Abstract
The present study investigates to what extent and by which time course prolonged strenuous exercise influences the plasma concentration of pro-inflammatory and inflammation responsive cytokines as well as cytokine inhibitors and anti-inflammatory cytokines.
Ten male subjects (median age 27.5 years, range 24–37) completed the Copenhagen Marathon 1997 (median running time 3:26 (h:min), range 2:40–4:20). Blood samples were obtained before, immediately after and then every 30 min in a 4 h post-exercise recovery period.
The plasma concentrations of tumour necrosis factor (TNF)α, interleukin (IL)-1β, IL-6, IL-1ra, sTNF-r1, sTNF-r2 and IL-10 were measured by enzyme-linked immunosorbent assay (ELISA). The highest concentration of IL-6 was found immediately after the race, whereas IL-1ra peaked 1 h post exercise (128-fold and 39-fold increase, respectively, as compared with the pre-exercise values). The plasma level of IL-1β, TNFα, sTNF-r1 and sTNF-r2 peaked in the first hour after the exercise (2.1-, 2.3-, 2.7- and 1.6-fold, respectively). The plasma level of IL-10 showed a 27-fold increase immediately post exercise.
In conclusion, strenuous exercise induces an increase in the pro-inflammatory cytokines TNFα and IL-1β and a dramatic increase in the inflammation responsive cytokine IL-6. This is balanced by the release of cytokine inhibitors (IL-1ra, sTNF-r1 and sTNF-r2) and the anti-inflammatory cytokine IL-10. The study suggests that cytokine inhibitors and anti-inflammatory cytokines restrict the magnitude and duration of the inflammatory response to exercise.
The integrated cytokine response to infection and injury is complex and tissue responses depend not only on absolute concentrations of tumour necrosis factor (TNF)α and interleukin (IL)-1β, but also on the simultaneous presence of naturally occurring cytokine inhibitors and anti-inflammatory cytokines. The local response to infection or tissue injury involves the production of cytokines which are released at the site of inflammation. These cytokines facilitate an influx of lymphocytes, neutrophils, monocytes and other cells, which participate in the clearing of antigens and healing of tissue. The local inflammatory response is accompanied by a systemic response known as the acute phase response. Injection of TNFα, IL-1β and IL-6 into laboratory animals (Dinarello, 1992) or humans (Richards & Gauldie, 1998) will produce most, if not all, aspects of the acute phase response. These cytokines are therefore usually referred to as ‘inflammatory’ or ‘pro-inflammatory’ cytokines, although it may be more reasonable to classify IL-6 as an ‘inflammation-responsive’ cytokine, since IL-6 alone does not induce inflammation. A pivotal advance in the past years has been the discovery and identification of at least two classes of biological inhibitors of the pro-inflammatory cytokines. These include the IL-1 receptor antagonist (IL-1ra), the soluble type of IL-1 receptors (sIL-1r(I) and sIL-1r(II)) and the two soluble receptors for TNF (sTNF-r1 and sTNF-r2). Furthermore the anti-inflammatory cytokine IL-10 inhibits the release of TNFα and IL-1β (Chernoff et al. 1995), and induces the production of IL-1ra (Jenkins et al. 1994; Cassatella et al. 1994).
Early studies demonstrated that exercise induced an increase in IL-1 (Cannon et al. 1986; Evans et al. 1986) although it was later pointed out that the biological assay used in the early studies did not distinguish between IL-1 and IL-6 (Bagby et al. 1996). Northof & Berg (1991) found increased levels of IL-6, but not IL-1β after a marathon. The finding of markedly elevated levels of IL-6 after exercise is consistent (Sprenger et al. 1992; Ullum et al. 1994a; Drenth et al. 1995; Nehlsen-Cannarella et al. 1997; Castell et al. 1997; Rohde et al. 1997; Hellsten et al. 1997; Bruunsgaard et al. 1997; Ostrowski et al. 1998), and so is the finding of no changes or only slightly elevated levels of IL-1β in plasma (Sprenger et al. 1992; Ullum et al. 1994a; Drenth et al. 1995; Bruunsgaard et al. 1997; Nehlsen-Cannarella et al. 1997; Ostrowski et al. 1998). Inconsistent findings have been reported for TNFα. Dufaux & Order (1989) and Espersen et al. (1990) reported increased plasma TNFα 2 h after completing a 2.5 h run (2 h 30 min) and 1 h after a 5 km race, respectively, but other studies have failed to detect TNFα after exercise (Rivier et al. 1994; Ullum et al. 1994a,b).
The present study provides measurements of the time course of TNFα, IL-1β and IL-6 in the post-exercise period. Furthermore, it was investigated to what extent exercise induces cytokine inhibitors (IL-1ra, sTNF-r1 and sTNF-r2) and the anti-inflammatory cytokine IL-10.
METHODS
Subjects
Ten male subjects (median age 28 years, range 24-37; median weight 79.5 kg, range 71-91; median VO2,max 61.2 ml min−1 kg−1, range 53.3-70.2) participated in the study. The experimental protocol was approved by the local ethics committee and all subjects were informed of the risks and purposes of the study before their written consent was obtained. The subjects participated in the Copenhagen Marathon, May 1997 (median running time 3:27 (h:min), range 2:40-4:20). Ambient temperature during running was 17°C.
Determination of maximum oxygen consumption (VO2,max)
For each subject VO2,max was determined prior to the experiment during an incremental exercise test on treadmill.
Sampling and analysis of blood
Blood samples were drawn from the antecubital vein 1 week prior to, immediately after (the delay from stopping running to the taking of the first blood sample did not exceed 10 min), and every 30 min in the 4 h rest period after the race. Blood was sampled 1 week prior to the race, in order not to influence the performance of the runners. The subjects prepared for the pre-exercise sample, in a similar way as they prepared for the marathon, which involved refraining from exercise for 2 days before the pre-sample was taken. The subjects were allowed to consume fluid and carbohydrate ad libitum before, during and after running. Two 10 ml blood samples were drawn into glass tubes containing 35 μmol dipotassium-EDTA and 1500 kallikrein inactivator units Trasylol (Bayer, Leverkusen, Germany). The tubes were kept on ice until centrifuged at 2 150 g for 15 min at 4°C. Plasma was separated from the cells and stored at -80°C until analysed by commercially available enzyme-linked immunosorbent assay (ELISA; R&D systems, Minneapolis, MN, USA).
ELISA
All measurements were performed in duplicate, and high sensitivity kits were used when available, which was the case for TNFα, IL-1β, IL-6 and IL-10. According to information provided by R&D Systems, the ELISA used for measuring IL-6 and TNFα are insensitive to the addition of the recombinant forms of the soluble receptors (sIL-6r, sTNF-r1 and sTNF-r2, respectively) and these measurements, therefore, probably correspond to both soluble and receptor-bound cytokine. Likewise, the ELISA for sTNF-r1 and sTNF-r2 are relatively insensitive to added TNFα or TNFβ, and these measurements, therefore, correspond to the total amount of the soluble receptor present in samples. The detection limit (DL) and the intra-assay coefficient of variation (c.v.) for the ELISA can be found in Table 1.
Table 1.
Detection limit (DL) and intra-assay coefficient of variation (c.v.) for the cytokine ELISA
| Assay | DL (pg ml−1) | CV (%) |
|---|---|---|
| TNFα(HS) | < 0.1 | 6.6 |
| IL-1β(HS) | < 0.1 | 7.8 |
| IL-6 (HS) | < 0.1 | 6.9 |
| IL-1ra | < 14 | 4.9 |
| sTNF-r1 | < 30 | 4.1 |
| sTNF-r2 | < 10 | 2.2 |
| IL-10(HS) | < 0.5 | 9.7 |
Correction for plasma volume shifts
Changes in plasma volume were calculated from measurements of haemoglobin and haematocrit according to the method described by Dill & Costill (1974), and cytokine measurements were corrected accordingly.
Statistical analysis
Cytokine measurements were tested for effects of ‘time’ and ‘subject’ in a two-way analysis of variance (ANOVA). The model used was:
If the effect of ‘time’ tested significant (P < 0.05) Tukey's test was used for comparison of the multiple measurements made during and after running, with the pre-exercise value. Before proceding with the statistical analysis, the residuals in the ANOVA were examined for a normal distribution through investigation of a histogram and a normal plot. If residuals were considered not to be normally distributed, data were log transformed and residuals were investigated again. This was the case for IL-1ra, IL-6, IL-1β and IL-10. After log transformation, residuals were considered to be normally distributed and thus for these measurements log-transformed data were used in the subsequent statistical analysis. The cytokine data found not to be normally distributed (IL-1β, IL-6, IL-1ra and IL-10) are plotted as geometric means with a 95 % confidence interval (c.i.). The rest (TNFα and the sTNF receptors) are plotted as means with 95 % c.i.
Statistical calculations were performed using SYSTAT 7.0 for Windows (SPSS Inc., Chicago USA).
RESULTS
The measured cytokines all changed with time (two-way ANOVA: P < 10−9 for all except for IL-1β and sTNF-r2: P < 5× 10−4).
The mean plasma concentration of TNFα peaked immediately after the race (2.3-fold increase compared with pre-race value) and declined slowly during the resting period. It remained significantly different from the pre-race value until 2.5 h post running (Fig. 1A). The level of IL-1β was slightly elevated (2.1-fold compared with the pre-race value) immediately after the race (Fig. 1B). Three of the subjects had approximately 10-fold higher IL-1β plasma levels than the remaining subjects, which is the reason for the wide confidence interval seen on the figure. The mean plasma concentration of IL-6 peaked immediately after exercise where it had increased 128-fold compared with the pre-race value. The level of IL-6 declined during the 4 h rest period, but remained significantly above the pre-race value (Fig. 1C). The plasma concentration of IL-1ra peaked 1 h after the race (39-fold increase compared with pre-race value; Fig. 1D). The levels of sTNF-r1 and sTNF-r2 increased 2.7- and 1.6-fold, respectively, above the pre-exercise levels within the first hour after exercise (Fig. 1E). The plasma concentration of IL-10 peaked immediately after the race, where it had increased 27-fold compared with the pre-race value. The level of IL-10 declined steadily in the resting period, but remained significantly above the pre-race value throughout the resting period (Fig. 1F).
Figure 1. Plasma cytokine concentrations measured before (Pre) and every half hour in the 4 h resting period after a marathon race.
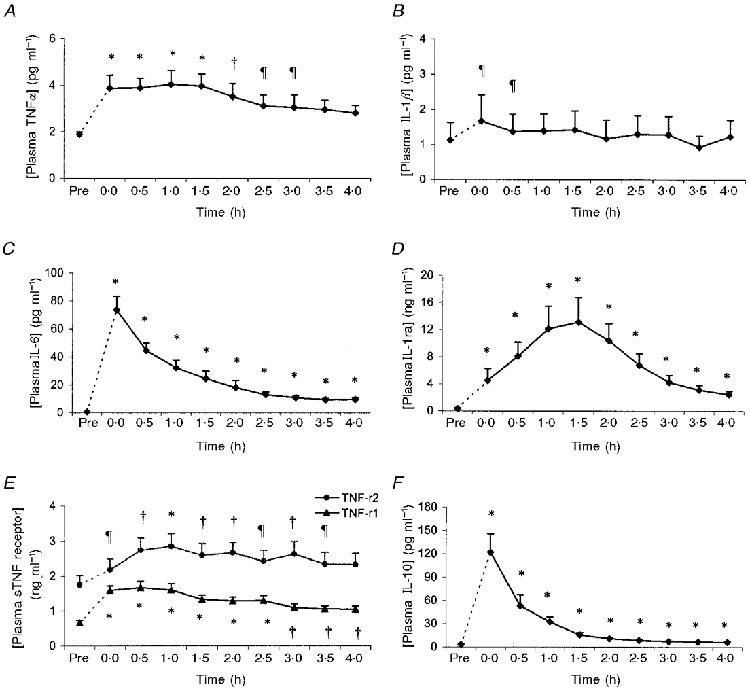
Data are plotted as geometric mean with 95 % confidence interval (c.i.) (IL-1β, IL-6, IL-1ra and IL-10) or as mean with 95 % c.i. (TNFα and sTNF receptors). Legend denotes difference from the pre-exercise value in the Tukey multiple comparison test (* P < 5 × 10−5; †P < 5× 10−3 and ¶P < 0.05, respectively).
DISCUSSION
The present study provides information about changes in the plasma concentration of the cytokines TNFα, IL-1β, IL-6, IL-1ra, sTNF-r1, sTNF-r2 and IL-10 in the 4 h recovery period after the marathon race. The small but significant increase in TNFα found after exercise is in accordance with other studies (Dufaux & Order, 1989; Espersen et al. 1990; Ostrowski et al. 1998), and so is the finding of high levels of IL-6 (Sprenger et al. 1992; Ullum et al. 1994a; Drenth et al. 1995; Bruunsgaard et al. 1997; Nehlsen-Cannarella et al. 1997; Castell et al. 1997; Rohde et al. 1997; Hellsten et al. 1997; Ostrowski et al. 1998). Furthermore, we found a significant increase in plasma IL-1β immediately after running. Previous studies demonstrated increased levels of IL-1 in plasma post exercise (Cannon & Kluger, 1983; Evans et al. 1986), but recent studies using more sensitive and specific assays have found only minor, if any, changes in the plasma concentration of IL-1 after exercise (Sprenger et al. 1992; Ullum et al. 1994a; Drenth et al. 1995; Bruunsgaard et al. 1997; Nehlsen-Cannarella et al. 1997; Castell et al. 1997; Ostrowski et al. 1998). This may be due to less intense exercise regimens studied, or alternatively, to the fact that IL-1β is produced locally and is rapidly cleared from the circulation. The recent finding of IL-1β mRNA in muscle biopsies obtained after strenuous exercise without increase in the IL-1β protein in plasma (Ostrowski et al. 1998) and the finding of IL-1β in the urine of runners (Sprenger et al. 1992) support this latter idea.
The present study demonstrates that exercise induces a cascade of cytokine inhibitors (IL-1ra, sTNF-r1 and sTNF-r2) and the anti-inflammatory cytokine IL-10. Since this was a field study, blood sampling during running was not possible, and an analysis of changes in plasma cytokines during exercise was therefore not done. It should be noted however, that IL-1ra peaked 1 h after IL-6, which is compatible with the knowledge that IL-6 induces an increase in the IL-1ra plasma concentration (Tilg et al. 1994; Jordan et al. 1995).
Carbohydrate intake was not controlled in the present study, but subjects can be assumed to be well loaded. It has been shown that carbohydrate loading diminishes the exercise-induced increase in IL-6 and IL-1ra (Nehlsen-Cannarella et al. 1997). Thus, with carbohydrate restriction, an even more pronounced increase in plasma cytokine levels might have been found.
Elevated levels of plasma IL-6 have been shown to induce C-reactive protein, but despite the high levels of IL-6 detected after strenuous exercise, only a modest increase in plasma C-reactive protein was detected after a marathon run (Castell et al. 1997). Furthermore, other biological effects of the TNF/IL-1/IL-6 system such as myocardium depression, vasodilatation, leukocyte aggregation and dysfunction of kidneys, livers, lungs and brain do not develop in response to exercise. In other words, although the initiation of the acute-phase response develops, exercise is not followed by a fully developed systemic response. The present study suggests that anti-inflammatory cytokines, soluble receptors and receptor antagonists restrict the magnitude and duration of the inflammatory response to exercise.
In conclusion, strenuous exercise induces an increase in the pro-inflammatory cytokines TNFα and IL-1β and a dramatic increase in the inflammation responsive cytokine IL-6. This release is balanced by the release of cytokine inhibitors (IL-1ra, sTNF-r1 and TNF-r2) and the anti-inflammatory cytokine IL-10.
Acknowledgments
The excellent technical assistance of Ruth Rousing and Hanne Villumsen is acknowledged. This study was supported by Team Danmark, Idrættens Forskningsråd and The Danish National Research Foundation.
References
- Bagby GJ, Crouch LD, Shepherd RE. Exercise and cytokines: Spontaneous and elicited response. In: Hoffman GL, editor. Exercise and Immune function. New York: CRC Press; 1996. pp. 55–78. [Google Scholar]
- Bruunsgaard H, Galbo H, Halkjaer KJ, Johansen TL, Maclean DA, Pedersen BK. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. The Journal of Physiology. 1997;499:833–841. doi: 10.1113/jphysiol.1997.sp021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JG, Evans WJ, Hughes VA, Meredith CN, Dinarello CA. Physiological mechanisms contributing to increased interleukin-1 secretion. Journal of Applied Physiology. 1986;61:1869–1874. doi: 10.1152/jappl.1986.61.5.1869. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Kluger MJ. Endogenous pyrogen activity in human plasma after exercise. Science. 1983;220:617–619. doi: 10.1126/science.6836306. [DOI] [PubMed] [Google Scholar]
- Cassatella MA, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. Journal of Experimental Medicine. 1994;179:1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell LM, Poortmans JR, Leclercq R, Brasseur M, Duchateau J, Newsholme EA. Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementation. European Journal of Applied Physiology and Occupational Physiology. 1997;75:47–53. doi: 10.1007/s004210050125. [DOI] [PubMed] [Google Scholar]
- Chernoff AE, Granowitz EV, Shapiro L, Vannier E, Lonnemann G, Angel JB, Kennedy JS, Rabson AR, Wolff SM, Dinarello CA. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. Journal of Immunology. 1995;154:5492–5499. [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. Journal of Applied Physiology. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Role of interleukin-1 in infectious diseases. Immunological Reviews. 1992;127:119–146. doi: 10.1111/j.1600-065x.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- Drenth JP, Van Uum SH, Van Deuren M, Pesman GJ, Van Der Ven Jongekrijg J, Van Der Meer JW. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-alpha and IL-1 beta production. Journal of Applied Physiology. 1995;79:1497–1503. doi: 10.1152/jappl.1995.79.5.1497. [DOI] [PubMed] [Google Scholar]
- Dufaux B, Order U. Plasma elastase-alpha 1-antitrypsin, neopterin, tumor necrosis factor, and soluble interleukin-2 receptor after prolonged exercise. International Journal of Sports Medicine. 1989;10:434–438. doi: 10.1055/s-2007-1024939. [DOI] [PubMed] [Google Scholar]
- Espersen GT, Elbaek A, Ernst E, Toft E, Kaalund S, Jersild C, Grunnet N. Effect of physical exercise on cytokines and lymphocyte subpopulations in human peripheral blood. APMIS. 1990;98:395–400. doi: 10.1111/j.1699-0463.1990.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Meredith CN, Cannon JG, Dinarello CA, Frontera WR, Hughes VA, Jones BH, Knuttgen HG. Metabolic changes following eccentric exercise in trained and untrained men. Journal of Applied Physiology. 1986;61:1864–1868. doi: 10.1152/jappl.1986.61.5.1864. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U, Orthenblad N, Sjodin B, Richter EA. Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. The Journal of Physiology. 1997;498:239–248. doi: 10.1113/jphysiol.1997.sp021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JK, Malyak M, Arend WP. The effects of interleukin-10 on interleukin-1 receptor antagonist and interleukin-1 beta production in human monocytes and neutrophils. Lymphokine and Cytokine Research. 1994;13:47–54. [PubMed] [Google Scholar]
- Jordan M, Otterness IG, Ng R, Gessner A, Rollinghoff M, Beuscher HU. Neutralization of endogenous IL-6 suppresses induction of IL-1 receptor antagonist. Journal of Immunology. 1995;154:4081–4090. [PubMed] [Google Scholar]
- Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, Bailey EM, Warren BJ, Utter A, Davis JM. Carbohydrate and the cytokine response to 2.5 h of running. Journal of Applied Physiology. 1997;82:1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- Northoff H, Berg A. Immunologic mediators as parameters of the reaction to strenuous exercise. International Journal of Sports Medicine. 1991;12(suppl. 1):S9–15. doi: 10.1055/s-2007-1024743. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. The Journal of Physiology. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CD, Gauldie J. Role of cytokines in acute-phase response. In: Aggarwal BB, Puri P, editors. Human cytokines: their Role in Disease and Therapy. Cambridge: Blackwell Science; 1998. pp. 253–269. [Google Scholar]
- Rivier A, Pene J, Chanez P, Anselme F, Caillaud C, Prefaut C, Godard P, Bousquet J. Release of cytokines by blood monocytes during strenuous exercise. International Journal of Sports Medicine. 1994;15:192–198. doi: 10.1055/s-2007-1021046. [DOI] [PubMed] [Google Scholar]
- Rohde T, Maclean DA, Richter EA, Kiens B, Pedersen BK. Prolonged submaximal eccentric exercise is associated with increased levels of plasma IL-6. American Journal of Physiology. 1997;273:E85–91. doi: 10.1152/ajpendo.1997.273.1.E85. [DOI] [PubMed] [Google Scholar]
- Sprenger H, Jacobs C, Nain M, Gressner AM, Prinz H, Wesemann W, Gemsa D. Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clinical Immunology and Immunopathology. 1992;63:188–195. doi: 10.1016/0090-1229(92)90012-d. 10.1016/0090-1229(92)90012-D. [DOI] [PubMed] [Google Scholar]
- Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- Ullum H, Haahr PM, Diamant M, Palmo J, Halkjaer KJ, Pedersen BK. Bicycle exercise enhances plasma IL-6 but does not change IL-1 alpha, IL-1 beta, IL-6, or TNF-alpha pre-mRNA in BMNC. Journal of Applied Physiology. 1994a;77:93–97. doi: 10.1152/jappl.1994.77.1.93. [DOI] [PubMed] [Google Scholar]
- Ullum H, Palmo J, Halkjaer KJ, Diamant M, Klokker M, Kruuse A, Laperriere A, Pedersen BK. The effect of acute exercise on lymphocyte subsets, natural killer cells, proliferative responses, and cytokines in HIV-seropositive persons. Journal of Acquired Immune Deficiency Syndromes. 1994b;7:1122–1133. [PubMed] [Google Scholar]


