Abstract
The contributions of specific residues in γ- and ɛ-subunits to the developmental changes in conductance and open time of Xenopus muscle acetylcholine receptors (AChRs) were investigated. This study was directed primarily at residues in the M2 domains of γ- and ɛ-subunits; however, the results of additional mutations in the extracellular region flanking M2 and in the amphipathic region between M3 and M4 are also described.
The M2 domains of γ- and ɛ-subunits differ at only three amino acid residues, two of which are adjacent to each other and located near the narrowest part of the pore. These two residues (NI in γ, SV in ɛ) were found to be major determinants of the difference in conductance and open time of AChRs bearing γ- or ɛ-subunits.
Mutation of N to S in the γ-subunit converted the long open time of receptors bearing the γ-subunit (γ-AChRs) to the brief open time characteristic of receptors bearing an ɛ-subunit (ɛ-AChRs). Conversely, ɛ-AChRs with SV mutated to NI in the ɛ-subunit exhibited a long open time characteristic of γ-AChRs.
Mutation of N to S in the γ-subunit increased the conductance of γ-AChRs but did not confer the full conductance of wild-type ɛ-AChRs. Conversely, mutation of SV to NI in the ɛ-subunit reduced the conductance of ɛ-AChRs, but not completely to the level of wild-type γ-AChRs.
During development of skeletal muscle in mammals and amphibia, the nicotinic acetylcholine receptor (AChR) acquires a higher conductance and briefer open time. These changes in function are due to replacement of a γ-subunit in the embryonic AChR (γ-AChR) by an ε-subunit in the mature receptor (ε-AChR) (Mishina et al. 1986; Murray et al. 1995). Like the other subunits of the AChR pentamer, γ- and ε-subunits each have four membrane-spanning domains, M1-M4 (Stroud et al. 1990). Their amino and carboxy termini are extracellular, and each subunit has a large cytoplasmic domain between M3 and M4. Studies of mammalian AChRs have identified regions of γ and ε which contribute to the developmental change in channel properties. In rat, all four membrane-spanning domains play a role in conferring the conductance differences between fetal and adult channels; however, the majority of the change is due to a few residues in and adjoining the pore-forming M2 region of γ- and ε-subunits (Herlitze et al. 1996). On the other hand, changes in channel open time of mammalian AChRs do not appear to involve M2, but instead reflect differences chiefly in a stretch of amphipathic helix, the HA region, which is part of the cytoplasmic domain between M3 and M4 (Bouzat et al. 1994).
In Xenopus, like rat, the M2 regions of γ and ε are highly homologous (Fig. 1; Baldwin et al. 1988; Murray et al. 1995). They differ at only three residues, two of which are found near the narrowest part of the pore. There are, however, some differences between mammalian and amphibian γ- and ε-subunits which suggest that different strategies might be used to achieve developmental changes in channel properties. For instance, in rat, a K to Q change in the position immediately adjacent to the extracellular side of M2 is important in bringing about the conductance increase of receptors (Herlitze et al. 1996). This is not the case in Xenopus since the residues at this position are identical in γ and ε. Furthermore, the residues of the M2 domain in rat AChRs that affect conductance are also identical in Xenopusγ and ε. It is of interest that the key M2 residues in rat (γT278 and εI277) that affect conductance differences probably do not face the lumen of the open channel (Unwin, 1995), whereas one of the residue sets in Xenopus is predicted to face the centre of the open channel where it could directly interact with cations traversing the pore.
Figure 1. Alignment of M2 regions of Xenopus and rat ε- and γ-subunits.
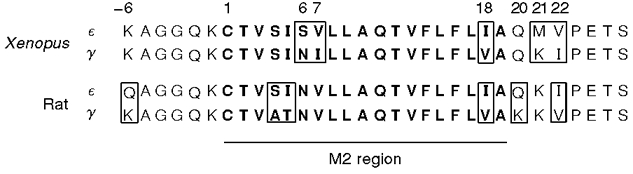
Boxed residues indicate differences between the two subunits within a species. Numbering begins with the first residue of M2.
We investigated the role of residues in M2 and flanking regions in governing the shift in conductance and open time of Xenopus nicotinic AChRs. Our data indicate that in Xenopus, unlike mammals, differences in the M2 region between γ- and ε-subunits confer developmental changes in open time as well as conductance of the AChR.
METHODS
The methods used in this study were similar to those used previously to examine properties of Xenopus AChRs expressed in oocytes (Kullberg et al. 1994; Murray et al. 1995).
cRNA preparation
Subunit cRNAs were prepared using Ambion mMessage mMachine SP6 and T3 kits from cDNA clones encoding Xenopus AChR α-, β-, δ-, γ- and ε-subunits. The transcripts were resuspended in nuclease-free water at a concentration of 200 ng μl−1 (α-subunit transcript) or 100 ng μl−1 (β-, δ-, γ- and ε-subunit transcripts) and aliquots were stored at -70°C until use. Stock solutions of each type of subunit cRNA were premixed to create injection solutions (α, 50 ng ml−1; β, 25 ng ml−1; δ, 25 ng ml−1; γ or ε, 25 ng ml−1). Site-directed mutagenesis of γ- and ε-subunits was done using either the Morph Mutagenesis Kit (5 Prime → 3 Prime, Inc., Boulder, CO, USA) or the method of Kunkel (Sambrook et al. 1989). Mutations were confirmed by dideoxy sequencing. In this report, residues are numbered with respect to the first position of the M2 domain.
Solutions
The following solutions were used: OR3 (50 % L-15 medium (Gibco), 15 mM Hepes, 1 mM L-glutamate, 20 mg ml−1 gentamicin; adjusted to pH 7.6); Ringer solution (120 mM NaCl, 1 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM Hepes; adjusted to pH 7.4); recording solution (120 mM NaCl, 1 mM KCl, 0.4 mM CaCl2, 1 mM MgCl2, 10 mM Hepes; adjusted to pH 7.4); and pipette solution (80 mM KF, 20 mM KCl, 10 mM K-EGTA, 10 mM Hepes; adjusted to pH 7.4).
Oocyte removal and injection
Adult female Xenopus laevis frogs were immersed in crushed ice. They were then killed by a blow to the head and the oocytes surgically removed. The anaesthetic and surgical procedures used in this study fall within the guidelines of our Institutional Animal Care and Use Committee. To facilitate the removal of the follicular membrane by microdissection, oocytes were exposed to a 8-15 min treatment in collagenase (Cooper Biomedical CLS III; 102 units mg−1, at a concentration of 15 mg ml−1 in Ringer solution), followed by washing in Ringer solution. Defolliculation was done in Ringer solution and the defolliculated oocytes were then stored in OR3 solution at 17°C.
Microinjection pipettes were pulled from capillary tubes and bevelled to an outer diameter of 18-20 μm. Pipettes were baked at 200°C for 2 h and stored in a metal box container to eliminate RNase contamination. Oocytes were immersed in OR3 and each oocyte was injected with 50 nl of cRNA using a Medical Systems PLI-100 injector. The oocytes were maintained at 17°C in OR3 solution. Oocytes were incubated for 2 days prior to recording to allow expression of the AChRs. All recordings were obtained within 1 week of injection.
Single channel recording
To facilitate removal of the vitelline membrane by microdissection, individual oocytes were exposed for 1.5-2 min to 0.05 % trypsin-EDTA (in Hanks' balanced salt solution without Ca2+ and Mg2+, Gibco BRL) and then rinsed thoroughly in Ringer solution. Patch electrodes were pulled to an outer diameter of 1-2 μm, fire polished, filled with pipette solution and coated with Sigmacote (Sigma). Outside-out patches were excised from the oocyte membrane and transferred to an adjacent trough through which recording solution could flow. By lowering the solution level in the trough, the recording solution was isolated from the oocyte chamber so that the oocyte was not exposed to ACh. The volume of the recording trough was 0.7 ml and the solution flow rate was 3-4 ml min−1. Patches were exposed to recording solution for at least 1 min to allow complete washout of Ringer solution before recording commenced. Complete washout was done because the Ringer solution has a higher Ca2+ concentration (1.8 mM) than the recording solution (0.4 mM), and we wanted to avoid possible effects of residual calcium on channel conductance. All data were obtained from outside-out patches in recording solution with concentrations of ACh ranging from 125 to 500 nM. Single channel events were recorded by a patch amplifier (EPC-7), filtered at 3 kHz, and continuously digitized at 10 kHz with an ITC-16 A/D converter (Instrutech Inc.). For kinetic analysis, records were acquired at a continuous applied pipette potential of -100 mV. For current- voltage (I-V) data, the pipette potential was stepped through potentials from -60 to -160 mV, in 20 mV steps, with 8 s of data acquired at each step.
Analysis
Digitized records were analysed with MAC-TAC analysis software (Skalar Instruments, Seattle, WA, USA). Prior to kinetic analysis, records were digitally filtered at 2683 Hz, giving an overall corner frequency of 2000 Hz. For the detection of channel openings, an amplitude threshold was set at about half full amplitude. Overlapping events were excluded from the analysis. The burst resolution was set at 0.3 ms, and for the purposes of this paper, the term ‘open time’ refers to the burst duration. For kinetic analysis, the average analysed sample size from each recording site was more than 500 events, and the minimum size was 100 events. The durations of brief events were corrected for the corner frequency of 2000 Hz. Log open time histograms were fitted with the sum of two exponential functions and the weighted mean channel open time of each histogram was calculated from the two exponential components. Channel open times are given as means ±s.d. (sample size); P < 0.05 was considered significant.For conductance analysis, records were digitally filtered at either 2683 or 1000 Hz. I-V plots were generated by measuring the amplitudes of events acquired at successive potentials from -60 to -160 mV. Typically, 5-15 events were measured at each potential in every patch. Amplitudes were measured in 11-24 patches for each construct. Only events which clearly reached full amplitude were used for I-V analysis. Some patches exhibited more than one class of channel amplitude. When low amplitude events were present, they were usually a minority class and in such cases, we confined our measurements to the class of events with the largest amplitude. The individual I-V plots corresponding to each γ- or ε-construct were pooled and the resulting average I-V plot was fitted with a quadratic polynomial. The derivative of the fitted curve was used to estimate the slope conductance at -100 mV.
RESULTS
The pore-forming M2 domains of γ- and ε-subunits of the Xenopus AChR differ at only three positions: 6, 7 and 18 (Fig. 1). The substitutions at positions 7 and 18 are conservative (valine for isoleucine or vice versa), whereas the difference at position 6 involves replacing an asparagine in γ with a serine in ε. Two further differences are found in the extracellular region flanking the M2 domain, at positions 21 and 22. We examined the effects of exchanging γ- and ε-residues at these positions on channel open time and conductance. The mutations performed for this study exclusively involved replacing ε-residues with their counterparts in γ, or vice versa.
Effects of mutations at positions 6 and 7 on channel open time
Channels containing wild-type γ-subunits have an open time which is about 3-fold longer than those containing wild-type ε-subunits. Single channel recordings, as illustrated in Fig. 2, indicated that residues at positions 6 and 7 in the M2 region of the γ- and ε-subunits were important determinants of channel open time. Open duration histograms were compiled from individual recording sites and fitted with double exponential functions (Fig. 3). For each histogram, the weighted mean open time was computed from the double exponential parameters and employed as a simple means of comparing the effects of different mutations. The effects of mutations in the M2 domain on the weighted mean open time are summarized in Fig. 4. The mean open time increased 1.7-fold following mutation of serine at position 6 in the ε-subunit to asparagine. When the residues at positions 6 and 7 in the ε-subunit were both mutated to their counterparts in the γ-subunit (ε S6N V7I), the mean channel open time increased 3.5-fold. The resultant mean open time was not significantly different from that of wild-type γ-AChRs, given the large variability in open time that is characteristic of these channels.
Figure 2. Single channel records illustrating the effects of mutations in the M2 domains of γ- and ε-subunits on channel open time.
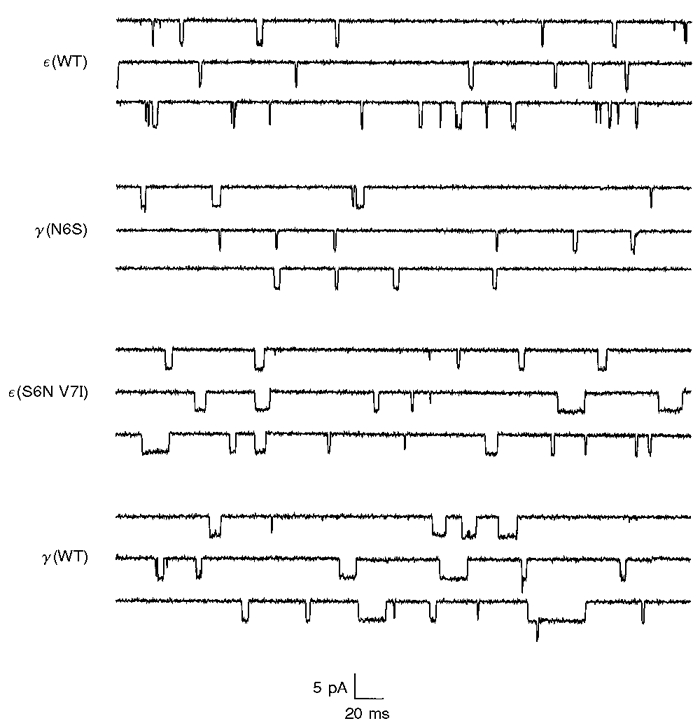
Recordings were obtained from outside-out patches at -100 mV and digitally filtered at 1 kHz. WT, wild-type.
Figure 3. Open duration histograms of single channel events, illustrating effects of M2 mutations on channel open time.
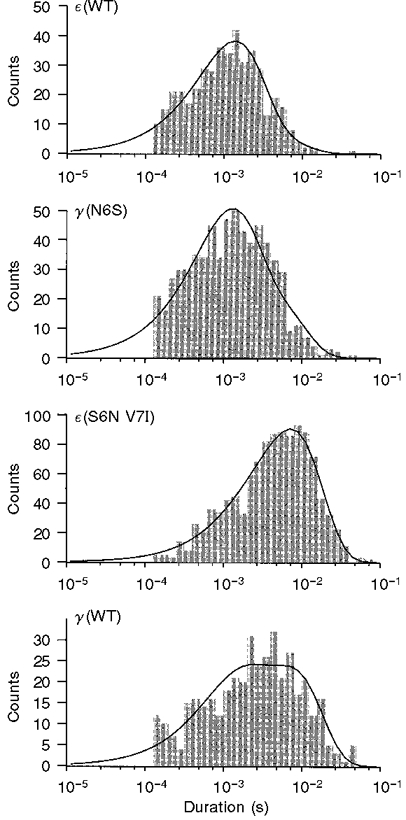
The curves are fitted with double exponential functions, which were used to compute the following weighted mean open times: ε(WT), 1.73 ms; γ(N6S), 2.09 ms; ε(S6N V7I), 7.28 ms; γ(WT), 5.36 ms.
Figure 4. Effect of M2 mutations on channel open time.
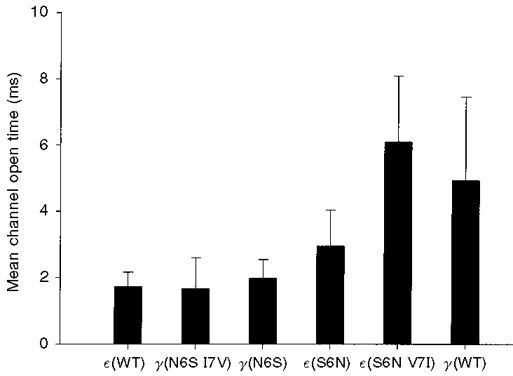
Each bar indicates the weighted mean channel open time obtained from several recording sites for each of the constructs listed on the abscissa. The double exponential parameters used to compute the weighted mean open times are summarized in Fig. 5. Data were obtained at -100 mV. The weighted mean channel open times (±s.d.) and sample sizes presented above were as follows: ε(WT), 1.72 ± 0.44 ms (23); γ(N6S I7V), 1.65 ± 0.94 ms (12); γ(N6S), 1.97 ± 0.57 ms (12); ε(S6N), 2.94 ± 1.09 ms (14); ε(S6N V7I), 6.08 ± 1.99 ms (16); γ(WT), 4.91 ± 2.52 ms (20). The difference between the open times of ε(S6N V7I) and γ(WT) was not statistically significant (Student's two-tailed t test, P = 0.092).
Conversely, mutation of amino acids at positions 6 and 7 in γ-subunits to their counterparts in ε-subunits reduced the open time (Fig. 4). When the asparagine at position 6 was replaced by serine (γ N6S), a 2.5-fold reduction in mean open time was observed, and the resultant open time was comparable to that of channels bearing the wild-type ε-subunit. Additional mutation of isoleucine at position 7 to valine (γ N6S I7V) caused no further significant reduction in open time.
Examination of the double exponential parameters indicated that the major effect of mutations at positions 6 and 7 on open time histograms was on the time constant of the slow component (Fig. 5). There was considerable relative variability in the fast component and in the areas of the two components, but these parameters exhibited no trend comparable to that of the slow component.
Figure 5. Effect of γ and ε M2 mutations on double exponential parameters.
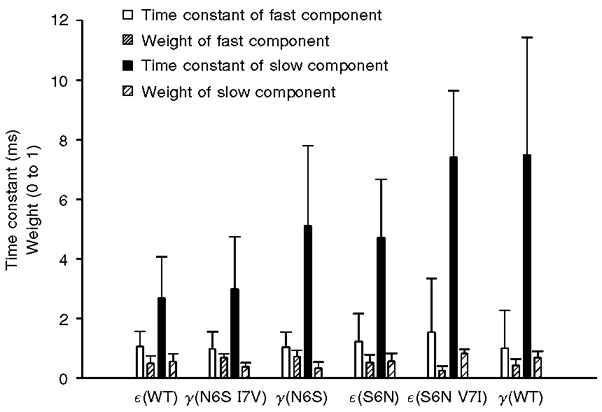
Individual open duration histograms were fitted with double exponential functions by the method of maximum likelihood. The estimated parameters from several recordings of each construct were averaged to produce the data above. Each bar shows the mean parameter ±s.d. These data were used to compute the weighted mean open times in Fig. 4.
We conclude that the developmental decrease in AChR channel open time, associated with the substitution of an ε-subunit for a γ-subunit, depends on the changes in residues at positions 6 and 7 of the M2 region. When these changes were eliminated in the ε-subunit, channels containing that subunit no longer exhibited the brief open times characteristic of mature AChR channels.
Effects of additional M2 and flanking domain mutations on channel open time
We also examined the effects of additional mutations at positions 18, 21 and 22. Channels bearing ε-subunits with mutations to γ-specific residues at positions 6, 7, 18, 21 and 22 had a mean open time of 6.0 ± 1.7 ms (9), which was nearly identical to that of channels with mutations at positions 6 and 7 alone (6.1 ± 2.0 ms (24)). We conclude that the additional mutations had no effect on channel open time.
On the other hand, γ-AChRs with ε-residues at positions 6, 7, 18, 21 and 22 had a mean open time of 0.9 ± 0.4 ms (8), which was significantly less than that of channels with mutations in γ at positions 6 and 7 alone (1.6 ± 0.9 ms (12). We did not determine which one or more of the residues at positions 18, 21 or 22 contributed to this further reduction in open time.
Effects of the HA region on channel open time
Bouzat et al. (1994) reported that replacement of γHA with εHA in mammalian γ-AChRs caused those receptors to acquire a brief open time, typical of adult ε-AChRs. Unlike Xenopus receptors, changes in the M2 region in mammalian AChRs were not found to contribute to the developmental change in channel kinetics. We examined the effects of HA mutations on channel kinetics in Xenopus to see whether this region influenced channel open time in amphibian receptors as well. When γHA was replaced by εHA, the channel open time of γ-AChRs was reduced 2.3-fold (Fig. 6), nearly to that of wild-type ε-AChRs. These results suggest that, in Xenopus, as in mammalian receptors, the HA region is also involved in the developmental change in channel kinetics.
Figure 6. Effects of HA substitution on channel open time.
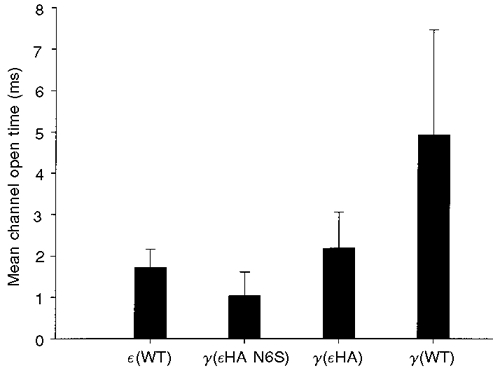
As in Fig. 4, each bar indicates the weighted mean channel open time obtained from several recording sites for each of the constructs listed on the abscissa. Data were obtained at -100 mV. The weighted mean channel open times (±s.d.) and sample sizes presented above were as follows: ε(WT), 1.72 ± 0.44 ms (23); γ(εHA N6S), 1.03 ± 0.58 ms (5); γ(εHA), 2.18 ± 0.86 ms (8); γ(WT), 4.91 ± 2.52 ms (20). The mean open time of ε(WT)-AChRs was significantly less than that of γ(εHA)-AChRs (one-tailed t test, P = 0.031) and significantly greater than that of AChRs bearing the γ(εHA N6S) subunit (P = 0.003).
Interestingly, the HA and M2 domains appeared to have additive effects on channel open time. The mutation γ (N6S) reduced the open time by more than 50 % in γ-AChRs with either native HA or εHA (Fig. 6). The mean open time of γ-AChRs bearing γ-subunits with both the N6S mutation and εHA was significantly briefer than that of wild-type ε-AChRs.
Effects of M2 mutations at positions 6 and 7 on channel conductance
In addition to gating, channel conductance was affected by mutations in the M2 domain (Figs 7 and 8). Channels containing wild-type ε-subunits had a slope conductance of 52 pS at -100 mV, in comparison to 37 pS for channels containing the wild-type γ-subunit. Mutation of S at position 6 in the ε-subunit to N caused a reduction in channel conductance to 45 pS (Fig. 7). Additional mutation of V at position 7 to I yielded an even lower conductance, 41 pS. Conversely, mutation of N at position 6 in the γ-subunit to S increased the channel conductance from 37 to 45 pS (Fig. 8); however, the additional mutation of I at position 7 to V caused little further change in conductance (46 pS).
Figure 7. Effect of ε-subunit M2 mutations on channel slope conductance.
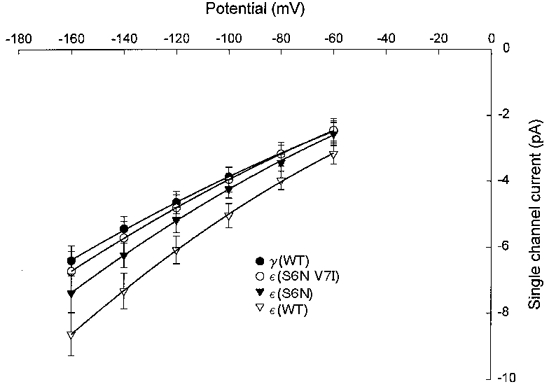
Each data point represents the mean ±s.d. channel current at a given potential from oocytes injected with wild-type α-, β- and δ-subunits plus the construct indicated. The pooled I-V data for each construct listed were fitted by 2nd order polynomial functions. The coefficients of the fitted curves were used to compute the slope conductance at -100 mV. The estimated slope conductances were: γ(WT), 37.3 pS; ε(S6N V7I), 40.8 pS; ε(S6N), 45.4 pS; ε(WT), 51.9 pS.
Figure 8. Effect of γ-subunit M2 mutations on channel slope conductance.
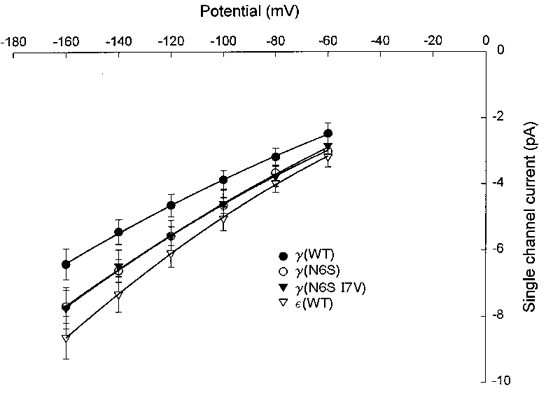
The constructs indicated were injected in combination with wild-type α-, β- and δ-subunits. See legend to Fig. 7 for curve fitting procedures. Note overlap of curves for γ(N6S) and γ(N6S I7V). The estimated slope conductances were: γ(WT), 37.3 pS; γ(N6S), 45.2 pS; γ(N6S I7V), 46.2 pS; ε(WT), 51.9 pS.
Effects of additional M2 and flanking domain mutations on channel conductance
Further mutations were made at positions 18, 21 and 22. Channels bearing ε-subunits with γ-specific residues at positions 6, 7, 18, 21 and 22 had a mean slope conductance of 38 pS, comparable to that of wild-type γ-AChRs. Surprisingly, γ-channels which had ε-residues at positions 6, 7, 18, 21 and 22 had conductances which were not as great as those achieved by mutating residues at positions 6 and 7 alone. The latter channels had a conductance of 46 pS, while addition of ε-residues at positions 18, 21 and 22 decreased the conductance to 42 pS. This result was also unexpected in view of a previous report by Murray et al. (1995) that insertion of the ε-domain between M1 and M2 into the γ-subunit caused γ-AChRs to acquire the full conductance of ε-AChRs. However, we have not been able to replicate that result and instead measured a mean slope conductance of 41 pS (17 patches) for channels bearing that construct, which is consistent with our present findings.
DISCUSSION
The data presented in this paper indicate that two residues in the pore-forming domain of γ- and ε-subunits play an important role in developmental changes of AChR function in amphibian muscle. γ-AChRs acquire the briefer open time of adult ε-AChRs by replacement of just the residue at position 6 with its counterpart from the ε-subunit. This replacement also produces about 60 % of the conductance increase between γ-AChRs and ε-AChRs. Conversely, replacement of residues at positions 6 and 7 in the ε-subunit with their counterparts from the γ-subunit causes ε-AChRs to acquire the longer open time of γ-AChRs and reverses about 75 % of the developmental increase in conductance.
The residue at position 6 (S in ε and N in γ) is predicted to play a key role in receptor function from imaging of the Torpedo AChR in its open state (Unwin, 1995). When the channel opens, the residues at positions 2, 6 and 10 of each subunit are turned towards the centre of the pore. The open channel becomes narrower at the cytoplasmic side. The narrowest constriction of the open channel may be formed by the residues at position 2 (Villarroel et al. 1991; Unwin, 1995), a ring of threonines in Xenopus, with their hydroxyl groups facing the lumen of the channel. Thus, the predicted location of the residue at position 6 is near the narrowest part of the pore and its side chain faces the lumen of the pore where it is in a favourable position to interact with cations passing through the channel.
Molecular modelling (Fig. 9) predicts that the amide oxygen of asparagine will project about 0.1 nm further into the channel pore than the hydroxyl oxygen of serine, increasing steric hindrance to ion movement through the channel. In addition, the carbonyl oxygen atom of an amide group bears about 0.5 negative charge (Abeles et al. 1992), as compared with a predicted negative charge of 0.3 on the hydroxyl oxygen of serine (Zumdahl, 1993). The greater electron density on the carbonyl oxygen would result in stronger hydrogen bonding to the hydration sphere of cations in the channel, possibly increasing the dwell time of hydrated cations in proximity to the carbonyl oxygen and thereby slowing their transit through the channel. Thus both charge and steric considerations favour lower conductance of the channel when it bears an asparagine rather than a serine.
Figure 9. Longitudinal and axial views of α-helical M2 domain showing ε(S6N V7I), ε(S6N) and ε(WT) constructs.
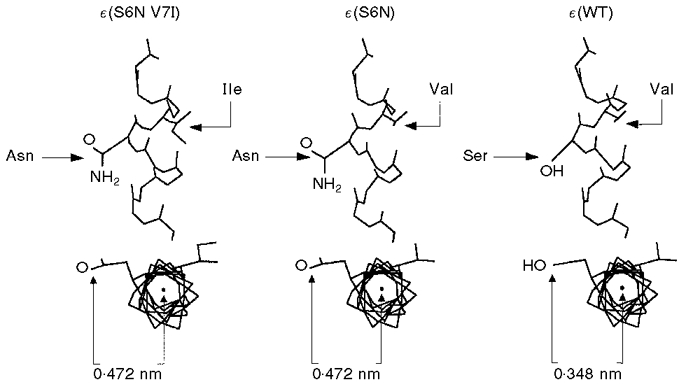
The asparagine residue projects 0.472 nm into the lumen of the channel, as compared with 0.348 nm for serine. SwissPdbViewer (Glaxo Inc.) was used to model the helix.
Unwin's (1995) model predicts that the residue at position 7 (ε V, γ I) does not face the pore. The difference between valine and isoleucine is the addition of one methyl group on the side chain in isoleucine. Molecular modelling predicts that this methyl group does not directly affect the position of the side chain on residue 6 with regard to the axis of the helix. The effect of this residue on conductance may result from its interaction with other membrane-spanning portions of the ε- or γ-subunit, perhaps resulting in a local distortion of the helix and a consequent alteration in diameter of the pore.
In mammalian receptors, residues facing the lumen of the open channel do not appear to be responsible for the difference in conductance between γ-AChRs and ε-AChRs. The M2 residues at position 6 are identical in rat γ- and ε-subunits (Herlitze et al. 1996), whereas dissimilarities are present at positions equivalent to 4 and 5, and exchanging these residues between ε- and γ-subunits alters conductance. The possibility that these residues affect channel conductance as a consequence of their interaction with other membrane-spanning components of γ- or ε-subunits is suggested by the fact that γ-AChR conductance can be fully converted to ε-AChR conductance only by swapping all four membrane-spanning domains (Herlitze et al. 1996).
Another noteworthy difference between mammalian and amphibian AChRs concerns the role of charged residues flanking the M2 domain. The conductance of rat AChRs is affected by charge differences in residues flanking M2 in γ- and ε-subunits at positions corresponding to -6 and 20 of Fig. 1. In both locations, the rat γ-subunit has positively charged lysines (K) and the ε-subunit has neutral glutamines (Q). Mutation of K to Q in γ at these locations increases channel conductance, and the converse mutation of Q to K in ε reduces conductance (Herlitze et al. 1996). In Xenopusγ- and ε-subunits, the residues at positions -6 and 20 are identical and thus do not contribute to conductance differences. There is a difference in charge at position 21 (Fig. 1); however, mutation of the residue at this position from K to M in Xenopusγ does not alter conductance (Murray et al. 1998). In the present study we did not specifically examine the contribution of flanking residues (positions 21 and 22) to channel conductance. However, we found that the conductance of ε-AChRs could be converted to that of γ-AChRs by mutation of residues at positions 6, 7, 18, 20 and 21 in the ε-subunit to their counterparts in the γ-subunit, whereas mutations at just positions 6 and 7 were insufficient to produce a complete conversion. Thus, one or more of the residues at positions 18, 20 and 21 contributes to the conductance difference between ε- and γ-AChRs.
Unexpectedly, mutation of residues at positions 6, 7, 18, 21 and 22 in the γ-subunit did not produce as much increase in conductance as mutation of residues at positions 6 and 7 alone. These five mutations produce a stretch of the γ-subunit which has the same sequence as the ε-subunit M2 and flanking regions, but not necessarily the same geometry. The mutant residues interact with other native γ-residues, possibly in other membrane-spanning domains, and such interactions will affect the channel geometry. Thus the ε-subunit M2 and flanking regions may not retain their native conformation when inserted into the γ-subunit. This inherent problem with mutagenesis experiments might underlie the fact that Herlitze et al. (1996) found that they had to replace all four membrane-spanning segments to completely switch conductance properties between γ- and ε-subunits.
The present study reveals significant differences between amphibian and mammalian AChRs with respect to the molecular determinants of developmental changes in channel open time. In Xenopus AChRs, we found that swapping residues between γ- and ε-subunits at positions 6 and 7 in M2 could convert embryonic channel open times to adult and vice versa, whereas Bouzat et al. (1994) found that M2 residues did not account for the different open times of mouse embryonic and adult AChRs. Instead, they found that the crucial region was a 30-residue stretch of amphipathic helix located in the cytoplasmic domain. In addition, two residues present in γM4 were essential to confer long channel openings on ε-AChRs. In view of their results, we were curious to see if the HA region in Xenopus also had an effect on open time. We found that moving εHA into the γ-subunit caused the open time to become briefer, in agreement with Bouzat et al. (1994). Thus, in Xenopus AChRs, it appears that differences between γ- and ε-subunits in both the M2 and HA regions could influence the development of channel open time. Moreover, the effects of these two sites on open time are additive. Addition of the N6S mutation to the γ-subunit bearing εHA caused a further 50 % reduction in open time, resulting in γ-AChRs with a briefer open time than wild-type ε-AChRs. Since both these substitutions occur naturally during development, the wild-type ε-subunit must somehow compensate for their additive effects. We conclude that other unidentified regions of the ε-subunit, in addition to M2 and HA, must contribute to the difference in open time between embryonic and adult receptors in developing Xenopus muscle.
Acknowledgments
The authors wish to express their gratitude to Kevin Ferris and Patrick Rood for their valuable assistance in the experiments. The authors are also grateful to Dr Ram Srinivasan, Chemistry Department, University of Alaska Anchorage, for his insights into the effects of polar residues on channel conductance. This work was supported by NIH grant NS24078 to R. W. K. and by the Biomedical Program at the University of Alaska Anchorage.
References
- Abeles RH, Frey PA, Jencks WP. Biochemistry. Boston: Jones and Bartlett Publishers; 1992. p. 218. [Google Scholar]
- Baldwin TJ, Yoshihara CM, Blackmer K, Kintner CR, Burden SJ. Regulation of acetylcholine receptor transcript expression during development in Xenopus laevis. Journal of Cell Biology. 1988;106:469–478. doi: 10.1083/jcb.106.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzat C, Bren N, Sine SM. Structural basis of the different gating kinetics of fetal and adult acetylcholine receptors. Neuron. 1994;13:1395–1402. doi: 10.1016/0896-6273(94)90424-3. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Villarroel A, Witzemann V, Koenen M, Sakmann B. Structural determinants of channel conductance in fetal and adult rat muscle acetylcholine receptors. The Journal of Physiology. 1996;492:775–787. doi: 10.1113/jphysiol.1996.sp021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg RW, Zheng YC, Todt W, Owens JL, Fraser SF, Mandel G. Structure and expression of the nicotinic acetylcholine receptor beta subunit of Xenopus laevis. Receptors and Channels. 1994;2:23–31. [PubMed] [Google Scholar]
- Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986;321:406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Murray N, Zheng YC, Mandel G, Brehm P, Bolinger R, Reuer Q, Kullberg R. A single site on the ε subunit is responsible for the change in ACh receptor channel conductance during skeletal muscle development. Neuron. 1995;14:865–870. doi: 10.1016/0896-6273(95)90230-9. 10.1016/0896-6273(95)90230-9. [DOI] [PubMed] [Google Scholar]
- Murray N, Zheng YC, Mandel G, Brehm P, Bolinger R, Reuer Q, Kullberg R. Retraction: A single site on the ε subunit is responsible for the change in ACh receptor channel conductance during skeletal muscle development. Neuron. 1998;20:1049. doi: 10.1016/s0896-6273(02)02053-6. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1989. pp. 15.74–15.79. [Google Scholar]
- Stroud RM, McCarthy MP, Shuster M. Nicotinic acetylcholine receptor superfamily of ligand-gated ion channels. Biochemistry. 1990;29:11009–11023. doi: 10.1021/bi00502a001. [DOI] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Villarroel A, Herlitze S, Koenen M, Sakmann B. Location of a threonine residue in the α-subunit M2 transmembrane segment that determines the ion flow through the acetylcholine receptor channel. Proceedings of the Royal Society. 1991;B 243:69–74. doi: 10.1098/rspb.1991.0012. [DOI] [PubMed] [Google Scholar]
- Zumdahl SS. Chemistry. Lexington, MA, USA: D. C. Heath and Company; 1993. p. 347.p. 360. [Google Scholar]


