Ca2+-ATPase is only a very small fraction and will be insignificant in many experimental purposes. For example, the slowing of the caffeine-sensitive component by CE is 0.06 s−1 compared with a mean initial value of the caffeine-sensitive component of 1.98 s−1. This is therefore only a 3 % effect.
The role of sarcolemmal Ca2+-ATPase in recovery of [Ca2+]i during the Na+-withdrawal response
Previous work (Allen et al. 1983) has shown that, when Na+-Ca2+ exchange is inhibited, resting [Ca2+]i is still regulated but at a higher level than normal. This suggests that the other mechanisms, such as sarcolemmal Ca2+-ATPase, by themselves are incapable of keeping resting [Ca2+]i at the normal level. In the present work, we have been able to inhibit Ca2+-ATPase to ask the converse question. Can Na+-Ca2+ exchange alone maintain the low resting [Ca2+]i? The data show that carboxyeosin increases resting [Ca2+]i and that, therefore, in addition to Na+-Ca2+ exchange, a functional sarcolemmal Ca2+-ATPase is required to maintain resting [Ca2+]i at the normal low resting level. Previous work on rabbit and ferret myocytes found no effect of carboxyeosin on resting [Ca2+]i (Bassani et al. 1995). It is possible that the greater effect in the rat reflects the fact that Na+-Ca2+ exchange plays a larger role than does the sarcolemmal Ca2+-ATPase in Ca2+ extrusion in these species compared with the rat (Bassani et al. 1994).
Removal of extracellular Na+ results in entry of Ca2+ into the cell on reverse Na+-Ca2+ exchange (accompanied by Na+ efflux). This results in a large increase in [Ca2+]i which then decays to a steady-state level (Fig. 5). This spontaneous decay of [Ca2+]i suggests a significant contribution of other mechanisms to Ca2+ removal when the Na+-Ca2+ exchange is inhibited. Previous studies have shown that the recovery of [Ca2+]i during Na+ withdrawal is an energy-dependent process (Allen et al. 1983) but could not distinguish between the effects of mitochondrial Ca2+ sequestration and sarcolemmal Ca2+-ATPase. Using carboxyeosin, we have shown that sarcolemmal Ca2+-ATPase is the major mechanism responsible for restoring diastolic [Ca2+]i to a relatively low level when the Na+-Ca2+ exchange is inhibited. As seen in Fig. 6A, when sarcolemmal Ca2+-ATPase is inhibited by carboxyeosin, the half-time of [Ca2+]i decay is prolonged approximately fourfold. In other words, more than 70 % of the Ca2+ is removed from the cytoplasm by the sarcolemmal Ca2+-ATPase. The fact that the remaining decrease in Ca2+ is inhibited by caffeine suggests that it results from uptake by the SR. It is worth noting that the caffeine-sensitive fraction of recovery of [Ca2+]i is only about 50 % of that due to the sarcolemmal Ca2+-ATPase showing that, under these conditions, sarcolemmal Ca2+-ATPase is more effective than is accumulation by the SR. This probably reflects the fact that the SR Ca2+ content is near the maximum level and therefore the ability of the SR to accumulate Ca2+ will be much diminished compared with that during systole. This inhibition of [Ca2+]i decay by carboxyeosin emphasizes the important role of sarcolemmal Ca2+-ATPase in the control of diastolic [Ca2+]i at least when Na+-Ca2+ exchange is inhibited. The abolition of [Ca2+]i decay by carboxyeosin plus caffeine during the Na+ withdrawal response emphasizes, again, that mitochondria make little contribution to Ca2+ removal in rat ventricular myocytes.
The role of sarcolemmal Ca2+-ATPase in the control of resting [Ca2+]i
The work described above has examined the role of sarcolemmal Ca2+-ATPase and other mechanisms in dealing with increases in [Ca2+]i produced either by electrical stimulation or caffeine. Other experiments showed the role of Ca2+-ATPase in the regulation of resting [Ca2+]i. When extracellular Ca2+ was increased over a low concentration range, resting Ca2+ could be controlled at approximately normal levels even in the absence of extracellular Na+ (i.e. Na+-Ca2+ exchange inhibited). If, however, the sarcolemmal Ca2+-ATPase was also inhibited then even modest increases in [Ca2+]o (from 0 to 0.1 mM) resulted in large increases in resting [Ca2+]i. This result shows that Ca2+-ATPase alone can regulate [Ca2+]i at a steady level. The experiments in Na+-free solutions identify Ca2+-ATPase as the mechanism responsible for removing Ca2+. As mentioned in the Introduction, it has been suggested that Ca2+-ATPase is a low capacity, high affinity system responsible more for the control of resting [Ca2+]i than for removing large Ca2+ loads. However, we have shown previously that the relative contribution of the two Ca2+ extrusion systems, i.e. sarcolemmal Ca2+-ATPase and Na+-Ca2+ exchange, does not change as [Ca2+]i declines (Lamont & Eisner, 1996). In agreement with this, inhibition of sarcolemmal Ca2+-ATPase with carboxyeosin simply decreases the rate constant of decay of the exponential fall of [Ca2+]i. In contrast, if Ca2+-ATPase was most important at low [Ca2+]i one would expect the initial part of the decay of a caffeine-evoked response to carboxyeosin to be less sensitive than the final component.
Acknowledgments
The work described in this paper was supported by a grant from The Wellcome Trust.
References
- Allen DG, Eisner DA, Lab MJ, Orchard CH. The effects of low sodium solutions on intracellular calcium concentration and tension in ferret ventricular muscle. The Journal of Physiology. 1983;345:391–407. doi: 10.1113/jphysiol.1983.sp014984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Eisner DA, Orchard CH. Factors influencing free intracellular calcium concentration in quiescent ferret ventricular muscle. The Journal of Physiology. 1984;350:615–630. doi: 10.1113/jphysiol.1984.sp015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani JWM, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependant differences in cellular mechanisms. The Journal of Physiology. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani RA, Bassani JWM, Bers DM. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. The Journal of Physiology. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani RA, Bassani JWM, Bers DM. Relaxation in ferret ventricular myocytes: role of the sarcolemmal Ca ATPase. Pflügers Archiv. 1995;430:573–578. doi: 10.1007/BF00373894. [DOI] [PubMed] [Google Scholar]
- Caroni P, Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Journal of Biological Chemistry. 1981;256:3263–3270. [PubMed] [Google Scholar]
- Chapman RA. Sodium/calcium exchange and intracellular calcium buffering in ferret myocardium: an ion-sensitive micro-electrode study. The Journal of Physiology. 1986;373:163–179. doi: 10.1113/jphysiol.1986.sp016040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RA, Tunstall J. The tension-depolarization relationship of frog atrial trabeculae as determined by potassium contractures. The Journal of Physiology. 1981;310:97–115. doi: 10.1113/jphysiol.1981.sp013539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo LM, Grantham C, Cannell MB. Kinetics, stoichiometry and role of the Na-Ca exchange mechanism in isolated cardiac myocytes. Nature. 1990;345:618–621. doi: 10.1038/345618a0. [DOI] [PubMed] [Google Scholar]
- Díaz ME, Trafford AW, O'Neill SC, Eisner DA. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. The Journal of Physiology. 1997;501:3–16. doi: 10.1111/j.1469-7793.1997.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R, Beaugé L. Physiological role of ATP-driven calcium pump in squid axon. Nature. 1979;278:271–273. doi: 10.1038/278271a0. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Nichols CG, O'Neill SC, Smith GL, Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. The Journal of Physiology. 1989;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto C, Hale CC, Milanik MA. Eosin, a potent inhibitor of the plasma membrane Ca pump, does not inhibit the cardiac Na-Ca exchanger. Biophysical Journal. 1994;66:A331. doi: 10.1021/bi00003a031. [DOI] [PubMed] [Google Scholar]
- Gatto C, Milanik MA. Inhibition of red blood cell calcium pump by eosin and other fluorescein analogues. American Journal of Physiology. 1993;264:C1577–1586. doi: 10.1152/ajpcell.1993.264.6.C1577. [DOI] [PubMed] [Google Scholar]
- Kim D, Okada A, Smith TW. Control of cytosolic calcium activity during low sodium exposure in cultured chick heart cells. Circulation Research. 1987;61:29–41. doi: 10.1161/01.res.61.1.29. [DOI] [PubMed] [Google Scholar]
- Lamont C, Eisner DA. The sarcolemmal mechanisms involved in the control of diastolic intracellular calcium in isolated rat cardiac trabeculae. Pflügers Archiv. 1996;432:961–969. doi: 10.1007/s004240050223. [DOI] [PubMed] [Google Scholar]
- Negretti N, O'Neill SC, Eisner DA. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovascular Research. 1993;27:1826–1830. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- O'Neill SC, Donoso P, Eisner DA. The role of [Ca2+]i and [Ca2+]i-sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. The Journal of Physiology. 1990;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SC, Valdeolmillos M, Lamont C, Donoso P, Eisner DA. The contribution of Na-Ca exchange to relaxation in mammalian cardiac muscle. Annals of the New York Academy of Sciences. 1991;639:444–452. doi: 10.1111/j.1749-6632.1991.tb17331.x. [DOI] [PubMed] [Google Scholar]
- Skou JC, Esmann M. Eosin, a fluorescent probe of ATP binding to the Na-K ATPase. Biochimica et Biophysica Acta. 1981;647:232–240. doi: 10.1016/0005-2736(81)90251-0. [DOI] [PubMed] [Google Scholar]
- Smith GL, Valdeolmillos M, Eisner DA, Allen DG. Effects of rapid application of caffeine on intracellular calcium concentration in ferret papillary muscles. Journal of General Physiology. 1988;92:351–368. doi: 10.1085/jgp.92.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A, Negretti N, Hester SB, Eisner DA. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflügers Archiv. 1993;423:158–160. doi: 10.1007/BF00374975. [DOI] [PubMed] [Google Scholar]


