Abstract
The effects of fetal intravenous treatment with phentolamine or a vasopressinergic V1-receptor antagonist on the fetal cardiovascular responses to acute hypoxaemia in the llama were investigated.
Six llama fetuses were surgically prepared between 60 and 70% of gestation under general halothane anaesthesia with vascular catheters and transit-time ultrasonic flow probes around a carotid artery and a femoral artery. At least 4 days after surgery all fetuses were subjected to a 3 h experiment: 1 h of normoxia, 1 h of hypoxaemia and 1 h of recovery while on slow i.v. infusion with saline. On separate days this experiment was repeated with fetal i.v. treatment with either phentolamine or a V1-receptor antagonist dissolved in saline.
During saline infusion all llama fetuses responded to acute hypoxaemia with intense femoral vasoconstriction. Phentolamine during normoxia produced hypotension, tachycardia and vasodilatation in both the carotid and the femoral circulations. During hypoxaemia, fetuses treated with phentolamine did not elicit the pronounced femoral vasoconstriction and all died within 20 min of the onset of hypoxaemia. A V1-receptor antagonist produced a femoral vasodilatation during normoxia but did not affect the fetal cardiovascular responses to acute hypoxaemia.
In conclusion, α-adrenergic and V1-vasopressinergic mechanisms contribute to a basal vasoconstrictor tone in the femoral circulation in the llama fetus. The enhanced femoral vasoconstriction during acute hypoxaemia in the llama fetus is not mediated by stimulation of V1-vasopressin receptors, but is dependent on α-adrenergic receptor stimulation. Such α-adrenergic efferent mechanisms are indispensable to fetal survival during hypoxaemia in the llama since their abolition leads to cardiovascular collapse and death.
One of the most common challenges to the developing mammalian fetus during pregnancy, and in particular in late gestation, is reduced oxygen supply. In lowland species, such as the sheep, acute fetal hypoxaemia elicits a transient bradycardia, a gradual increase in arterial blood pressure and a redistribution of the combined ventricular output shunting blood flow away from the periphery towards the cerebral, myocardial and adrenal circulations (see Giussani et al. 1994b for review).
Control of the fetal cardiovascular responses during hypoxaemia involves carotid chemoreflexes and slower endocrine components. The bradycardia and initial femoral vasoconstriction during hypoxaemia are abolished by carotid sinus nerve section (Bartelds et al. 1993; Giussani et al. 1993). Fetal bradycardia is effected by vagal efferents and the initial peripheral vasoconstriction by α-adrenergic efferents since they are prevented by muscarinic (Parer, 1984; Giussani et al. 1993) and α-adrenergic receptor blockade (Giussani et al. 1993), respectively.
Once initiated, the fetal peripheral vasoconstriction is maintained during hypoxaemia by slower release of vasoactive hormones into the fetal circulation. These include catecholamines (Jones & Wei, 1985) and arginine vasopressin (Alexander et al. 1974; Giussani et al. 1994a). Treatment of sheep fetuses with adrenergic α1- (Reuss et al. 1982; Giussani et al. 1993) or vasopressinergic V1- (Perez et al. 1989) receptor antagonists reduces the delayed peripheral vasoconstriction and attenuates the increase in fetal arterial blood pressure during acute hypoxaemia.
We have previously reported that the fetal llama, a species adapted to the chronic hypobaric hypoxaemia of pregnancy at altitude, demonstrates an intense femoral vasoconstriction during acute hypoxaemia which is, even at 60 to 70 % of gestation, 4-5 times greater in magnitude than that measured in fetal sheep in late pregnancy (Giussani et al. 1996). By analogy with fetal sheep, the similarity in the pattern of the femoral haemodynamic response to acute hypoxaemia suggests that it is mediated via similar mechanisms. The difference in the magnitude of the response suggests a potent carotid chemoreflex and/or a stronger endocrine vasoconstrictor response. However, section of the carotid sinus nerves in the llama fetus does not alter the fetal peripheral vasoconstriction during acute hypoxaemia (Giussani et al. 1996). This suggests that the intense fetal femoral vasoconstriction in the llama fetus is mediated via mechanisms independent of a carotid chemoreflex and favours a potent endocrine response. Indeed, the increase in fetal plasma vasopressin during acute hypoxaemia in the llama fetus is 7-8 times greater than that measured in the sheep fetus (Giussani et al. 1996). Although there is a substantial increase in plasma catecholamine concentrations during acute hypoxaemia in fetal sheep (Jones & Robinson, 1975), comparison of the magnitude of this increase with that in llama fetuses is not possible. Changes in fetal plasma catecholamine concentrations during hypoxaemia have not been measured in the llama to date.
In this study we have tested the hypothesis that the intense femoral vasoconstriction during acute hypoxaemia in the llama fetus has a potent α-adrenergic or V1-vasopressinergic component by investigating the effects of adrenergic α- or vasopressin V1-receptor blockade on the cardiovascular responses to acute hypoxaemia.
METHODS
Use of animals
Six pregnant llamas between 60 and 70 % of gestation (gestational age estimated from fetal body weights; 3517 ± 915 g, mean ±s.d. where term is 7000-8000 g; ca 350 days; Fowler, 1989) were obtained from the University of Chile farm at the Rinconada de Maipú, at 580 m above sea level. Upon arrival in Santiago (585 m above sea level), the llamas were housed in an open yard with access to food and water ad libitum and they were familiarized with the study metabolic cage and the laboratory conditions for 1-2 weeks prior to surgery.
Surgical preparation
Maternal and fetal surgeries were carried out on consecutive days using well established techniques previously described in detail (Giussani et al. 1996, Riquelme et al. 1998). In brief, following food and water deprivation for 24 h, the llamas were pre-medicated with atropine (1 mg, i.m., Atropina sulfato, Laboratorio Chile, Santiago, Chile). Polyvinyl catheters (1.3 mm i.d.) were placed in the maternal descending aorta and inferior vena cava via a hindlimb artery and vein under light general anaesthesia (ketamine, 10 mg kg−1i.m., Ketostop, Drug Pharma-Invetec, Santiago, Chile) with additional local infiltration of lidocaine (2 % lidocaine hydrochloride, Dimecaína, Laboratorio Beta, Santiago, Chile). The catheters were then tunnelled subcutaneously to exit at the maternal flank.
The following day the fetuses were surgically prepared under maternal general anaesthesia (5-7 mg kg−1 sodium thiopentone, Tiopental Sódico, Laboratorio Astorga, Santiago, Chile for induction and 1 % halothane in 50:50 O2 and N2O for maintenance). Following a mid-line laparotomy a fetal hindlimb was withdrawn through a small hysterotomy. Polyvinyl catheters (i.d. 0.8 mm) were inserted into the fetal aorta via a hindlimb artery and into the inferior vena cava via a hindlimb vein, and an ultrasonic flow transducer (2RS Transonic Inc., Ithaca, NY, USA) was implanted around one femoral artery (Giussani et al. 1993, 1996). The fetal head was exposed through a second hysterotomy and a catheter (i.d. 0.8 mm) was inserted into a carotid artery and a second Transonic flow probe (2RS) was implanted around the contralateral carotid artery. Another catheter was placed in the amniotic cavity. The uterine incisions were closed in layers and all vascular catheters were filled with heparinized saline (1000 i.u. heparin ml−1 0.9 % NaCl), plugged with a copper pin, exteriorized through a maternal flank and kept in a pouch sewn onto the maternal skin.
During surgery all animals were continuously hydrated with warm 0.9 % NaCl solution (15-20 ml kg−1 h−1) to compensate for any fluid loss. At the end of surgery, and daily after surgery for 5 days, 1 × 106 u penicillin (Penicilina G Sodica, Laboratorio Chile) and 500 mg kanamycin (Canamicina Sulfato, Laboratorio Chile) were administered in the amniotic fluid via the amniotic catheter. After surgery the animals were returned to the yard and at least 4 days of postoperative recovery were allowed before the beginning of the experiments. Vascular catheters were maintained patent by daily flushing with heparinized (200 i.u. heparin ml−1) saline.
All animal care procedures and experimentation were conducted in accordance with The Guiding Principles for Research Involving Animals and Human Beings of the American Physiological Society and the British Animals (Scientific Procedures) Act, 1986.
Experimental procedure
Only one experiment was conducted each day. All experiments were based on a 3 h protocol divided into three periods of 60 min: 1 h normoxia, 1 h hypoxaemia and 1 h recovery. A transparent polyethylene bag was placed over the llama's head into which known concentrations of O2, N2 and CO2 were passed at a rate of ca 35 l min−1. In all six preparations, following 1 h of breathing air (normoxia), hypoxia (9 % O2 with 2-3 % CO2 in N2) was induced in the mother, which reduced maternal Pa,O2 from ∼90 to ∼ 35 mmHg and haemoglobin saturation from ∼94 to ∼70 %. This, in turn, reduced fetal carotid Pa,O2 to ∼12 mmHg (hypoxaemia) and fetal descending aortic haemoglobin saturation to < 30 %. After the hour of fetal hypoxaemia the llama was returned to breathing air for a further 60 min (recovery).
On subsequent days, the experimental protocol was repeated after pre-treatment of all six fetuses with either a vasopressin V1 antagonist (d-(CH2)51-Tyr(Me)2,Arg8)-vasopressin, Bachem, Saffron Walden, UK; 240 μg kg−1i.v. bolus followed by i.v. infusion of 4 μg kg−1 min−1) dissolved in heparinized saline (n = 6), or the α-adrenergic antagonist phentolamine (Rogitine, Ciba, Horsham, UK; 20 mg kg−1i.v. bolus followed by i.v. infusion of 0.4 mg kg−1 min−1) dissolved in heparinized saline (n = 6). Fetal treatment with either phentolamine or V1 antagonist started 15 min before the onset of hypoxaemia and it ran continuously until the end of the hypoxaemic challenge. Fetal treatment with saline or the V1 antagonist was randomized; fetal treatment with phentolamine always followed either of these two protocols because of fetal mortality. Complete α-adrenergic and vasopressinergic V1 blockade was verified by the lack of a pressor and vasopressor response to an i.v. injection of 100 μg of phenylephrine (The Boots Company, plc, Nottingham, UK) or of 60-100 ng of the vasopressin analogue Pitressin (Bachem), respectively, 10 min after the onset of the antagonist infusion. The agonist and antagonist doses used in the llama fetus were scaled up from our previous observations in fetal sheep (Perez et al. 1989; Giussani et al. 1993).
During any 3 h experimental protocol, arterial blood samples (0.5 ml) were taken from the mother and fetus after 15 and 45 min of normoxia, after 5, 15 and 45 min of hypoxia and after 15 and 45 min of recovery, to determine pHa, Pa,O2 and Pa,CO2 (BMS 3 MK2 Blood Microsystem and PHM 73/Blood Gas Monitor, Radiometer, Copenhagen, Denmark; measurements corrected to 39°C), percentage saturation of haemoglobin (%satHb) and haemoglobin concentration ([Hb]; OSM2 Haemoximeter, Radiometer). Calibrated fetal arterial, venous and amniotic pressures were measured using disposable clinical pressure transducers and pressure amplifiers developed at the University of Maastricht. Fetal heart rate was derived from the arterial blood pressure pulse. Carotid and femoral blood flows were measured on a T206 flow box (Transonic Inc.). All cardiovascular variables were recorded continuously using a data acquisition system which sampled all signals at a rate of 500 Hz. Cardiovascular analogue data were digitized, displayed and stored on disk by custom software (HDAS developed at the University of Maastricht) running on an IBM compatible computer. Digital files were subsequently analysed using Microsoft Excel spreadsheets.
On completion of the experiments the llama was anaesthetized under halothane and nitrous oxide as previously described (Giussani et al. 1996). A Caesarean section was performed and the fetus was removed, injected with sodium thiopentone i.v. (1 g Tiopental Sódico, Laboratorio Chile) and killed with saturated KCl injected i.v. The llama fetus was then dissected and the flow probe removed after verification of the correct surgical implantation sites. The maternal tissues were closed and the adult llama was left to recover.
Measurements and calculations
Fetal arterial and venous pressures were corrected for amniotic pressure. Fetal carotid and femoral vascular resistances were calculated by dividing fetal perfusion pressure (A-V) by fetal carotid and femoral blood flows, respectively. Cardiovascular data collected at 500 Hz were averaged over every minute.
Maternal and fetal arterial blood oxygen content (O2,cont) and capacity (O2,cap) and oxygen delivery (O2,del) were calculated using eqns (1), (2) and (3), respectively:
| (1) |
| (2) |
| (3) |
Statistical analyses
Values for all variables are expressed as means ±s.e.m. Data were analysed first by the summary of measures method (Matthews et al. 1990) to focus the number of comparisons. All measured variables were compared between normoxia and hypoxaemia or recovery using one-way ANOVA for repeated measures followed by the Student-Newman-Keuls test or using Student's t test for paired data with the Bonferroni correction. Comparisons between groups of data were made using Student's t test for unpaired data. For all statistical comparisons differences were considered significant when P < 0.05.
RESULTS
Survival
While treatment of llama fetuses with the V1-receptor antagonist d-(CH2)51-Tyr(Me)2,Arg8)-vasopressin did not affect their capacity to withstand the 1 h period of acute hypoxaemia, when fetuses were treated with phentolamine they all died within 20 min of the onset of the hypoxaemic challenge. When the fetuses were treated with phentolamine the pattern of cardiovascular collapse during hypoxaemia was similar. The length of time during which this pattern occurred varied from fetus to fetus but in all of them a time was reached during which there was a parallel fall in fetal arterial blood pressure and fetal heart rate leading to cardiovascular collapse (Fig. 1).
Figure 1. Fetal cardiovascular responses to acute hypoxaemia.
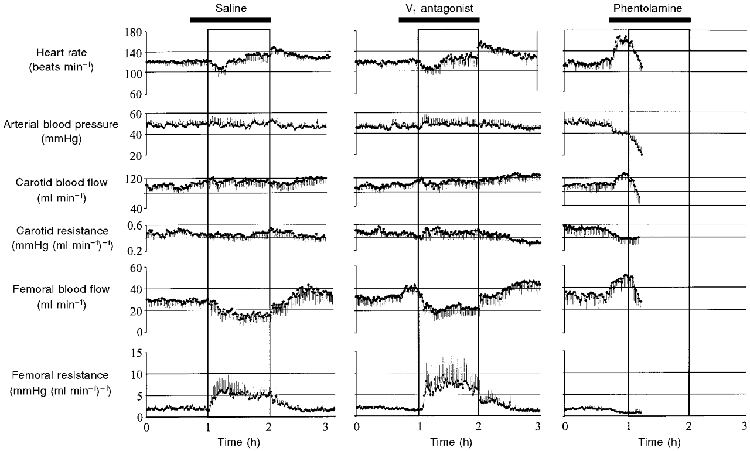
Values are means ±s.e.m. of every minute for cardiovascular variables during the experimental protocol when the fetuses were infused with saline (n = 6), V1 antagonist (n = 6), or phentolamine (n = 6). The boxes represents the episode of acute hypoxaemia. Saline or antagonist infusion was started 15 min prior to hypoxaemia and ran continuously until the end of the hypoxaemic challenge (bar). All 6 fetuses died during hypoxaemia when treated with phentolamine. Because the time course of fetal cardiovascular collapse in hypoxaemia during phentolamine treatment was different from fetus to fetus, the last data point shown for all cardiovascular variables in phentolamine-treated fetuses represents the mean ±s.e.m. when all 6 fetuses were still alive.
Arterial blood gas status
Maternal
Changes in maternal arterial blood gas status are shown in Table 1. All values pertinent to maternal blood gas status measured during saline infusion were unchanged by fetal treatment with either antagonist during normoxia. Therefore, pre-infusion values and values during infusion for maternal arterial blood gases and pH were pooled for the baseline period. During hypoxaemia, maternal Pa,O2 and %satHb were reduced to similar levels during saline, V1 antagonist or phentolamine infusion. Maternal Pa,CO2 values measured in hypoxaemia during saline and V1 antagonist infusion were lower than respective values measured in normoxia. Maternal O2,cap during hypoxaemia increased from baseline during V1 antagonist infusion.
Table 1.
Pregnant llama arterial blood gases and acid/base status
| Normoxia | Hypoxia | Recovery | |
|---|---|---|---|
| pHa | |||
| Saline | 7.43 ± 0.02 | 7.49 ± 0.02 | 7.46 ± 0.02 |
| V1 antagonist | 7.46 ± 0.02 | 7.50 ± 0.02 | 7.49 ± 0.02 |
| Phentolamine | 7.44 ± 0.01 | 7.46 ± 0.05 | — |
| Pa,CO2(mmHg) | |||
| Saline | 36.8 ± 1.1 | 32.8 ± 0.8* | 34.1 ± 1.6 |
| V1 antagonist | 36.4 ± 0.6 | 31.9 ± 1.1* | 33.7 ± 1.5 |
| Phentolamine | 33.9 ± 3.4 | 34.7 ± 3.1 | — |
| Pa,O2(mmHg) | |||
| Saline | 89.8 ± 1.7 | 34.5 ± 1.7* | 86.0 ± 3.4 |
| V1 antagonist | 88.8 ± 3.1* | 32.4 ± 2.5* | 81.4 ± 3.9 |
| Phentolamine | 85.3 ± 5.6 | 39.0 ± 5.9* | — |
| %satHb | |||
| Saline | 93.5 ± 1.6 | 74.6 ± 2.3* | 93.5 ± 1.5 |
| V1 antagonist | 94.5 ± 1.2 | 71.3 ± 3.8* | 94.3 ± 1.6 |
| Phentolamine | 93.2 ± 2.1 | 68.7 ± 13.5* | — |
| [Hb](g dl−1) | |||
| Saline | 9.8 ± 0.3 | 11.0 ± 0.4 | 10.2 ± 0.3 |
| V1 antagonist | 9.9 ± 0.4 | 11.7 ± 0.5 | 10.2 ± 0.4 |
| Phentolamine | 9.8 ± 0.5 | 10.9 ± 0.4 | — |
| O2 capacity (vol. % O2) | |||
| Saline | 13.1 ± 0.4 | 14.7 ± 0.5 | 13.6 ± 0.4 |
| V1 antagonist | 13.3 ± 0.6 | 15.6 ± 0.6* | 13.6 ± 0.5 |
| Phentolamine | 13.1 ± 0.7 | 14.7 ± 0.5 | — |
| O2 content (vol. % O2) | |||
| Saline | 12.3 ± 0.5 | 11.0 ± 0.6 | 12.8 ± 0.5 |
| V1 antagonist | 12.6 ± 0.6 | 11.1 ± 0.8 | 12.9 ± 0.7 |
| Phentolamine | 12.3 ± 0.9 | 10.1 ± 2.2 | — |
| Base excess (mequiv l−1) | |||
| Saline | −0.6 ± 1.1 | 1.7 ± 1.3 | 0.9 ± 1.4 |
| V1 antagonist | 1.9 ± 1.2 | 1.3 ± 0.9 | 2.3 ± 0.6 |
| Phentolamine | −1.1 ± 1.7 | 1.0 ± 2.5 | — |
Values are means ± s.e.m. of all values obtained during each of the experimental hours when the fetuses were infused with saline, V1 antagonist, or phentolamine. pHa, arterial pH; Pa,CO2, arterial CO2 pressure; Pa,O2, arterial O2 pressure; %satHb, percentage saturation of Hb. Significant differences (P < 0.05)
hypoxia or recovery vs. normoxia (ANOVA + Student-Newman-Keuls test).
Fetal
Changes in fetal arterial blood gas status are shown in Table 2. In normoxia, values for fetal arterial blood gases and acid/base status during saline infusion were unchanged following treatment with either V1-receptor antagonist or phentolamine. Therefore, pre-infusion values and values during infusion for fetal arterial blood gases and pH were also pooled for the baseline period. In hypoxaemia, fetal Pa,O2, %satHb and O2,cont were significantly reduced from baseline during saline, V1 antagonist or phentolamine infusion. Five minutes following the onset of hypoxaemia, fetal Pa,O2 was similarly reduced in all three groups (from 22.8 ± 1.4 to 12.2 ± 0.8 mmHg during saline infusion; from 22.3 ± 1.2 to 12.4 ± 1.9 mmHg during V1-receptor antagonist infusion; from 20.0 ± 2.0 to 12.2 ± 2.0 mmHg during phentolamine infusion). However, while the reductions in Pa,O2, %satHb and O2,cont remained similar for the duration of the hypoxaemic challenge during saline and V1-receptor antagonist infusion, values for these variables fell to a greater extent in phentolamine-treated fetuses 15 min following the onset of the hypoxaemic challenge (Table 2). Accordingly, fetuses treated with phentolamine developed pronounced acidosis during hypoxaemia as indexed by significant falls in arterial pH and in base excess and a tendency for Pa,CO2 to increase before cardiovascular collapse. In contrast, a small reduction in fetal Pa,CO2 occurred during hypoxaemia when fetuses were either infused with saline or treated with V1 antagonist. During recovery fetal arterial pH and acid/base excess became significantly reduced from values in normoxia following either saline or V1 antagonist infusion. Values for fetal Pa,CO2 and %satHb remained altered from values in normoxia by the end of the experimental protocol when the fetuses were infused with saline.
Table 2.
Fetal llama arterial blood gases and acid/base status
| Normoxia | Hypoxia | Recovery | |
|---|---|---|---|
| pHa | |||
| Saline | 7.36 ± 0.03 | 7.34 ± 0.02 | 7.24 ± 0.03* |
| V1 antagonist | 7.36 ± 0.02 | 7.33 ± 0.02 | 7.24 ± 0.04* |
| Phentolamine | 7.35 ± 0.02 | 7.17 ± 0.02*† | — |
| Pa,CO2 (mmHg) | |||
| Saline | 46.0 ± 1.2 | 39.7 ± 1.0* | 40.9 ± 1.8* |
| V1 antagonist | 44.9 ± 1.0 | 41.0 ± 1.1* | 41.9 ± 1.4 |
| Phentolamine | 43.4 ± 3.5 | 48.8 ± 4.1 | — |
| Pa,O2(mmHg) | |||
| Saline | 22.8 ± 1.4 | 12.4 ± 0.7* | 20.8 ± 1.5 |
| V1 antagonist | 22.3 ± 1.2 | 12.0 ± 0.4* | 20.2 ± 0.9 |
| Phentolamine | 20.0 ± 2.0 | 9.8 ± 0.3*† | — |
| %satHb | |||
| Saline | 52.6 ± 3.5 | 24.1 ± 3.3* | 41.5 ± 3.5* |
| V1 antagonist | 51.4 ± 4.5 | 24.4 ± 2.8* | 42.9 ± 3.4 |
| Phentolamine | 42.8 ± 6.2 | 15.7 ± 3.2*† | — |
| [Hb](g dl−1) | |||
| Saline | 11.5 ± 0.5 | 12.3 ± 0.3 | 11.9 ± 0.5 |
| V1 antagonist | 11.6 ± 0.6 | 12.8 ± 0.5 | 11.4 ± 0.7 |
| Phentolamine | 11.5 ± 0.8 | 10.1 ± 0.8 | — |
| O2 capacity (vol. % O2) | |||
| Saline | 15.4 ± 0.6 | 16.4 ± 0.4 | 15.9 ± 0.7 |
| V1 antagonist | 15.5 ± 0.8 | 17.1 ± 0.7 | 15.2 ± 0.9 |
| Phentolamine | 15.3 ± 1.0 | 13.5 ± 1.1† | — |
| O2 content (vol. % O2) | |||
| Saline | 8.1 ± 0.5 | 4.0 ± 0.6* | 6.8 ± 0.7 |
| V1 antagonist | 7.9 ± 0.5 | 4.1 ± 0.5* | 6.5 ± 0.4 |
| Phentolamine | 6.7 ± 1.2 | 2.0 ± 0.3*† | — |
| Base excess (mequiv l−1) | |||
| Saline | −0.3 ± 1.5 | −4.0 ± 1.5 | −8.9 ± 1.7* |
| V1 antagonist | −0.9 ± 0.6 | −4.7 ± 1.3 | −8.3 ± 2.0* |
| Phentolamine | −2.0 ± 0.8 | −10.8 ± 1.4*† | — |
Values are means ± s.e.m. of all values obtained during each of the experimental hours when the fetuses were infused with either saline or V1 antagonist. Values for phentolamine-treated fetuses during hypoxaemia represent the mean ± s.e.m. of the last blood sample taken during acute hypoxaemia prior to death. pHa, arterial pH; Pa,CO2, arterial CO2 pressure; Pa,O2, arterial O2 pressure; %satHb, percentage saturation of Hb. Significant differences (P < 0.05):
hypoxia or recovery vs. normoxia (ANOVA + Student-Newman-Keuls test)
V1 antagonist or phentolamine vs. saline (Student's t test for unpaired data).
Cardiovascular variables
Normoxia
Pre-infusion values for all measured cardiovascular variables were similar for all treatment groups. While treatment of llama fetuses with the V1 antagonist during normoxia had no effect on fetal heart rate, fetal arterial blood pressure or fetal carotid haemodynamics, a significant increase in fetal femoral blood flow and a significant fall in fetal femoral vascular resistance occurred during normoxia immediately following the onset of V1 antagonist infusion (Fig. 1 and Fig. 2). In contrast, treatment of fetuses with phentolamine during normoxia produced significant hypotension and tachycardia, in addition to significant vasodilatation in both the carotid and the femoral circulations (Fig. 1 and Fig. 2).
Figure 2. Statistical summary of fetal cardiovascular responses to acute hypoxaemia.
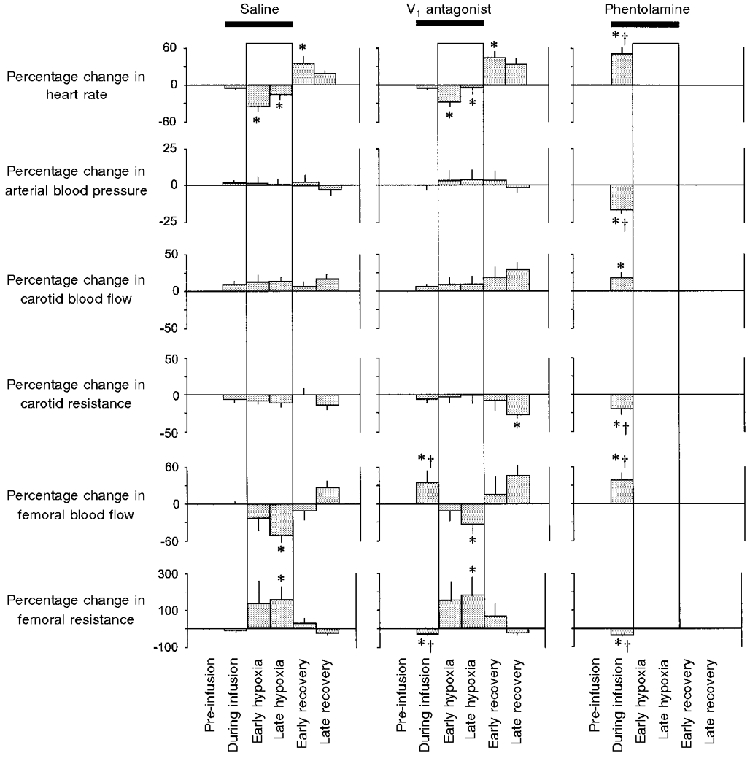
Histograms are the means ±s.e.m. of the 45 min prior to the onset of infusion during normoxia (pre-infusion period), the 15 min of infusion in normoxia (during infusion period), the 15 min following the onset of hypoxaemia (early hypoxia period), the remaining 45 min of hypoxaemia (late hypoxia period), the 15 min following the end of hypoxia (early recovery period), and the remaining 45 min of the experimental protocol (late recovery period). Histograms are percentage increment or decrement from the pre-infusion period. The boxes represents the episode of acute hypoxaemia. Saline or antagonist infusion was started 15 min prior to hypoxaemia and ran continuously until the end of the hypoxaemic challenge (bar). Significant differences (P < 0.05): * all periods vs. pre-infusion period (ANOVA + Student-Newman-Keuls test); † antagonist vs. saline (Student's t test for unpaired data).
Hypoxaemia
When the llama fetuses were infused with saline they showed typical cardiovascular responses to the acute hypoxaemic challenge (Giussani et al. 1996). These included a transient bradycardia, after which fetal heart rate returned to, or to above, pre-hypoxaemic levels, and an intense fetal femoral vasoconstriction which was maintained throughout the duration of the hypoxaemia (Fig. 1 and Fig. 2). Pre-treatment of the llama fetuses with the V1 antagonist did not alter these fetal cardiovascular responses to acute hypoxaemia (Fig. 1 and Fig. 2). In contrast, pre-treatment of the llama fetuses with phentolamine not only abolished the increase in fetal femoral vascular resistance but prevented survival of all fetuses during hypoxaemia (Fig. 1 and Fig. 2).
Recovery
Following the end of hypoxaemia, when the llama fetuses had been infused with saline, a significant increase in fetal heart rate occurred which returned to baseline levels by the end of the recovery period. Similarly, the fall in fetal femoral blood flow and the increase in fetal femoral vascular resistance returned to baseline during recovery (Fig. 1 and Fig. 2). Treatment of the llama fetuses with the V1 antagonist did not alter the rebound tachycardia during early recovery but a significant fall in carotid vascular resistance occurred by the end of the recovery period when the llama fetuses had been treated with the V1 antagonist (Fig. 1 and Fig. 2).
DISCUSSION
This study tested the hypothesis that the intense femoral vasoconstriction during acute hypoxaemia in the llama fetus has potent α-adrenergic or V1-vasopressinergic components. A V1 antagonist did not affect the fetal cardiovascular responses to acute hypoxaemia, but treatment of fetal llamas with the α-adrenergic antagonist phentolamine not only abolished the femoral vasoconstriction but prevented fetal survival during acute hypoxaemia.
Although the fetal cardiovascular responses to a single episode of acute hypoxaemia in late gestation have been well characterized (see Giussani et al. 1994b), little evidence is available on how these cardiovascular defence mechanisms may be modified by fetal pre-exposure to chronic hypoxaemia earlier in gestation. We have addressed some of these questions in the llama, a species that has evolved under the influence of the chronic hypobaric hypoxia of life at altitude, and one that demonstrates genetic adaptations that persist even when born and living at sea level. For example, we and others have previously demonstrated that the adult llama has a high blood oxygen affinity, which is typical of mammals genotypically adapted to hypoxia. More importantly, this affinity does not change whether the adult llama has been reared at high or low altitude (Banchero & Grover, 1972; Moraga et al. 1996). Furthermore, when the llama fetus is subjected to acute hypoxaemia, it responds with an intense peripheral vasoconstriction which is 4-5 times greater, even at 60-70 % of gestation, than that measured in late gestation fetal sheep subjected to the same degree and duration of hypoxaemia (Giussani et al. 1994b, 1996). This intense peripheral vasoconstriction during acute hypoxaemia persists in llama fetuses even when carried by mothers born and living at sea level (Giussani et al. 1996). Differences in the cardiovascular responses to acute hypoxaemia between the fetal sheep and the fetal llama are likely to result from chronic adaptation to the environment, rather than from species differences alone, since the fetal cardiovascular response to acute hypoxaemia in other lowland species such as the human and the chick fetus resemble that of sheep, and not that of the llama. For example, while human (Wladimiroff et al. 1986), sheep (Giussani et al. 1994b) and chick (Mulder et al. 1998) fetuses increase blood flow to the brain during hypoxaemia, cerebral blood flow remains unaltered in the llama fetus during acute hypoxaemia (Giussani et al. 1996).
Interestingly, the intense peripheral vasoconstriction during acute hypoxaemia in the llama fetus is unaltered by section of the carotid sinus nerves (Giussani et al. 1996). These findings imply that the intense peripheral vasoconstriction during acute hypoxaemia in the fetal llama is mediated through chemoreflex-independent, possibly endocrine mechanisms. We have reported that in the llama fetus at 60-70 % of gestation, vasopressin reaches plasma concentrations 7-8 times greater than those measured in late gestation fetal sheep during acute hypoxaemia (Giussani et al. 1994b, 1996). However, our results show that pre-treatment with the V1 antagonist did not alter the fetal femoral vascular resistance response to acute hypoxaemia in the llama. On the other hand, α-adrenergic blockade during acute hypoxaemia not only abolished the femoral vasoconstriction, but prevented fetal survival during hypoxaemia in the llama fetus. This is in marked contrast to the sheep fetus in which α-adrenergic blockade also abolished the femoral vasoconstriction, but only prevented fetal survival during hypoxaemia when combined with carotid sinus nerve section (Giussani et al. 1993).
Therefore, taken together, previous reports in the literature suggest that sustained hypoxaemia, such as that associated with pregnancy at altitude, may increase the relative importance of α-adrenergic components in the fetal cardiovascular response. Secondly, in lowland species, such as the sheep, α-adrenergic mechanisms in combination with carotid chemoreflexes are indispensable to fetal survival during acute hypoxaemia. In contrast, enhancement of endocrine vs. chemoreflex contributions to fetal cardiovascular responses to acute hypoxaemia in high altitude species renders the llama fetus more vulnerable than the sheep fetus to α-adrenergic blockade during acute hypoxaemia.
These contentions are of paramount clinical importance to pregnancy both at altitude and at sea level since under situations of adverse intrauterine conditions, α-adrenergic compensation may determine fetal survival in subsequent acute hypoxaemia, such as that associated with labour and delivery (Airede & Weerasinghe, 1995; Rurak et al. 1997). One previous study investigated the cardiovascular responses to acute hypoxaemia of chronically hypoxaemic, growth-retarded fetal sheep, secondary to embolization of the uteroplacental vascular bed (Block et al. 1984). Interestingly, in the Discussion the authors state: ‘We have not published our results in regard to α-adrenergic activity, mainly because of our lack of success in keeping alive a growth-retarded sheep fetus in whom we instituted α-adrenergic blockade. All such fetuses have died.’
The mechanism mediating enhanced α-adrenergic responses during acute hypoxaemia in the peripheral vasculature of the llama fetus remains unclear. In sheep fetal cerebral and uterine arteries, long-term high altitude exposure is associated with a decrease in both α1-adrenoreceptor density and receptor-second messenger coupling (Zhang, 1998). Any information available on the effects of chronic hypoxaemia on peripheral vasoreactivity is conflicting. Investigators have reported both increased (Aoki & Robinson, 1969; Harrison et al. 1986) and decreased (Doyle & Walker, 1991) peripheral vasoreactivity to α-adrenergic agonists in the chronically hypoxic rat and guinea-pig. Information on peripheral vasoreactivity in response to vasoconstrictor/vasodilator agents in either the llama fetus or the chronically hypoxic sheep fetus is not available to date.
Given the significant elevations in circulating plasma vasopressin during acute hypoxaemia in the llama fetus and the lack of an effect of V1-receptor blockade on this response, the functional role of this hormone during acute hypoxaemia in the fetal llama is not clear. One possibility is that the dose of V1-receptor antagonist used in the present study was insufficient to block the actions of endogenous circulating vasopressin in the llama fetus during acute hypoxaemia, although the dose of V1 antagonist we used did block the potent vasoconstrictor effects of doses of synthetic vasopressin. Another possibility is that vasopressin mediates vasoconstriction in the llama fetus in peripheral vascular beds other than those represented by changes in femoral vascular resistance. Alternatively, increased concentrations of vasopressin may compensate for V1-receptor downregulation following exposure to sustained hypoxaemia, as suggested by the blunted systemic pressor response to vasopressin in hypoxia-adapted rats compared with control rats (Jin et al. 1989).
In the current manuscript treatment of fetal llamas with the V1-receptor antagonist or phentolamine also produced significant cardiovascular changes during normoxia. Both antagonists induced femoral vasodilatation, but only phentolamine induced a fall in carotid vascular resistance during normoxia. These findings suggest significant basal V1 vasoconstrictor tone to the femoral vascular bed and α-adrenergic vasoconstrictor tone to both the carotid and femoral circulations. A fall in fetal arterial blood pressure occurred following the onset of phentolamine, but not V1 antagonist, treatment. This difference may emphasize a greater α-adrenergic contribution to vasoconstrictor tone not only during hypoxia but also during normoxia in the llama fetus. Significant tachycardia after phentolamine during normoxia may result from a combination of a baroreflex and enhanced synaptic noradrenaline release due to pre-synaptic α2-adrenergic blockade by phentolamine (see Langer, 1977; Giussani et al. 1993). While the exact cause of death of the llama fetus during phentolamine treatment in hypoxaemia is unknown, it is likely that peripheral vasodilatation following phentolamine treatment may have led to pronounced hypotension, myocardial hypoperfusion and heart failure.
In conclusion, the data reported in this manuscript do not support the hypothesis that the enhanced femoral vasoconstriction during acute hypoxaemia in the llama fetus is mediated by vasopressin. These data rather emphasize not only that femoral vasoconstriction during acute hypoxaemia in the llama fetus is dependent on α-adrenergic pathways, but also that α-adrenergic efferent mechanisms are indispensable to fetal survival during hypoxaemia in the llama since their abolition leads to cardiovascular collapse and death.
Acknowledgments
We wish to thank Miss Tania Fergusson, Mr Emilio Herrera, Mr Criatian Gajardo, Mr Carlos Brito and Mr Hernán Riquelme for their collaboration on these studies and Mrs Margaret Bardy for her help with the manuscript. These studies were funded by The Wellcome Trust and Fondecyt 197-0236.
References
- Airede AI, Weerasinghe HD. Birth asphyxia. East African Medical Journal. 1995;72:252–257. [PubMed] [Google Scholar]
- Alexander DP, Bashore RA, Britton HG, Forsling ML. Maternal and fetal arginine vasopressin in the chronically catheterised sheep. Biology of the Neonate. 1974;25:242–248. doi: 10.1159/000240696. [DOI] [PubMed] [Google Scholar]
- Aoki VS, Robinson SM. Hindquarters vascular responses in chronically hypoxic rats. American Journal of Physiology. 1969;217:661–665. doi: 10.1152/ajplegacy.1969.217.3.661. [DOI] [PubMed] [Google Scholar]
- Banchero N, Grover RF. Effect of different levels of simulated altitude on O2 transport in the llama and in the sheep. American Journal of Physiology. 1972;222:1239–1245. doi: 10.1152/ajplegacy.1972.222.5.1239. [DOI] [PubMed] [Google Scholar]
- Bartelds B, van Bel F, Teitel DF, Rudolph AM. Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatric Research. 1993;34:51–55. doi: 10.1203/00006450-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Block BS, Llanos AJ, Creasy RK. Responses of the growth-retarded fetus to acute hypoxemia. American Journal of Obstetrics and Gynecology. 1984;148:878–885. doi: 10.1016/0002-9378(84)90529-5. [DOI] [PubMed] [Google Scholar]
- Doyle MP, Walker BR. Attenuation of systemic vasoreactivity in chronically hypoxic rats. American Journal of Physiology. 1991;260:R1114–1122. doi: 10.1152/ajpregu.1991.260.6.R1114. [DOI] [PubMed] [Google Scholar]
- Fowler ME. Medicine & Surgery of South American Camelids: Llama, Alpaca, Vicuña, Guanaco. Ames, IA, USA: Iowa State University Press; 1989. pp. 126–127. [Google Scholar]
- Giussani DA, McGarrigle HHG, Spencer JAD, Moore PJ, Bennet L, Hanson MA. Effect of carotid denervation on plasma vasopressin levels during acute hypoxia in the late-gestation sheep fetus. The Journal of Physiology. 1994a;477:81–87. doi: 10.1113/jphysiol.1994.sp020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Spencer J, Hanson MA. Fetal cardiosvascular reflex responses to hypoxaemia. Fetal and Maternal Medicine Review. 1994b;6:17–37. [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. The Journal of Physiology. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Riquelme RA, Moraga FA, McGarrigle HHG, Gaete CR, Sanhueza EM, Hanson MA, Llanos AJ. Chemoreflex and endocrine components of cardiovascular responses to acute hypoxemia in the llama fetus. American Journal of Physiology. 1996;271:R73–83. doi: 10.1152/ajpregu.1996.271.1.R73. [DOI] [PubMed] [Google Scholar]
- Harrison GL, McMurtry IF, Moore LG. Meclofenamate potentiates vasoreactivity to α-adrenergic stimulation in chronically hypoxic guinea pigs. American Journal of Physiology. 1986;251:H496–501. doi: 10.1152/ajpheart.1986.251.3.H496. [DOI] [PubMed] [Google Scholar]
- Jin H, Yang R-H, Chen Y-F, Thornton RM, Jackson RM, Oparil S. Hemodynamic effects of arginine vasopressin in rats adapted to chronic hypoxia. Journal of Applied Physiology. 1989;66:151–160. doi: 10.1152/jappl.1989.66.1.151. [DOI] [PubMed] [Google Scholar]
- Jones CT, Robinson RO. Plasma catecholamines in fetal and adult sheep. The Journal of Physiology. 1975;285:381–393. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CT, Wei G. Adrenal-medullary activity and cardiovascular control in the fetal sheep. In: Kunzel W, editor. Fetal Heart Rate Monitoring. Berlin: Springer-Verlag; 1985. pp. 127–135. [Google Scholar]
- Langer SZ. Presynaptic receptors and their role in the regulation of transmitter release. Sixth Gaddum Memorial Lecture. National Institute for Medical Research. British Journal of Pharmacology. 1977;60:481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. British Medical Journal. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga F, Monge C, Riquelme R, Llanos AJ. Fetal and maternal blood oxygen affinity: A comparative study in llamas and sheep. Comparative Biochemistry and Physiology. 1996;115A:111–115. doi: 10.1016/0300-9629(96)00016-3. [DOI] [PubMed] [Google Scholar]
- Mulder ALM, van Golde JC, Prinzen FW, Blanco CE. Cardiac output distribution in response to hypoxia in the chick embryo in the second half of incubation time. The Journal of Physiology. 1998;508:281–287. doi: 10.1111/j.1469-7793.1998.281br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parer JT. Effect of atropine on heart rate and oxygen consumption of the hypoxic fetus. American Journal of Obstetrics and Gynecology. 1984;148:1118–1122. doi: 10.1016/0002-9378(84)90638-0. [DOI] [PubMed] [Google Scholar]
- Perez R, Espinoza M, Riquelme R, Parer JT, Llanos AJ. Arginine vasopressin mediates cardiovascular responses to hypoxemia in fetal sheep. American Journal of Physiology. 1989;256:R1011–1018. doi: 10.1152/ajpregu.1989.256.5.R1011. [DOI] [PubMed] [Google Scholar]
- Reuss ML, Parer JT, Harris JL, Kreuger TR. Hemodynamic effects of alpha-adrenergic blockade during hypoxia in fetal sheep. American Journal of Obstetrics and Gynecology. 1982;142:410–415. doi: 10.1016/s0002-9378(16)32381-x. [DOI] [PubMed] [Google Scholar]
- Riquelme RA, Giussani DA, McGarrigle HHG, Sanhueza EM, Hanson MA, Llanos JA. Chemoreflex contribution to adrenocortical function during acute hypoxemia in the llama fetus at 0.6–0.7 of gestation. Endocrinology. 1998;139:2564–2570. doi: 10.1210/endo.139.5.6010. [DOI] [PubMed] [Google Scholar]
- Rurak DW, Tan W, Riggs KW, Stobbs KE, Kwan E, Hall C. Circulatory and metabolic changes in fetal sheep during labor. Journal of the Society for Gynecologic Investigation. 1997;4:166A. [Google Scholar]
- Wladimiroff JW, Tonge HM, Stewart PA. Doppler ultrasound assessment of cerebral blood flow in the human fetus. British Journal of Obstetrics and Gynecology. 1986;93:471–475. [PubMed] [Google Scholar]
- Zhang LB. Adaptation of pharmacomechanical coupling of vascular smooth muscle to chronic hypoxia. Comparative Biochemistry and Physiology. 1998;119A:661–667. doi: 10.1016/s1095-6433(98)01002-2. A. [DOI] [PubMed] [Google Scholar]


