Abstract
The effect of A1-adenosine receptor antagonism via 8-cyclopentyl-1,3-dipropyl-xanthine (CPDPX) on the stimulation of skeletal muscle glucose uptake by contractions and hypoxia was investigated in isolated perfused rat hindquarters. The standard perfusate contained either no insulin or a submaximal insulin concentration at 100 μU ml−1.
Muscles were stimulated to contract for 45 min by intermittent tetanic stimulation of the sciatic nerve. Hypoxia was induced by reducing perfusate haematocrit from 30% to 10% on the one hand, and by switching the gassing of the perfusate from a 35% to a 0% O2 mixture for 60 min on the other hand. The effect of contractions and hypoxia alone, or in combination, was investigated.
Hypoxia-induced muscle glucose uptake was not altered by CPDPX in the absence or presence of insulin. In contrast, contraction-induced glucose uptake was reduced by ≈25% (P < 0.05) by exposure of muscles to CPDPX. CPDPX did not affect hindlimb glucose uptake either before or after contractions.
The increment of muscle glucose uptake during hypoxia combined with contractions was greater (P < 0.05) than the effect of hypoxia alone.
The current findings provide evidence that the mechanism by which hypoxia stimulates muscle glucose uptake is, at least in part, different from the mechanism of glucose uptake stimulation by contractions, because (i) A1-adenosine receptors regulate insulin-mediated glucose uptake in muscle during contractions but not during hypoxia and (ii) submaximal hypoxia and contractions are additive stimuli to muscle glucose uptake.
In a state of hypoxia, skeletal muscle primarily relies on anaerobic glycolysis to generate ATP, because the reduced oxygen availability uncouples fat and carbohydrate oxidation. As a consequence, glycogenolysis (Ren et al. 1992) and the uptake of blood-borne glucose (Randle & Smith, 1958; Cartee et al. 1991; Bashan et al. 1992; Youn et al. 1994; Azevedo et al. 1995; Wojtaszewski et al. 1998) in hypoxic muscle is markedly enhanced in order to maintain adequate energy production. However, the precise cellular mechanisms underlying stimulation of muscle glucose uptake by hypoxia remain largely unknown. In 1991, Cartee etal. showed for the first time that hypoxia, similar to contractions (Douen et al. 1989; Fushiki et al. 1989; King et al. 1989), stimulates muscle membrane glucose transport by increasing sarcolemmic glucose transporter 4 (GLUT4) content, presumably by translocation of GLUT4 from an intracellular pool to the cell surface (Cartee et al. 1991). In addition, they demonstrated that the stimulatory effect of hypoxia on glucose transport is additive to the effect of insulin, but not to the effect of contractions. Hence they concluded that hypoxia and contractions stimulate glucose transport by the same cellular mechanisms (Cartee et al. 1991). Although clear evidence for the validity of such a hypothesis is still lacking, hypoxia has often been used as a model of muscle glucose transport stimulation by contractions (Youn et al. 1994; Azevedo et al. 1995; Brozinick et al. 1997; Hansen et al. 1997). However, Wojtaszewski et al. (1998) have recently provided evidence that the signalling pathway for GLUT4 translocation due to hypoxia is different from stimulation by contractions.
It is well documented that adenosine is produced in skeletal muscle in response to decreased oxygen supply-to-demand ratio, e.g. during contractions (Tominaga et al. 1980; Ballard et al. 1987; Achike & Ballard, 1993) or hypoxia (Rubio et al. 1973; Mian & Marshall, 1991; Marshall et al. 1993; Skinner & Marshall, 1996; Mo & Ballard, 1997). Part of the adenosine produced accumulates in the interstitium where it may act as a ‘local hormone’ by binding to various types of adenosine receptors on the surface of adjacent cells. Thus, interstitial adenosine probably contributes to local vasodilatation in muscles during both contractions (Schwartz & McKenzie, 1990) and hypoxia (Neylon & Marshall, 1991). Interestingly, our recent findings in perfused rat muscles have also indicated that, during contractions, stimulation of A1-adenosine receptors in oxidative muscle enhances insulin-mediated glucose uptake on the one hand (Vergauwen et al. 1994), but may reduce glycogen breakdown on the other (Vergauwen et al. 1997). Accordingly, a recent stable isotope study in humans has shown peripheral glucose uptake during exercise to be reduced and lactate production to be enhanced by the administration of theophylline, a potent but non-specific adenosine receptor antagonist (Raguso et al. 1996).
The possible role of adenosine in the regulation of glucose metabolism in muscle during hypoxia has not yet been investigated. Furthermore, if hypoxia and contractions do indeed stimulate muscle glucose transport by the same mechanism (Cartee et al. 1991), then it is reasonable to hypothesize that adenosine plays a role in the regulation of insulin-stimulated glucose uptake during hypoxia similar to the role we have previously described during contractions (Vergauwen et al. 1994).
Therefore, the present study was undertaken to compare the effects of selective A1-adenosine receptor antagonism, using 8-cyclopentyl-1,3-dipropyl-xanthine (CPDPX; Bruns et al. 1987) on glucose uptake in contracting and hypoxic skeletal muscle. In addition, the presumed additive effect of contractions and hypoxia on muscle glucose uptake was reinvestigated by using a more physiological degree of hypoxia than has been used in previous analogous studies (Cartee et al. 1991; Wojtaszewski et al. 1998).
METHODS
Animals
Male Wistar rats, weighing 200-250 g, were used in this study. They were purchased from the laboratory animal-breeding centre at the University of Leuven. Rats were maintained on a constant light-dark cycle (12 h:12 h) and received normal rat chow ad libitum until the morning the experiments were carried out. Experimental procedures were approved by the Ethics Committee of the Belgian National Research Foundation (Nationaal Fonds voor Wetenschappelijk Onderzoek) and carried out according to the guidelines of the local animal welfare committee.
Experimental procedures
The rats were anaesthetized by an intraperitoneal injection of pentobarbital sodium (5 mg (100 g body wt)−1) and prepared surgically for hindquarter perfusion, as previously described (Ruderman et al. 1971). Before insertion of the perfusion catheters, the rat was heparinized with 125 i.u. of heparin in the inferior vena cava. The rat was killed with an intracardiac injection of pentobarbital sodium immediately before being placed in the perfusion cabinet.
The initial perfusate (200 ml) consisted of Krebs-Henseleit solution, 1- to 3-day-old washed bovine erythrocytes, 5 % bovine serum albumin (Cohn fraction V, Sigma), 6 mM glucose, 0.15 mM pyruvate and 0.3-0.5 mM lactate originating from the erythrocytes. During normoxia experiments, erythrocytes were added to the perfusion mix to a haematocrit of 30 %. The arterial perfusate was gassed with a mixture of 35 % O2, 62 % N2 and 3 % CO2, which yielded arterial pH values of 7.25-7.50, PO2 values of 100-240 mmHg and PCO2values of 30-45 mmHg during perfusions (see Results for more details). In hypoxia experiments, perfusate haematocrit was reduced to 10 %. This low haematocrit was chosen because at higher values (20-30 %) normal hindlimb oxygen uptake was maintained for > 30 min even during hypoxic gassing. Thus in addition to low haematocrit (10 %), hypoxia was induced by gassing the perfusate with an O2-free gas mixture composed of 95 % N2 and 5 % CO2, yielding arterial pH, PO2 and PCO2 values of 7.20-7.35, 15- 25 mmHg and 35-45 mmHg, respectively (see Results for more details). During the experiments arterial perfusate glucose concentration (6 mM) was prevented from decreasing by continuous infusion of a 40 % glucose solution into the perfusate reservoir by means of a microinfusion pump (Harvard model 11, South Natick, MA, USA) the speed of which was adjusted to the estimated rate of glucose uptake by the hindquarter.
The first 25 ml of perfusate that passed through the hindquarter were discarded, whereupon the perfusate was recirculated. Thereafter, different perfusion protocols were used for experiments intended to study muscle glucose uptake during contractions in the absence of hypoxia (protocol A), during hypoxia in the absence of contractions (protocol B) and during contractions in combination with hypoxia (protocol C).
In protocol A, hindquarters were first perfused at a flow of 12.5 ml min−1 during an initial 20 min equilibration period. Subsequently, the common iliac vessels supplying the left leg were tied off, and a clamp was fixed tightly around the proximal part of the leg. Perfusion flow to the right hindlimb was then reduced to 9 ml min−1, which resulted in similar perfusion pressures as for bilateral perfusion at a flow of 12.5 ml min−1. The right lower leg was immobilized onto the perfusion platform by two pins. The first pin was slid under the patella tendon. The second pin was a hook pin that was positioned around the Achilles' tendon and connected to an isometric muscle tension transducer. The immobilized hindlimb was then allowed to rest for an additional 15 min, after which perfusate samples were taken from the venous and arterial tubing for baseline determinations. Immediately after this, a hook-electrode was placed around the sciatic nerve and connected to a locally constructed impulse generator. The resting length of the gastrocnemius-soleus-plantaris muscle group was adjusted to obtain maximum active tension upon electrical stimulation, whereupon a period of muscle contractions was begun. Isometric muscle contractions were induced by stimulating intermittently the sciatic nerve with supramaximal (20-30 V) trains composed of 1 ms square wave pulses firing at 100 Hz. Fifty millisecond trains were delivered at a rate of 20 min−1 for 45 min. At the start of the contractions the flow of perfusate was increased from 9 to 15 ml min−1. During stimulation, tension developed by the gastrocnemius-soleus-plantaris muscle group was measured by a self-constructed isometric muscle tension transducer and recorded by a pen writer (Kipp & Zonen, Delft, The Netherlands). At the end of the contraction period, perfusate flow was returned from 15 to 9 ml min−1 and perfusion was continued in the resting hindquarter for an additional 30 min. Arterial and venous perfusate samples were taken after 15 and 45 min of electrical stimulation and at 30 min post-contractions. Perfusate flow rate was measured immediately preceding any sampling of perfusate, using timed collections of the venous effluent from the hindlimb. Perfusion pressures were read from a manometer, which was connected to the arterial tubing of the perfusion apparatus.
In protocol B, a fixed flow rate of 15 ml min−1 to both hindlimbs was maintained throughout the experiment. This high flow was necessary to obtain adequate perfusion pressures (> 35 mmHg) at the lower perfusate haematocrit (10 %) used in these hypoxia experiments in resting hindlimbs. After the 20 min equilibration period, hypoxia was induced by switching the gassing of the perfusate from the normal O2-CO2-N2 mixture to the anoxic N2-CO2 mixture for 60 min. Immediately thereafter oxygenation was restored during a 30 min normoxia period. Arterial and venous perfusates were sampled at the end of the 20 min equilibration period, after 30 and 60 min of hypoxia and after 30 min of reoxygenation.
Protocol C perfusions in the initial stage were identical to protocol B experiments. However, after the 20 min equilibration period followed by 45 min of hypoxia, circulation to the left leg was tied off (see protocol A) and flow rate was reduced to 9 ml min−1 for 15 min. Finally, normoxia was restored during 5 min whereupon a 5 min period of electrical stimulation (see protocol A) was started. In order to exclude stimulation of muscle glucose uptake by elevated flow rate (Hespel et al. 1995), the perfusate flow was maintained at 9 ml min−1 during the contractions. Arterial and venous perfusate samples were taken after 60 min of hypoxia, 5 min after restoration of normoxia, and after 2 and 5 min of electrical stimulation. The perfusate was gassed with the N2-CO2 mixture during the 60 min hypoxia period and with the normal O2-CO2-N2 gas mixture for the last 10 min of perfusion, including during the contractions. Separate experiments were performed to evaluate whether hypoxia beyond 60 min of exposure caused a further rise of muscle glucose uptake. In these experiments, hypoxia was applied for 90 min according to protocol B procedures and perfusate samples for glucose determination were taken before and after 30, 60 and 90 min of hypoxia.
Experimental conditions
All experiments were performed according to the standard procedures described above. Different experimental conditions were induced, in separate groups of animals, by addition (or not) of insulin (human monocomponent insulin, Novo Nordisk, Bagsværd, Denmark) and/or CPDPX (Research Biochemicals Inc., Natick, MA, USA) to the perfusate reservoir at the start of each experiment. All perfusion experiments, designed for mutual comparisons, were carried out in a randomized order.
To study the effect of selective antagonism of A1-adenosine receptors (A1 receptors) on muscle glucose uptake during protocols A and B, CPDPX was added to the perfusate reservoir at a concentration of 75 μmol l−1 of perfusate plasma, as previously described (Vergauwen et al. 1994). Because CPDPX was dissolved in ethanol, a similar volume of ethanol (250 μl) was added to control perfusate media. In protocol B, the effects of adenosine receptor antagonism were studied in the presence of either 0 or 100 μU ml−1 of insulin in perfusate plasma. The arterial insulin level was set at 100 μU ml−1 in protocol A, and no insulin was added to the perfusate in protocol C experiments.
Analyses, calculations and statistics
Perfusate samples were analysed for glucose in at least duplicate within 1 h, using a YSI type 2300 STAT automatic glucose analyser (Yellow Springs, OH, USA). Haemoglobin concentration, O2 saturation of haemoglobin, PO2, PCO2 and pH were determined immediately after sampling using an ABL 510 acid-base laboratory (Radiometer, Copenhagen, Denmark). Rates of glucose uptake and oxygen uptake by the hindquarter were calculated by multiplying the respective arterio-venous concentration differences by the flow rate, and were expressed per gram of perfused muscle per hour. Perfused muscle mass was considered to be 8.3 and 16.6 % of body weight in one-leg and two-leg perfusions, respectively (Richter et al. 1982). During electrical stimulation and recovery from stimulation, uptake is expressed relative to stimulated muscle mass, while unstimulated muscle was assumed to have the same uptake as prior to electrical stimulation. The stimulated muscle mass was considered to be 56 % of the total perfused muscle mass during one-leg perfusions (Spriet et al. 1985). Muscle tension at the beginning and at the end of the contraction period was read from the tension curves recorded during stimulation.
In order to estimate the additive effects of hypoxia plus insulin, and contractions plus insulin (see Fig. 3), we used the following calculations. Basal glucose uptake was calculated as glucose uptake in resting hindlimbs perfused at a flow of 9 ml min−1 in the absence of insulin (as in protocol B). Insulin-stimulated glucose uptake was calculated as glucose uptake, measured in resting hindlimbs perfused with 100 μU ml−1 insulin, minus basal glucose uptake (as in protocol B). Contraction- and flow-stimulated glucose uptake was calculated as glucose uptake, measured after 15 min of electrical stimulation in one-leg perfusions at a flow of 15 ml min−1, minus basal glucose uptake (data from Hespel et al. 1995). Hypoxia-stimulated glucose uptake was calculated as glucose uptake after 60 min of hypoxia minus basal glucose uptake (as in protocol B).
Figure 3. Effects of hypoxia plus insulin and contractions plus insulin on glucose uptake in perfused rat muscles.
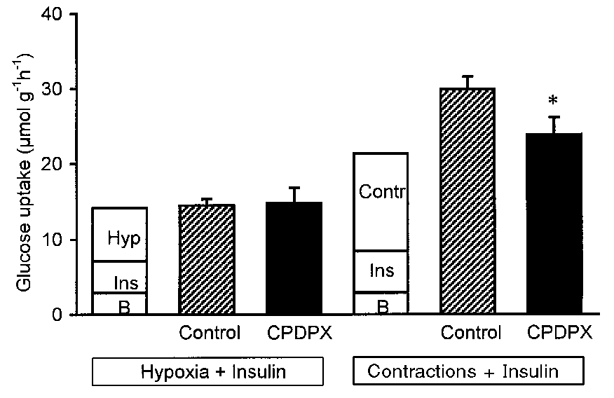
Estimated (see Methods section for details) versus measured rates of muscle glucose uptake during stimulation by hypoxia (left) and contractions (right), in hindlimbs exposed to insulin. Open bars represent glucose uptake rate calculated as the sum of the stimulatory actions of insulin (Ins, 100 μU ml−1) and either hypoxia (Hyp) or contractions (Contr), added to basal glucose uptake rate (B).  and ▪, glucose uptake rate measured after 60 min of hypoxia (data from Fig. 1) and after 45 min of contractions (data from Fig. 2) in hindquarters perfused with perfusate containing 100 μU ml−1 insulin and 0 (
and ▪, glucose uptake rate measured after 60 min of hypoxia (data from Fig. 1) and after 45 min of contractions (data from Fig. 2) in hindquarters perfused with perfusate containing 100 μU ml−1 insulin and 0 ( ) or 75 μmol l−1 CPDPX (▪). Values are means ±s.e.m. of 8-14 observations. *P < 0.05 compared with control. See Methods for further details.
) or 75 μmol l−1 CPDPX (▪). Values are means ±s.e.m. of 8-14 observations. *P < 0.05 compared with control. See Methods for further details.
In protocols A and B, data were analysed by either Student's unpaired t tests or two-way repeated-measures ANOVA, where appropriate. For analysis of data obtained using protocol C, one-way ANOVA was used. Data are presented as means ±s.e.m. A probability level (P) of 0.05 was chosen as the criterion for acceptance of statistical significance.
RESULTS
Oxygen uptake during hypoxia and contractions
Hindquarter oxygen uptake was not affected by CPDPX in either experimental condition. In protocol A experiments, using 30 % haematocrit perfusates, oxygen uptake in resting hindquarters (n = 27) was 24.9 ± 1.1 μmol g−1 h−1, increasing about fourfold to 93.4 ± 5.4 μmol g−1 h−1 during electrical stimulation (Fig. 2, upper panel). In protocol B perfusions, 10 % haematocrit perfusates were used, which, during normoxia, resulted in a lower (P < 0.05) resting hindlimb oxygen uptake (17.4 ± 0.5 μmol g−1 h−1) than was obtained with 30 % haematocrit perfusates (protocol A). After 60 min of hypoxia, induced by anoxic gassing of the perfusate, hindlimb oxygen uptake (n = 34) was reduced to as low as 3.2 ± 0.5 μmol g−1 h−1 (Fig. 1, upper panel). However, restoration of normal oxygen delivery to the hindlimb returned oxygen uptake to baseline level (18.4 ± 0.7 μmol g−1 h−1) within 30 min.
Figure 2. Effect of A1-receptor antagonism on glucose uptake in perfused rat muscles during and after contractions.
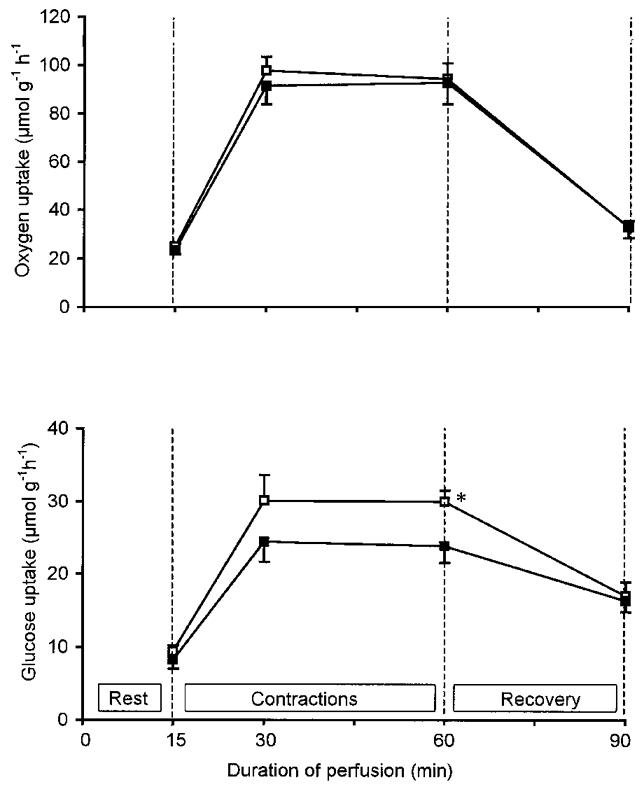
Effect of CPDPX on muscle oxygen (upper panel) and glucose (lower panel) uptake in perfused rat hindquarters before, during and after 45 min of electrical stimulation is shown. Values are means ±s.e.m. of 13-14 observations. Hindquarters were perfused with vehicle (□) or 75 μmol l−1 CPDPX (▪) in the presence of 100 μU ml−1 insulin. *P < 0.05 compared with control.
Figure 1. Effect of A1-receptor antagonism on glucose uptake in perfused rat muscles during and after hypoxia.
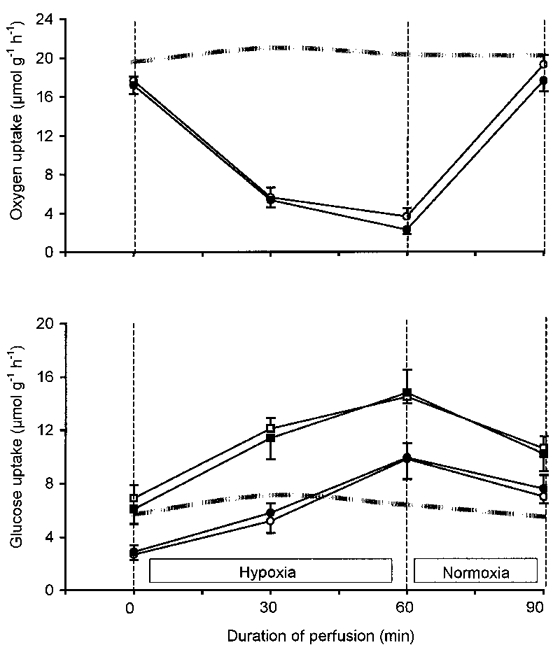
The effect of CPDPX on muscle oxygen (upper panel) and glucose (lower panel) uptake in perfused rat hindquarters at rest, after 30 and 60 min of hypoxia, and after 30 min of re-oxygenation is shown. Hindquarters were perfused with vehicle (open symbols) or 75 μmol l−1 CPDPX (filled symbols) at 0 (circles) and at 100 μU ml−1 (squares) insulin. Values are means ±s.e.m. of 8-9 observations (lower panel) and 17 observations (upper panel, data with and without insulin were pooled). The dashed lines indicate the time course of oxygen (upper panel) and glucose (lower panel) uptake in control experiments with continuous normoxia for 90 min at a perfusate insulin concentration of 100 μU ml−1 (n = 5).
Effects of contractions (protocol A)
The effect of CPDPX on muscle glucose uptake during contractions was studied only in hindquarters exposed to insulin (Vergauwen et al. 1994). As shown in Fig. 2, glucose uptake at rest was similar in CPDPX-treated and control hindquarters. At the onset of electrical stimulation, glucose uptake markedly increased in all hindlimbs. However, the contraction-induced increment in glucose uptake was attenuated (P < 0.05) by the addition of CPDPX to the perfusion mix. Compared with controls, hindquarter glucose uptake over the 45 min stimulation period was 20-25 % lower in CPDPX-treated hindquarters (P < 0.05). After cessation of the electrical stimulation, hindlimb glucose uptake decreased at a faster rate in controls than in hindlimbs perfused with CPDPX (P < 0.05). Thus hindlimb glucose uptake rate 30 min post contractions was still markedly higher (P < 0.05) than baseline level, but was similar in CPDPX-treated and control hindquarters. The rate of lactate release by the hindlimb was not altered by CPDPX. For the total group of rats (n = 27), lactate release was 4.8 ± 0.6 μmol g−1 h−1 at rest and increased to 9.8 ± 1.8 μmol g−1 h−1 after 45 min of electrical stimulation (P < 0.05).
Effects of hypoxia (protocol B)
Baseline glucose uptake during normoxia was 2.7 ± 0.4 μmol g−1 h−1 in the absence and 6.9 ± 1 μmol g−1 h−1 in the presence of 100 μU ml−1 perfusate insulin, and was not altered by CPDPX (2.9 ± 0.5 and 6.1 ± 1.1 μmol g−1 h−1, respectively). As shown in Fig. 1, the fall in hindlimb O2 uptake induced by hypoxia was associated with a marked stimulation of muscle glucose uptake, irrespective of whether insulin was added to the perfusate. Accordingly, during 30 min of re-oxygenation glucose uptake decreased in both groups yet remained above baseline level (P < 0.05). CPDPX had no effect on hindlimb glucose uptake before, during or after hypoxia in either the presence or absence of insulin. Thus, compared with baseline, during 60 min of hypoxia, muscle glucose uptake increased by 7.6 ± 1.0 and by 8.6 ± 1.9 μmol g−1 h−1 in control and CPDPX-treated hindquarters, respectively. Corresponding values in the absence of insulin were 6.9 ± 1.3 and 7.5 ± 1.2 μmol g−1 h−1 in control and CPDPX-treated hindquarters, respectively. Lactate release by the hindquarters was not altered by the addition of CPDPX to the perfusion mix, in either the absence or presence of insulin. In the total group of rats (n = 34) lactate release was 6.9 ± 0.6 μmol g−1 h−1 before and 23.3 ± 1.3 μmol g−1 h−1 after 60 min of hypoxia.
Interaction of insulin with hypoxia and contractions
In Fig. 3, the way in which insulin interacts with hypoxia and contractions to stimulate muscle glucose uptake was evaluated. Measured rates of muscle glucose uptake during combined stimulation with insulin and either contractions or hypoxia were compared with values calculated from the sum of the independent effects of insulin, contractions and hypoxia (see Methods for details on calculations). Glucose uptake in hindquarters that were simultaneously exposed to hypoxia and insulin was similar to the mathematical sum of the separate insulin- and hypoxia-stimulated muscle glucose uptakes, irrespective of whether CPDPX was added to the perfusate. Thus, in any experimental condition considered, stimulation of muscle glucose uptake by insulin and by hypoxia was found to be additive. In contrast, in the absence, but not in the presence, of CPDPX, the effects of insulin and contractions together on stimulation of muscle glucose uptake were synergistic. Thus, with no CPDPX in the perfusate, the rate of glucose uptake in muscles exposed to insulin during electrical stimulation was higher than just the sum of the separate effects of insulin and contractions on glucose uptake.
Interaction of hypoxia with contractions (protocol C)
The effect of adding electrical stimulation to stimulation of muscle glucose uptake by hypoxia was studied only in hindquarters perfused in the absence of insulin (Fig. 4). Muscle glucose uptake increased from 2.8 ± 0.4 μmol g−1 h−1 at baseline to 10.2 ± 0.9 μmol g−1 h−1 at the end of the 60 min period of hypoxia. This degree of stimulation of glucose uptake was maintained (10.5 ± 0.8 μmol g−1 h−1) during 5 min of normoxic recovery following the hypoxia. Subsequent electrical stimulation of the muscles, leading to contractions, caused a further rise of glucose uptake rate to 14.1 ± 1.9 μmol g−1 h−1 after 2 min and to 16.3 ± 2.1 μmol g−1 h−1 after 5 min of stimulation (P < 0.05). Conversely, continuation of the hypoxic stimulus beyond 60 min did not cause hindlimb glucose uptake to increase further: glucose uptake was 9.6 ± 1.5 μmol g−1 h−1 after 90 min of hypoxia versus 10.2 ± 0.9 μmol g−1 h−1 after 60 min (Fig. 4).
Figure 4. Additive effect of contractions and hypoxia on glucose uptake in perfused rat hindquarters.
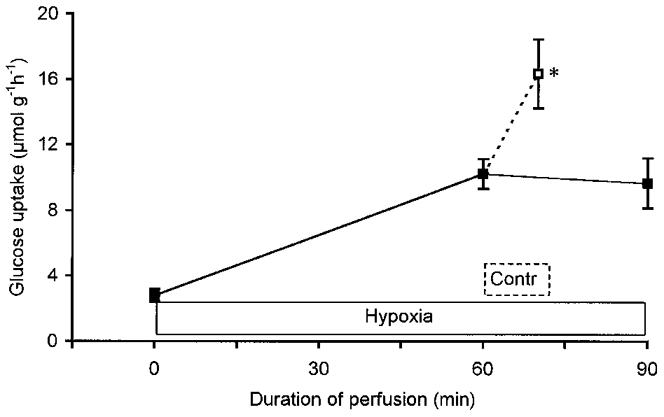
Glucose uptake was measured at rest and after 60 min of hypoxia (n = 17). Thereafter, hypoxic perfusion was either continued for another 30 min (▪—▪, n = 3) or a 5 min electrical stimulation was started (—□, n = 14). Hindquarters were perfused with no CPDPX or insulin added to the perfusate. Values are means ±s.e.m.*P < 0.05 compared with 60 min of hypoxia.
pH, muscle tension and perfusion pressure
Perfusate pH, perfusion pressures, and isometric muscle tension during electrical stimulation were not affected by adenosine receptor antagonism in either experimental condition. In the contraction experiments using 30 % haematocrit perfusate mixtures (protocol A; n = 27), arterial and venous pH values were 7.37 ± 0.01 and 7.33 ± 0.01 at rest, respectively, versus 7.35 ± 0.01 and 7.32 ± 0.01 after 45 min of stimulation. During hypoxia protocols (protocol B; n = 34), where perfusates at 10 % haematocrit were used, arterial and venous pH values were 7.47 ± 0.01 and 7.40 ± 0.01, respectively, before hypoxia, decreasing (P < 0.05) to 7.30 ± 0.01 and 7.22 ± 0.02 after 60 min of hypoxia. Thus, compared with 45 min of contractions, arterial and venous pH values were lower (P < 0.05) after 60 min of hypoxia (P < 0.05). In protocol A (contractions plus normoxia), muscle tension at the onset of electrical stimulation was 842 ± 24 g, decreasing to 389 ± 19 g or 46 % of initial tension after 45 min of contractions. In protocol C experiments (contractions plus hypoxia), muscle tensions were markedly lower (P < 0.05) than in protocol A. Muscle tension was 488 ± 39 g at the onset and 168 ± 39 g, or 31 % of initial tension, after 5 min of contractions. Electrical stimulation (protocol A) increased perfusion pressure from 54 ± 2 mmHg at rest (flow rate = 9 ml min−1) to 79 ± 3 mmHg during contractions (flow rate = 15 ml min−1). Due to the lower perfusate haematocrit, perfusion pressure in resting hindlimbs was lower (P < 0.05) during hypoxia experiments (35 ± 1 mmHg with protocol B; 32 ± 2 mmHg with protocol C). Perfusate flow was not increased during electrical stimulation in protocol C, which resulted in an unchanged perfusion pressure (34 ± 2 mmHg) compared with control.
DISCUSSION
Insulin, contractions and hypoxia are the major stimuli to glucose transport in skeletal muscle. We have recently demonstrated that adenosine is critical to stimulation of insulin-mediated glucose uptake in muscle during contractions (Vergauwen et al. 1994). Furthermore, because the stimulatory effects of maximally effective contractions and maximal hypoxia on muscle glucose transport were not additive, it was proposed that hypoxia and contractions stimulate glucose transport in skeletal muscle via identical cellular mechanisms (Cartee et al. 1991). The current findings demonstrate that stimulation of insulin-mediated glucose uptake in skeletal muscle during hypoxia, in contrast to the effects of insulin applied during contractions (Vergauwen et al. 1994), occurs independently of A1-adenosine receptors. In addition, submaximal contractions and submaximal hypoxia were found to act as additive stimuli to muscle glucose uptake. Thus, the present findings indicate that the cellular mechanism underlying activation of muscle glucose transport by hypoxia and contractions must be at least partly different.
It has been known for a long time that insulin and contractions act as synergistic rather than additive stimuli to muscle glucose uptake during exercise (Defronzo et al. 1981; Turinsky, 1987; Vergauwen et al. 1994). We have recently demonstrated that stimulation of A1-receptors by endogenous adenosine stimulates insulin-mediated glucose transport in oxidative muscle fibres during contractions, but not at rest. This mechanism generates the aforementioned synergistic action of insulin and contractions (Vergauwen et al. 1994). In the present study, A1-receptor antagonism via CPDPX has once more been shown to totally counteract this synergistic action (see Fig. 3). In addition, the effect of CPDPX was found to be rapidly reversed upon cessation of electrical stimulation (see Fig. 2). Thus, our current data demonstrate even more clearly than previous findings that the stimulatory action of adenosine on insulin-mediated glucose uptake in muscle is strictly confined to the period of contractile activity. Because it is currently often postulated that contractions and hypoxia stimulate muscle glucose transport via identical mechanisms (Cartee et al. 1991; Youn et al. 1994; Azevedo et al. 1995), we hypothesized that adenosine exerts an identical action on insulin-mediated glucose uptake in muscles exposed to hypoxia. Therefore, we investigated the effect of CPDPX on hindlimb glucose uptake during hypoxia. In accordance with earlier reports (Cartee et al. 1991; Wojtaszewski et al. 1998), hypoxia markedly elevated the rate of muscle glucose uptake. However, in contrast with earlier data from our laboratories which showed that insulin facilitates contraction-induced muscle glucose uptake (Hespel et al. 1995), the magnitude of the hypoxia-induced increase in glucose uptake was independent of the presence of insulin in the perfusate (see Figs 1 and 3). Furthermore, and again in contrast to its effect on insulin-mediated and electrical stimulation of glucose uptake (Vergauwen et al. 1994 and Fig. 1), CPDPX did not affect insulin-mediated glucose uptake in hindlimbs exposed to hypoxia. Thus, as shown in Fig. 3, because adenosine did not enhance insulin-mediated glucose uptake in hypoxic muscles, the combined effects of insulin and hypoxia on muscle glucose uptake, in contrast to the interaction of insulin and contractions, was additive, not synergistic. Based on the above findings, we conclude that the mechanisms underlying stimulation of muscle glucose transport by hypoxia and contractions cannot be identical. Clearly regulation by both insulin and adenosine of contraction-stimulated muscle glucose uptake is different from that of hypoxia-stimulated uptake.
Cartee et al. (1991) investigated for the first time the combined effects of contractions and hypoxia on muscle glucose uptake. They found in rat epitrochlearis muscles, in which glucose uptake was first maximally stimulated by hypoxia, that maximally effective contractions did not cause a further elevation of glucose uptake rate. In these experiments, hypoxia was induced by incubating muscles in an oxygen- and cell-free perfusate for 60 min. Because, in our opinion, in the latter and other recent studies (Bashan et al. 1992; Azevedo et al. 1995; Wojtaszewski et al. 1998) the effect of anoxia rather than hypoxia on muscle glucose transport was investigated, the relevance of the above observations to regulation of muscle glucose transport by contractions and hypoxia in vivo may be questioned. In contrast to these studies, in the present experiments on isolated perfused rat hindquarters, the level of hypoxia was less severe and therefore more appropriate for studying the interaction of hypoxia and contractions in the regulation of muscle glucose uptake. First, as shown in Fig. 1, oxygen uptake was indeed markedly reduced during hypoxia, yet was not completely abolished, due to the presence of erythrocytes in the perfusate supplying the intact muscle vasculature. In fact, the combination of a low perfusate haematocrit (10 %) and gassing the perfusate with an O2-free gas mixture cut down oxygen delivery to the hindquarters from ∼90 μmol min−1 (normoxia) to barely 7 μmol min−1 during the final stage of hypoxia. Yet this dramatic fall in oxygen delivery (∼12-fold) caused the rate of oxygen uptake by the hindquarters to decrease only about 6-fold (Fig. 1, upper panel). Second, the increase of muscle glucose uptake and the decrease of oxygen uptake caused by hypoxia were found to rapidly (< 30 min) revert towards normal levels upon re-oxygenation. This indicates that the hypoxia did not cause muscle damage, at least not to the extent that oxidative metabolism was irreversibly disturbed. In addition, muscles still were able to produce significant tension upon electrical stimulation after the hypoxia. In fact, muscles were allowed to re-oxygenate for 5 min between the hypoxia protocol and electrical stimulation without the maximal degree of stimulation of muscle glucose uptake by hypoxia being lost. Third, although the degree of hypoxia used here is termed ‘submaximal’, the rate and magnitude of stimulation of muscle glucose uptake found was to be similar to earlier observations in either perfused (Wojtazsewski et al. 1998) or incubated (Cartee et al. 1991) rat skeletal muscles stimulated by anoxia. Under the aforementioned experimental conditions, and as shown in Fig. 4, contractions produced a higher level muscle glucose uptake than that produced after stimulation by hypoxia alone. Thus, in contrast to earlier findings which indicated that anoxia and contractions are not additive (Cartee et al. 1991; Wojtazsewski et al. 1998), our present observations prove that hypoxia and contractions may indeed act as additive stimuli to muscle glucose uptake. Based on this finding, it is tempting to speculate that local hypoxia per se, which is probably most prominent in fast glycolytic muscle fibres, might account for a significant fraction of muscle glucose uptake stimulation during high intensity exercise. In addition, the finding that stimulation of muscle glucose uptake by contractions and hypoxia is additive is not compatible with a theory that states that the pathway for stimulation of muscle glucose transport by contractions and hypoxia is identical (Cartee et al. 1991).
It might be argued that quantitative data on the rate of muscle adenosine production during normoxia, hypoxia and contractions are lacking in the current studies. However, a number of pilot experiments in a subgroup of rats used in this study showed adenosine to be undetectable (HPLC assay with detection limit of 100 pmol ml−1; see Hellsten & Frandsen, 1997) in the venous effluent from the perfused hindlimb, both during contractions and during hypoxia (Y. Hellsten, personal communication). This is probably due to the fact that the small amounts of adenosine being locally produced in oxidative muscle (Schwartz & McKenzie, 1990; Mian & Marshall, 1991; Achike & Ballard, 1993) of the perfused rat hindquarter, during contractions and/or hypoxia, are excessively diluted by the supraphysiological rates of perfusate flow used in this preparation. Second, a very high rate of uptake and metabolism of adenosine by the circulating erythrocytes probably reduces the half-life of perfusate plasma adenosine to a few seconds (Moser et al. 1989). Moreover, if indeed the venous adenosine concentration were detectable, it would not provide information that is relevant to the adenosine concentrations existing in the interstitial medium surrounding the different muscle fibres. In this respect it is important to notice that the rat hindlimb is largely composed of fast-twitch muscle fibres (Delp & Duan, 1996), whereas adenosine production is most prominent in their oxidative counterparts (Schwartz & McKenzie, 1990; Achike & Ballard, 1993). However, studies using experimental models other than the perfused rat hindquarter have clearly demonstrated that adenosine production is markedly enhanced during both muscle contractions (Tomaniga et al. 1980; Ballard et al. 1987; Achike & Ballard, 1993) and hypoxia (Marshall et al. 1993; Skinner & Marshall, 1996). In fact, it has been shown that muscle adenosine release is closely correlated with venous lactate concentration and pH (Ballard, 1995; Mo & Ballard, 1997). In the current experiments, venous lactate concentration was markedly elevated during both contractions and hypoxia, whereas venous pH was significantly depressed only during hypoxia.
The current data suggest that the signalling pathway for stimulation of muscle glucose transport by contractions must include at least some steps that are different from the hypoxia-induced pathway. Over the last decade, multiple regulators of contraction-induced glucose transport in skeletal muscle have been postulated (for a recent review on this topic see Hayashi et al. 1997). Thus Ca2+ release from the sarcoplasmic reticulum, activation of protein kinase C, rate of glycogenolysis, and a number of autocrine and paracrine mechanisms including nitric oxide and adenosine, have been implicated in the regulation of membrane glucose transport during contractions. There is some evidence that sarcoplasmic calcium concentration, like contractions, is probably also important for glucose transport regulation during hypoxia. Yet our current findings indicate that adenosine is probably not involved in a Ca2+-dependent step of glucose transport regulation, because A1-receptor antagonism is shown here to reduce muscle glucose uptake rate during contractions but not during hypoxia (Fig. 3). With regard to protein kinase C, some recent evidence has been presented which indicates that the kinase is involved in contraction-induced, but not hypoxia-induced, glucose transport stimulation (Wojtaszewski et al. 1998). Interestingly in this respect, a recent study in rat adipocytes has suggested that A1-adenosine receptor stimulation may activate protein kinase C (Lai et al. 1998).
In conclusion, the present paper demonstrates that hypoxia, in contrast to contractions, stimulates muscle glucose uptake independently of A1-adenosine receptor stimulation. Furthermore, and again in contrast to contractions, hypoxia and insulin stimulate muscle glucose uptake in an additive, not a synergistic manner. Conversely, the synergistic effect of contractions and insulin on muscle glucose uptake is markedly attenuated by adenosine receptor antagonism. Finally, submaximal hypoxia and contractions may act as additive stimuli to muscle glucose uptake. Thus, evidence is provided that at least some steps in the pathway for stimulation of muscle glucose uptake by contractions and by hypoxia must be different.
Acknowledgments
The authors wish to express their gratitude to Professor E. A. Richter (August Krogh Institute, Copenhagen, Denmark) for his constructive criticism and inspiration throughout this work. We are indebted to Monique Ramaekers for providing skilled technical assistance during the experiments. This study was supported by grants from the ‘Onderzoeksraad K.U.-Leuven’ (grant no. OT94/31) and from the Belgian ‘Nationaal Fonds voor Wetenschappelijk Onderzoek’ (grant no. F297/1994, Krediet aan Navorsers).
References
- Achike FI, Ballard HJ. Influence of stimulation parameters on the release of adenosine, lactate and CO2 from contracting dog gracilis muscle. The Journal of Physiology. 1993;463:107–121. doi: 10.1113/jphysiol.1993.sp019586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo JL, Jr, Carey JO, Pories WJ, Morris PG, Dohm GL. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes. 1995;44:695–698. doi: 10.2337/diab.44.6.695. [DOI] [PubMed] [Google Scholar]
- Ballard HJ. The role of intracellular pH in the control of adenosine output from red skeletal muscle. Biological Signals. 1995;4:168–173. doi: 10.1159/000109437. [DOI] [PubMed] [Google Scholar]
- Ballard HJ, Cotterrell D, Karim F. Appearance of adenosine in venous blood from the contracting gracilis muscle and its role in vasodilatation in the dog. The Journal of Physiology. 1987;387:401–413. doi: 10.1113/jphysiol.1987.sp016580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan N, Burdett E, Hundal HS, Klip A. Regulation of glucose transport and GLUT1 glucose transporter expression by O2 in muscle cells in culture. American Journal of Physiology. 1992;262:C682–690. doi: 10.1152/ajpcell.1992.262.3.C682. [DOI] [PubMed] [Google Scholar]
- Brozinick JT, Jr, McCoid SC, Reynolds TH, Wilson CM, Stevenson RW, Cushman SW, Gibbs EM. Regulation of cell surface GLUT4 in skeletal muscle of transgenic mice. Biochemical Journal. 1997;321:75–81. doi: 10.1042/bj3210075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LA, Hartman JD, Hays SJ, Huang CC. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn-Schmiedeberg's Archives of Pharmacology. 1987;335:59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. Journal of Applied Physiology. 1991;70:1593–1600. doi: 10.1152/jappl.1991.70.4.1593. [DOI] [PubMed] [Google Scholar]
- Defronzo R, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. Journal of Clinical Investigation. 1981;68:1468–1474. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibres and citrate synthase activity of rat muscle. Journal of Applied Physiology. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Douen A, Ramlal T, Klip A, Young D, Cartee G, Holloszy J. Exercise-induced increase in glucose transporters in plasma membranes of rat skeletal muscle. Endocrinology. 1989;124:449–454. doi: 10.1210/endo-124-1-449. [DOI] [PubMed] [Google Scholar]
- Fushiki T, Wells J, Tapscott E, Dohm G. Changes in glucose transporters in muscle in response to exercise. American Journal of Physiology. 1989;256:E580–587. doi: 10.1152/ajpendo.1989.256.5.E580. [DOI] [PubMed] [Google Scholar]
- Hansen PA, Corbett JA, Holloszy JO. Phorbol esters stimulate muscle glucose transport by a mechanism distinct from the insulin and hypoxia pathways. American Journal of Physiology. 1997;273:E28–36. doi: 10.1152/ajpendo.1997.273.1.E28. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Wojtaszewski J, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. American Journal of Physiology. 1997;273:E1039–1051. doi: 10.1152/ajpendo.1997.273.6.E1039. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U. Adenosine formation in contracting primary rat skeletal muscle cells and endothelial cells in culture. The Journal of Physiology. 1997;504:695–704. doi: 10.1111/j.1469-7793.1997.695bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespel P, Vergauwen L, Vandenberghe K, Richter EA. Important role of insulin and flow in stimulating glucose uptake in contracting skeletal muscle. Diabetes. 1995;44:210–215. doi: 10.2337/diab.44.2.210. [DOI] [PubMed] [Google Scholar]
- King PA, Hirshman MF, Horton ED, Horton ES. Glucose transport in skeletal muscle membrane vesicles from control and exercised rats. American Journal of Physiology. 1989;257:C1128–1134. doi: 10.1152/ajpcell.1989.257.6.C1128. [DOI] [PubMed] [Google Scholar]
- Lai CW, Hsu FL, Cheng JT. Stimulatory effect of paeoniflorin on adenosine A-1 receptors to increase the translocation of protein kinase C (PKC) and glucose transporter (GLUT4) in isolated rat white adipocytes. Life Science. 1998;62:1591–1595. doi: 10.1016/s0024-3205(98)00112-x. 10.1016/S0024-3205(98)00112-X. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Thomas T, Turner L. A link between adenosine, ATP-sensitive K+ channels, potassium and muscle vasodilatation in the rat in systemic hypoxia. The Journal of Physiology. 1993;472:1–9. doi: 10.1113/jphysiol.1993.sp019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R, Marshall JM. The role of adenosine in dilator responses induced in arterioles and venules of rat skeletal muscle by systemic hypoxia. The Journal of Physiology. 1991;443:499–511. doi: 10.1113/jphysiol.1991.sp018847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo FM, Ballard HJ. Intracellular lactate controls adenosine output from dog gracilis muscle during moderate systemic hypoxia. American Journal of Physiology. 1997;272:H318–324. doi: 10.1152/ajpheart.1997.272.1.H318. [DOI] [PubMed] [Google Scholar]
- Moser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. American Journal of Physiology. 1989;256:C799–806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- Neylon M, Marshall JM. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. The Journal of Physiology. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguso CA, Coggan AR, Sidossis LS, Gastaldelli A, Wolfe RR. Effect of theophylline on substrate metabolism during exercise. Metabolism. 1996;45:1153–1160. doi: 10.1016/s0026-0495(96)90016-5. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Smith GH. Regulation of glucose uptake in muscle: 1. The effect of insulin, anaerobiosis and cell poisons on the uptake of glucose and release of potassium by isolated rat diaphragm. Biochemical Journal. 1958;70:490–508. doi: 10.1042/bj0700490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JM, Gulve EA, Cartee GD, Holloszy JO. Hypoxia causes glycogenolysis without an increase in percent phosphorylase a in rat skeletal muscle. American Journal of Physiology. 1992;263:E1086–1091. doi: 10.1152/ajpendo.2006.263.6.E1086. [DOI] [PubMed] [Google Scholar]
- Richter EA, Ruderman NB, Gavras H, Belur ER, Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. American Journal of Physiology. 1982;242:E25–32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- Rubio R, Berne RM, Dobson JG. Sites of adenosine production in cardiac and skeletal muscle. American Journal of Physiology. 1973;225:938–953. doi: 10.1152/ajplegacy.1973.225.4.938. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Houghton CR, Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochemical Journal. 1971;124:639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz LM, Mckenzie JE. Adenosine and active hyperaemia in soleus and gracilis muscle of cats. American Journal of Physiology. 1990;259:H1295–1304. doi: 10.1152/ajpheart.1990.259.4.H1295. [DOI] [PubMed] [Google Scholar]
- Skinner MR, Marshall JM. Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. The Journal of Physiology. 1996;495:553–560. doi: 10.1113/jphysiol.1996.sp021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriet L, Matsos C, Peters S, Heigenhauser GJ, Jones N. Muscle metabolism and performance in perfused rat hindquarter during heavy exercise. American Journal of Physiology. 1985;248:C109–118. doi: 10.1152/ajpcell.1985.248.1.C109. [DOI] [PubMed] [Google Scholar]
- Tominaga S, Curnish RR, Belardinelli L, Rubio R, Berne RM. Adenosine release during early and sustained exercise of canine skeletal muscle. American Journal of Physiology. 1980;238:H156–163. doi: 10.1152/ajpheart.1980.238.2.H156. [DOI] [PubMed] [Google Scholar]
- Turinsky J. Glucose and amino acid uptake by exercising muscles in vivo: effect of insulin, fibre population, and denervation. Endocrinology. 1987;121:528–535. doi: 10.1210/endo-121-2-528. [DOI] [PubMed] [Google Scholar]
- Vergauwen L, Hespel P, Richter EA. Adenosine receptors mediate synergistic stimulation of glucose uptake and transport by insulin and by contractions in rat skeletal muscle. Journal of Clinical Investigation. 1994;93:974–981. doi: 10.1172/JCI117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergauwen L, Richter EA, Hespel P. Adenosine exerts a glycogen-sparing action in contracting rat skeletal muscle. American Journal of Physiology. 1997;272:E762–768. doi: 10.1152/ajpendo.1997.272.5.E762. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Laustsen JL, Derave W, Richter EA. Hypoxia and contractions do not utilise the same signalling mechanism in stimulating skeletal muscle glucose transport. Biochimica et Biophysica Acta. 1998;1380:396–404. doi: 10.1016/s0304-4165(98)00011-7. [DOI] [PubMed] [Google Scholar]
- Youn JH, Gulve EA, Henriksen EJ, Holloszy JO. Interactions between effects of W-7, insulin, and hypoxia on glucose transport in skeletal muscle. American Journal of Physiology. 1994;267:R888–894. doi: 10.1152/ajpregu.1994.267.4.R888. [DOI] [PubMed] [Google Scholar]


