Abstract
Hyperoxia can cause local vasoconstriction in adult animal organs as a protective mechanism against hyperoxia-induced toxicity. It is not known at what time during development this vasoconstrictor capacity is present. Therefore, we measured the cardiac output (CO) distribution in different organs during a period of acute hyperoxia (100% O2) in the developing chick embryo.
Fertile eggs were divided into five incubation time groups (10 and 11, 12 and 13, 14 and 15, 16 and 17, and 18 and 19 days of a normal incubation time of 21 days). Eggs were opened at the air cell and a catheter was inserted into a branch of the chorioallantoic vein for injections of 15 μm fluorescent microspheres during normoxia and at the end of 5 min (test group 1; n = 39) or 20 min (test group 2; n = 21) of hyperoxia exposure (100% O2). The fraction of CO to an organ was calculated as the fluorescence of the organ sample divided by the sum of the fluorescence of all organs.
Only in 18- and 19-day-old embryos did hyperoxia cause a decrease in the fractions of CO to the heart and carcass, and an increase in those to the yolk-sac and chorioallantoic membrane. This response was more pronounced after 20 min (test group 2) than after 5 min (test group 1) of hyperoxia with an additional decrease in the fractions of CO to the brain, intestine and liver (test group 2).
These data indicate that local mechanisms for hyperoxia-induced vasoconstriction in the heart, brain, liver, intestine and carcass develop late, during the final 15% of the incubation period, in the developing chick embryo.
The response to hypoxia in the developing fetal lamb (for review see Hanson, 1988; Jensen et al. 1991; Giussani et al. 1994) is characterized by hypertension, bradycardia and cardiac output (CO) redistribution to the heart, brain and adrenals at the expense of peripheral organs. A comparable response has been observed in the developing chick embryo when exposed to anoxia (van Golde et al. 1997; Mulder et al. 1998). Organs that are crucial to fetal survival - brain, heart and adrenals - respond directly to local changes in PO2 to maintain O2 delivery at the expense of delivery to other areas of the body. Blood flow to the peripheral organs decreases secondary to increases in activity of the sympathetic nervous system and increases in catecholamine and vasopressin concentrations (Hanson, 1988). In contrast, as described in adults, the response to hyperoxia is the opposite of that to hypoxia. Increases in arterial PO2 cause a decrease in the fractions of the CO to the brain, heart and adrenals, probably as a result of local vasoconstriction (Peeters et al. 1979; Ashwal et al. 1984; Iwamoto et al. 1987; Jackson et al. 1987; Blanco et al. 1988; Gleason et al. 1988; Bendeck & Langille 1992; Menke et al. 1993; Lundstrøm et al. 1995). However, this response was observed in adults and in late gestation fetuses, and the time at which vasoconstriction occurs in response to hyperoxia exposure during development is not known.
Oxygen is not only life giving but might also be toxic during hyperoxic conditions due to increased production of reactive oxygen species (ROS). Therefore it is important to regulate the oxygen supply. Hyperoxia and the resulting increase in aerobic metabolism are known to generate ROS that can interact with proteins, DNA and lipids resulting in cellular oxidative damage (Frank, 1985; Saugstad, 1990). Several harmful effects of hyperoxia have been observed after oxygen treatment of newborn babies, such as bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis and patent ductus arteriosus (Saugstad, 1990). A vasoconstrictor response during hyperoxia might serve two goals: regulation of tissue oxygen supply (Iwamoto et al. 1987) and protection against an increase in metabolic rate (Frank, 1985).
Because the chick embryo has been shown to be a feasible model in fetal physiology due to easily controllable gas exchange, and rapid development compared with the fetal sheep (van Golde et al. 1997; Mulder et al. 1998), this model was used to determine the changes in the fractions of CO to different organs in response to an increased arterial PO2 level, during the second half of the incubation period. The first aim was to determine the age at which the vasoconstriction response to 5 min of hyperoxia was present. The second aim was to determine the temporal effects of the response by applying two different durations of hyperoxia exposure (5 or 20 min exposure to 100 % O2) in the older groups.
METHODS
Animals
This work was performed in accordance with Dutch law for animal experimentation. Fertilized White Leghorn eggs were incubated at 38°C and 60 % humidity. During incubation, eggs were turned hourly along their long axis to prevent adhesions between the embryo and its membranes and to avoid abnormal development (Tazawa, 1981; Pearson et al. 1996). Chick embryos were studied during the second half of the incubation period, corresponding to stages 36-44, according to Hamburger & Hamilton (1951). To document the response to 5 min of hyperoxia we studied 39 chick embryos (test group 1) at five different incubation periods: 10 and 11 days (n = 6), 12 and 13 days (n = 8), 14 and 15 days (n = 8), 16 and 17 days (n = 6) and 18 and 19 days (n = 11). The response to 20 min of hyperoxia was studied in 21 chick embryos (test group 2) at two different incubation periods: 16 and 17 days (n = 7) and 18 and 19 days (n = 14).
Preparation
The measurements were done inside a clinical infant incubator at a temperature of 38°C and 60 % humidity. After the air cell at the blunt side of the egg had been opened with an electric saw, the egg was placed in a small Plexiglass holder, through which there was a continuous flow of a N2-O2 mixture (5 l min−1), at 38°C and 60 % humidity. A catheter was inserted into a branch of the chorioallantoic vein for injection of fluorescent microspheres, as described previously (Mulder et al. 1997).
Protocol
For each injection, 0.2 ml of a suspension of microspheres (100 000 microspheres ml−1) in 0.05 % Tween 80 was used (sphere diameter, 15 μm; Fluospheres, Molecular Probes Inc., Eugene, OR, USA). Five minutes after insertion of the catheter, yellow-green fluorescent microspheres were injected (normoxia level). Three minutes later the chick embryo was exposed to hyperoxia by application of 100 % O2 (5 l min−1, at 38°C and 60 % humidity) to the Plexiglass holder. In test group 1, the second microsphere injection (orange) was performed during the last minute of a 5 min hyperoxia period. In test group 2, the second injection was performed during the last minute of a 20 min hyperoxia period. After the experiment, chick embryos were removed from the egg shell and membranes and immediately decapitated. Afterwards, the CAM (chorioallantoic membrane, placenta equivalent), brain, heart, lungs, intestine, liver and yolk-sac were dissected.
In another group of chick embryos, on days 16 and 17 (n = 10) of the incubation period blood samples were obtained for blood gas analysis of the experimental conditions. The pH, PO2 and PCO2 were determined after 5 min (n = 5) and 20 min (n = 5) of hyperoxia (100 % O2). The embryos were exposed to the same environmental conditions as the experimental group. To obtain the samples, the chorioallantoic artery was carefully lifted with forceps and a curved 30 gauge needle was inserted contrary to the blood stream. Blood samples (0.2 ml) were collected in a heparinized syringe and analysed at 38°C (Radiometer ABL3, Copenhagen). Values were corrected for embryonic temperature.
Analysis of microsphere distribution
Fluorescence in whole organs (CAM, brain, heart, lungs, intestine, liver and yolk-sac) and in the remaining carcass was determined. The organs were digested in a 2 M ethanol-KOH solution for 48 h at 60°C. The microspheres were isolated from the homogenate by centrifugation, as described by van Oosterhout et al. (1995). The dye was extracted from the final pellet by addition of 3 ml 2-(2-ethoxyethoxy)ethylacetate (100 %) and the fluorescence was determined by fluorimetry using a LS-50B fluori-spectrometer (Perkin Elmer). No correction for spectral overlap was used since the excitation and emission spectra of the injected microsphere dyes were well separated from each other (dye characteristics: yellow- green: 490 nm excitation maximum, 506 emission maximum; orange: 530 nm excitation maximum, 552 emission maximum). During fluorimetry all samples had the same volume (3 ml). The fraction of CO to an organ was calculated as the level of the fluorescence in the tissue, after correction for background, divided by the sum of the fluorescence of all organs.
Statistical analysis
The data are expressed as median with interquartile range (p25-p75). Significant differences in CO distribution during hyperoxia (100 % O2) compared with distribution during normoxia were analysed by a non-parametric sample test (Wilcoxon signed-rank test). Differences were considered significant at P < 0.05.
RESULTS
Exposure of eggs to 100 % O2 caused an increase in chorioallantoic artery oxygen tension, while pH and arterial PCO2 remained unchanged. Median PO2 values were 5.65 kPa (range, 5.04-7.71 kPa) after 5 min hyperoxia and 6.56 kPa (range, 6.03-8.13 kPa) after 20 min hyperoxia, compared with the PO2 range 3.12-6.02 kPa previously reported during normoxia (van Golde et al. 1996).
During normoxic conditions, in both test groups a large fraction of the CO was directed to the CAM and a relatively small fraction to the heart, brain, intestine and liver (Tables 1 and 2). In both groups, no significant changes in the fractions of CO in response to hyperoxia were observed in chick embryos less than 18 days old. In 18- and 19-day-old embryos, a significant decrease in the fractions of CO directed to the heart (-50.43 % of normoxia level) and carcass (-31.47 %), and a significant increase in those to the CAM (+40.09 %) and yolk-sac (+58.29 %) were observed after 5 min of hyperoxia (test group 1; two-related sample test, *P < 0.05vs. normoxia; Table 1). When the duration of exposure to hyperoxia was prolonged to 20 min (test group 2), a significant decrease in the fractions of CO to the heart (-61.71 %) and carcass (-25.69 %) was again observed, but in addition the fractions of CO to the brain (-32.86 %), intestine (-47.09 %) and liver (-30.09 %) also decreased significantly. In these embryos, the fractions of CO to the lungs (+42.75 %), CAM (+38.28 %) and yolk-sac (+25.0 %) increased significantly (Wilcoxon signed-rank test, *P < 0.05vs. normoxia; Table 2). Figure 1 illustrates the relative percentage changes in CO distribution after 20 min hyperoxia.
Table 1.
Fraction of the CO distribution (%) directed to different organs during normoxia and 5 min of hyperoxia (test group 1) in the developing chick embryo from days 10 to 19
| Heart | Brain | Intestine | Liver | Carcass | CAM | Lungs | Yolk-sac | |
|---|---|---|---|---|---|---|---|---|
| Days 10 and 11 (n = 6) | ||||||||
| Normoxia | 1.34 | 3.20 | 1.87 | 1.07 | 28.88 | 54.84 | 0.41 | 13.65 |
| (0.78–1.77) | (1.25–3.56) | (0.784–2.11) | (0.36–1.49) | (17.49–34.06) | (44.95–59.74) | (0.12–0.80) | (6.57–18.89) | |
| Hyperoxia | 1.51 | 3.22 | 2.11 | 1.38 | 27.99 | 46.69 | 0.72 | 17.36 |
| (1.07–2.0) | (1.65–4.86) | (1.40–2.61) | (0.44–1.93) | (21.56–31.87) | (38.78–64.10) | (0.50–0.86) | (5.77–19.6) | |
| Days 12 and 13 (n = 8) | ||||||||
| Normoxia | 2.90 | 3.81 | 2.83 | 2.3 | 26.81 | 48.32 | 0.76 | 11.9 |
| (2.41–3.39) | (2.5–5.65) | (1.75–3.75) | (1.66–2.65) | (24.03–31.92) | (44.33–51.66) | (0.5–1.12) | (8.81–16.86) | |
| Hyperoxia | 3.75 | 4.29 | 3.23 | 2.5 | 28.14 | 47.6 | 0.58 | 14.02 |
| (2.43–4.39) | (3.68–5.71) | (2.26–4.83) | (1.91–3.29) | (19.17–34.13) | (33.9–51.47) | (0.48–0.96) | (7.84–15.4) | |
| Days 14 and 15 (n = 8) | ||||||||
| Normoxia | 3.22 | 3.60 | 2.75 | 3.26 | 36.87 | 44.45 | 0.82 | 7.71 |
| (1.94–4.29) | (2.66–4.34) | (1.88–3.43) | (1.36–5.64) | (28.53–38.38) | (35.15–49.76) | (0.44–1.15) | (5.0–12.63) | |
| Hyperoxia | 3.01 | 3.34 | 2.25 | 2.77 | 34.69 | 42.84 | 0.88 | 11.96 |
| (2.12–3.44) | (2.11–4.41) | (1.66–3.98) | (1.09–3.92) | (26.26–41.81) | (30.96–51.69) | (0.41–1.42) | (7.36–12.89) | |
| Days 16 and 17 (n = 6) | ||||||||
| Normoxia | 3.26 | 3.67 | 4.4 | 2.83 | 34.72 | 48.26 | 0.74 | 3.7 |
| (2.8–4.34) | (2.3–6.06) | (0.73–5.84) | (0.85–6.03) | (19.57–52.46) | (20.43–60.83) | (0–1.45) | (2.21–7.04) | |
| Hyperoxia | 2.18 | 3.85 | 3.19 | 2.48 | 33.14 | 41.28 | 0.58 | 5.13 |
| (0.54–5.24) | (1.43–4.67) | (0.62–5.4) | (0.54–6.51) | (24.73–43.19) | (24.55–70.85) | (0–3.64) | (3.32–8.69) | |
| Days 18 and 19 (n = 11) | ||||||||
| Normoxia | 4.6 | 6.82 | 4.99 | 2.12 | 42.84 | 35.72 | 0.75 | 2.11 |
| (3.19–5.67) | (5.16–8.09) | (3.88–7.03) | (1.93–3.53) | (25.13–42.05) | (30.6–40.39) | (0.43–1.3) | (1.08–3.31) | |
| Hyperoxia | 2.28 | 6.23 | 5.94 | 1.39 | 29.36 | 50.04 | 0.74 | 3.34 |
| (1.9–3.09)* | (4.3–6.48) | (4.3–6.77) | (1.12–2.37) | (25.1–42.05)* | (32.97–56.14)* | (0.47–1.4) | (2.02–4.16)* | |
Data are expressed as the median with the interquartile range in parentheses.
Significant difference compared with normoxia (P < 0.05). CAM, chorioallantoic membrane.
Table 2.
Fraction of the CO distribution (%) directed to different organs during normoxia and 20 min of hyperoxia (test group 2) in the developing chick embryo from days 16 to 19
| Heart | Brain | Intestine | Liver | Carcass | CAM | Lungs | Yolk-sac | |
|---|---|---|---|---|---|---|---|---|
| Days 16 and 17 (n = 7) | ||||||||
| Normoxia | 4.38 | 4.13 | 3.92 | 4.48 | 37.11 | 40.33 | 1.01 | 3.49 |
| (1.53–5.35) | (3.0–6.65) | (2.68–5.06) | (2.26–6.07) | (34.48–48.44) | (35.13–46.76) | (0.58–1.77) | (1.75–8.05) | |
| Hyperoxia | 2.96 | 5.09 | 5.41 | 3.1 | 29.69 | 36.16 | 2.29 | 8.93 |
| (1.3–4.64) | (3.66–6.8) | (2.11–8.57) | (1.76–5.7) | (25.65–50.05) | (18.71–46.17) | (0.31–3.89) | (0.6–15.75) | |
| Days 18 and 19 (n = 14) | ||||||||
| Normoxia | 4.03 | 5.63 | 6.54 | 4.11 | 42.32 | 28.79 | 1.38 | 3.28 |
| (2.93–6.36) | (4.99–10.57) | (3.39–9.81) | (3.36–4.94) | (34.0–54.89) | (20.15–36.13) | (0.53–1.95) | (2.11–3.74) | |
| Hyperoxia | 1.43 | 3.78 | 3.46 | 2.84 | 31.45 | 39.81 | 1.97 | 4.1 |
| (1.14–2.59)* | (2.37–5.64)* | (2.79–6.23)* | (1.84–4.32)* | (22.55–43.39)* | (25.46–54.39)* | (1.1–2.5)* | (2.66–7.31)* | |
Data are expressed as the median with the interquartile range in parentheses.
Significant difference compared with normoxia (P < 0.05).
Figure 1. Magnitude of response to 20 min hyperoxia at incubation days 18 and 19.
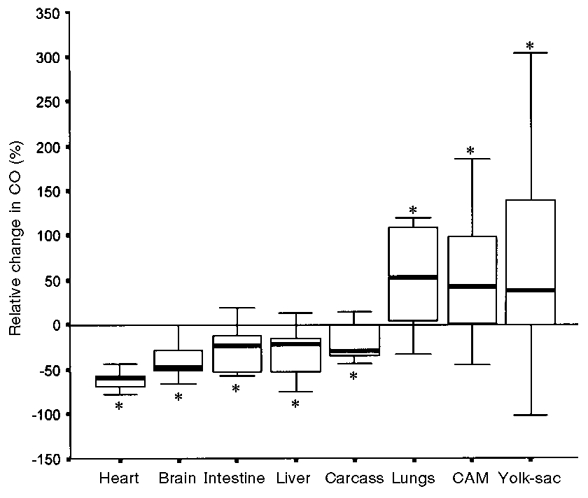
The bars represent the median change expressed as a percentage of normoxia level with the interquartile range (p25-p75). * Significant difference compared with normoxia (P < 0.05). Error bars indicate ±s.d.
DISCUSSION
CO distribution during normoxic conditions was similar to data reported previously from a larger group of chick embryos (Mulder et al. 1997) and from the late gestation sheep fetus (Jensen et al. 1991). A large fraction of the CO was directed to the CAM and a relatively small fraction to the brain, heart, liver and intestine. This study showed that exposure to hyperoxia for 5 or 20 min, late in the incubation period, resulted in a decrease in the fractions of CO to the heart and carcass of the chick embryo, whereas the fractions to the CAM and yolk-sac increased. When the hyperoxia exposure was prolonged to 20 min, the response was augmented, and a decrease in CO fractions to the brain, intestine and liver was also observed. Hyperoxia had no effect on organ perfusion before the final 15 % of the incubation period of the developing chick embryo.
At birth, PO2 increases from about 25 to 85 mmHg, which reduces the perfusion demands, and may contribute to decreased blood flow to skeletal muscles, brain, skin, bone, carcass and adrenal glands, mediated by local vasoconstriction (Bendeck & Langille, 1992; Lundstrøm et al. 1995). Regional blood flow is also redistributed during maternal hyperoxygenation late in gestation (Niijima et al. 1988; Almstrom & Sonesson, 1996). Studies on chronically catheterized fetal sheep (Peeters et al. 1979; Iwamoto et al. 1987; Blanco et al. 1988; Gleason et al. 1988) and fetal rhesus monkey (Jackson et al. 1987) showed a decreased blood flow to the adrenals, brain and heart with increased oxygenation. All these studies were performed late in gestation or in the newborn. To our knowledge, the effects of hyperoxia on organ perfusion have not been investigated early in gestation to determine the point at which hyperoxia causes vasoconstriction. In the present study, the developing chick embryo was used to investigate the maturation of the vasoconstriction response to hyperoxia. Our study demonstrates that in the developing chick embryo redistribution of the CO in response to hyperoxia does not occur before the final 15 % of the incubation period.
Development of the response to hypoxia is better described than that to hyperoxia. In the developing chick embryo, anoxia (100 % N2, causing isocapnic hypoxaemia with mean arterial PO2 of 1.2 kPa) causes CO redistribution in favour of the heart and brain at the expense of the intestine, liver, yolk-sac and carcass from day 14 (Mulder et al. 1998). From studies in fetal sheep, it is known that this response is mediated by neurohormonal mechanisms (Hanson, 1988). Increased levels of catecholamines, but also vasopressin, opioids and prostaglandins, cause vasoconstriction in vascular smooth muscle cells (Jones & Robinson, 1975; Millard et al. 1979; Faucher et al. 1987; Hanson, 1988; Espinoza et al. 1989).
Vasoconstriction during hyperoxia exposure has been attributed to a direct effect of oxygen on the vessel wall (Lewis, 1968; Sparks, 1980) or to a release of vasoconstrictor factors, such as leukotrienes, prostaglandin F2α and thromboxane A2 (Stuart et al. 1984; Gurtner et al. 1985; Wagerle & Mishra, 1988). Furthermore, increased release of ROS during hyperoxia, generated by cyclooxygenase-1 in endothelial cells, might produce vasoconstriction by inhibiting the synthesis or the action of vasodilatory components such as prostacyclin (PGI2) or nitric oxide (Stuart et al. 1984). In contrast, it is also reported that ROS could reduce vascular reactivity by inhibition of calcium transport and reduction of the vasoconstrictor response to thromboxane (which is dependent upon Ca2+ influx) (Gurtner et al. 1985; Hardy et al. 1994). These conflicting reports do not help to explain the role of ROS in the vasomotor responses in our model, but further research should clarify this. Furthermore, differences in vasoconstrictor response after changes in oxygen tension at different stages of development and in different organs might be explained by different ion channel densities in vascular smooth muscle cells (Weir et al. 1997).
In summary, we can conclude that hyperoxia causes a decrease in the fractions of CO to the brain, heart, liver, intestine and carcass in the chick embryo, but only after 18 days of incubation. The diversity of response in vascular smooth muscle cells to changes in oxygen tension at different stages of development and in different organs might be determined by the capacity for production of vasoconstrictor factors, the distribution of a variety of ion channels in the vascular smooth muscle cells and the production of ROS. Further studies are needed to determine the exact mechanisms underlying the vascular response to hyperoxia.
References
- Almstrom H, Sonesson SE. Doppler echocardiographic assessment of fetal blood flow redistribution during maternal hyperoxygenation. Ultrasound in Obstetrics and Gynecology. 1996;8:256–261. doi: 10.1046/j.1469-0705.1996.08040256.x. 10.1046/j.1469-0705.1996.08040256.x. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Dale PS, Longo LD. Regional cerebral blood flow. Studies in the fetal lamb during hypoxia, hypercapnia, acidosis and hypotension. Pediatric Research. 1984;18:1309–1316. doi: 10.1203/00006450-198412000-00018. [DOI] [PubMed] [Google Scholar]
- Bendeck MP, Langille BL. Changes in blood flow distribution during the perinatal period in fetal sheep and lambs. Canadian Journal of Physiology and Pharmacology. 1992;70:1576–1582. doi: 10.1139/y92-226. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Martin CB, Rankin J, Landauer M, Phernetton T. Changes in fetal organ flow during intrauterine mechanical ventilation with or without oxygen. Journal of Developmental Physiology. 1988;10:53–62. [PubMed] [Google Scholar]
- Espinoza M, Riquelme R, Germain AM, Tevah J, Parer JT, Llanos AJ. Role of endogenous opioids in the cardiovascular responses to asphyxia in fetal sheep. American Journal of Physiology. 1989;256:1063–1068. doi: 10.1152/ajpregu.1989.256.5.R1063. [DOI] [PubMed] [Google Scholar]
- Faucher DJ, Lowe TW, Magness RR, Laptook AR, Porter JC, Rosenfeld CR. Vasopressin and catecholamine secretion during metabolic acidemia in the ovine fetus. Pediatric Research. 1987;21:38–43. doi: 10.1203/00006450-198701000-00010. [DOI] [PubMed] [Google Scholar]
- Frank L. Effects of oxygen on the newborn. Federation Proceedings. 1985;44:2328–2334. [PubMed] [Google Scholar]
- Giussani DA, Spencer JAD, Hanson MA. Fetal cardiovascular reflex responses to hypoaemia. Fetal and Maternal Medicine Review. 1994;6:17–37. [Google Scholar]
- Gleason CA, Jones MD, Traystman RJ, Notter RH. Fetal cerebral responses to ventilation and oxygenation in utero. American Journal of Physiology. 1988;255:R1049–1054. doi: 10.1152/ajpregu.1988.255.6.R1049. [DOI] [PubMed] [Google Scholar]
- Gurtner GH, Michael JR, Farrukh IS, Sciuto AM, Adkinson NF. Mechanism of hyperoxia-induced pulmonary vascular paralysis: effect of antioxidant pretreatment. Journal of Applied Physiology. 1985;59:953–958. doi: 10.1152/jappl.1985.59.3.953. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Hanson MA. The importance of baro- and chemoreflexes in the control of the fetal cardiovascular system. Journal of Developmental Physiology. 1988;10:491–511. [PubMed] [Google Scholar]
- Hardy P, Abran D, Li D, Fernandez H, Varma DR, Chemtob S. Free radicals in retinal and choroidal blood flow autoregulation in the piglet: Interaction with prostaglandins. Investigative Ophthalmology and Visual Science. 1994;35:580–591. [PubMed] [Google Scholar]
- Iwamoto HS, Teitel D, Rudolph AM. Effect of birth-related events on blood flow distribution. Pediatric Research. 1987;22:634–640. doi: 10.1203/00006450-198712000-00004. [DOI] [PubMed] [Google Scholar]
- Jackson BT, Piasecki GJ, Novy MJ. Fetal responses to altered maternal oxygenation in rhesus monkey. American Journal of Physiology. 1987;252:R94–101. doi: 10.1152/ajpregu.1987.252.1.R94. [DOI] [PubMed] [Google Scholar]
- Jensen A, Roman C, Rudolph AM. Effects of reducing uterine blood flow on fetal blood flow distribution and oxygen delivery. Journal of Developmental Physiology. 1991;15:309–323. [PubMed] [Google Scholar]
- Jones CT, Robinson RO. Plasma catecholamines in foetal and adult sheep. The Journal of Physiology. 1975;248:15–33. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BV. The response of isolated sheep and human umbilical arteries to oxygen and drugs. British Journal of Obstetrics and Gynaecology. 1968;75:87–91. doi: 10.1111/j.1471-0528.1968.tb00126.x. [DOI] [PubMed] [Google Scholar]
- Lundstrøm KE, Pryds O, Greisen G. Oxygen at birth and prolonged cerebral vasoconstriction in preterm infants. Archives of Disease in Childhood. 1995;73:F81–86. doi: 10.1136/fn.73.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke J, Michel E, Rabe H, Bresser BW, Grohs B, Schmitt RM, Jorch G. Simultaneous influence of blood pressure, PCO2, and PO2 on cerebral blood flow velocity in preterm infants of less than 33 weeks' gestation. Pediatric Research. 1993;34:173–177. doi: 10.1203/00006450-199308000-00014. [DOI] [PubMed] [Google Scholar]
- Millard RW, Baig H, Vatner SF. Prostaglandin control of the renal circulation in response to hypoaemia in the lamb in utero. Circulation Research. 1979;45:172–179. doi: 10.1161/01.res.45.2.172. [DOI] [PubMed] [Google Scholar]
- Mulder ALM, van Golde JC, Prinzen FW, Blanco CE. Cardiac output distribution in response to hypoxia in the chick embryo in the second half of the incubation time. The Journal of Physiology. 1998;508:281–287. doi: 10.1111/j.1469-7793.1998.281br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder TLM, van Golde JC, Prinzen FW, Blanco CE. Cardiac output distribution in the chick embryo from stage 36 to 45. Cardiovascular Research. 1997;34:525–528. doi: 10.1016/s0008-6363(97)00065-5. [DOI] [PubMed] [Google Scholar]
- Niijima S, Shortland DB, Levene MI, Evans DH. Transient hyperoxia and cerebral blood flow velocity in infants born prematurely and at full term. Archives of Disease in Childhood. 1988;63:1126–1130. doi: 10.1136/adc.63.10_spec_no.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JT, Haque MA, Hou PCL, Tazawa H. Developmental patterns of O2 consumption, heart rate and O2 pulse in unturned eggs. Respiration Physiology. 1996;103:83–87. doi: 10.1016/0034-5687(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Peeters LLH, Sheldon RE, Douglas MD, Jones MD, Makowski EL, Meschia G. Blood flow to fetal organs as a function of arterial oxygen content. American Journal of Obstetrics and Gynecology. 1979;135:637–646. doi: 10.1016/s0002-9378(16)32989-1. [DOI] [PubMed] [Google Scholar]
- Saugstad OD. Oxygen toxicity in the neonatal period. Acta Paediatrica Scandinavica. 1990;79:881–892. doi: 10.1111/j.1651-2227.1990.tb11348.x. [DOI] [PubMed] [Google Scholar]
- Sparks HV. Effect of local metabolic factors on vascular smooth muscle. In: Bohr DF, Somylo AP, Sparks HV, editors. Handbook of Physiology, The Cardiovascular System. II. Bethesda, MD, USA: American Physiological Society; 1980. pp. 475–513. [Google Scholar]
- Stuart MJ, Setty BNY, Walenga RW, Graeber JE, Ganley C. Effects of hyperoxia and hypoxia on vascular prostacyclin formation in vitro. Pediatrics. 1984;74:548–553. [PubMed] [Google Scholar]
- Tazawa H. Adverse effect of failure to turn the avian egg on the embryo oxygen exchange. Respiration Physiology. 1981;41:137–142. doi: 10.1016/0034-5687(80)90047-x. [DOI] [PubMed] [Google Scholar]
- van Golde J, Mulder T, Blanco CE. Changes in mean chorioallantoic artery blood flow and heart rate produced by hypoxia in the developing chick embryo. Pediatric Research. 1997;42:293–298. doi: 10.1203/00006450-199709000-00008. [DOI] [PubMed] [Google Scholar]
- van Golde J, Mulder T, van Straaten H, Blanco CE. The chorioallantoic artery blood flow of the chick embryo from stage 34 to 43. Pediatric Research. 1996;40:867–871. doi: 10.1203/00006450-199612000-00016. [DOI] [PubMed] [Google Scholar]
- van Oosterhout MFM, Willigers HMM, Reneman RS, Prinzen FW. Fluorescent microspheres to measure organ perfusion: validation of a simplified sample processing technique. American Journal of Physiology. 1995;269:H725–733. doi: 10.1152/ajpheart.1995.269.2.H725. [DOI] [PubMed] [Google Scholar]
- Wagerle LC, Mishra OP. Mechanism of CO2 response in cerebral arteries of the newborn pig: role of phospholipase, cyclooxygenase and lipoxygenase pathways. Circulation Research. 1988;62:1019–1026. doi: 10.1161/01.res.62.5.1019. [DOI] [PubMed] [Google Scholar]
- Weir EK, Reeve HL, Cornfield DN, Tristani-Firouzi M, Peterson DA, Archer SL. Diversity of response in vascular smooth muscle cells to changes in oxygen tension. Kidney International. 1997;51:462–466. doi: 10.1038/ki.1997.62. [DOI] [PubMed] [Google Scholar]


