Abstract
An outwardly rectifying Cl− (ORCl) current of murine osteoclasts was activated by hypotonic stimulation. The current was characterized by rapid activation, little inactivation, strong outward rectification, blockage by DIDS and permeability to organic acids (pyruvate and glutamate).
The hypotonically activated ORCl current was inhibited by intracellular dialysis with an ATP-free pipette solution, but not by replacement of ATP with a poorly hydrolysable ATP analogue adenosine 5′-O-(3-thiotriphosphate). The current amplitude was reduced when intracellular alkalinity increased over the pH range 6.6–8.0.
Intracellular application of cytochalasin D occasionally activated the ORCl current without hypotonic stress, but inhibited activation of the ORCl current by hypotonic stimulation. The hypotonically activated ORCl current was unaffected by a non-actin-depolymerizing cytochalasin, chaetoglobosin C, but partially inhibited by deoxyribonuclease I.
Removal of extracellular Ca2+ inhibited activation of the ORCl current by hypotonic shock, but did not reduce the current once activated. The hypotonically activated ORCl current was partially decreased by intracellular dialysis with 20 mm EGTA.
With 10 mm Ca2+ in the extracellular medium, the ORCl current was activated in response to more minor decreases in osmolarity than with 1 mm Ca2+. The increased sensitivity to hypotonicity was mimicked by increasing the intracellular Ca2+ level (pCa 6.5).
These results suggest that hypotonic stimulation and a rise in the extracellular Ca2+ level synergistically activate the ORCl channel of murine osteoclasts, and that the activating process is modified by multiple intracellular factors (pH, ATP and actin cytoskeletal organization).
Osteoclasts secrete protons and various enzymes to degrade bone matrix, and play a crucial role in bodily Ca2+ homeostasis. Both organic and inorganic bone degradation products are transcytosed through osteoclasts and liberated into the extracellular space (Nesbitt & Horton, 1997; Salo et al. 1997). During the bone resorption cycle, osteoclasts face dynamic changes in diverse extracellular/intracellular environmental factors, such as osmolarity, Ca2+ levels, pH, metabolic activities and organization of cytoskeletons (Zaidi et al. 1993; Lakkakorpi & Väänänen, 1996). The ruffled membrane proton secretion via a vacuolar-type H+-ATPase requires anion efflux through the Cl− channel. Resultant accumulation of HCO3− and reduction of intracellular Cl− are compensated for via the HCO3−-Cl− exchanger in the basal membrane (Schlesinger et al. 1994). Transmembrane Cl− flux is thus closely related to the functional state of osteoclasts. Chloride channels have been demonstrated in rat (Sims et al. 1991), avian (Blair & Schlesinger, 1990), rabbit (Kelly et al. 1994) and murine osteoclasts (Shibata et al. 1997), but still little is known about their role in osteoclast functions.
So far, the only known activating extracellular stimulus for Cl− channels of osteoclasts is either a rise in the extracellular Ca2+ concentration ([Ca2+]o) (Fujita et al. 1996; Shibata et al. 1997) or hypotonic stress (Kelly et al. 1994). Resorbing activities are inhibited by a rise in [Ca2+]o possibly via a low-affinity receptor-like [Ca2+]o-sensing molecule (Zaidi et al. 1995). Cell swelling induced by hypotonic shock may also lead to changes in various cellular events, such as ion transport through the plasma membrane, organization of the cytoskeleton and metabolic activities (Lang et al. 1998). Osteoclasts would be exposed to both a rise in [Ca2+]o and an osmotic imbalance during the bone resorption. We have shown that a rise in [Ca2+]o activates an outwardly rectifying Cl− (ORCl) channel in in vitro-generated murine osteoclasts (Shibata et al. 1997) which have the characteristics of mature osteoclasts (Akatsu et al. 1992; Shioi et al. 1994), but the hypotonic effects on their Cl− channels remain to be solved.
This study aimed at characterizing hypotonically activated Cl− channels in murine osteoclasts and studying the co-operative action of the two stimuli, a rise in [Ca2+]o and osmotic perturbation. We show that the hypotonically activated Cl− current shares common electrophysiological features with the ORCl current activated by a rise in [Ca2+]o, and that a rise in [Ca2+]o shifts the osmolarity set-point for the current activation to respond to a minor change in osmolarity. In addition, we provide evidence that the hypotonically activated ORCl current requires extracellular Ca2+ and intracellular ATP and is inhibited by intracellular alkalization and perturbation of the actin cytoskeleton. A preliminary report has been published (Sakai et al. 1997).
METHODS
Cell culture
Osteoclasts were generated from a co-culture of bone marrow cells of male, 5- to 8-week-old mice (C3H/HeN) with a marrow-derived stromal cell line (ST2; Riken Cell Bank, Tsukuba, Japan). The mice were killed by cervical dislocation. Bone marrow cells obtained from the femurs and tibias were centrifuged at 300 g for 7 min at 4°C, and incubated in α-minimum essential medium (α-MEM) supplemented with 10 % fetal calf serum (FCS), streptomycin (0.1 mg ml−1) and penicillin (100 u ml−1) at 37°C in a 95 % air-5 % CO2 atmosphere overnight. Non-adherent cells were collected by centrifugation at 300 g at 4°C for 7 min and incubated in a phosphate-buffered saline solution containing 0.02 % pronase and 1.5 mM EDTA for 15 min at 37°C. The pronase reaction was stopped by heat-inactivated horse serum (0.2 ml per 10 ml pronase solution), and the cell suspension was layered on ice-cold horse serum. After 15 min of sedimentation at unit gravity in the ice-cold horse serum, the uppermost part of the layers was collected, transferred into cold horse serum and then centrifuged at 800 g at 4°C for 10 min. The bone marrow cell pellet was suspended in fresh α-MEM supplemented with 10 % FCS at 1 × 106 cells ml−1 and co-cultured with ST2 cells (1 × 105 cells ml−1) in the presence of 1α,25(OH)2D3 (10−8 M) and dexamethasone (10−7 M) at 37°C in a 95 % air-5 % CO2 atmosphere. The total medium was changed twice a week. ST2 cells were removed by incubation with 0.1 % bacterial collagenase (collagenase S-1)-0.1 % bovine serum albumin (BSA) in α-MEM for 10-30 min at 37°C before recordings were made.
Identification of cells
After culturing for 5-7 days, osteoclasts were identified as multinucleated cells with a unique morphology (a flattened cell body, developed lamellipodia and retraction fibres) and tartrate-resistant acid phosphatase (TRAP) activity (Shioi et al. 1994). Actin rings and podosomes containing F-actin were identified by staining with rhodamine-conjugated phalloidin.
Solutions
The standard external solution contained (mM): 145 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 0.1 % BSA and 10 Hepes-NaOH (pH 7.3) (299 ± 2 mosmol l−1, n = 5 solutions). The osmolarity of solutions was measured using a freezing-point depression osmometer (OS osmometer, Fiske, MA, USA). Hypotonic solutions were made by reducing NaCl concentration. In the high Ca2+ (10-40 mM) or K+-free solutions, the concentration of NaCl was changed to adjust the osmolarity to the required value. The Cl− concentration of the medium containing 10 mM Ca2+ was 3 mM higher than that containing 1 mM Ca2+. The low Cl− hypotonic solutions were made by replacing NaCl with sodium isethionate, sodium pyruvate or sodium glutamate. The standard pipette solution contained (mM): 150 potassium glutamate, 3 MgCl2, 1 EGTA, 1 Na2ATP and 10 Hepes-KOH (pH 7.3). To examine the effects of intracellular pH on the currents, BAPTA (1 mM) was substituted for EGTA to stabilize the free Ca2+ concentration at different pH values. EGTA (20 mM) was added to keep the intracellular Ca2+ concentration ([Ca2+]i) low in some experiments. To investigate the effects of the elevated [Ca2+]i on current activities, pipette solutions containing high Ca2+ (pCa 6.5) were prepared with the appropriate amounts of EGTA and CaCl2, which were estimated using the CHELATOR program (Schoenmakers et al. 1992). Potassium glutamate was replaced by caesium methanesulphonate to block K+ currents. Alkaline pipette solutions (pH 7.8-8.0) were made by adjusting the pH with KOH, and the acidic pipette solution (pH 6.6) was buffered with 120 mM Mes. In the solutions containing high Mes or EGTA, potassium glutamate was reduced to compensate for the osmolarity. The osmolarity of these pipette solutions was maintained at between 280 and 290 mosmol l−1.
Electrophysiological recordings
Recordings were made from osteoclasts cultured for 5-14 days. Culture dishes were placed on the stage of an inverted microscope (Diaphot-TMD, Nikon, Japan), and cells were monitored with a CCD camera (KY-F55MD, Olympus, Japan). The borosilicate glass pipettes had a resistance of 5-8 MΩ. Series resistance compensation (70-90 %) was conducted to reduce the voltage error. The reference electrode was a Ag-AgCl wire connected to the bath solution through a Ringer-agar bridge. The zero current potential before formation of the gigaseal was taken as 0 mV.
Whole-cell currents were recorded by an amplifier (Axopatch 200A, Axon Instruments) at room temperature (20-24°C). Current signals were digitized at 2 kHz with an analog-to-digital converter (Maclab/4S, ADInstruments, New South Wales, Australia), stored and analysed by a personal computer. Voltage ramps (0.24 mV ms−1 from -120 to +120 mV) were applied at a holding potential of -60 mV every 10 s. Leak current was determined from the linear portion of the current-voltage (I-V) relationship when either inward or outward current was absent or when the currents were eliminated by the blockers. The outward conductance was obtained from the I-V relationship between +80 and +100 mV, after subtraction of the leak current. Within this voltage range, the conductance estimated from the voltage ramp method was almost identical to that obtained by measuring the peak current amplitude evoked by voltage steps. The hypotonically activated conductance was obtained by subtracting the data before hypotonic stimulation from the maximum conductance evoked by the hypotonic stimulation. Data are expressed as means ±s.e.m., and were tested using Student's unpaired t test (unless otherwise stated). Values of P < 0.05 were considered to be significant. The P values between 0.05 and 0.2 are described in the figure legends.
Chemicals
Mes and BAPTA were purchased from Dojindo Laboratories (Kumamoto, Japan), collagenase S-1 from Nitta Gelatin Co. (Osaka, Japan), and 1α,25(OH)2D3 from Solway Duphar (Weesp, The Netherlands). All other chemicals were obtained from Sigma Chemical Co. A concentrated stock solution of Na2ATP (500 mM) was prepared in 1 M Tris-Cl, stored in a freezer, and added to the internal medium before use. Adenosine 5′-O-(3-thiotriphosphate) (ATPγS) and deoxyribonuclease I (DNase I) were dissolved in distilled water. Cytochalasin D, chaetoglobosin C and DIDS were dissolved in DMSO. The final DMSO concentration was less than 0.1 %, which itself did not affect the membrane currents.
RESULTS
Hypotonic stimulation activates an outwardly rectifying Cl− current
The resting membrane potential of osteoclasts was -58 ± 2 mV (n = 72 osteoclasts) and whole-cell capacitance was 85 ± 3 pF (n = 145). In the standard solution, an inwardly rectifying K+ (IRK) current was exhibited in most in vitro -generated murine osteoclasts, but outward currents were generally small or negligible (Shibata et al. 1996, 1997). We previously reported that a rise in [Ca2+]o activated an outwardly rectifying Cl− (ORCl) current (Shibata et al. 1997). A similar OR current was activated by hypotonic stimulation. Figure 1A shows whole-cell currents evoked by voltage steps applied at a holding potential of -60 mV. When the osmolarity of the external medium was reduced to 60 % of the control, a rapidly activating and little-inactivating current was evoked by depolarization (Fig. 1A, middle). The reduction of the peak current during a 500 ms-long depolarization (+80 or +100 mV) was 6.5 ± 2.0 % (n = 10). A Cl− channel blocker, DIDS, blocked the outward current (Fig. 1A, right). The percentage inhibition of the hypotonically activated OR current by 50 and 100 μM DIDS was 97 ± 3 % (minimum-maximum, 84-100 %; n = 6) and 98 ± 1 % (92-100 %; n = 9). Swelling of cells during hypotonic challenges was confirmed under the microscope, although this was not quantified. Figure 1B (left) shows a time course of changes in the outward conductance when the cell was perfused with the hypotonic solution (70 % of control osmolarity) at about 0.1 ml s−1 (volume of the recording chamber, 2 ml). To eliminate the IRK current, K+ was omitted from the extracellular medium. Exposure to the hyposmotic solution reversibly activated the outward conductance. The current-voltage (I-V) relationships obtained by voltage ramps before (a), during (b) and after (c) exposure to the hypotonic solution are superimposed in Fig. 1B, right. The OR conductance was decreased by 30-100 % of the maximum within 5-10 min after washout of the hypotonic solution. Immediately after switching from the hypotonic to the normotonic solution, OR currents transiently increased in 10 of 37 cells.
Figure 1. Hypotonically activated ORCl current of murine osteoclast.
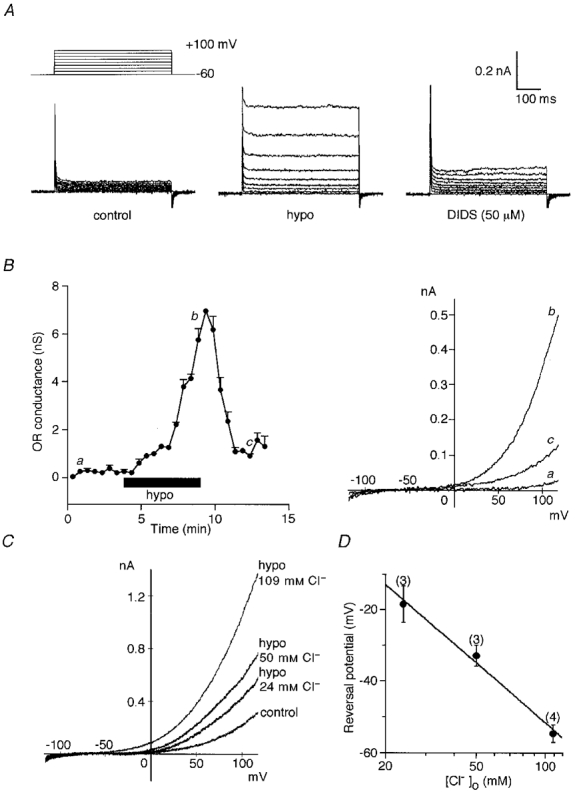
A, whole-cell currents evoked by a series of 500 ms voltage steps in control (left), during exposure to hypotonic solution (middle) and after addition of DIDS (right). B, time course of outward conductance in a cell exposed to hypotonic shock (left). The conductance was calculated from I-V relationships obtained by voltage ramps. Each point represents the mean of three consecutive responses at an interval of 10 s. Some error bars are smaller than the actual symbols. The I-V relationships at times indicated by a, b and c are superimposed (right). C, I-V relationship of the hypotonically activated currents in a cell recorded with different [Cl−]o. D, semilogarithmic plot of the reversal potential (means ±s.e.m.) of the hypotonically activated current against [Cl−]o. The number of cells examined is given in parentheses. The line indicates a least-squares fit of the data. The osmolarity of the hypotonic solution was 180 mosmol l−1 (60 % of control osmolarity) in A and 210 mosmol l−1 (70 %) in B-D. The extracellular medium was K+ free in B and C. Leak currents were subtracted in B (right) and C, but not in A.
Figure 1C shows the I-V relationships of the hypotonically activated currents recorded in the K+-free extracellular medium. The OR current was decreased in amplitude and activated at more positive potentials by lowering the extracellular Cl− concentration of the hypotonic solution. A least-squares fit of a semilogarithmic plot of the reversal potential had a slope of 55 mV per tenfold change in [Cl−]o (Fig. 1D). The hypotonically activated OR current was also observed when intracellular K+ was totally replaced by Cs+ (data not shown). These results suggest that the hypotonically activated OR current is carried mainly by Cl− and shares common features with the ORCl current activated by a rise in [Ca2+]o (Shibata et al. 1997). The permeability ratio between monovalent cations and Cl− (Pcation/PCl), calculated from the reversal potentials using the Goldman-Hodgkin-Katz equation, was 0.09 ± 0.02 (n = 9). When the organic ions pyruvate− and glutamate− were substituted for the Cl− of the hypotonic solution (70 %, 109 mM anions), the reversal potential was shifted in a positive direction by 28.7 ± 1.6 mV (n = 3) and 50.9 ± 1.9 mV (n = 4), respectively, giving a permeability ratio Ppyruvate/PCl of 0.32 ± 0.02 (n = 3) and Pglutamate/PCl of 0.13 ± 0.01 (n = 4). The hypotonically activated OR conductance, calculated from the I-V relationship between +80 and +100 mV positive to the reversal potential, was reduced to 39.4 ± 8.7 % (n = 3) of the Cl− conductance with pyruvate− and 8.5 ± 3.8 % (n = 4) with glutamate−.
The relationship between the extracellular osmolarity and the OR conductance is summarized in Fig. 2. The OR conductance, normalized by the cell capacitance, was obtained from seven cells which were first suspended in the standard solution and then subsequently challenged with various hypotonic media. The OR conductance recorded at 100, 90, 80 and 70 % of control osmolarity was 5.4 ± 0.8, 4.9 ± 1.4, 12.5 ± 5.3 and 121.3 ± 30.8 pS pF−1 (n = 7), respectively. In later experiments, the hypotonically activated ORCl current was examined by exposure to the solution at 70 % of control osmolarity for about 5 min unless described otherwise.
Figure 2. Osmolarity-conductance relationship for hypotonically activated OR current.
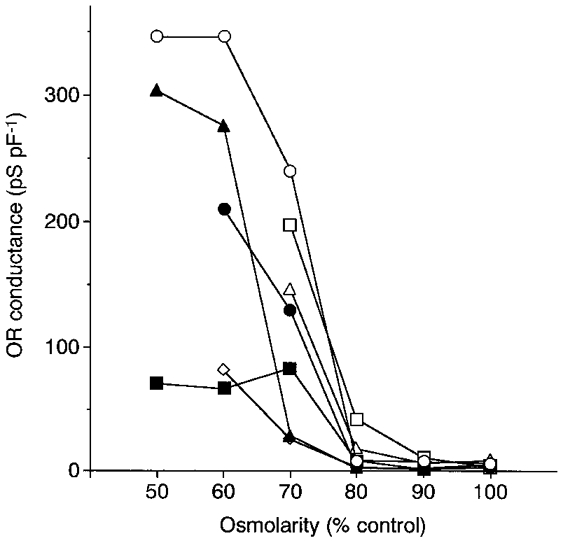
The hypotonically activated OR conductance in seven cells is plotted against the osmolarity of the extracellular medium. The conductance was normalized by the cell capacitance. The osmolarity is expressed as a percentage of the control (100 %, 299 mosmol l−1). The osmolarity of the extracellular medium was subsequently decreased from 100 % and data were obtained at about 5 min following perfusion of each hypotonic solution.
Hypotonic activation of the ORCl current depends on intracellular ATP and pH
The rate of ORCl current activation varied among cells. In some cells, the hypotonically activated ORCl current spontaneously decreased despite continued hypotonic stimulation. On average, the ORCl current developed gradually for at least 3-5 min with the standard pipette solution containing 1 mM ATP (Fig. 3A, ○; n = 22). The current activation was inhibited by dialysis with an ATP-free solution (□, n = 6), but not by replacing ATP with 1 mM ATPγS, a poorly hydrolysable ATP analogue (▵, n = 6). Figure 3B summarizes the maximum hypotonically activated OR conductance during exposure to the hypotonic solution, obtained by subtracting the conductance before stimulation. The conductance with 1 mM ATP was 79.5 ± 11.4 pS pF−1 (n = 37) (leftmost column in Fig. 3B). Inhibitory effects of depletion of ATP on the activation were not evident when the hypotonic shock was applied 3 min after formation of the whole-cell configuration (50.2 ± 23.8 pS pF−1, n = 6), but were significant when the stimulus was applied at 10 min (15.3 ± 6.8 pS pF−1, n = 6). Pretreatment with 5 mM sodium azide for 20-80 min followed by dialysis with the ATP-free solution for 10 min did not further inhibit the OR current (22.2 ± 17.8 pS pF−1, n = 6) (Fig. 3B). Most (84.8 ± 6.7 %, n = 5) of this small current was blocked by 100 μM DIDS, but the remaining DIDS-insensitive current, characterized by slow activation kinetics on depolarization, seemed to differ from the ORCl current. The second conductance, unidentified at this moment, was occasionally seen before hypotonic stimulation, but the amplitude was small even at potentials greater than +100 mV and was not increased by hypotonic stimulation. The hypotonically activated ORCl current with 1 mM ATPγS was 89.8 ± 34.2 pS pF−1 (n = 4), significantly greater than that with the ATP-free solution (P < 0.05), so that the major portion of the activated ORCl current seems to depend on non-hydrolytic ATP binding.
Figure 3. ATP dependence of hypotonically activated OR current.
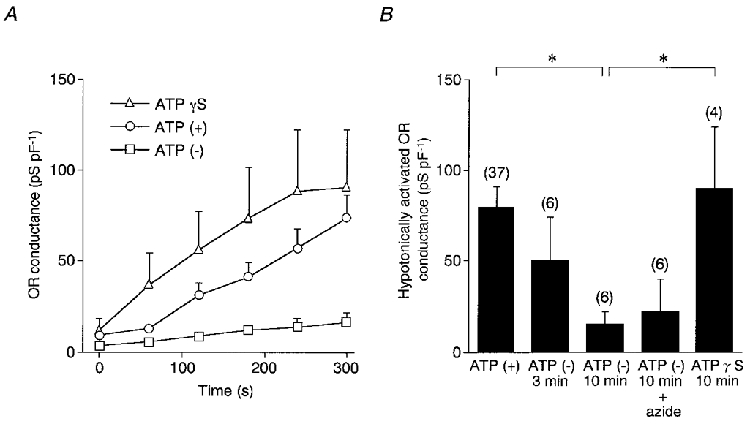
A, time courses of the OR conductance following hypotonic stimulation with ATP-containing (○, n = 22), ATPγS (1 mM)-containing (▵, n = 4) and ATP-free (□, n = 6) pipette solutions. Cells were dialysed for 10 min with ATP-free or ATPγS-containing pipette solutions and then exposed to the hypotonic solution (70 % of control osmolarity for 5 min) at time zero. B, the hypotonically activated OR conductance. Pretreatment with 5 mM sodium azide for 20-80 min was followed by dialysis with ATP-free solution for 10 min. Data are means and s.e.m. Number of cells examined is given in parentheses. *P < 0.05 for ATP (+) and ATPγS versus ATP (-) for 10 min. The P value for ATP (-) plus sodium azide versus ATP (+) was 0.06.
The hypotonic activation of the ORCl current was also inhibited by increasing the pH of the pipette solution (Fig. 4A). To minimize the difference in the Ca2+-buffering action at a different pH, 1 mM BAPTA was substituted for 1 mM EGTA in the pipette solutions (Schoenmakers et al. 1992). The maximum ORCl conductance activated during exposure to hypotonic stimulation was 182.2 ± 31.9 pS pF−1 (n = 7) at pHi 6.6, 112.2 ± 30.8 pS pF−1 (n = 13) at pHi 7.3, and 43.2 ± 9.6 pS pF−1 (n = 12) at pHi 7.8-8.0 (Fig. 4B). The conductance with the alkaline solution was significantly larger than that at pHi 7.3 (P < 0.05) and pHi 6.6 (P < 0.005), suggesting that pHi is crucial for the hypotonic activation of the ORCl current. The effects of pHi were evident even when the cell was exposed to the hypotonic solution within 5 min following the rupture of the patch membrane.
Figure 4. pH dependence of hypotonically activated OR current.
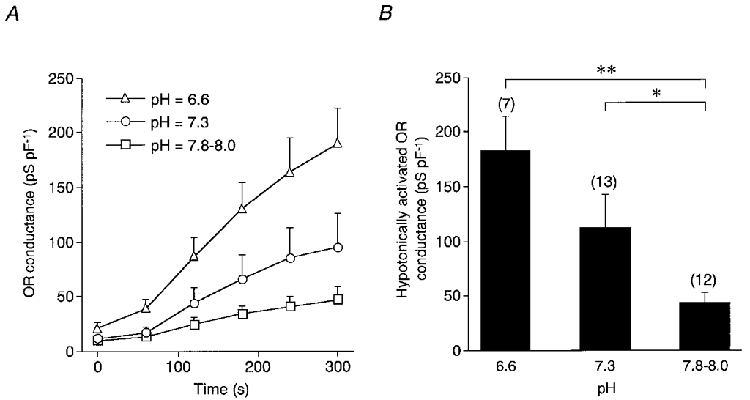
A, time courses of the OR conductance following hypotonic stimulation at different intracellular pH values. Cells were dialysed with pipette solutions of variable pH for 10 min, and then exposed to the hypotonic solution (70 % of control osmolarity for 5 min) at time zero. The pH of the standard pipette solution was 7.3. The pipette solutions contained 1 mM BAPTA, as a substitute for EGTA, to minimize the difference in free Ca2+ concentration at different pH values. B, the hypotonically activated OR conductances at different pH values. Data are means and s.e.m. Number of cells examined is given in parentheses. *P < 0.05. **P < 0.005. The P value for pH 6.6 versus pH 7.3 was 0.16.
Cytochalasin D inhibits the hypotonically activated ORCl current
Intracellular dialysis with cytochalasin D (1 μM) gradually activated the ORCl current without hypotonic stimulation in 7 out of 11 cells, often in association with bleb formation (Fig. 5A and B). The cytochalasin D-activated current shared common electrophysiological features with the hypotonically activated ORCl current. With pipette solutions containing 0.1, 0.3 and 1 μM cytochalasin D, the OR conductance recorded at 3-4 min was 6.5 ± 1.7 (n = 5), 18.1 ± 2.1 (n = 4) and 41.7 ± 13.4 pS pF−1 (n = 11), respectively (Fig. 5C). The hypotonically activated ORCl current was significantly inhibited by dialysing cells for 3-4 min with cytochalasin D at both 0.1 μM (15.6 ± 5.2 pS pF−1, n = 5) and 1 μM (9.9 ± 3.0 pS pF−1, n = 4), where the current activation was negligible without hypotonic stimulation (Fig. 5D). Chaetoglobosin C (1 μM), a cytochalasin without depolymerizing action, did not affect the hypotonically activated ORCl current (79.4 ± 25.4 pS pF−1, n = 5). Dialysis for 10 min with 10 μM DNase I, which inhibits actin polymerization by binding to globular actin, did not affect the hypotonically activated ORCl conductance (64.9 ± 21.5 pS pF−1, n = 8). With 30 μM DNase I, however, the ORCl conductance was smaller than 18 pS pF−1 (n = 4) except for one cell (101.2 pS pF−1) (29.3 ± 18.1 pS pF−1; n = 5). Neither chaetoglobosin C nor DNase I (10-30 μM) activated the OR current without hypotonic stimulation.
Figure 5. Effects of cytochalasins and DNase I on hypotonically activated OR current.
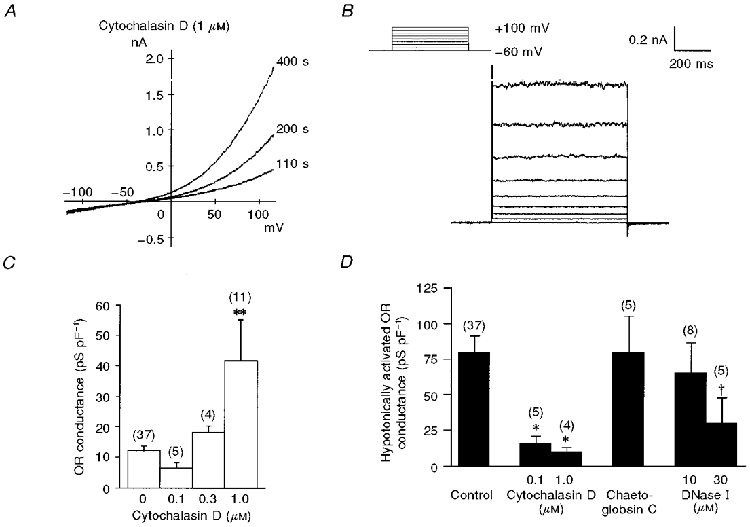
A, I-V relationship recorded at 110, 200 and 400 s following intracellular dialysis with a pipette solution containing 1 μM cytochalasin D. B, a family of the cytochalasin D-induced currents evoked by voltage steps at around 300 s. C, the OR conductances in cells dialysed with cytochalasin D (0.1-1.0 μM for 3-4 min) without hypotonic stimulation. **P < 0.005 compared with the control (0 μM cytochalasin D). The P value for 0 versus 0.1 μM cytochalasin D was 0.18. D, the OR conductance activated by hypotonic stimulation (70 % of control osmolarity for 5 min) in cells dialysed with cytochalasin D (0.1 and 1.0 μM for 3-10 min), chaetoglobosin C (1 μM, 10 min) and DNase I (10 and 30 μM, 10 min). Data are means and s.e.m. Number of cells examined is given in parentheses. With 30 μM DNase I, hypotonic stimulation failed to activate the current in 4 of 5 cells. *P < 0.05 and †P = 0.12 compared with control.
Extracellular Ca2+ is required for the hypotonically activated ORCl current
Figure 6A shows the ORCl conductance of a cell which was successively exposed to nominally Ca2+-free, Ca2+-free hypotonic, and Ca2+ (1 mM)-containing hypotonic solutions. The ORCl current was not activated by hypotonic stimulation in the absence of Ca2+. Subsequent perfusion with the Ca2+-containing hypotonic solution reversibly activated the ORCl current. Figure 6B summarizes the results of similar experiments in seven cells. The hypotonically activated ORCl current in the absence of Ca2+ was significantly smaller (11.2 ± 4.7 pS pF−1, n = 7) than that in the presence of Ca2+ (55.8 ± 13.3 pS pF−1, n = 7) (P < 0.01 with Student's paired t test). Removal of the extracellular Ca2+, however, did not reduce, but rather increased the current once activated (n = 7, data not shown).
Figure 6. Extracellular Ca2+ dependence of hypotonically activated OR current.
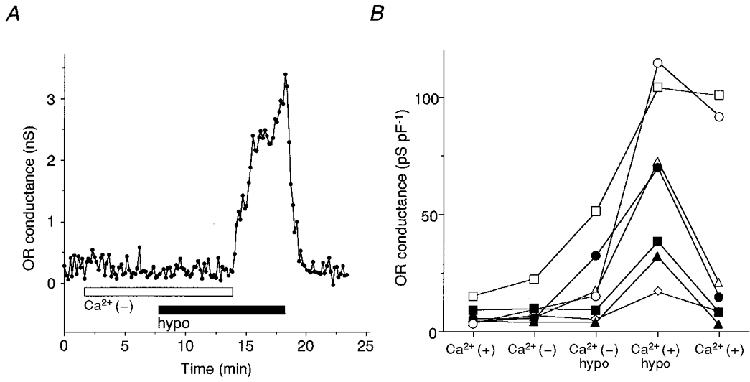
A, representative time course of the OR conductance when a cell was successively exposed to nominally Ca2+-free, Ca2+-free hypotonic or Ca2+ (1 mM)-containing hypotonic solutions. The osmolarity of the hypotonic solutions was 70 % of control. B, the ORCl conductances induced by similar experiments in seven cells. Data were obtained at about 5 min following perfusion of each solution (sequentially, from left to right).
When cells were exposed to hypotonic shock 10 min after dialysis with the pipette solution containing 20 mM EGTA (n = 7), the hypotonically activated ORCl conductance was generally small (24.1 ± 5.4 pS pF−1, n = 6) except for one cell (111.9 pS pF−1). Thus a low basal [Ca2+]i seems to be required for the hypotonic activation of the ORCl current. It was noted that the ORCl current was not decreased by washout of the hypotonic solution, possibly because of profound cellular disturbances due to chelating intracellular Ca2+.
Synergetic activation of the ORCl current by hypotonic stimulation and a rise in [Ca2+]o
A rise in the [Ca2+]o activates the ORCl current (Shibata et al. 1997). The amplitude of the ORCl current in the presence of 10 and 40 mM Ca2+ was 20.6 ± 5.3 pS pF−1 (n = 18) and 116.9 ± 28.5 pS pF−1 (n = 18) (Fig. 7A). Figure 7B represents a typical experiment in which hypotonic stimulation was applied in the presence of 10 mM Ca2+. Even with 90 % of the control osmolarity, exposure to the solution activated the ORCl current. The hypotonically activated ORCl conductance at 90, 80 and 70 % of control osmolarity was 23.7 ± 11.2, 64.2 ± 24.3 and 102.9 ± 30.7 pS pF−1 (n = 7) in the 10 mM Ca2+-containing external medium (Fig. 8, ▵), and 1.3 ± 1.1, 7.6 ± 5.3 and 115.8 ± 30.8 pS pF−1 (n = 7) in the standard medium containing 1 mM Ca2+ (○). Values at 90-80 % were significantly larger with 10 mM Ca2+ although there was no difference in those at 70 %, suggesting that a rise in [Ca2+]o is likely to sensitize the channel to respond to more minor decreases in osmolarity.
Figure 7. Effects of high extracellular [Ca2+] on hypotonically activated OR current.
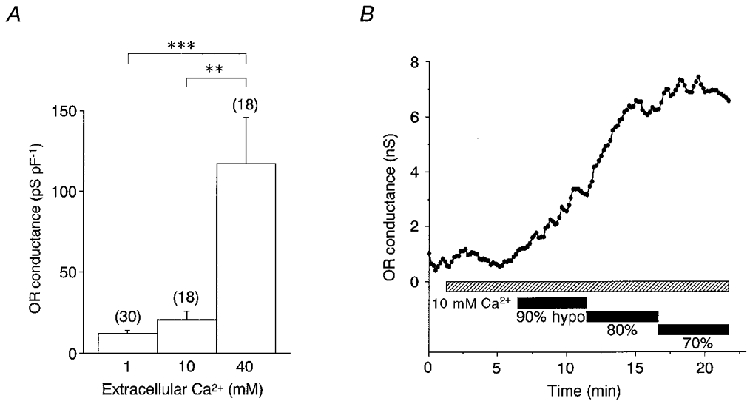
A, OR conductance (means and s.e.m.) produced by a rise in [Ca2+]o in the normotonic solutions. The standard extracellular medium contained 1 mM Ca2+. **P < 0.005. ***P < 0.0005. The P value for 1 mM Ca2+versus 10 mM Ca2+ was 0.09. B, time course of the OR conductance when a cell was exposed to a series of hypotonic solutions (90-70 % of control osmolarity) in the presence of 10 mM Ca2+.
Figure 8. Effects of [Ca2+]o and [Ca2+]i on the osmolarity-conductance relationship.
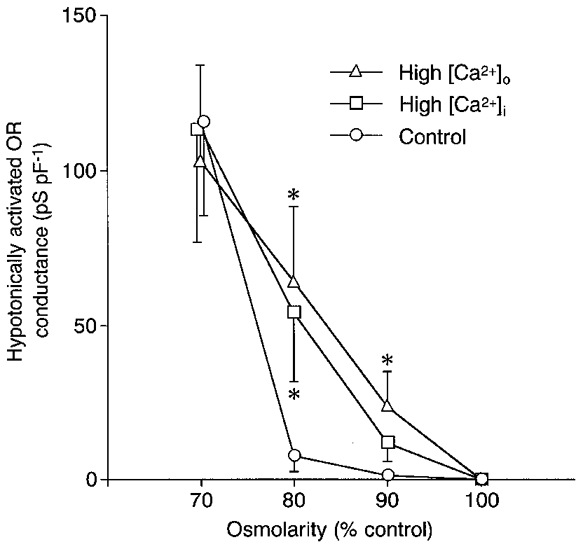
The hypotonically activated OR conductance (means and s.e.m.) in control (○, n = 7), with high [Ca2+]o (10 mM) (▵, n = 7) and with high [Ca2+]i (pCa 6.5) (□, n = 7-9). The [Ca2+]o was 1 mM and the [Ca2+]i was < 10 nM in the control condition. After treatment with these solutions for 10 min, the osmolarity of the extracellular medium was decreased. Data were obtained at about 5 min following the perfusion of each solution. *P < 0.05 compared with control.
A rise in [Ca2+]o leads to elevation of [Ca2+]i in osteoclasts (Miyauchi et al. 1990; Zaidi et al. 1993). To examine the role of a rise of [Ca2+]i in increasing sensitivity to osmolarity, hypotonic stimulation was applied to cells dialysed for 10 min with a pipette solution containing high Ca2+ (pCa 6.5, 316 nM). The extracellular medium contained 1 mM Ca2+. Intracellular dialysis with the high Ca2+ pipette solution itself did not activate the OR current (6.5 ± 0.8 pS pF−1, n = 11). The hypotonically activated ORCl conductance at 90, 80 and 70 % of control osmolarity was 11.8 ± 6.0 (n = 8), 54.1 ± 22.4 (n = 7) and 113.2 ± 36.1 pS pF−1 (n = 9) (Fig. 8, □). The values at 80 % were significantly larger than the control, while there was no significant difference in those at 90 and 70 %. Thus an increase in [Ca2+]i did not activate the ORCl current by itself, but might shift the osmolarity set-point for the activation to a more minor hypotonicity.
DISCUSSION
Hypotonically activated ORCl current in murine osteoclasts
In murine osteoclasts, a strong elevation of [Ca2+]o (≤ 20 mM) activates a sizable ORCl current (Shibata et al. 1997). The present study provides evidence that hypotonic stress also activates an ORCl current. The hypotonically activated Cl− current and the high [Ca2+]o-activated Cl− current share the same kinetics (rapid activation and little inactivation on depolarization), strong outward rectification and sensitivity to DIDS, suggesting that the same class of ORCl channel underlies the two currents. In rabbit osteoclasts an ORCl current characterized by similar kinetics and pharmacology is activated by hypotonic stimulation (Kelly et al. 1994). The properties of hypotonically activated Cl− currents of osteoclasts differ from those of either the ClC-2-type Cl− channels or the maxi-Cl− channels (Strange et al. 1996).
ATP dependence of the hypotonically activated ORCl current
A deficiency of intracellular ATP inhibited hypotonic activation of the ORCl current of murine osteoclasts. The inhibition was not apparent when the hypotonic challenge was applied at 3 min following formation of the whole-cell configuration. Considering that the inhibitory effects of dialysis with either alkaline or cytochalasin D-containing pipette solutions were evident after a few minutes, ATP deficiency is likely to need a certain period to be effective. In rabbit osteoclasts, the hypotonically activated ORCl current was not inhibited by ATP depletion, although the period for the dialysis was not stated (Kelly et al. 1994). As substitution of ATPγS for ATP did not affect the hypotonically activated ORCl current, non-hydrolytic ATP binding seems to be responsible for the activation, although it is not known whether ATP binds to the channel or to unidentified intracellular regulatory molecules. In the present study, even when cells were dialysed with an ATP-free pipette solution for 10 min after pretreatment with 5 mM sodium azide for 20-80 min, a small but significant ORCl current was activated by hypotonic shock. Thus we cannot exclude the possibility that ATP-insensitive pathways are partially involved in the activating process, although the major portion of the hypotonically activated ORCl current indeed depends on non-hydrolytic ATP binding. The requirement for non-hydrolysing ATP for the hypotonic activation of Cl− channels is reported in many mammalian cells (Jackson et al. 1994; Oike et al. 1994). As organic acids (pyruvate and glutamate) can permeate through the ORCl channel, this ATP dependency may work as a negative feedback mechanism to prevent loss of metabolic intermediate anions when cellular energy production is low (Jackson et al. 1994: Okada, 1997).
Effects of intracellular pH on the hypotonically activated ORCl current
In many cell types, cell swelling leads to cytosolic acidification (Lang et al. 1998). The present study showed that the hypotonically activated ORCl current was larger at a lower pHi. During bone resorption, osteoclasts actively secrete protons into the resorbing pit and generate a load of cytoplasmic equivalent, primarily as HCO3−. The pHi is influenced by the culture substrate (Lehenkari et al. 1997), extracellular pH (Teti et al. 1989; Nordström et al. 1997), and activities of diverse pHi regulatory mechanisms including the HCO3−-Cl− exchanger, the H+ channel and the Na+-H+ exchanger (Schlensinger et al. 1994; Nordström et al. 1997). It is conceivable that the ORCl current activity dependent on changes in pHi may modulate resorbing actions (Teti et al. 1991).
Involvement of actin cytoskeleton in the hypotonically activated ORCl current
Actin filaments are depolymerized or disrupted during osmotic cell swelling in a variety of cells (Lang et al. 1998). Activation of volume-sensitive Cl− currents is often accompanied by reorganization of F-actin cytoskeleton (Strange et al. 1996; Okada, 1997). Cytochalasins have a number of actions on actin filaments (Sampath & Pollard, 1991), including inhibition of both polymerization and depolymerization of F-actin and disruption of F-actin, suggesting that the effects of cytochalasin D may arise from multiple combinations of these actions. Intracellular application of 1 μM cytochalasin D activated the ORCl current in 7 out of 11 cells. Intense reorganization of actin filaments by cytochalasin D may underlie this activation, as the current activation was often accompanied by bleb formation. DNase I, which inhibits polymerization of F-actin by binding to G-actin, did not activate the current by itself. The hypotonically activated ORCl current was inhibited by even 0.1 μM cytochalasin D, which did not activate the ORCl current by itself, but not by chaetoglobosin C, a cytochalasin without actin-depolymerizing action. Cytochalasins have been reported to inhibit activation of Cl− channels by hypotonic stimulation in other cell types (Fatherazi et al. 1994; Schwiebert et al. 1994). DNase I also inhibited the hypotonically activated ORCl current partially, but the inhibitory action seems to be moderate compared with that of cytochalasin D, since a rather high concentration (30 μM) of DNase I was needed for the inhibition. Taking these findings together, organization of actin filaments would be involved in the current activation machinery. Depletion of intracellular ATP alters actin cytoskeleton (Molitoris et al. 1991), and actin polymerization is inhibited as alkalinity increases over the pH range 6.6-8.3 (Sampath & Pollard, 1991). This may partly explain the inhibitory effect of ATP deficiency and intracellular alkalization on the hypotonically activated ORCl current.
Osteoclasts have organized membrane domains and change their shape and polarity according to functional states (Teti et al. 1991; Aubin, 1992). Osteoclasts form F-actin-containing podosomes or actin rings even on the culture dishes and disruption of actin rings suppresses the pit-forming activity (Zhang et al. 1995; Lakkakorpi & Vänäänen, 1996). A scattered punctate pattern of podosomes in the lamellipodia was often decreased by hypotonic stimulation in association with cell swelling (authors' unpublished observation), so hypotonic shock could inhibit bone resorption via reorganization of the actin cytoskeleton.
Effects of [Ca2+]o and [Ca2+]i on the hypotonically activated ORCl current
In some cell types, regulatory volume decrease or activation of Cl− channels by hypotonic shock depends on [Ca2+]o (McCarty & O'Neil, 1992; Strange et al. 1996). The hypotonically activated ORCl current of murine osteoclasts was inhibited in the nominally Ca2+-free solution, contrary to rabbit osteoclasts (Kelly et al. 1994). Removal of extracellulsr Ca2+, however, did not reduce the current once activated, as reported in pancreatic duct cells (Verdon et al. 1995). Thus extracellular Ca2+ seems to be required for activation of the current, but not for sustained activity. Cell swelling leads to a rise in [Ca2+]i in a variety of cells and, in some cell types, the rise in [Ca2+]i due to Ca2+ influx is suggested to activate Cl− channels (McCarty & O'Neil, 1992; Lang et al. 1998). Addition of high concentrations of EGTA to the pipette solutions partially inhibited the hypotonically activated ORCl current in murine osteoclasts, as in bovine vascular endothelial cells (Szücs et al. 1996) and rat carotid body cells (Carpenter & Peers, 1997). However, neither the introduction of 0.3-10 μM Ca2+ into the pipette solution nor the addition of ionomycin into the extracellular medium activated the ORCl current (Shibata et al. 1997). As reported in bovine vascular endothelial cells in which the hypotonically activated Cl− current is unaffected by [Ca2+]i > 50 nM (Szücs et al. 1996), it is likely that a rise in [Ca2+]i does not activate the ORCl channel directly but that a low basal [Ca2+]i is needed for the activation.
During bone resorption, [Ca2+]o in the resorptive pit is estimated to increase up to 40 mM (Silver et al. 1988), which results in suppression of the resorptive activity of osteoclasts by retraction of lamellipodia, reduction of podosome expression, de-adhesion of osteoclasts and inhibition of release of resorptive enzymes (Miyauchi et al. 1990; Zaidi et al. 1993). Sensitivity to increased [Ca2+]o depends on their activation phases (Lakkakorpi et al. 1996). In the present study, a rise in [Ca2+]o changed the osmolarity set-point for the current activation. The increased sensitivity to hypotonicity was mimicked by increasing [Ca2+]i (pCa 6.5). A rise in [Ca2+]o increases [Ca2+]i to ≤ 200-300 nM by evoking Ca2+ release from the internal stores and Ca2+ influx through the plasma membrane in osteoclasts (Miyauchi et al. 1990; Zaidi et al. 1993). Actin filaments depolymerize by binding Ca2+ to gelsolin (Lang et al. 1998), which could modify the intracellular signalling for the activation of the ORCl channel. Thus an elevation in [Ca2+]i would participate in the sensitization of the channel to hypotonicity at least partly, but the entire intracellular machinery underlying the synergetic action of a rise in [Ca2+]o and hypotonicity on the ORCl current remains to be proved.
Physiological implications
Several roles of Cl− channels can be postulated in osteoclasts. First, the membrane potential would be changed by the activity of the channel, although the direction and the magnitude depend on [Cl−]i. The membrane potential may be crucial in determining the [Ca2+]i during bone resorption (Shankar et al. 1995). Second, the Cl− channels may be responsible for Cl− efflux through the ruffled membrane to maintain electroneutral HCl secretion into the resorptive lacuna. The ruffled border Cl− channel incorporated in planar lipid bilayers has a current with similar properties to the hypotonically activated ORCl current (sensitivity to stilbene sulfonate and strongly outwardly rectified conductance) (Schlesinger et al. 1997). Third, the Cl− channels may contribute to [Cl−]i homeostasis by compensating for changes in [Cl−]i due to the massive Cl− flux during bone resorption. Fourth, the Cl− channels may work as for organic solute transport, as suggested in swelling-activated organic osmolyte and anion channels (Strange et al. 1996). Fifth, the Cl− channels could be involved in morphological changes during the resorption cycle, since they are one of the most efficient mechanisms of regulating cell volume (Okada, 1997).
In conclusion, we have demonstrated that hypotonicity and a rise in [Ca2+]o synergistically activate the ORCl current of murine osteoclasts. Both stimuli could be inhibitory signals for resorbing activity, so that activation of the ORCl channel would rescue osteoclasts by the above mechanisms. As activation processes are modulated by pHi, ATP and the organization of F-actin, the channel activities seem to depend greatly on the cellular functional states. The degree of swelling in response to hypotonicity was also varied among cells, although the change in volume was not quantified. The large variability of the current size may be explained partly by diversity in either the regulatory mechanisms or the amount of swelling. We previously suggested that the ORCl current could be activated only in a severe resorbing phase because efficient activation of the ORCl current needed a much higher [Ca2+]o (> 20 mM) than that required for inhibition of the IRK current (Shibata et al. 1997). The present study indicates that sizable ORCl currents may be activated in response to a minor change in osmolarity and a small increase in [Ca2+]o if the two stimuli co-exist.
Acknowledgments
We thank Dr S. Matsuura for critically reading the manuscript, and Ms J. Kawawaki and Ms C. H. Kim for preparation of this manuscript. This work was supported by grants from The Assistant Program of Graduate Student Fellowships of Osaka City University, The Hoansha Foundation, and a Grant-in-Aid for Scientific Research from The Ministry of Education, Science and Culture, Japan.
References
- Akatsu T, Tamura T, Takahashi N, Udagawa N, Tanaka S, Sasaki T, Yamaguchi A, Nagata N, Suda T. Preparation and characterization of a mouse osteoclast-like multinucleated cell population. Journal of Bone and Mineral Research. 1992;7:1297–1306. doi: 10.1002/jbmr.5650071109. [DOI] [PubMed] [Google Scholar]
- Aubin JE. Osteoclast adhesion and resorption: The role of podosomes. Journal of Bone and Mineral Research. 1992;7:365–368. doi: 10.1002/jbmr.5650070402. [DOI] [PubMed] [Google Scholar]
- Blair HC, Schlesinger PH. Purification of a stilbene sensitive chloride channel and reconstitution of chloride conductivity into phospholipid vesicles. Biochemical and Biophysical Research Communications. 1990;171:920–925. doi: 10.1016/0006-291x(90)90771-e. [DOI] [PubMed] [Google Scholar]
- Carpenter E, Peers C. Swelling- and cAMP-activated Cl− currents in isolated rat carotid body type I cells. The Journal of Physiology. 1997;503:497–511. doi: 10.1111/j.1469-7793.1997.497bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatherazi S, Izutsu KT, Wellner RB, Belton CM. Hypotonically activated chloride current in HSG cells. Journal of Membrane Biology. 1994;142:181–193. doi: 10.1007/BF00234940. [DOI] [PubMed] [Google Scholar]
- Fujita H, Matsumoto T, Kawashima H, Ogata E, Fujita T, Yamashita N. Activation of Cl− channels by extracellular Ca2+ in freshly isolated rabbit osteoclasts. Journal of Cellular Physiology. 1996;169:217–225. doi: 10.1002/(SICI)1097-4652(199610)169:1<217::AID-JCP22>3.0.CO;2-8. ;10.1002/(SICI)1097-4652(199610)169:1<217::AID-JCP22>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. American Journal of Physiology. 1994;267:C1203–1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- Kelly MEM, Dixon SJ, Sims SM. Outwardly rectifying chloride current in rabbit osteoclasts is activated by hyposmotic stimulation. The Journal of Physiology. 1994;475:377–389. doi: 10.1113/jphysiol.1994.sp020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkakorpi PT, Lehenkari PP, Rautiala TJ, Väänänen HK. Different calcium sensitivity in osteoclasts on glass and bone and maintenance of cytoskeletal structures on bone in the presence of high extracellular calcium. Journal of Cellular Physiology. 1996;168:668–677. doi: 10.1002/(SICI)1097-4652(199609)168:3<668::AID-JCP19>3.0.CO;2-V. 10.1002/(SICI)1097-4652(199609)168:3<668::AID-JCP19>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi PT, Väänänen HK. Cytoskeletal changes in osteoclasts during the resorption cycle. Microscopy Research and Technique. 1996;33:171–181. doi: 10.1002/(SICI)1097-0029(19960201)33:2<171::AID-JEMT7>3.0.CO;2-W. 10.1002/(SICI)1097-0029(19960201)33:2<171::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiological Reviews. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lehenkari PP, Laitala-Leinonen T, Linna T-J, Väänänen HK. The regulation of pHi in osteoclasts is dependent on the culture substrate and on the stage of the resorption cycle. Biochemical and Biophysical Research Communications. 1997;235:838–844. doi: 10.1006/bbrc.1997.6894. 10.1006/bbrc.1997.6894. [DOI] [PubMed] [Google Scholar]
- McCarty NA, O'Neil RG. Calcium signaling in cell volume regulation. Physiological Reviews. 1992;72:1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Miyauchi A, Hruska KA, Greenfield EM, Duncan R, Alvarez J, Barattolo R, Colucci S, Zambonin-Zallone A, Teitelbaum SL. Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. Journal of Cell Biology. 1990;111:2543–2552. doi: 10.1083/jcb.111.6.2543. 10.1083/jcb.111.6.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA, Geerdes J, McIntosh JR. Dissociation and redistribution of Na+, K+-ATPase from its surface membrane actin cytoskeletal complex during cellular ATP depletion. Journal of Clinical Investigation. 1991;88:462–469. doi: 10.1172/JCI115326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt SA, Horton MA. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science. 1997;276:266–269. doi: 10.1126/science.276.5310.266. 10.1126/science.276.5310.266. [DOI] [PubMed] [Google Scholar]
- Nordström T, Shrode LD, Rotstein OD, Romanek R, Goto T, Heersche JNM, Manolson MF, Brisseau GF, Grinstein S. Chronic extracellular acidosis induces plasmalemmal vacuolar type H+ ATPase activity in osteoclasts. Journal of Biological Chemistry. 1997;272:6354–6360. doi: 10.1074/jbc.272.10.6354. 10.1074/jbc.272.10.6354. [DOI] [PubMed] [Google Scholar]
- Oike M, Droogmans G, Nilius B. The volume-activated chloride current in human endothelial cells depends on intracellular ATP. Pflügers Archiv. 1994;427:184–186. doi: 10.1007/BF00585960. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Sakai H, Shibata T, Nakamura F, Kuno M. Osmotic stress alters K+ and Cl− conductances in murine osteoclasts. The 33rd International Congress of Physiological Sciences. 1997:P002.20. abstract. [Google Scholar]
- Salo J, Lehenkari P, Mulari M, Metsikkö K, Väänänen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- Sampath P, Pollard TD. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry. 1991;30:1973–1980. doi: 10.1021/bi00221a034. [DOI] [PubMed] [Google Scholar]
- Schlesinger PH, Blair HC, Teitelbaum SL, Edwards JC. Characterization of the osteoclast ruffled border chloride channel and its role in bone resorption. Journal of Biological Chemistry. 1997;272:18636–18643. doi: 10.1074/jbc.272.30.18636. [DOI] [PubMed] [Google Scholar]
- Schlesinger PH, Mattsson JP, Blair HC. Osteoclastic acid transport: mechanism and implications for physiological and pharmacological regulation. Mineral and Electrolyte Metabolism. 1994;20:31–39. [PubMed] [Google Scholar]
- Schoenmakers TJM, Visser GJ, Flick G, Theuvenet APR. CHELATOR: An improved method for computing metal ion concentrations in physiological solutions. BioTechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- Schwiebert EM, Mills JW, Stanton BA. Actin-based cytoskeleton regulates a chloride channel and cell volume in a renal cortical collecting duct cell line. Journal of Biological Chemistry. 1994;269:7081–7089. [PubMed] [Google Scholar]
- Shankar VS, Huang CL-H, Adebanjo O, Simon B, Alam ASMT, Moonga BS, Pazianas M, Scott RH, Zaidi M. Effect of membrane potential on surface Ca2+ receptor activation in rat osteoclasts. Journal of Cellular Physiology. 1995;162:1–8. doi: 10.1002/jcp.1041620102. [DOI] [PubMed] [Google Scholar]
- Shibata T, Sakai H, Nakamura F. Membrane currents of murine osteoclasts generated from bone marrow/stromal cell co-culture. Osaka City Medical Journal. 1996;42:93–107. [PubMed] [Google Scholar]
- Shibata T, Sakai H, Nakamura F, Shioi A, Kuno M. Differential effect of high extracellular Ca2+ on K+ and Cl− conductances in murine osteoclasts. Journal of Membrane Biology. 1997;158:59–67. doi: 10.1007/s002329900243. [DOI] [PubMed] [Google Scholar]
- Shioi A, Ross FP, Teitelbaum SL. Enrichment of generated murine osteoclasts. Calcified Tissue International. 1994;55:387–394. doi: 10.1007/BF00299320. [DOI] [PubMed] [Google Scholar]
- Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Experimental Cell Research. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Sims SM, Kelly MEM, Dixon SJ. K+ and Cl− currents in freshly isolated rat osteoclasts. Pflügers Archiv. 1991;419:358–370. doi: 10.1007/BF00371118. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Szücs G, Heinke S, Droogmans G, Nilius B. Activation of the volume-sensitive chloride current in vascular endothelial cells requires a permissive intracellular Ca2+ concentration. Pflügers Archiv. 1996;431:467–469. doi: 10.1007/BF02207289. [DOI] [PubMed] [Google Scholar]
- Teti A, Blair HC, Schlesinger P, Zambonin-Zallone A, Kahn AJ, Teitelbaum SL, Hruska KA. Extracellular protons acidify osteoclasts, reduce cytosolic calcium, and promote expression of cell-matrix attachment structures. Journal of Clinical Investigation. 1989;84:773–780. doi: 10.1172/JCI114235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti A, Marchisio PC, Zambonin-Zallone A. Clear zone in osteoclast function: role of podosomes in regulation of bone-resorbing activity. American Journal of Physiology. 1991;261:C1–7. doi: 10.1152/ajpcell.1991.261.1.C1. [DOI] [PubMed] [Google Scholar]
- Verdon B, Winpenny JP, Whitfield KJ, Argent BE, Gray MA. Volume-activated chloride currents in pancreatic duct cells. Journal of Membrane Biology. 1995;147:173–183. doi: 10.1007/BF00233545. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Alam ASMT, Huang CL-H, Pazianas M, Bax CMR, Bax BE, Moonga BS, Bevis PJR, Shankar VS. Extracellular Ca2+ sensing by the osteoclast. Cell Calcium. 1993;14:271–277. doi: 10.1016/0143-4160(93)90048-b. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Shankar VS, Tunwell R, Adebanjo OA, Mackrill J, Pazianas M, O'Connell D, Simon BJ, Rifkin BR, Venkitaraman AR, Huang CL-H, Lai FA. A ryanodine receptor-like molecule expressed in the osteoclast plasma membrane functions in extracellular Ca2+ sensing. Journal of Clinical Investigation. 1995;96:1582–1590. doi: 10.1172/JCI118197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Udagawa N, Nakamura I, Murakami H, Saito S, Yamasaki K, Shibasaki Y, Morii N, Narumiya S, Takahashi N, Suda T. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. Journal of Cell Science. 1995;108:2285–2292. doi: 10.1242/jcs.108.6.2285. [DOI] [PubMed] [Google Scholar]


