Abstract
Head and gaze movements are usually highly co-ordinated. Here we demonstrate that under certain circumstances they can be controlled independently and we investigate the role of anticipatory activity in this process.
In experiment 1, subjects tracked, with head and eyes, a sinusoidally moving target. Overall, head and gaze trajectories were tightly coupled. From moment to moment, however, the trajectories could be very different and head movements were significantly more variable than gaze movements.
Predictive head and gaze responses can be elicited by repeated presentation of an intermittently illuminated, constant velocity target. In experiment 2 this protocol elicited a build-up of anticipatory head and gaze velocity, in opposing directions, when subjects made head movements in the opposite direction to target movement whilst maintaining gaze on target.
In experiment 3, head and gaze movements were completely uncoupled. Subjects followed, with head and gaze, respectively, two targets moving at different, harmonically unrelated frequencies. This was possible when both targets were visual, and also when gaze followed a visual target at one frequency whilst the head was oscillated in time with an auditory tone modulated at the second frequency.
We conclude that these results provide evidence of a visuomotor predictive mechanism that continuously samples visual feedback information and stores it such that it can be accessed by either the eye or the head to generate anticipatory movements. This overcomes time delays in visuomotor processing and facilitates time-sharing of motor activities, making possible the performance of two tasks simultaneously.
In many everyday activities, humans carry out more than one motor task simultaneously, for example whilst driving a car. However, in the laboratory this is not often tested and many experimental situations tend to elicit synchronized motor behaviour. An example of this is the production of combined head and gaze movements. Under most circumstances, movements of the head and eyes appear highly co-ordinated, particularly during gaze shifting. Indeed, as a result of the examination of behavioural responses in humans (Barnes, 1979; Zangemeister & Stark, 1982a,b; Guitton & Volle, 1987; Ron & Berthoz, 1991) and more basic neurophysiology in other species (Bizzi et al. 1971; Grantyn & Berthoz, 1988; Guitton & Munoz, 1991), much attention has been given to the possibility that common drive mechanisms may be responsible for this co-ordination. More recently, evidence has emerged (Ron & Berthoz, 1991) that the head and eyes may be dissociated in less predictable conditions. We have previously shown that prediction plays a large part in the control of head and gaze movements during head-free pursuit (Barnes & Grealy, 1992). In the experiments described here we specifically sought to establish what role this predictive process might play when head and gaze are deliberately dissociated.
A major problem in attempting to study co-ordinated head and gaze movements is that they are inevitably coupled, because rotation of the head provides an angular acceleration stimulus to the semi-circular canals of the inner ear. In darkness, this stimulus would evoke a vestibulo-ocular reflex (VOR) response that would drive the eyes with a velocity close to that of the head, but in the opposite direction, so that the eyes would be stabilized in space. During head-free pursuit of a continuously moving target, this VOR response would be counter-productive, as it would drive the eye in the opposite direction to the target. Under these circumstances, it would be advantageous to suppress the VOR so that the eye could move with the head. But, if head movement does not completely match target movement, an additional pursuit response must be generated so that gaze velocity (eye velocity in space) can match target velocity.
Although this process might appear complex, it is likely that both VOR suppression and pursuit components are controlled simultaneously by the same mechanism during head-free pursuit. The complexities involved, such as changes in behaviour with changes in stimulus frequency and velocity, have been reviewed elsewhere (Barnes, 1993) and for the purpose of the current study will not be considered further. In summary, there is strong evidence (Barnes et al. 1978; Lau et al. 1978; Koenig et al. 1978; Koenig et al. 1986) that the neurological mechanisms, derived from visual feedback, that are responsible for the head-fixed pursuit response are also responsible for visual suppression of the VOR. On this basis, head-free pursuit could be thought of as a process in which the VOR continuously nulls gaze velocity, leaving the pursuit mechanisms to control gaze velocity. This has the conceptual advantage that gaze control would be unaffected by differing levels of head movement and thus gaze and head movements could be quite independent. This situation would only be true if VOR gain were unity (i.e. if it exactly compensated for head movement) and all VOR suppression were carried out by visual feedback mechanisms. During active head rotations VOR gain is generally close to unity (Barnes, 1993), but the evidence regarding VOR suppression is more difficult to interpret. There is considerable evidence to support the existence of non-visual VOR suppression mechanisms both in humans (Gauthier & Robinson, 1975; Barr et al. 1976; McKinley & Peterson, 1985; Barnes & Eason, 1988) and in monkeys (Lisberger, 1990; Cullen et al. 1991), but the relevance to head-free pursuit is not clear. It has been argued (Robinson, 1982) that a non-visual VOR suppression mechanism may be of advantage during head-free pursuit. Under natural conditions, a combined movement of the head and eyes is usually induced when target displacement is large and hence target velocity is likely to be high (> 60 deg s−1). It has been shown that the head-fixed pursuit response begins to deteriorate at such velocities due to non-linear saturation effects in the visual feedback system (Lisberger et al. 1981; Barnes, 1993). So, if the VOR were suppressed by non-visual mechanisms and pursuit only had to make up the difference in velocity between the head and target, these saturation effects would become less significant. However, when head-free and head-fixed pursuit tasks have been directly compared (Barnes & Lawson, 1989) no evidence has been found to support this concept.
One feature that is clear is that prediction can play a very large part in VOR suppression. This was demonstrated by Barnes & Grealy (1992) when subjects fixated a head-fixed target during passive whole-body rotation on a turntable. The turntable moved sinusoidally but the head-fixed target was only visible to the subject for very brief durations as the turntable passed through the centre of its trajectory (i.e. at peak head velocity). A similar protocol had been used previously (Barnes & Asselman, 1991) to examine the predictive mechanisms involved in head-fixed pursuit of an intermittently illuminated target. It elicits a highly reproducible response - smooth eye velocity builds up over the first few presentations of the target and becomes progressively more phase advanced with respect to the onset of target appearance. After three or four presentations of the target a steady-state response is established, in which smooth eye velocity is initiated well in advance of the appearance of the target, i.e. the response has become predictive. When viewing a briefly illuminated head-fixed target during passive whole-body rotation on a turntable (Barnes & Grealy, 1992), a similar build-up of predictive activity was seen in the VOR suppression signal. Moreover, the authors showed that this stereotyped predictive activity is also seen in both head and gaze signals during head-free pursuit of such a stimulus.
As a result of these experiments it was suggested that pursuit and VOR suppression share a common predictive mechanism. It was postulated that eye movement control involves two feedback pathways - one which provides straightforward feedback of retinal velocity error and another which samples and stores the pre-motor drive signal (derived from this afferent velocity error signal) and subsequently uses it to boost the drive to the extra-ocular muscles. According to this hypothesis, it is this second pathway that is responsible for the build-up and maintenance of anticipatory activity with repeated presentation of the stimulus. During head-fixed pursuit this pre-motor drive signal generates eye velocity directly, whereas during head-free pursuit it is used both to suppress the vestibular drive from the semi-circular canals and to provide any additional pursuit required. Given this hypothesis, it would be of interest to determine what evidence of predictive characteristics might emerge if head and gaze were dissociated. That is the subject of the current experiments.
The experiments had several specific aims. First, we wanted to assess the extent to which head and gaze are uncoupled from moment to moment during normal co-ordinated head-free pursuit. Although this has been noted previously (Gresty & Leech, 1977; Lanman et al. 1978), the effect has not been quantified. Second, we wanted to use the technique employed previously by Barnes & Grealy (1992) to examine more closely the build-up of anticipatory head and gaze responses. Specifically, we wanted to investigate whether it would be possible to build up independent anticipatory activity for head and gaze, in opposing directions, by instructing the subject to make head and gaze movements in opposite directions. In such a task, the VOR would drive the eye in the opposite direction to the head, but at best this would only achieve stabilization of the eye in space (i.e. zero gaze velocity). To drive gaze in the opposite direction to the head would therefore require an extra predictive pursuit component in the opposite direction to head movement. Having established that this could be done, our third objective was to investigate whether independent gaze and head control could be achieved under circumstances in which the head and gaze trajectories were very different. To achieve this, we devised a protocol that required subjects to produce simultaneous head and gaze movements at two independent frequencies. Overall, our objective was to show that anticipatory head and gaze movements might be used to facilitate time-sharing of motor control processes during tasks which require the simultaneous execution of both types of movement.
In all experiments, we are concerned principally with gaze and head movements because they are the components of movement most relevant to the tracking task. However, we will attempt to interpret the results in terms of the underlying implications for the pursuit and VOR mechanisms, based on the arguments set out above.
METHODS
Nine healthy subjects (five male, four female) participated in this study after giving their informed written consent. Their mean age was 34.0 years (range 25-51 years, s.d. 7.6 years). The study was performed in accordance with the Declaration of Helsinki and had local ethics committee approval.
Equipment
Subjects were seated in a darkened room at the centre of a semi-circular screen with a radius of 1.5 m. Eye movements were recorded from the left eye using an infra-red limbus tracking technique (Skalar Iris), with a resolution of 5-10 min of arc. The eye movement recorders were mounted on a lightweight helmet; a bite bar was used to ensure that the helmet and recorders were immobilized with respect to the head. Head rotation was transduced by a continuous turn potentiometer which was coupled to the top of the helmet by a flexible bellows coupling that was non-compliant in torsion.
Visual targets were projected onto the screen via a servomotor-controlled mirror; their motion was controlled by a computer-generated waveform. Target visibility was controlled by an electromagnetic shutter - targets could only be seen when the shutter was open. This ensured that the timing of target presentation could be precisely controlled. Target 1 consisted of a white circle, the radius of which subtended 50 min of arc at the eye, with superimposed cross-hairs. This target was used in experiments 1, 2 and 3 and was also the calibration target. Target 2 consisted of a green circle with the same dimensions as target 1. Target 2 was used in experiment 3, where it was displayed 3 deg above target 1. An auditory timing signal was provided in experiment 3 by a modulated audio signal which was delivered to the subject via a stationary loud speaker.
Protocols
Experiment 1
Subjects performed head-free pursuit of target 1, which was constantly visible and moved sinusoidally. The run was divided into five sectors, each consisting of a number of cycles of target movement (between 5 and 11, depending on target frequency). In each sector, the target moved at one of the following frequencies: 0.16, 0.32, 0.66, 0.96, or 1.25 Hz. Peak amplitude of the target was held constant at ± 30 deg for all sectors; peak velocity varied from ± 30.2 to ± 235.6 deg s−1, depending on frequency. Head and eye displacement signals were sampled at a frequency of 100 Hz.
Experiment 2
The motion of target 1 was a constant velocity ramp, alternating in direction every 2.16 s. The target was illuminated intermittently (controlled by the shutter), such that it was only visible for 240 ms as it passed through the centre of its trajectory. The run was divided into five sectors, each consisting of a number of cycles of target movement (either 5 or 6, in a randomized pattern). In each sector the target moved at one of the following velocities (randomized): 8, 16, 24, 32 or 40 deg s−1. Successive sectors were separated by one blank cycle, in which the target was not visible. Head and eye displacement signals were sampled at a frequency of 100 Hz. Two conditions were carried out using this target configuration. In the first condition (TOG), subjects pursued the target under head-free conditions with eyes and head following the motion of the target. In the second condition (OPP), subjects were instructed to move their head at the same frequency as the target but in the opposite direction to its motion, whilst pursuing the target motion with their gaze. Subjects were given one practice run with each protocol.
Experiment 3
Visual targets were constantly illuminated and moved sinusoidally with a peak velocity of 40 deg s−1. Two conditions were examined:
(a) 2VIS. In this condition, targets 1 and 2 were illuminated simultaneously. They moved at different, harmonically unrelated frequencies (0.4 and 0.65 Hz). Subjects were instructed to pursue target 2 initially with both their head and gaze, but after two cycles to transfer their gaze to target 1 whilst maintaining head movements appropriate to target 2. The frequency of target 2 was reinforced using the modulated audio timing signal. Each subject performed the 2VIS protocol twice, once with target 1 moving at 0.4 Hz and target 2 at 0.65 Hz (target configuration 1), and once with the frequencies reversed (target 1 at 0.65 Hz and target 2 at 0.4 Hz - target configuration 2).
(b) 1VIS. This condition was similar to the 2VIS condition, except that only one visual target (target 1) was presented. Head movement frequency was controlled by the audio timing signal, which was simultaneously presented with target 1 and was modulated at a harmonically unrelated frequency. Subjects were instructed to concentrate initially on matching the frequency of their head movements with the frequency at which the audio signal was being modulated and after two cycles to transfer their gaze to target 1 whilst maintaining head movements appropriate to the audio signal. Note that the audio signal did not provide any cues about desired head displacement, it just provided frequency information. As for the 2VIS condition, the1VIS protocol was carried out twice, once with target configuration 1 and once with target configuration 2.
In both 1VIS and 2VIS conditions, subjects were not given specific instructions regarding the amplitude of head displacement. However, they all tended to match the amplitude of head movement to the amplitude of target movement. Subjects were given one practice run for each condition. Head and eye displacement signals were sampled at a frequency of 125 Hz.
Data analysis
Eye and head displacement signals were summated to obtain gaze displacement, from which gaze velocity was obtained by digital differentiation. The fast phase components were then removed using an interactive graphics procedure (Barnes, 1982). In the following description of results, we use the term gaze velocity to describe the velocity of the remaining slow phase components of the response.
In experiments 1 and 3, the relationship between head and gaze signals and the target signal was assessed by performing multiple regression analyses. Specifically, head displacement and gaze displacement gain and phase were derived using head displacement or gaze displacement as the dependent variables and target velocity and target displacement as the independent variables (Barnes, 1982). Gaze velocity gain and phase were similarly calculated, using gaze velocity (i.e. with saccades removed) as the dependent variable. A comparable measure for head velocity gain and phase was not derived because of the inability to identify clearly saccade-like components in the head velocity trace. For each subject, these gain and phase measures were calculated using data from the entire response and also for each individual cycle of the response. This latter analysis allowed the standard deviation (across cycles) to be calculated. The standard deviation of gaze and head gains gave an indication of the cycle-by-cycle variability in the magnitude of the tracking response. The standard deviation of the phase values gave an indication of the cycle-by-cycle variability in the timing of the head and gaze tracking responses.
For experiment 2, the build-up of anticipatory activity in both TOG and OPP conditions was assessed by examining head and gaze velocity profiles for the first four presentations of the target at each target velocity and comparing them with the steady-state head or gaze velocity profile at that target velocity. The steady-state response was calculated by averaging the velocity profiles obtained in response to the remaining presentations, i.e. the 5th presentation onwards. This represented an average of either four or six responses, depending on the number of cycles presented at each target velocity.
For experiment 3, a Fast Fourier Transform (FFT) analysis was performed on the last 16 s of the head and gaze velocity traces recorded in 1VIS and 2VIS conditions for both target frequency configurations. In order to assess whether any head or gaze response made at the inappropriate target frequency was significant, multiple regression analyses were performed using head velocity or gaze velocity as the dependent variables and target velocity and target displacement as the independent variables. This was carried out using each individual subject's data for the 1VIS and 2VIS conditions at target configurations 1 and 2. In order to determine whether head and gaze responses at their respective target frequencies were predictive, the time delay associated with the overall phase angle was calculated by dividing the mean displacement phase (across cycles) by 360 and multiplying by the period of the appropriate stimulus.
Statistical analysis
All statistical analyses were performed using the SPSS software package. All variables were tested for normality using the Shapiro-Wilk statistic. For the analyses of variance, each factor was tested for sphericity using the Mauchly test. If the assumption of sphericity was violated, a Greenhouse-Geisser correction was applied.
For experiment 1, multiple regression coefficients for head and gaze were compared using the non-parametric Wilcoxon signed rank test. At each target frequency, head displacement phase and gaze displacement phase (calculated from each subject's entire response) were compared by performing Student's paired t tests (5 in total - 1 for each frequency). Standard deviation (across cycles for each subject) of gaze and head displacement gain and gaze and head displacement phase were compared at each frequency using paired t tests (5 in total).
For experiment 2, a two-way repeated-measures analysis of variance (ANOVA) (target velocity (8, 16, 24, 32, 40 deg s−1) × presentation (1st, 2nd, 3rd, 4th, steady state)) was carried out for head and gaze velocity measured 100 ms after target onset (V100), in both TOG and OPP conditions (four separate ANOVAs in total). Differences between target velocities and between presentations were assessed using a priori contrasts. To examine the effect of target velocity on V100 specifically for the steady-state response, four further one-way repeated-measures ANOVAs (target velocity (8, 16, 24, 32, 40 deg s−1)) were carried out and differences between target velocities were assessed using a priori contrasts.
For experiment 3, the gain of the response was compared using a 4-way repeated-measures ANOVA (condition (1VIS, 2VIS) × target configuration (1, 2) × frequency (0.4, 0.65) × gain (head displacement, gaze displacement, gaze velocity)). Specific differences in gain at each frequency and for each target configuration were assessed in a series of post hoc one-way ANOVAs. Displacement phase, for the head and gaze signals, respectively, was compared for the 0.65 Hz frequency component of experiment 3 (the mean taken across 1VIS and 2VIS conditions) and the 0.66 Hz stimulus of experiment 1 by performing a paired t test. A paired t test was also performed on the standard deviation (across cycles) of phase, for head and gaze, respectively.
RESULTS
Experiment 1
Head, eye and gaze trajectories in response to different target frequencies
A typical response to the sinusoidal movement of target 1 is shown in Fig. 1. At the lowest frequency (0.16 Hz, Fig. 1A) this subject produced hypometric head movements. This meant that the visuomotor mechanisms had not only to suppress the VOR (which would have driven the eyes with a velocity close to that of the head but in the opposite direction), but also had to generate a pursuit movement of the eyes in order to maintain gaze on target. This pursuit activity is shown by the eye displacement signal in Fig. 1A, which is of similar amplitude to the head movement, but lags well behind it. The precise matching of gaze velocity to target velocity (Fig. 1A) demonstrates how effective the oculomotor system was at maintaining gaze on target at this frequency, despite the mismatch, in both amplitude and phase, between head and eye displacement. Figure 1B shows that at the higher target frequency (1.25 Hz) this subject's head movements were no longer hypometric and matched target displacement more precisely (although this was not the case for all subjects - see Fig. 2A). Suppression of the VOR was not totally effective at this relatively high frequency; hence the eye displacement trace exhibits the characteristics of vestibular nystagmus, with slow-phase eye velocity acting in the opposite direction to head displacement. But, the peak velocity of the eye was considerably less than that of the head, indicating that substantial, although not total, suppression of the VOR had been achieved. This is also reflected in the velocity traces (Fig. 1B), which show that although head velocity closely matched target velocity, the gaze velocity signal was slightly hypometric.
Figure 1. Single subject's response to a target moving sinusoidally (experiment 1).
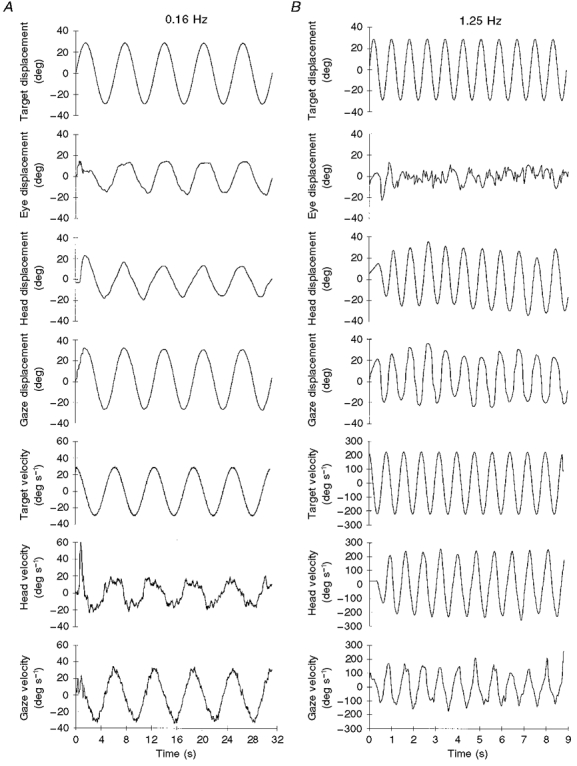
Eye, head and gaze displacement and head and gaze velocity, in response to a target moving at 0.16 Hz (A) or 1.25 Hz (B).
Figure 2. Head and gaze responses in experiment 1.
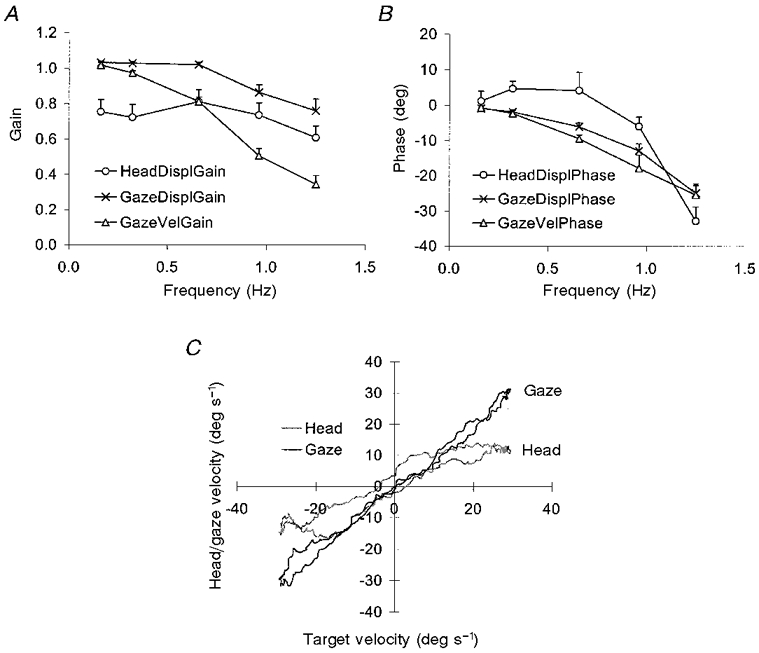
Gain (A) and phase (B) of head displacement, gaze displacement and gaze velocity at each target frequency, mean of 9 subjects, +1 s.e.m.C, head and gaze velocity plotted against target velocity for a single subject's response to a target moving at 0.16 Hz.
Overall, the averaged head and gaze trajectories were very closely associated and varied similarly with frequency. This is illustrated in Fig. 2A, which shows the effect of target frequency on the gain of head displacement, gaze displacement (which includes both fast and slow phase components of the response) and gaze velocity (which refers just to the slow phase component of the response). The data represent the mean of all nine subjects. Head displacement and gaze displacement gains were similar, in that they did not decline a great deal with frequency. However, the ratio of head to gaze gain increased from 0.7 at a target frequency of 0.16 Hz to 0.8 at a target frequency of 1.25 Hz. Gaze velocity gain decreased more sharply with increasing target frequency. This reflects the fact that, unlike the head and gaze displacement traces, saccadic components had been removed from the gaze velocity trace. The overall pattern of phase errors (Fig. 2B) also exhibited a similar trend for head and gaze signals, with phase lag generally increasing with increasing target frequency. However, head displacement phase differed significantly from gaze displacement phase at 0.32 Hz (P = 0.02) and 0.66 Hz (P = 0.002) and almost reached significance at 0.96 Hz (P = 0.051), thus illustrating the temporal dissociation between head and gaze movements at these frequencies. In fact, head displacement exhibited a net phase advance at the lowest frequencies (0.16, 0.32 and 0.66 Hz), suggesting some prediction in this component.
Despite the overall similarities between gaze and head trajectories, from moment to moment the signals could be very different. This is illustrated in Fig. 3, which shows the variability, across cycles, in head and gaze displacement gain (Fig. 3A) and head and gaze displacement phase (Fig. 3B). Variability in gain, which reflects cycle-by-cycle variability in the magnitude of the tracking response, was greater (P < 0.014) for the head response than for the gaze response at the three lowest target frequencies. Variability in phase, which reflects cycle-by-cycle variability in the timing of the tracking response, was greater (P < 0.007) for the head response than for the gaze response at all five target frequencies. In addition to this greater variability, there was also evidence that the head response was less linear than the gaze response. This is illustrated in Fig. 2C, which shows an example of one subject's response to the target moving at 0.16 Hz. Head and gaze trajectories (plotted against target velocity) are similar, but the gaze velocity signal appears to be slightly more linear than the head velocity signal, which appears to show a saturation effect. This was typical of all subjects, as revealed by a multiple regression analysis of either head or gaze velocity against both target velocity and target displacement (in order to take into account phase errors, which are evident in Fig. 2C). For the data shown in Fig. 2C, this analysis yielded multiple correlation coefficients of 0.992 for the gaze signal and 0.933 for the head signal. Comparison of the data from all subjects showed that the correlation coefficients for gaze were significantly greater (P = 0.008) than those for the head.
Figure 3. Cycle-by-cycle analysis - experiment 1.
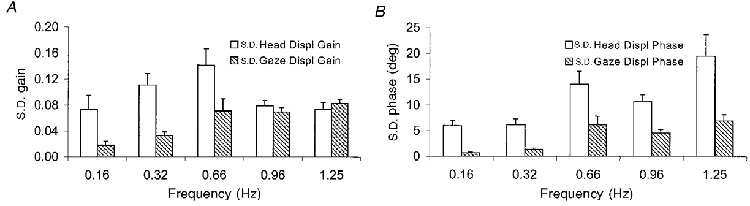
Standard deviation across cycles (between 4 and 9, depending on frequency) for gain (A) and phase (B), for head and gaze displacement at each target frequency, mean of 9 subjects, +1 s.e.m.
Taking all these measures into account, it is evident that although there are global similarities in head and gaze control, there are also marked differences in the magnitude (gain), timing (phase) and moment-to-moment trajectories of head and gaze movement. It should be noted that peak target amplitude was kept constant in this protocol to facilitate the natural generation of head movements across the frequency range.
Experiment 2
Head, eye and gaze trajectories
In response to intermittent target presentation, subjects were able to maintain their gaze on the target regardless of whether the head was moved with the eyes (TOG condition) or in the opposite direction (OPP condition). The results for the TOG condition are very similar to those obtained previously by Barnes & Grealy (1992) using a very similar protocol. A typical response for a single subject is shown in Fig. 4. Figure 4A shows eye, head and gaze responses for the TOG condition when the target moved at 32 deg s−1. Overall, gaze and head displacement were smaller than target displacement, but this was not unexpected since the target was only visible for 240 ms as it passed through the centre of its trajectory. The eye displacement trace was confined within a few degrees of centre and consisted of both smooth and saccadic components. Overall, VOR suppression was very effective, but in places (especially when the head moved to the right in Fig. 4A) there was evidence of residual VOR, with smooth eye movements occurring in the opposite direction to head movements. However, this accounted for only a small fraction of the trace, indicating the overall effectiveness of VOR suppression. This is also reflected by the close correspondence between gaze and head velocity trajectories.
Figure 4. Response of a single subject to a constant velocity, intermittently illuminated target moving at 32 deg s−1 (experiment 2).
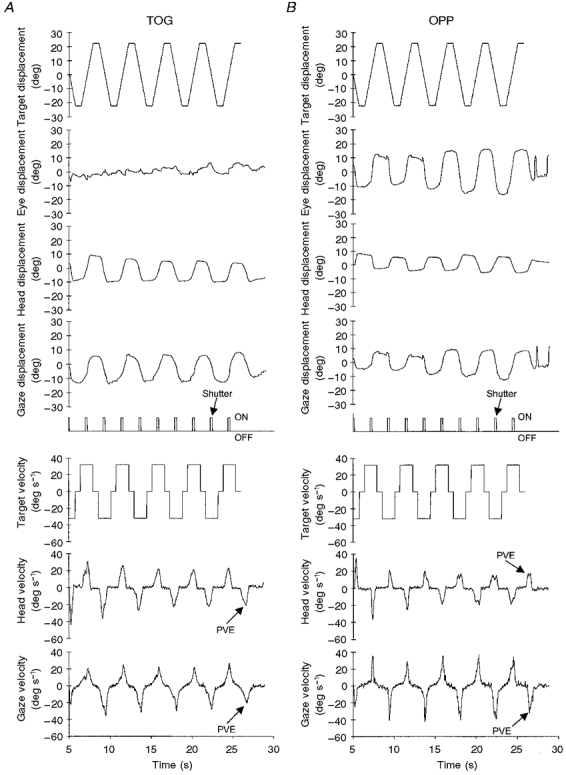
A, eye, head and gaze displacement and head and gaze velocity in the TOG condition - both gaze and head followed the motion of the target. B, eye, head and gaze displacement and head and gaze velocity in the OPP condition - gaze followed the target, head moved with the same frequency in the opposite direction. In both conditions the target was visible only whilst the shutter signal was ON - i.e. for 240 ms as it passed through centre. PVE = predictive velocity estimate that occurs when the target fails to appear when expected.
The response of the same subject to the OPP condition is shown in Fig. 4B. This subject was clearly able to produce head and gaze movements in opposite directions whilst maintaining gaze on target during its brief period of illumination. This task does not require that the VOR be suppressed, rather it requires that it be supplemented by an additional smooth eye movement component. This additional eye movement component can be clearly seen in Fig. 4B. The effective separation of the gaze and head responses is also demonstrated by their opposing velocity traces.
The graphs in Fig. 4 also show that when the target failed to appear at the end of the sequence, the subject, unaware that the target would not be appearing, continued to make a predictive, but inappropriate, head movement. Without modification of the VOR, gaze velocity would be close to zero at this time, but in both TOG and OPP conditions this was not the case. In the TOG condition the close similarity of head and gaze trajectories (labelled PVE in Fig. 4A) indicates that the VOR was being predictively suppressed. By contrast, in the OPP condition, head and gaze were driven in opposing directions (labelled PVE in Fig. 4B), indicating that a predictive smooth eye movement drive was effectively supplementing the action of the VOR.
Changes in response with repeated stimulation
In both the TOG and OPP conditions the timing of the subjects' responses at each target velocity changed with repeated exposure to the target's motion. Typically, head and gaze responses to the first target presentation were not initiated until after the target became visible (i.e. after the shutter had opened). Latencies for these first responses were comparable to those obtained previously (Barnes & Grealy, 1992) and will not be discussed here. With repeated presentation of the target, head and smooth gaze movements occurred before target onset (i.e. they were anticipatory). This anticipatory activity tended to build up over the first few presentations, before reaching a steady-state level. This build-up in anticipatory velocity is shown in Fig. 5. Figure 5A and B shows the gaze and head velocity profiles for the first four presentations and the steady-state response for the TOG condition at a target velocity of 32 deg s−1. For both head and gaze trajectories, the response to the first presentation was not initiated until well after the shutter had opened. For the second presentation, some anticipatory velocity had built up, allowing the response to be initiated earlier, but this anticipatory activity was still lower than that of the steady-state response. By the third presentation anticipatory head and gaze activity had reached the steady-state level, and both responses were initiated well in advance of the opening of the shutter. For the OPP condition, the velocity build-up was similar (Fig. 5C and D), except that gaze velocity did not reach its steady-state level until the fourth presentation of the target.
Figure 5. Build-up in anticipatory head and gaze activity with repeated presentation of an intermittently illuminated target moving at 32 deg s−1 (experiment 2).
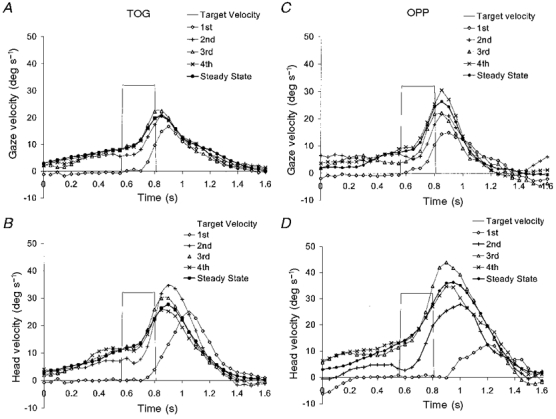
Mean of 9 subjects in both TOG (A and B) and OPP (C and D) conditions. The response to the 1st, 2nd, 3rd and 4th presentation of the target is plotted along with the steady-state response (the mean of responses to presentation 5 onwards). A non-zero target velocity signal denotes the time for which the target was visible.
This build-up in anticipatory activity can be quantified by looking at the velocity of the response 100 ms after target onset (V100). We have shown previously (Ohashi & Barnes, 1996) that V100 provides a robust measure of anticipatory activity because it represents an internally generated response produced prior to the onset of visual feedback (Carl & Gellman, 1987). For all target velocities, V100 for head and gaze velocity for the first presentation of the target was negligible in both TOG and OPP conditions (Fig. 6) and was significantly lower than the steady-state value (P < 0.005). For head velocity in the TOG condition, and both head and gaze velocity in the OPP condition, V100 in response to the 2nd presentation of the target was also less than V100 in the steady-state condition (P < 0.03). For all conditions, there was no difference between V100 in response to either the 3rd or 4th presentation compared with the steady-state response. This supports the conclusion that steady-state V100 values were attained in all conditions within three presentations of the target.
Figure 6. Head and gaze velocity 100 ms after target onset (V100) - experiment 2.
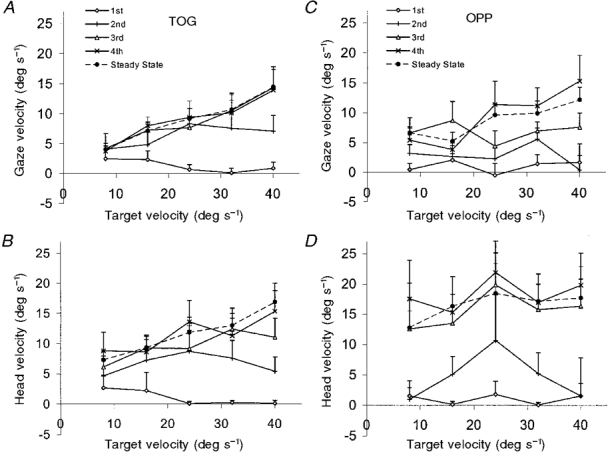
Mean of 9 subjects +1 s.e.m. in both TOG (A and B) and OPP (C and D) conditions. For each target velocity, V100 for the 1st, 2nd, 3rd, 4th and steady-state responses is shown.
It is apparent from the graphs in Fig. 6 that the general trend was for V100 to increase with increasing target velocity. For the steady-state response this increase in V100 was significant for head (P < 0.005) and gaze (P < 0.01) velocity in the TOG condition and for gaze (P < 0.01) velocity in the OPP condition. This scaling of anticipatory activity to target velocity is also shown in Fig. 7, which shows the steady-state head and gaze velocity profiles in both TOG and OPP conditions at all target velocities. Both head and gaze velocity profiles for the TOG condition (Fig. 7A) and the gaze velocity profile for the OPP condition (upper 5 traces in Fig. 7B) clearly show this velocity scaling in the period between the onset of the target (the opening of the shutter) and the time at which visual feedback becomes effective. For head velocity in the OPP condition (lower 5 traces in Fig. 7B) this scaling is not present for V100, although, interestingly, it was present for peak head velocity, despite the fact that the target was moving in the opposite direction to the head.
Figure 7. Steady-state gaze and head velocity at each target velocity (experiment 2).
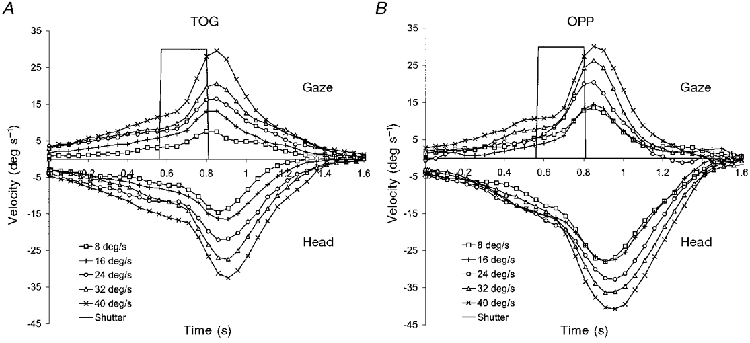
Mean of 9 subjects in TOG (A) and OPP (B) conditions. For the sake of clarity, head velocities are displayed as negative values for both conditions, whereas in the TOG condition they were actually positive. The time for which the target was visible is denoted by a non-zero shutter signal.
Experiment 3
Head and gaze trajectories
All nine subjects were able to produce simultaneous head and gaze movements at two independent frequencies. They were able to follow both frequency combinations - i.e. either gaze at 0.4 Hz and head at 0.65 Hz or gaze at 0.65 Hz and head at 0.4 Hz. The example response in Fig. 8 shows gaze and head displacement and velocity for a single subject carrying out the 2VIS condition with her gaze tracking target 1 at 0.65 Hz and her head tracking target 2 at 0.4 Hz. The displacement traces (Fig. 8A and C) show that head and gaze movements were maintained at an amplitude and frequency that were appropriate to the target that they were following. This is confirmed in the velocity traces (Fig. 8B and 8D) by the close correspondence of gaze velocity to the velocity of target 1, and head velocity to the velocity of target 2. However, as shown later by the results from the FFT and gain analyses, although all subjects were able to match the frequency of head movement to the frequency of target 2, this subject was unusual in her ability to match head displacement to that of target 2. Note that for the first two cycles of the response gaze movements were out of phase with target 1. This represents the period during which the subject was just concentrating on target 2, before shifting her gaze to target 1.
Figure 8. Response of a single subject carrying out the 2VIS condition with her gaze tracking target 1 moving at 0.65 Hz and her head moving at the frequency of target 2 (0.4 Hz) - experiment 3.
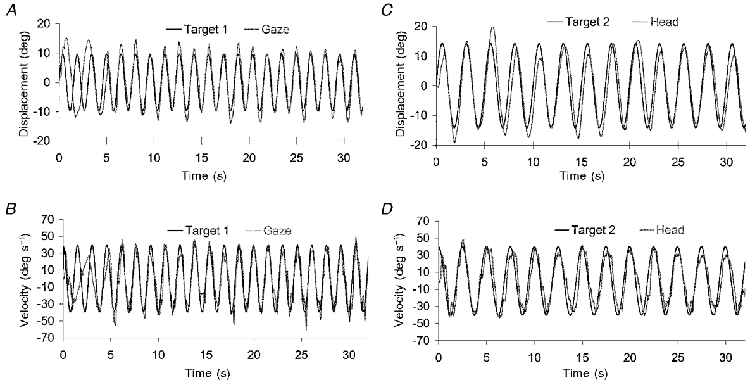
A, gaze displacement; B, gaze velocity; C, head displacement; D, head velocity. Note that the displacement traces for head and gaze are more variable than their respective velocity traces. For the head signal, variability in the displacement trace is due to the occasional shifting of the trace with respect to zero, which does not result in variability in the velocity trace. For the gaze signal, however, differences between the displacement and velocity traces demonstrate the effect of removing the fast phase (saccadic) components of the response prior to calculating gaze velocity.
Accuracy of gaze and head movement frequency
The accuracy with which head and gaze movements followed their respective target frequencies was revealed by the FFT analysis of the velocity signals, the results of which are shown (mean of 9 subjects) for the 1VIS condition in Fig. 9A and B and for the 2VIS condition in Fig. 9C and D. The results for the two conditions were almost identical. When gaze followed target 1 moving at 0.4 Hz, the velocity response was well matched to the target velocity signal in both frequency and amplitude, although there also appeared to be a very small response around the frequency of target 2 (0.65 Hz). The velocity profile for the head response was also well matched to target frequency, but was hypermetric. When gaze followed target 1 moving at 0.65 Hz, the response was well matched in amplitude and frequency, but again there was a small amount of activity at the frequency of target 2 (0.4 Hz). The head simultaneously following target 2 was hypometric.
Figure 9. Root mean square head and smooth component gaze velocity derived by FFT analysis (experiment 3).
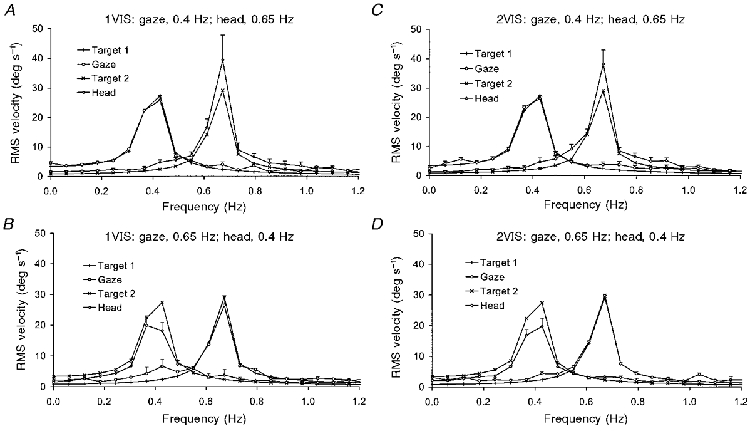
Mean of 9 subjects +1 s.e.m.A, 1VIS condition, gaze follows target 1 at 0.4 Hz, head follows target 2 at 0.65 Hz; B, 1VIS condition, gaze follows target 1 at 0.65 Hz, head follows target 2 at 0.4 Hz. C and D show the response to the 2VIS condition, with the same target frequency configurations as A and B.
This pattern of results is confirmed by comparison of the gain of head and gaze signals at each target frequency, derived from the regression analysis. The four-way ANOVA showed that the main effect of condition (1VIS versus 2VIS) was not significant (P = 0.611) and all interactions between condition and the other three factors were also non-significant. This confirmed the impression from the FFT analysis that there was no difference between 1VIS and 2VIS target conditions. The data were therefore collapsed across target condition and a second repeated-measures ANOVA was performed (target configuration (1, 2) × frequency (0.4, 0.65) × gain (head displacement, gaze displacement, gaze velocity)). This yielded a significant three-way interaction between target configuration, frequency and gain, so the data were not collapsed further. Instead, four one-way ANOVAs were performed and a priori contrasts were used to compare the gain of head displacement, gaze displacement and gaze velocity at each frequency for each target configuration.
The gain of head and gaze signals for each target frequency configuration is shown, in Fig. 10, averaged across target conditions (1VIS and2VIS). These graphs demonstrate, again, the degree to which the frequency of gaze and head movements were separated. For both target configurations, at each frequency there was always a highly significant difference (P < 0.0005) between head displacement gain and gaze displacement gain. Both graphs in Fig. 10 show relatively high head or gaze gain (between 0.7 and 1.3) at the appropriate frequency (i.e. at the frequency that was being followed by head or gaze) and relatively low gain (less than 0.15) at the frequency that was not being intentionally followed. For example, with gaze following the 0.4 Hz signal and head following the 0.65 Hz signal (Fig. 10A), gaze displacement gain (mean of 9 subjects and mean of 1VIS and 2VIS conditions) was 0.95 at 0.4 Hz and 0.13 at 0.65 Hz, whereas head displacement gain was 0.09 at 0.4 Hz and 1.24 at 0.65 Hz. This confirms the observation from the FFT analysis that although most of the head or gaze activity was occurring at its respective target frequency, there was a small amount of activity occurring at the inappropriate target frequency. The multiple regression analyses performed on the head and gaze velocity signals (for each subject for the 1VIS and 2VIS conditions at target configurations 1 and 2) showed that although the regression coefficients at the inappropriate frequency were very small (between 0.014 and 0.226) the majority (78 %) of the analyses were significant (P < 0.05). Figure 10 also demonstrates the hypermetria of head displacement when following the target moving at 0.65 Hz (Fig. 10A), and its hypometria when following the target moving at 0.4 Hz (Fig. 10B).
Figure 10. Gain of gaze and head responses (experiment 3).
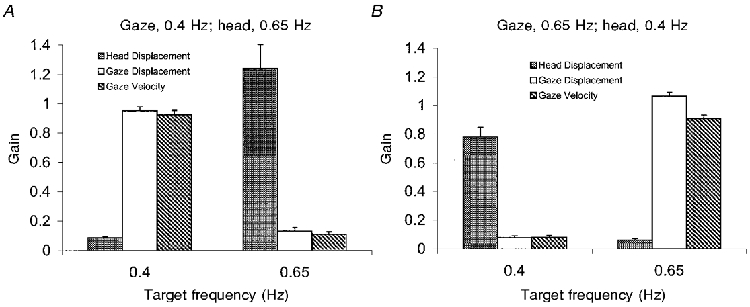
Averaged across 1VIS and 2VIS conditions, mean of 9 subjects +1 s.e.m.A, gaze follows target 1 at 0.4 Hz, head follows target 2 at 0.65 Hz. B, gaze follows target 1 at 0.65 Hz, head follows target 2 at 0.4 Hz.
The pattern of hyper- and hypometric head movements, both in terms of velocity (as revealed by the FFT analysis) and displacement (as revealed by the gain analysis) suggests that subjects were tending to match their head movement amplitude, although not frequency, to target 1 (the gaze target) rather than to target 2 (the head target). This was made more apparent by the results of an FFT analysis performed using gaze, head and target displacement (rather than velocity) signals. Targets 1 and 2 had the same velocity, but they differed in their frequency and hence in their displacement, with the 0.4 Hz target having a peak displacement 1.625 times greater than the 0.65 Hz target. When the head was moving at a frequency of 0.65 Hz, it had an amplitude approaching that of the 0.4 Hz stimulus and was therefore hypermetric (both in terms of displacement and velocity) with respect to the 0.65 Hz target. The reverse situation, with the head moving at 0.4 Hz, produced hypometric movements (Figs 9 and 10).
Gaze displacement gain - which is the sum of eye and head displacement signals prior to the removal of the fast phase components - is also shown in Fig. 10. When gaze was following the lower frequency target (Fig. 10A) there was no difference (P = 0.103) between gaze displacement gain and gaze velocity gain at this target frequency, indicating that most of the response was made up of the smooth velocity component. When gaze followed the higher frequency (Fig. 10B) gaze displacement gain was significantly (P < 0.0005) greater than gaze velocity gain at that target frequency, demonstrating that at this higher frequency more corrective saccadic responses were required to keep gaze on target.
Predictive head and gaze activity
In order to determine whether head and gaze responses at their respective target frequencies were predictive, the time delay associated with the overall phase angle was calculated. This yielded values (mean of 9 subjects, averaged across 1VIS and 2VIS conditions) of -1.4 ms for gaze following the 0.4 Hz target, -2.0 ms for gaze following the 0.65 Hz target, 17.7 ms for the head following the 0.4 Hz target and 7.2 ms for the head following the 0.65 Hz target. Although the values for gaze displacement represent a phase lag, the responses can still be regarded as predictive since the delay is considerably less than 100 ms, the approximate time required for visual feedback to become effective (Carl & Gellman, 1987).
Response timing in experiment 3 compared with experiment 1
In order to assess whether the simultaneous production of head and gaze movements at different frequencies impaired temporal performance, two measures (mean displacement phase and standard deviation (across cycles) of displacement phase) were compared for the response to the 0.65 Hz component in experiment 3 and the response to the single 0.66 Hz stimulus in experiment 1. This showed that there was no difference in the mean phase of either the head or gaze displacement signals in the two experiments. Standard deviation of gaze displacement phase also did not differ between the two experiments, but head displacement phase was more variable from cycle to cycle in experiment 3 than in experiment 1 (P = 0.04). In other words, there was greater cycle-by-cycle variability in the timing of the head response, but not the gaze response, when simultaneously making movements at two different frequencies.
DISCUSSION
Dissociation of eye and head movements
These experiments confirm that although under natural conditions head and gaze movements are usually closely associated, they can be controlled independently and can, under certain circumstances, become dissociated and even completely uncoupled. This result contrasts with the commonly held view that there is always a tight coupling between head and gaze. Such a coupling may often be observed in co-ordinated gaze shifts (Bizzi et al. 1971; Guitton & Munoz, 1991), but even under these circumstances there is often a temporal disparity in head and eye control depending on the stimulus conditions (Barnes, 1979; Ron & Berthoz, 1991). Of course, during a single transient gaze shift, dissociation might not be expected because responses tend to be reactive rather than pre-programmed, so there is little opportunity to plan independent head and eye movements in advance as there is in the experiments described here. However, even during continuous head-free pursuit, previous evidence has pointed towards head and gaze being controlled by similar mechanisms. For example, in an earlier study (Barnes & Lawson, 1989) it was shown that both head and gaze exhibit similar non-linear changes in gain and phase when pursuing pseudo-random target motion stimuli composed of mixed sinusoids. These changes were dependent on the frequency composition of the stimulus. However, similarity of frequency response need not imply that control is achieved by a high level of moment-to-moment correlation between head and gaze. This is confirmed by the results of experiment 1. Overall, head and gaze responses were highly correlated, as shown by the similar pattern of gain and phase for gaze and head. However, this correlation was not achieved through the slavish coupling of the two responses on a moment-to-moment basis. This is shown by the cycle-by-cycle analysis of the head and gaze gains and phases, which showed greater variability in the head response than in the gaze response. The multiple regression analysis also revealed small, but significant, differences between the head and gaze regression coefficients, which reflected the greater moment-to-moment variability in the head movement trace. This supports the observation by Lanman et al. (1978) that during head-free pursuit the head trajectory contains many irregularities which are compensated for by the eye movement response in order to keep gaze on target.
The results from experiments 2 and 3 demonstrate how independent control can be accomplished under certain circumstances through the use of temporarily stored information. In experiment 2 this was made evident by the fact that subjects could initiate movements of the head and gaze in opposing directions prior to the appearance of the moving target. These movements were therefore not controlled by any visual feedback component, at least for the first 100 ms after target onset. Note that the eye had to be driven in the opposite direction to the head at a velocity, relative to the head, that was much higher than head velocity itself to achieve this effect. Most importantly, these anticipatory movements were generally scaled according to target velocity, showing that they are truly predictive as well as anticipatory. Therefore, they must have been derived from previously stored information that was played out in anticipation of the target motion stimulus. The exception to this was that anticipatory head movement was not scaled in the OPP condition, although peak head velocity was scaled. This latter finding is interesting because in the OPP condition subjects were merely instructed to make head movements in the opposite direction to target movement, and were given no explicit instructions regarding head movement amplitude. Despite this, they appear to have naturally produced head movements of a greater amplitude (and hence velocity) for higher velocity target movements. This could be taken to imply that common velocity drive information is being used to control head and gaze but that the direction in which the drive is applied can be independently controlled.
The use of stored information is also evident in experiment 3. Here the strategy was for the subject to follow the first two cycles of one stimulus with head and eyes and then to maintain the head movement component of that response whilst transferring gaze to the other target. The results demonstrate the remarkable ability of the subjects to memorize the timing of the drive to the neck muscles that is required to maintain the frequency of that head movement. What we cannot be sure about is the degree to which subjects were able to, or needed to, monitor the timing of the head target whilst simultaneously attending to the gaze target. It is clear, however, that subjects were able to produce predictive head and gaze responses under these circumstances.
Although most subjects found it relatively easy to respond at two different frequencies, the resultant subjective perception was that the target that was being pursued (with gaze) was moving in a pseudo-random manner rather than in a sinusoidal one. The resultant gaze signal was, however, sinusoidal and largely confined to a single frequency (Fig. 8), confirming a previous observation (Worfolk & Barnes, 1992) that the subjective perception of eye movement can be very different from the actual eye movement trajectory. The ability to respond at two different frequencies was fairly robust and was achievable in both 1VIS and 2VIS conditions. This shows that subjects did not rely on having a visual target with which to match the frequency of their head movements. The remarkable similarity in the response to the 1VIS and 2VIS conditions probably reflects the fact that subjects tended to use target 1 (the gaze target) to set the amplitude of their head movements, although they clearly matched head movement frequency with the frequency of target 2.
The role of stored information in time-shared activities
The idea that subjects are able quickly to learn and reproduce head movements of a prescribed frequency and amplitude is almost taken for granted and has been used in many experiments as a means of reproducing a pattern of head or hand movement (O'Leary & Davis, 1992; Vercher et al. 1996). Recently, Miall et al. 1995 showed that the fidelity of this store of information for hand movements tends to deteriorate over a period of a few seconds. We have previously provided strong evidence that anticipatory smooth eye movements are also generated from internally stored information. In early experiments (Barnes & Asselman, 1991) we established that there was a gradual build-up of anticipatory smooth eye movement with repeated stimulation. A similar build-up of both head and gaze movements was observed by Barnes & Grealy (1992) and these results are now confirmed by the results from experiment 2 (Fig. 5). This is suggestive of the build-up of an internal store of information. The burst of predictive gaze velocity (PVE in Fig. 4) that occurs, in both TOG and OPP conditions, when the target unexpectedly disappears in experiment 2 is a manifestation of the release of this stored information. We have also demonstrated that subjects can continue to replicate amplitude and frequency of eye movement in the same way as they can for the head movement when retinal error is eliminated by stabilizing the image of the target on the fovea (Barnes et al. 1995). Recently we have demonstrated that this store of information appears to be charged by afferent visual motion information, not an efference copy of the eye movement itself (Barnes et al. 1997). This last finding implies that the stored information is not necessarily exclusive to eye movement but could be made available to other motor effectors.
Our general hypothesis then is that visual feedback information is continuously sampled and stored in a way that allows it to be accessed by the head or eye or, indeed, the hand (Xia & Barnes, 1997). Once captured in this way it can be played out independently to head or eye and thus achieve the temporal dissociation observed. Such a system would allow the movements to be initiated in a sequential (or time-shared) manner, independent of immediate visual feedback, with only the requirement that visual feedback be monitored to check the validity of the internal estimates. However, because the stored information was originally dependent on a common source of visual feedback, performance will be dependent ultimately on the non-linear predictive characteristics of that feedback, giving the similarity of non-linear frequency characteristics observed experimentally (Barnes et al. 1987; Barnes & Lawson, 1989; Xia & Barnes, 1997).
Implications for theories of VOR suppression
The mechanism of VOR suppression has long been contentious. Although most evidence points to the dominant role of visual feedback (pursuit) in this process, there is also strong evidence of central suppression (Cullen et al. 1991; Barnes, 1993). However, the results presented here cannot easily be explained by the theory that during head-free pursuit an efference copy of the head movement signal is used to cancel the VOR (Robinson, 1982). In experiment 3, simultaneous head and gaze movements were produced at two independent (harmonically unrelated) frequencies. If a copy of the head signal were used to suppress the VOR, even if not completely, this would result in gaze being driven at the wrong frequency for pursuit. So the pursuit system, far from being helped, would have to deal with the suppression of an inappropriate internally created signal as well as carrying out the pursuit task. Actually, the best situation would be for VOR gain to be unity, thus nulling gaze velocity at this frequency. It might be argued that non-visual suppression would not be used in the complex conditions of experiment 3, but this would imply the presence of a specialized switching mechanism for which there appears little evidence.
The results from experiment 2 provide further evidence of the highly reproducible stereotyped predictive behaviour that has been observed previously (Barnes & Asselman, 1991; Kao & Morrow, 1994; Ohashi & Barnes, 1996). The protocol used in experiment 2 was originally devised to isolate the predictive component of the smooth eye movement response during head-fixed pursuit. Our results for the TOG condition confirm the finding of Barnes & Grealy (1992) that during head-free pursuit the head and gaze velocity trajectories were very similar to the smooth eye velocity trajectory seen during head-fixed pursuit. In particular, the gradual build-up of predictive behaviour in the head and gaze signals over the first few presentations of the target resembled very closely the build-up in predictive smooth eye velocity previously reported during head-fixed pursuit (Barnes & Asselman, 1991).
Further evidence of the reproducibility of this predictive response is provided by the results from the OPP condition. In this condition, head and gaze responses were produced at the same frequency, but in opposite directions. As in the TOG condition, both head and gaze signals exhibited a similar build-up in predictive velocity. This indicates just how ubiquitous the predictive response is and provides further evidence for a common mechanism for the build-up and subsequent release of a predictive velocity signal during head-fixed and head-free pursuit of a moving target. The results for the OPP condition also provide a convincing demonstration that head and gaze responses can easily be independently controlled, even though under natural circumstances they are almost always closely coupled. The observation of opposing head and gaze predictive activity when the target is extinguished is particularly strong evidence of this mechanism. In the TOG condition this mechanism generated predictive VOR suppression whereas in the OPP condition it resulted in predictive supplementation of the normal VOR. In either case, an unmodified VOR would have produced negligible gaze velocity at this time. This finding should alert us to the power of predictive mechanisms, which in these circumstances occur a long time after the previous visual stimulus and therefore may easily be misinterpreted.
Timing control
The temporal aspects of carrying out two motor tasks simultaneously have previously been studied by Klapp and his colleagues in a series of studies. These studies compared the simultaneous performance of two timing tasks with the performance on each of the tasks performed separately. For example, Klapp (1979) used a protocol which required subjects to press and release a response key in synchrony with a tone (a metronome). The task was carried out either with each hand separately or with both hands simultaneously (each in synchrony with a different metronome). Klapp found that when responding with both hands simultaneously, there was no interference in temporal performance when the hands were responding in phase or completely out of phase, i.e. when the period was the same for both hands. However, if the period was different for the two hands then temporal performance was impaired compared with the performance with one hand alone. Similar results have been reported (Morasso et al. 1977) during the simultaneous performance of saccades and head movements. Our results for experiment 3 show a very high degree of synchrony of head and gaze signals with their respective target frequencies, which suggests that there was little temporal interference caused by carrying out the two tasks simultaneously. However, there was a small, but significant, component of head and gaze movement produced at the inappropriate target frequency in a large proportion (78 %) of responses. In addition, our comparison of timing variability for the 0.65 Hz component of experiment 3 and the 0.66 Hz stimulus of experiment 1 suggests that timing variability was increased for the head, although not for gaze, when responses were made simultaneously at two different frequencies. How these indications of minor temporal inco-ordination relate to the previous work of Klapp is not clear, but in future experiments we intend to explore these matters in more depth. It is perhaps worth noting that there are differences in the tasks employed in the previous experiments - in which subjects attempted to make discrete motor responses separated by an interval that matched the metronome interval - and in our own, where the task was essentially a tracking one and information about the target movement was continuously available to subjects.
Conclusions
This work demonstrates what is perhaps the most important role of predictive mechanisms in motor control. Their role is not merely to overcome time delays in visuomotor processing, although that is obviously an important function. Rather, their most important role is, arguably, in allowing the time-sharing of motor activities to take place. By allowing stored information to be used in a predictive manner these activities can be initiated with differing timing or in opposing directions. This would not be possible with simple reflexive sensory feedback mechanisms, especially when large time delays are involved in sensory processing. The power of this process is revealed in these experiments by the ability to predictively suppress or supplement the VOR, depending on the particular requirements dictated by the visual motion stimulus.
References
- Barnes GR. Vestibulo-ocular function during co-ordinated head and eye movements to acquire visual targets. The Journal of Physiology. 1979;287:127–147. doi: 10.1113/jphysiol.1979.sp012650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR. A procedure for the analysis of nystagmus and other eye movements. Aviation Space and Environmental Medicine. 1982;53:676–682. [PubMed] [Google Scholar]
- Barnes GR. Visual-vestibular interaction in the control of head and eye movement: the role of visual feedback and predictive mechanisms. Progress in Neurobiology. 1993;41:435–472. doi: 10.1016/0301-0082(93)90026-o. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Asselman PT. The mechanism of prediction in human smooth pursuit eye movements. The Journal of Physiology. 1991;439:439–461. doi: 10.1113/jphysiol.1991.sp018675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Benson AJ, Prior ARJ. Visual-vestibular interaction in the control of eye movement. Aviation Space and Environmental Medicine. 1978;49:557–564. [PubMed] [Google Scholar]
- Barnes GR, Donnelly SF, Eason RD. Predictive velocity estimation in the pursuit reflex response to pseudo-random and step displacement stimuli in man. The Journal of Physiology. 1987;389:111–136. doi: 10.1113/jphysiol.1987.sp016649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Eason RD. Effects of visual and non-visual mechanisms on the vestibulo-ocular reflex during pseudo-random head movements in man. The Journal of Physiology. 1988;395:383–400. doi: 10.1113/jphysiol.1988.sp016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Goodbody SJ, Collins S. Volitional control of anticipatory ocular pursuit responses under stabilized image conditions in humans. Experimental Brain Research. 1995;106:301–317. doi: 10.1007/BF00241126. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Grealy MA. Predictive mechanisms of head-eye coordination and vestibulo-ocular reflex suppression in humans. Journal of Vestibular Research. 1992;2:193–212. [PubMed] [Google Scholar]
- Barnes GR, Grealy MA, Collins S. Volitional control of anticipatory ocular smooth pursuit after viewing, but not pursuing, a moving target: evidence for a re-afferent velocity store. Experimental Brain Research. 1997;116:445–455. doi: 10.1007/pl00005772. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Lawson JF. Head-free pursuit in the human of a visual target moving in a pseudo-random manner. The Journal of Physiology. 1989;410:137–155. doi: 10.1113/jphysiol.1989.sp017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CC, Scultheis LW, Robinson DA. Voluntary, non-visual control of the vestibulo-ocular reflex. Acta Otolaryngologica. 1976;81:365–375. doi: 10.3109/00016487609107490. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Tagliasco V. Eye-head coordination in monkeys: evidence for centrally patterned organization. Science. 1971;173:452–454. doi: 10.1126/science.173.3995.452. [DOI] [PubMed] [Google Scholar]
- Carl JR, Gellman RS. Human smooth pursuit: stimulus-dependent responses. Journal of Neurophysiology. 1987;57:1446–1463. doi: 10.1152/jn.1987.57.5.1446. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Belton T, McCrea RA. A non-visual mechanism for voluntary cancellation of the vestibulo-ocular reflex. Experimental Brain Research. 1991;83:237–252. doi: 10.1007/BF00231150. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Robinson DA. Adaptation of the human vestibuloocular reflex to magnifying lenses. Brain Research. 1975;92:331–335. doi: 10.1016/0006-8993(75)90279-6. 10.1016/0006-8993(75)90279-6. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Berthoz A. The role of the tectoreticulospinal system in the control of head movement. In: Peterson BW, Richmond FJ, editors. Control of Head Movement. Oxford: Oxford University Press; 1988. pp. 224–244. [Google Scholar]
- Gresty M, Leech J. Coordination of the head and eyes in pursuit of predictable and random target motion. Aviation Space and Environmental Medicine. 1977;48:741–744. [PubMed] [Google Scholar]
- Guitton D, Munoz DP. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. 1. Identification, localization and effects of behavior on sensory responses. Journal of Neurophysiology. 1991;66:1605–1623. doi: 10.1152/jn.1991.66.5.1605. [DOI] [PubMed] [Google Scholar]
- Guitton D, Volle M. Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. Journal of Neurophysiology. 1987;58:427–459. doi: 10.1152/jn.1987.58.3.427. [DOI] [PubMed] [Google Scholar]
- Kao GW, Morrow MJ. The relationship of anticipatory smooth eye movement to smooth pursuit initiation. Vision Research. 1994;34:3027–3036. doi: 10.1016/0042-6989(94)90276-3. 10.1016/0042-6989(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Klapp S. Doing two things at once: The role of temporal compatibility. Memory and Cognition. 1979;7:375–381. [Google Scholar]
- Koenig E, Allum J, Dichgans J. Visual-vestibular interaction upon nystagmus slow phase velocity in man. Acta Otolaryngologica. 1978;85:397–410. doi: 10.3109/00016487809121468. [DOI] [PubMed] [Google Scholar]
- Koenig E, Dichgans J, Dengler W. Fixation suppression of the vestibulo-ocular reflex (VOR) during sinusoidal stimulation in humans as related to the performance of the pursuit system. Acta Otolaryngologica. 1986;102:423–431. doi: 10.3109/00016488609119427. [DOI] [PubMed] [Google Scholar]
- Lanman J, Bizzi E, Allum J. The coordination of eye and head movement during smooth pursuit. Brain Research. 1978;153:39–53. doi: 10.1016/0006-8993(78)91127-7. 10.1016/0006-8993(78)91127-7. [DOI] [PubMed] [Google Scholar]
- Lau CGY, Honrubia V, Jenkins HA, Baloh RW, Yee RD. Linear model for visual-vestibular interaction. Aviation Space and Environmental Medicine. 1978;49:880–885. [PubMed] [Google Scholar]
- Lisberger SG. Visual tracking in monkeys: evidence for short-latency suppression of the vestibuloocular reflex. Journal of Neurophysiology. 1990;63:676–688. doi: 10.1152/jn.1990.63.4.676. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Evinger C, Johanson GW, Fuchs AF. Relationship between eye acceleration and retinal image velocity during foveal smooth pursuit in man and monkey. Journal of Neurophysiology. 1981;46:229–249. doi: 10.1152/jn.1981.46.2.229. [DOI] [PubMed] [Google Scholar]
- McKinley PA, Peterson BW. Voluntary modulation of the vestibuloocular reflex in humans and its relation to smooth pursuit. Experimental Brain Research. 1985;60:454–464. doi: 10.1007/BF00236931. [DOI] [PubMed] [Google Scholar]
- Miall RC, Haggard PN, Cole JD. Evidence for a limited visuo-motor memory used in programming wrist movements. Experimental Brain Research. 1995;107:267–280. doi: 10.1007/BF00230047. [DOI] [PubMed] [Google Scholar]
- Morasso P, Sandini G, Tagliasco V, Zaccaria R. Control strategies in the eye-head coordination system. IEE Transactions on Systems, Man, and Cybernetics. 1977;7:639–651. [Google Scholar]
- Ohashi N, Barnes GR. A comparison of predictive and non-predictive ocular pursuit under active and passive stimulation conditions in humans. Journal of Vestibular Research. 1996;6:261–276. 10.1016/0957-4271(96)00022-5. [PubMed] [Google Scholar]
- O'Leary DP, Davis LL. Orienting and stabilizing eye and head movements. In: Berthoz A, Vidal PP, Graf W, editors. The Head-Neck Sensory Motor System. Oxford: Oxford University Press; 1992. pp. 404–407. [Google Scholar]
- Robinson DA. A model of cancellation of the vestibulo-ocular reflex. In: Lennerstrand G, Zee DS, Keller EL, editors. Functional Basis of Ocular Motility Disorders. Oxford: Pergamon Press; 1982. pp. 5–13. [Google Scholar]
- Ron S, Berthoz A. Eye and head coupled and dissociated movements during orientation to a double step visual target displacement. Experimental Brain Research. 1991;85:196–207. doi: 10.1007/BF00230001. [DOI] [PubMed] [Google Scholar]
- Vercher J-L, Gauthier GM, Guedon O, Blouin J, Cole J, Lamarre Y. Self-moved target eye tracking in control and deafferented subjects: Roles of arm motor command and proprioception in arm-eye coordination. Journal of Neurophysiology. 1996;76:1133–1144. doi: 10.1152/jn.1996.76.2.1133. [DOI] [PubMed] [Google Scholar]
- Worfolk R, Barnes GR. Interaction of active and passive slow eye movement systems. Experimental Brain Research. 1992;90:589–598. doi: 10.1007/BF00230943. [DOI] [PubMed] [Google Scholar]
- Xia R, Barnes GR. Amplitude factors affecting oculo-manual tracking responses to pseudo-random target motion stimuli. The Journal of Physiology. 1997;504.P:112–113. P. [Google Scholar]
- Zangemeister WH, Stark L. Gaze latency: variable interactions of head and eye latency. Experimental Neurology. 1982a;75:389–406. doi: 10.1016/0014-4886(82)90169-8. 10.1016/0014-4886(82)90169-8. [DOI] [PubMed] [Google Scholar]
- Zangemeister WH, Stark L. Types of gaze movement: variable interactions of eye and head movements. Experimental Neurology. 1982b;77:563–577. doi: 10.1016/0014-4886(82)90228-x. 10.1016/0014-4886(82)90228-X. [DOI] [PubMed] [Google Scholar]


